Cell-Membrane Biomimetic Indocyanine Green Liposomes for Phototheranostics of Echinococcosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
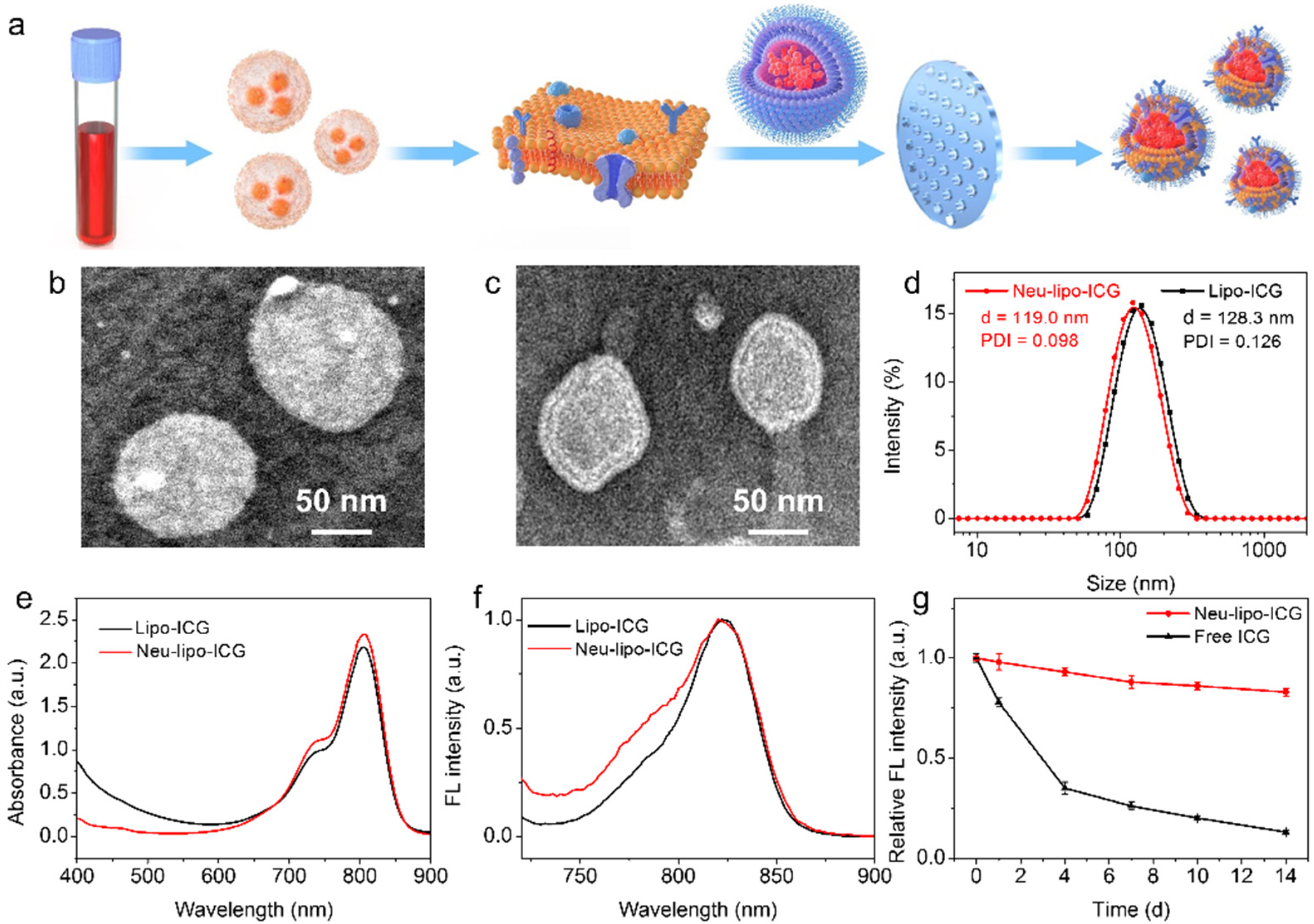
3.1. Synthesis and Characterization of Neu-Lipo-ICG
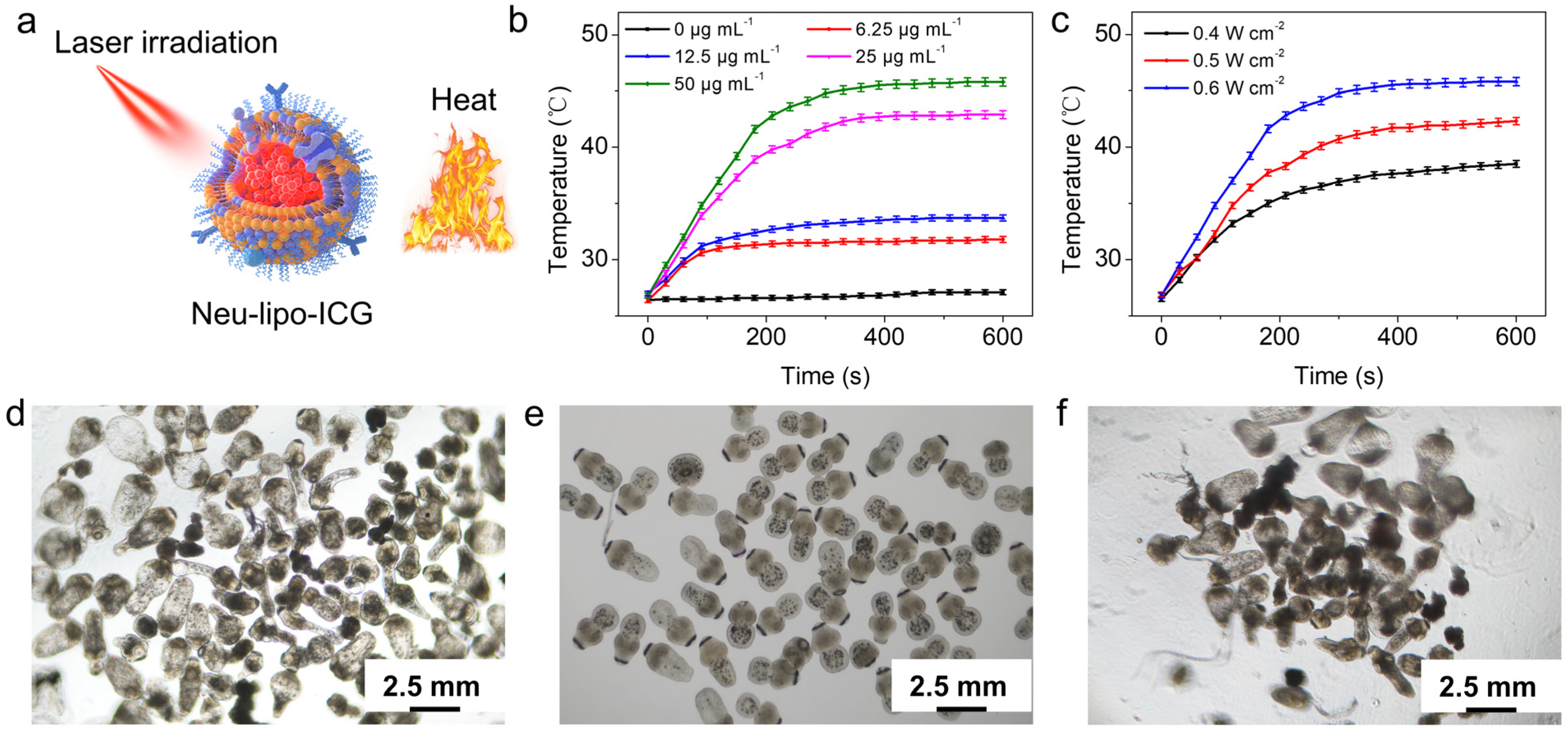
3.2. In-Vitro Photothermal Performance of Neu-Lipo-ICG
3.3. In-Vivo Biocompability of Neu-Lipo-ICG
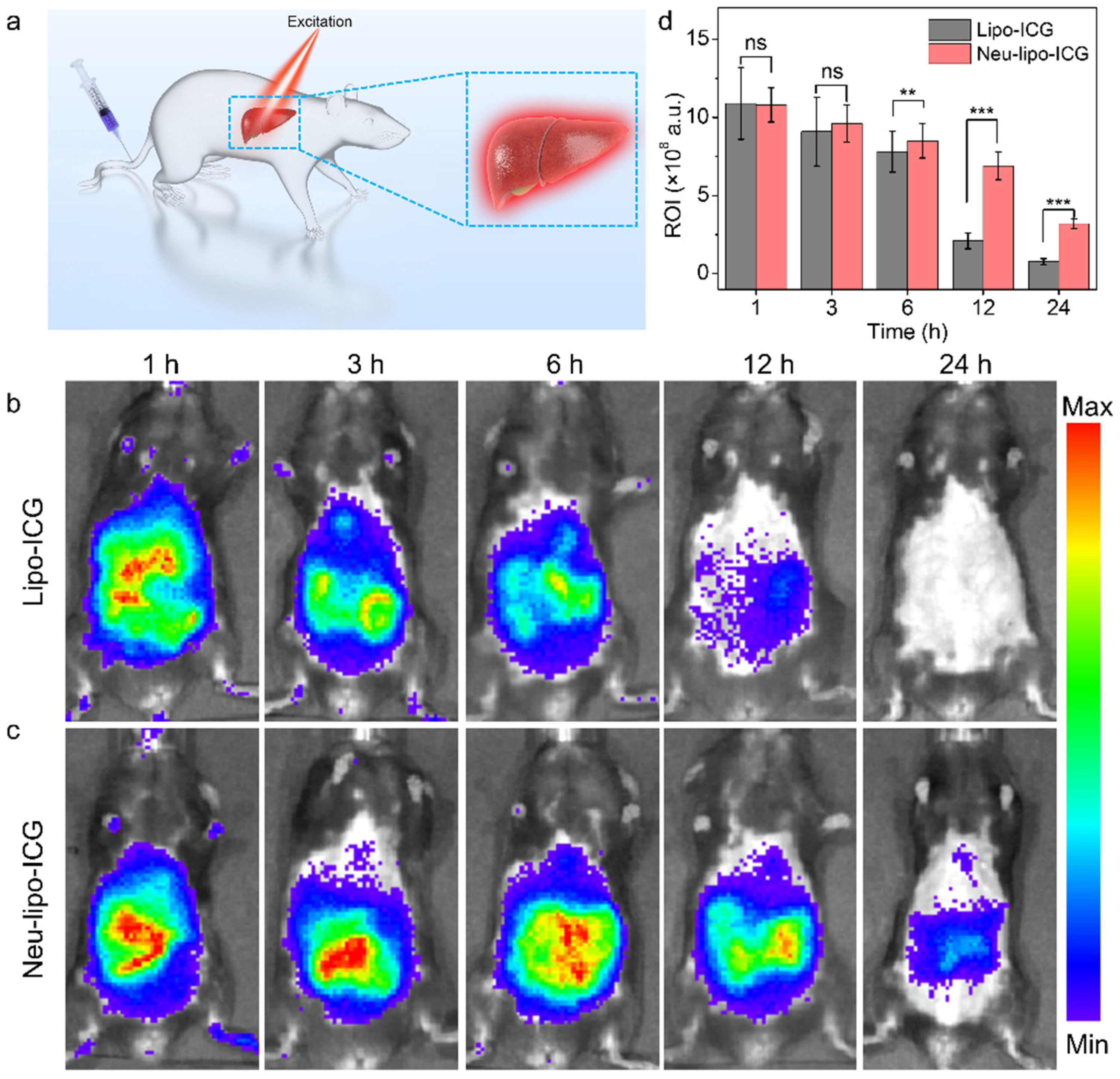
3.4. In-Vivo Near-Infrared Fluorescence Imaging of Neu-Lipo-ICG
3.5. In-Vivo Photothermal Treatment of Neu-Lipo-ICG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, P.S.; McManus, D.P.; Lightowlers, M.W.; Chabalgoity, J.A.; Garcia, H.H.; Gavidia, C.M.; Gilman, R.H.; Gonzalez, A.E.; Lorca, M.; Naquira, C.; et al. Prevention and control of cystic echinococcosis. Lancet Infect. Dis. 2007, 7, 385–394. [Google Scholar] [CrossRef]
- McManus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef]
- Craig, P.S.; Hegglin, D.; Lightowlers, M.W.; Torgerson, P.R.; Wang, Q. Chapter Two–Echinococcosis: Control and Prevention. Adv. Parasitol. 2017, 96, 55–158. [Google Scholar] [PubMed]
- Mandal, S.; Mandal, M.D. Human cystic echinococcosis: Epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac. J. Trop. Med. 2012, 5, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Baumann, S.; Shi, R.; Liu, W.; Bao, H.; Schmidberger, J.; Kratzer, W.; Li, W.; Barth, T.F.E.; Bloehdorn, J.; Fischer, I.; et al. Worldwide literature on epidemiology of human alveolar echinococcosis: A systematic review of research published in the twenty-first century. Infection 2019, 47, 703–727. [Google Scholar] [CrossRef] [Green Version]
- Gavidia, C.M.; González, A.E.; Zhang, W.; McManus, D.P.; Lopera, L.; Ninaquispe, B.; García, H.H.; Rodriguez, S.; Verastegui, M.; Calderón, C.; et al. Diagnosis of Cystic Echinococcosis, Central Peruvian Highlands. Emerg. Infect. Dis. 2008, 14, 260–266. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Fan, X.; Duan, Y.; Xiao, T.; Du, G.; Fu, Y.; Liu, H.; Wen, H. Robust Phase-Retrieval-Based X-ray Tomography for Morphological Assessment of Early Hepatic Echinococcosis Infection in Rats. PLoS ONE 2017, 12, e0183396. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, W.; Wang, J.; Xing, Y. Extrahepatic alveolar echinococcus on multi-slice computed tomography and magnetic resonance imaging. Sci. Rep. 2021, 11, 9409. [Google Scholar] [CrossRef]
- Yangdan, C.-R.; Wang, C.; Zhang, L.-Q.; Ren, B.; Fan, H.-N.; Lu, M.-D. Recent advances in ultrasound in the diagnosis and evaluation of the activity of hepatic alveolar echinococcosis. Parasitol. Res. 2021, 120, 3077–3082. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Gong, Y.; Yu, M.; Xu, E.; Aimaiti, W.; Ma, R.; Xing, L.; Wen, H.; Wang, J.; et al. Breaking-then-curing strategy for efficient cystic echinococcosis therapy. Chin. Chem. Lett. 2022, 1001–8417. [Google Scholar] [CrossRef]
- Hillenbrand, A.; Beck, A.; Kratzer, W.; Graeter, T.; Barth, T.F.E.; Schmidberger, J.; Möller, P.; Henne-Bruns, D.; Gruener, B. Impact of affected lymph nodes on long-term outcome after surgical therapy of alveolar echinococcosis. Langenbeck’s Arch. Surg. 2018, 403, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-F.; Tang, Y.-Y.; Wang, R.; Fang, D.; Chen, J.-H.; Zeng, Y.; Li, B.; Wen, T.-F.; Wang, W.-T.; Wu, H.; et al. The Choose of Different Surgical Therapies of Hepatic Alveolar Echinococcosis: A Single-Center Retrospective Case-Control Study. Medicine 2018, 97, e0033. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, R.; Chen, X.; Duan, B.; Xiong, L.; Yang, X.; Fan, H.; Ni, D. Remote Intelligent Assisted Diagnosis System for Hepatic Echinococcosis. In Proceedings of the Medical Ultrasound, and Preterm, Perinatal and Paediatric Image Analysis, Lima, Peru, 4–8 October 2020; pp. 3–12. [Google Scholar]
- Zheng, X.; Wu, G.; Lv, G.; Yin, L.; Luo, B.; Lv, X.; Chen, C. Combining derivative Raman with autofluorescence to improve the diagnosis performance of echinococcosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119083. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Doughty, A.C.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Qing, G.; Zhao, X.; Gong, N.; Chen, J.; Li, X.; Gan, Y.; Wang, Y.; Zhang, Z.; Zhang, Y.; Guo, W.; et al. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat. Commun. 2019, 10, 4336. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Jangili, P.; Son, S.; Ji, M.S.; Won, M.; Kim, J.S. Fluorescent Diagnostic Probes in Neurodegenerative Diseases. Adv. Mater. 2020, 32, 2001945. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal Therapy. J. Control Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and Bioactive Engineered Nanomaterials for Targeted Tumor Photothermal Therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Jeong, C.; Kim, W.J. Synergistic Nanomedicine by Combined Gene and Photothermal Therapy. Adv. Drug Deliv. Rev. 2016, 98, 99–112. [Google Scholar] [CrossRef]
- Xi, D.; Xu, N.; Xia, X.; Shi, C.; Li, X.; Wang, D.; Long, S.; Fan, J.; Sun, W.; Peng, X. Strong π−π Stacking Stabilized Nanophotosensitizers: Improving Tumor Retention for Enhanced Therapy to Large Tumor in Mice. Adv. Mater. 2022, 34, 2102797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Q.; Xiao, M.; Ramu, V.; Hilgendorf, J.; Li, X.; Papadopoulou, P.; Siegler, M.A.; Kros, A.; Sun, W.; Bonnet, S. The Self-Assembly of a Cyclometalated Palladium Photosensitizer into Protein-Stabilized Nanorods Triggers Drug Uptake In Vitro and In Vivo. J. Am. Chem. Soc. 2020, 142, 10383–10399. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Lee, H.-S.; Lim, J.-Y.; Park, J.-H. Liposomal Indocyanine Green for Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5683–5691. [Google Scholar] [CrossRef]
- Gupta, N.; Chan, Y.-H.; Saha, S.; Liu, M.-H. Recent Development in Near-Infrared Photothermal Therapy Based on Semi-conducting Polymer Dots. ACS Appl. Polym. Mater. 2020, 2, 4195–4221. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; et al. Smart Human Serum Albumin-Indocyanine Green Nanoparticles Generated by Programmed Assembly for Dual-Modal Imaging-Guided Cancer Synergistic Phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Hu, D.; Xue, M.; He, M.; Gong, P.; Cai, L. Indocyanine Green Nanoparticles for Theranostic Applications. Nano-Micro Lett. 2013, 5, 145–150. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, J.; Gao, G.; Sheng, Z.; Cui, H.; Cai, L. Indocyanine Green-Loaded Polydopamine-Reduced Graphene Oxide Nanocomposites with Amplifying Photoacoustic and Photothermal Effects for Cancer Theranostics. Theranostics 2016, 6, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Burnier, P.; Niddam, J.; Bosc, R.; Hersant, B.; Meningaud, J.-P. Indocyanine green applications in plastic surgery: A review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 814–827. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine Green-incorporating Nanoparticles for Cancer Theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR Imaging Tracking of Inflammation-activatable Engineered Neutrophils for Targeted Therapy of Surgically Treated Glioma. Nat. Commun. 2018, 9, 4777. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Li, J.; Gao, D.; Sheng, Z.; Zheng, H.; Liu, W. Cell-Membrane Biomimetic Indocyanine Green Liposomes for Phototheranostics of Echinococcosis. Biosensors 2022, 12, 311. https://doi.org/10.3390/bios12050311
Xiong X, Li J, Gao D, Sheng Z, Zheng H, Liu W. Cell-Membrane Biomimetic Indocyanine Green Liposomes for Phototheranostics of Echinococcosis. Biosensors. 2022; 12(5):311. https://doi.org/10.3390/bios12050311
Chicago/Turabian StyleXiong, Xinxin, Jun Li, Duyang Gao, Zonghai Sheng, Hairong Zheng, and Wenya Liu. 2022. "Cell-Membrane Biomimetic Indocyanine Green Liposomes for Phototheranostics of Echinococcosis" Biosensors 12, no. 5: 311. https://doi.org/10.3390/bios12050311
APA StyleXiong, X., Li, J., Gao, D., Sheng, Z., Zheng, H., & Liu, W. (2022). Cell-Membrane Biomimetic Indocyanine Green Liposomes for Phototheranostics of Echinococcosis. Biosensors, 12(5), 311. https://doi.org/10.3390/bios12050311




