An Ultrasensitive and Selective Determination of Cadmium Ions at ppt Level Using an Enzymic Membrane with Colorimetric and Electrochemical Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Preparation of the Enzymic Membrane
2.4. Enzymatic Assays
2.4.1. Spectrophotometric Assay
2.4.2. Electrochemical Horseradish Peroxidase Assay
2.5. Electrochemical Measurements
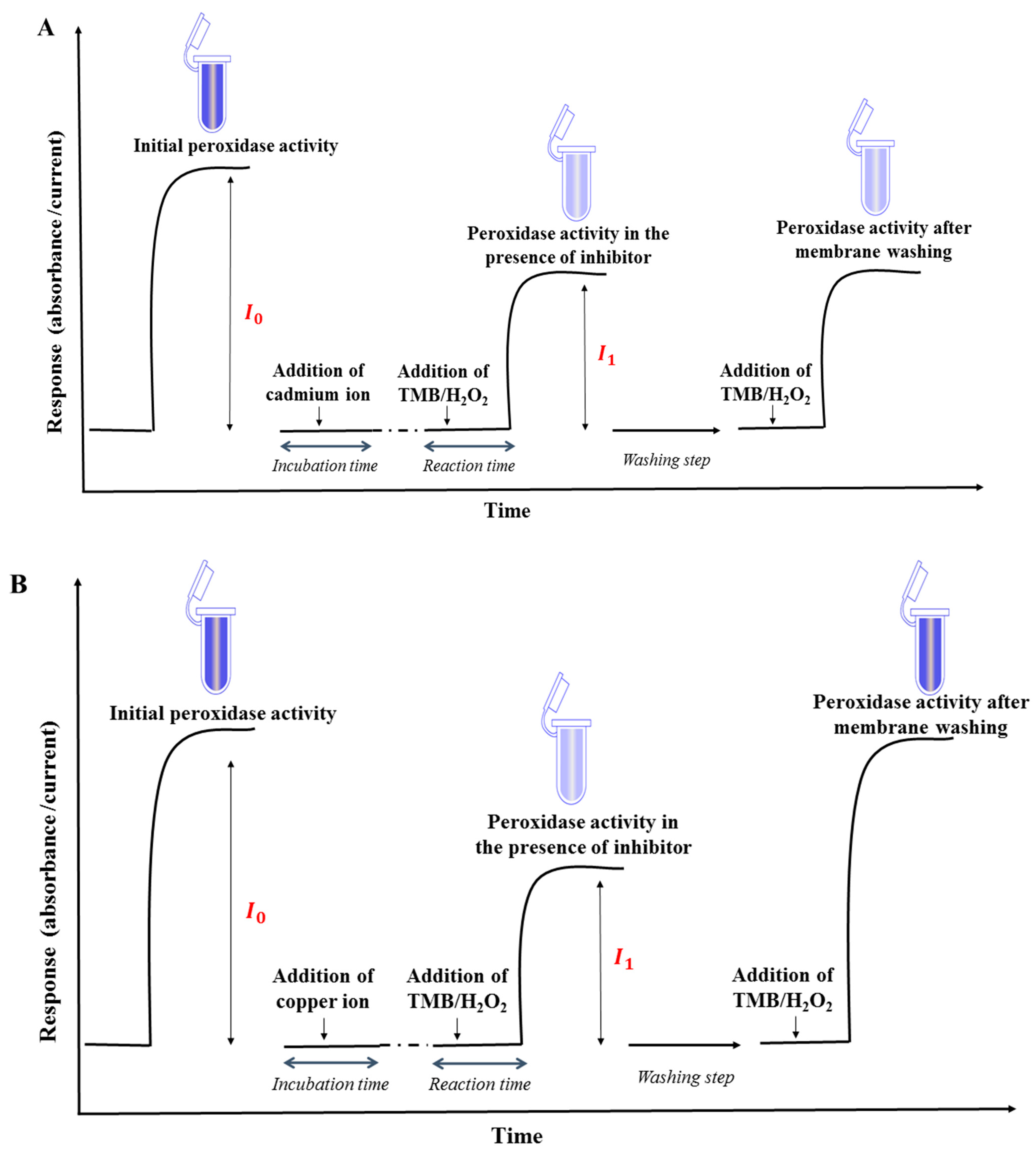
2.6. Reversible and Irreversible Inhibition of Horseradish Peroxidase
2.7. Medium Exchange Procedure
3. Results and Discussion
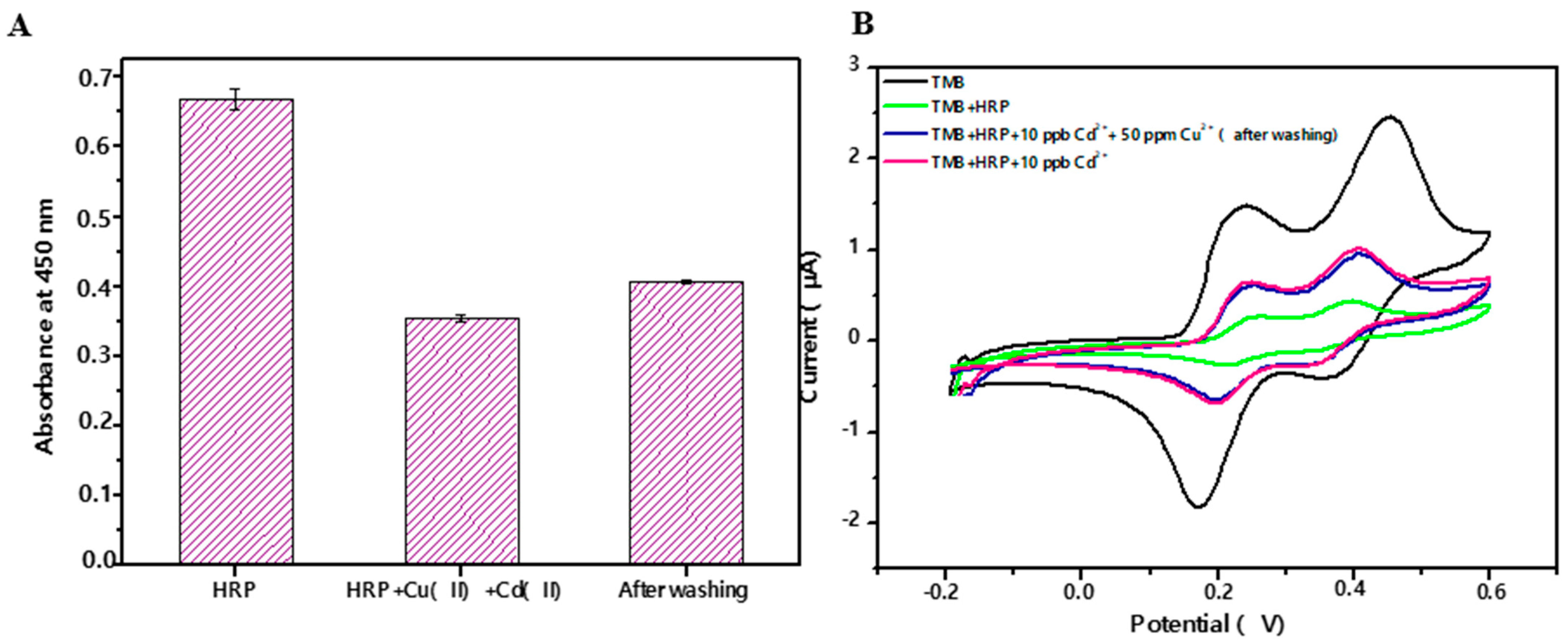
3.1. Spectrophotometric Assay
3.2. Electrochemical Biosensor
3.3. Selectivity Study
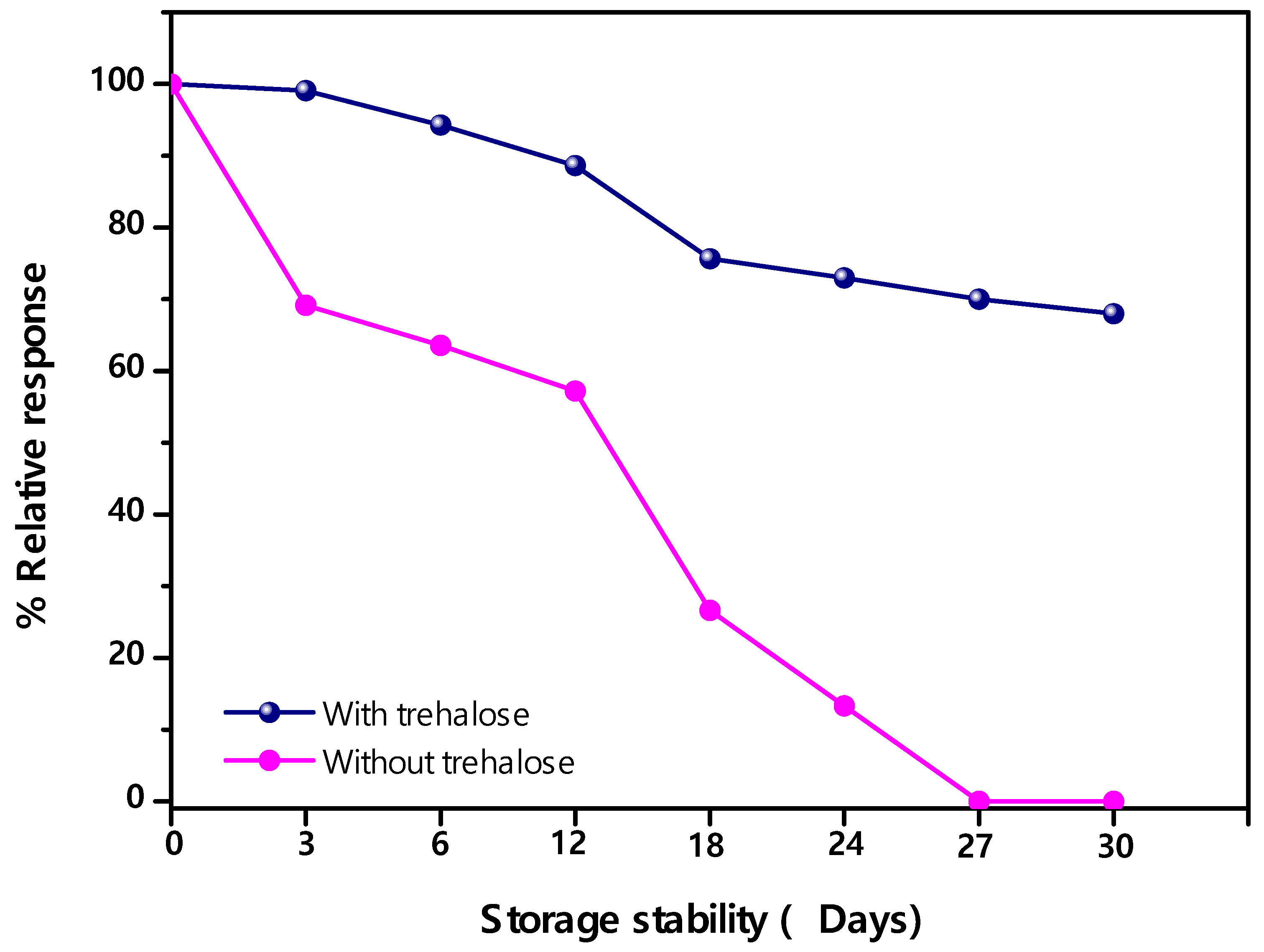
3.4. Stability of Enzymic Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, H.; Da, L.; Yang, L.; Chu, S.; Yang, F.; Yu, S.; Jiang, C. Colorimetric fluorescent paper strip with smartphone platform for quantitative detection of cadmium ions in real samples. J. Hazard. Mater. 2020, 392, 122506. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, Y. Heavy metals in indoor dust: Spatial distribution, influencing factors, and potential health risks. Sci. Total Environ. 2021, 755, 142367. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Li, Z.; Huang, X.; Zhang, W.; Zhang, D.; Zou, X.; Shi, J. A smartphone-integrated ratiometric fluorescence sensor for visual detection of cadmium ions. J. Hazard. Mater. 2021, 408, 124872. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Salman, S.; Islam, A.; Znad, H.; Hasan, M. Sustainable composite sensor material for optical cadmium(II) monitoring and capturing from wastewater. Microchem. J. 2021, 161, 105800. [Google Scholar] [CrossRef]
- Pavlaki, M.; Morgado, R.; Ferreira, V.; Rocha, R.; Soares, A.; Calado, R.; Loureiro, S. Cadmium Accumulation and Kinetics in Solea senegalensis Tissues under Dietary and Water Exposure and the Link to Human Health. Water 2021, 13, 522. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Diaz, D.; Ujueta, F.; Mansur, G.; Lamas, G.A.; Navas-Acien, A.; Arenas, I.A. Low-Level Cadmium Exposure and Atherosclerosis. Curr. Environ. Health Rep. 2021, 8, 42–53. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation strategies for heavy metals contaminated ecosystem: A review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 6 April 2022).
- Garrison, G.E.; Ader, O.L. Sodium in Drinking Water. Arch. Environ. Health Int. J. 1966, 13, 551–553. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, Y.; Zhang, Z.; Wu, Y.; Zhu, G. Recent developments in electrochemical detection of cadmium. Trends Environ. Anal. Chem. 2022, 33, 152. [Google Scholar] [CrossRef]
- Sun, R.; Ma, G.; Duan, X.; Sun, J. Determination of cadmium in seawater by chelate vapor generation atomic fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 141, 22–27. [Google Scholar] [CrossRef]
- Zarezade, V.; Behbahani, M.; Omidi, F.; Abandansari, H.S.; Hesam, G. A new magnetic tailor made polymer for separation and trace determination of cadmium ions by flame atomic absorption spectrophotometry. RSC Adv. 2016, 6, 103499–103507. [Google Scholar] [CrossRef]
- Vyhnanovský, J.; Sturgeon, R.E.; Musil, S. Cadmium Assisted Photochemical Vapor Generation of Tungsten for Detection by Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2019, 91, 13306–13312. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Biosensors and Bioassays Based on Lipases, Principles and Applications, a Review. Molecules 2019, 24, 616. [Google Scholar] [CrossRef] [Green Version]
- Attaallah, R.; Amine, A. The Kinetic and Analytical Aspects of Enzyme Competitive Inhibition: Sensing of Tyrosinase Inhibitors. Biosensors 2021, 11, 322. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. Highly selective and sensitive detection of cadmium ions by horseradish peroxidase enzyme inhibition using a colorimetric microplate reader and smartphone paper-based analytical device. Microchem. J. 2021, 172, 106940. [Google Scholar] [CrossRef]
- Attar, A.; Aguilera, L.C.; Naranjo-Rodríguez, I.; de Cisneros, J.L.H.H.; Palacios-Santander, J.M.; Amine, A. Amperometric inhibition biosensors based on horseradish peroxidase and gold sononanoparticles immobilized onto different electrodes for cyanide measurements. Bioelectrochemistry 2015, 101, 84–91. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ma, R.-T.; Shi, Y.-P. “Recent advances on support materials for lipase immobilization and applicability as biocatalysts in inhibitors screening methods”—A review. Anal. Chim. Acta 2020, 1101, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. 2011, 13, 1609–1623. [Google Scholar] [CrossRef]
- Luo, X.; Xia, J.; Jiang, X.; Yang, M.; Liu, S. Cellulose-Based Strips Designed Based on a Sensitive Enzyme Colorimetric Assay for the Low Concentration of Glucose Detection. Anal. Chem. 2019, 91, 15461–15468. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Rajput, S.S.; Das, M.; Laha, S.; Choudhuri, I.; Bhattacharyya, N.; Das, A.; Samanta, B.C.; Alam, M.M.; Maity, T. Easy, selective and colorimetric detection of Zn(II), Cu(II), F− ions by a new piperazine based Schiff base chemosensor along with molecular logic gate formation and live cell images study. J. Photochem. Photobiol. A Chem. 2022, 427, 113817. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kurkuri, M.; Losic, D.; Kigga, M.; Altalhi, A. New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Anal. Chim. Acta 2021, 1159, 338439. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Malmagro, J.; García-Molina, G.; De Lacey, A. Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations. Sensors 2020, 20, 3393. [Google Scholar] [CrossRef] [PubMed]
- Corporation, P.; Membranes, P. Membrane Quick Selection Guide. Available online: http://wolfson.huji.ac.il/purification/PDF/Others/Pall/PALL_ProteinSamplePrepManual3.pdf (accessed on 6 April 2022).
- Ricci, F.; Amine, A.; Tuta, C.S.; Ciucu, A.A.; Lucarelli, F.; Palleschi, G.; Moscone, D. Prussian Blue and enzyme bulk-modified screen-printed electrodes for hydrogen peroxide and glucose determination with improved storage and operational stability. Anal. Chim. Acta 2003, 485, 111–120. [Google Scholar] [CrossRef]
- A Single Component-Soluble Substrate for Kinetic and Endpoint Assays of Horseradish Peroxidase. Available online: https://www.sigmaaldrich.com/specification-sheets/157/638/T0440-BULK________SIGMA____.pdf (accessed on 2 March 2017).
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2016, 76, 180–194. [Google Scholar] [CrossRef]
- Amine, A.; El Harrad, L.; Arduini, F.; Moscone, D.; Palleschi, G. Analytical aspects of enzyme reversible inhibition. Talanta 2014, 118, 368–374. [Google Scholar] [CrossRef]
- Goka, A.K.J.; Farthing, M.J.G. The use of 3, 3′, 5, 5′-tetramethylbenzidine as a peroxidase substrate in microplate enzyme-linked immunosorbent assay. J. Immunoassay 1987, 8, 29–41. [Google Scholar] [CrossRef]
- Baldrich, E.; del Campo, F.J.; Muñoz, F.X. Biosensing at disk microelectrode arrays. Inter-electrode functionalisation allows formatting into miniaturised sensing platforms of enhanced sensitivity. Biosens. Bioelectron. 2009, 25, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyo, M.; Okonkwo, J.O. Horseradish peroxidase biosensor based on maize tassel-MWCNTs composite for cadmium detection. Sens. Actuators B Chem. 2014, 193, 515–521. [Google Scholar] [CrossRef]
- Sreekanth, S.P.; Alodhayb, A.; Assaifan, A.K.; Alzahrani, K.E.; Muthuramamoorthy, M.; Alkhammash, H.I.; Pandiaraj, S.; Alswieleh, A.M.; Van Le, Q.; Mangaiyarkarasi, R.; et al. Multi-walled carbon nanotube-based nanobiosensor for the detection of cadmium in water. Environ. Res. 2021, 197, 111148. [Google Scholar] [CrossRef] [PubMed]
- Dali, M.; Zinoubi, K.; Chrouda, A.; Abderrahmane, S.; Cherrad, S.; Jaffrezic-Renault, N. A biosensor based on fungal soil biomass for electrochemical detection of lead (II) and cadmium (II) by differential pulse anodic stripping voltammetry. J. Electroanal. Chem. 2018, 813, 9–19. [Google Scholar] [CrossRef]
- Silwana, B.; van der Horst, C.; Iwuoha, E.; Somerset, V. Inhibitive Determination of Metal Ions Using a Horseradish Peroxidase Amperometric Biosensor. State Art Biosens.-Environ. Med. Appl. 2013, 105–119. [Google Scholar]
- Nomngongo, P.N.; Ngila, J.C.; Nyamori, V.O.; Songa, E.A.; Iwuoha, E.I. Determination of selected heavy metals using amperometric horseradish peroxidase (HRP) inhibition biosensor. Anal. Lett. 2011, 44, 2031–2046. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Isfahani, Z.N. A simple optical sensor for cadmium ions assay in water samples using spectrophotometry. J. Anal. Chem. 2011, 66, 151–157. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Amperometric detection in TMB/HRP-based assays. Anal. Bioanal. Chem. 2005, 382, 297–302. [Google Scholar] [CrossRef]
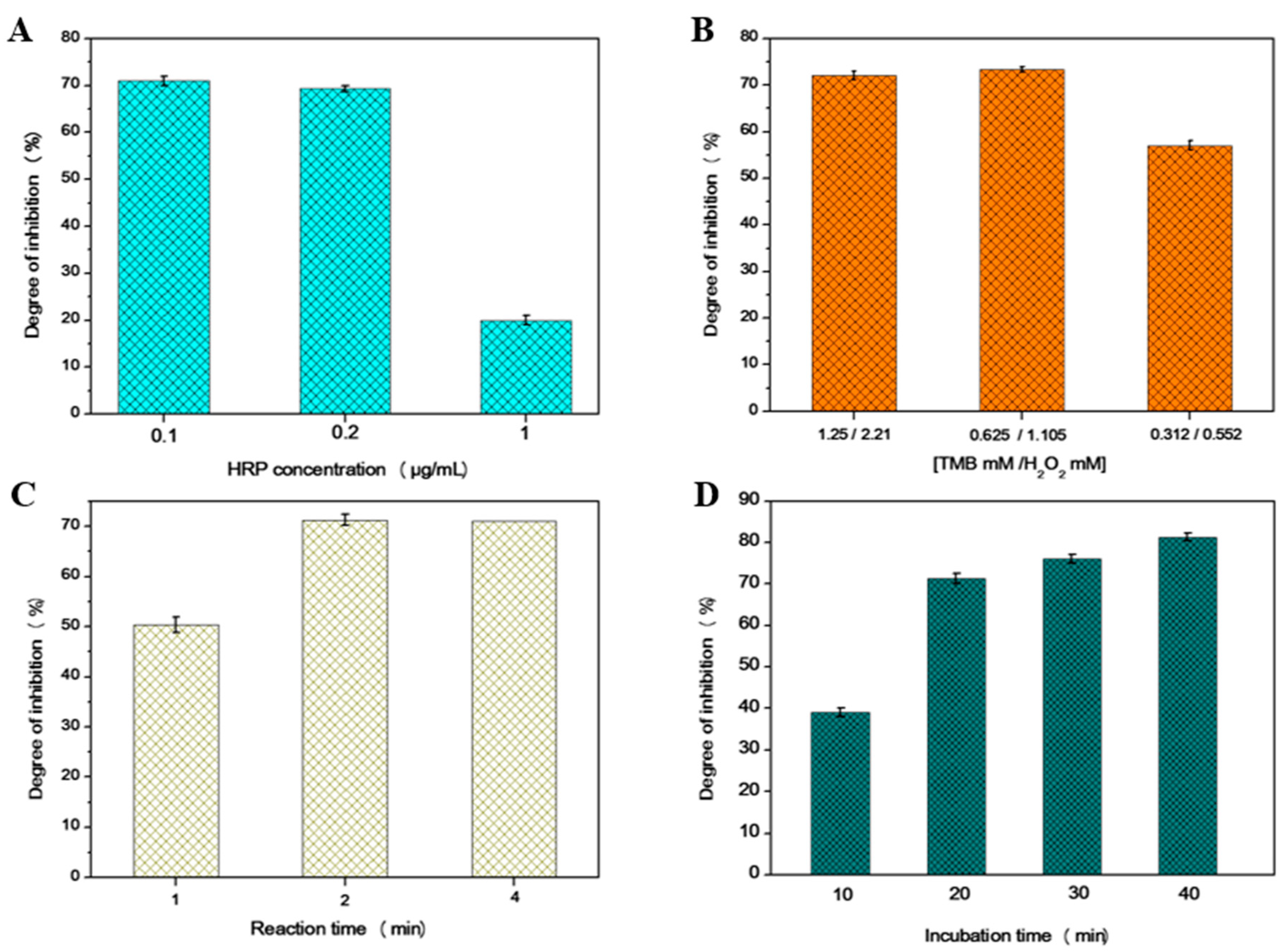
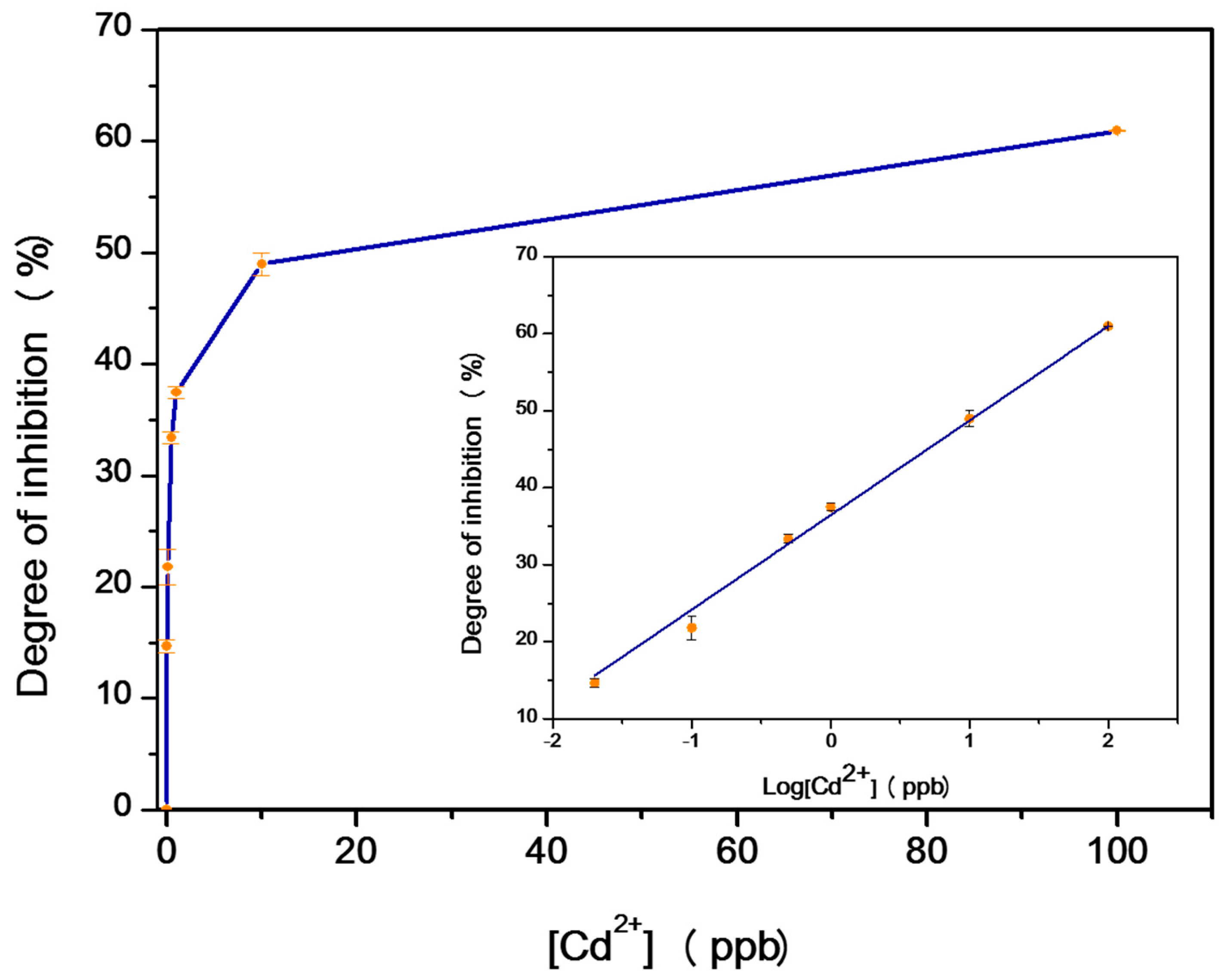

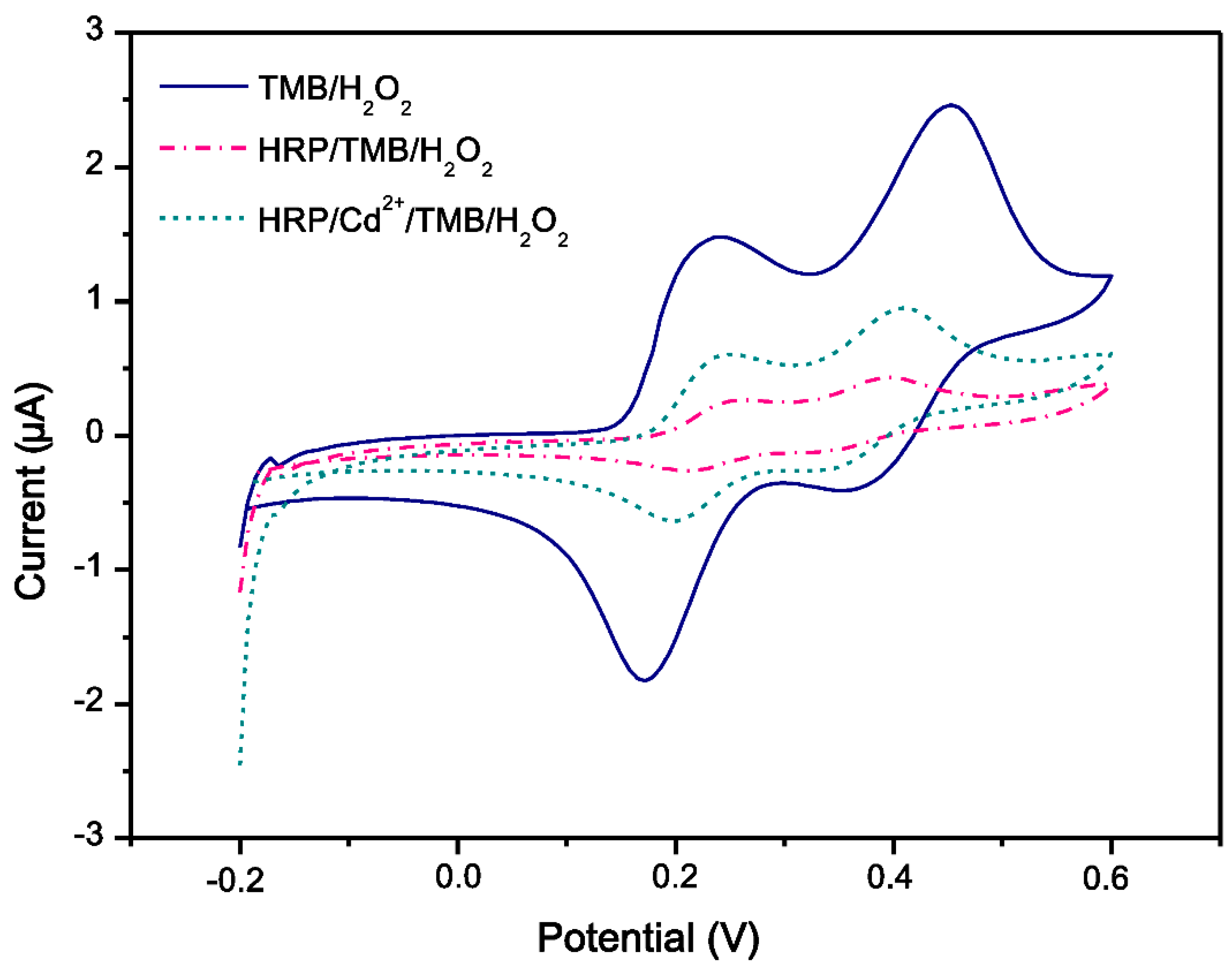

| Enzyme | Degree of Inhibition of 10 ppb | Degree of Inhibition of 100 ppb |
|---|---|---|
| HRP (type VI-A) | 51 | 70 |
| HRP (type XII) | 46 | 61 |
| Ab-HRP | 35 | 45 |
| Parameter | Tested Range | Selected Value |
|---|---|---|
| (TMB mM/H2O2 mM) | 1.25/2.21–0.25/0.442 | 0.25/0.442 |
| (HRP) (µg/mL) | 0.05–1 | 0.05 |
| Reaction time (min) | 1–5 | 5 |
| Incubation time (min) | 10–60 | 20 |
| Platform | Linear Range (ppb) | LOD (ppb) | Method | Ref. |
|---|---|---|---|---|
| MT/MWCNT-HRP | 2–30 | 0.51 | Electrochemical | [35] |
| MWCNT-DNA | -- | 0.22 | Electrochemical | [36] |
| Paper-HRP | -- | 1 | Colorimetric | [18] |
| SWCNTs/Biomass | -- | 0.1 | Electrochemical | [37] |
| Pt/PANI-HRP | 1.5–4 | 0.05 | Electrochemical | [38] |
| PANI-HRP | 4.76–55.3 | 0.09 | Electrochemical | [39] |
| ACDA-triacetylcellulose membrane | 0.33–3.81 | 0.2 | Colorimetric | [40] |
| Immunodyne membrane-HRP | 0.02–100 | 0.02 | Colorimetric | This work |
| Immunodyne membrane-HRP | 0.05–100 | 0.05 | Electrochemical | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attaallah, R.; Amine, A. An Ultrasensitive and Selective Determination of Cadmium Ions at ppt Level Using an Enzymic Membrane with Colorimetric and Electrochemical Detection. Biosensors 2022, 12, 310. https://doi.org/10.3390/bios12050310
Attaallah R, Amine A. An Ultrasensitive and Selective Determination of Cadmium Ions at ppt Level Using an Enzymic Membrane with Colorimetric and Electrochemical Detection. Biosensors. 2022; 12(5):310. https://doi.org/10.3390/bios12050310
Chicago/Turabian StyleAttaallah, Raouia, and Aziz Amine. 2022. "An Ultrasensitive and Selective Determination of Cadmium Ions at ppt Level Using an Enzymic Membrane with Colorimetric and Electrochemical Detection" Biosensors 12, no. 5: 310. https://doi.org/10.3390/bios12050310
APA StyleAttaallah, R., & Amine, A. (2022). An Ultrasensitive and Selective Determination of Cadmium Ions at ppt Level Using an Enzymic Membrane with Colorimetric and Electrochemical Detection. Biosensors, 12(5), 310. https://doi.org/10.3390/bios12050310





