Time Domain (TD) Proton NMR Analysis of the Oxidative Safety and Quality of Lipid-Rich Foods
Abstract
:1. Introduction
2. Current Analytical Methods for Determining the Presence of Unhealthy Food Components and Food Safety
3. Low-Field Proton NMR (LF 1H NMR) Analysis for Food Safety
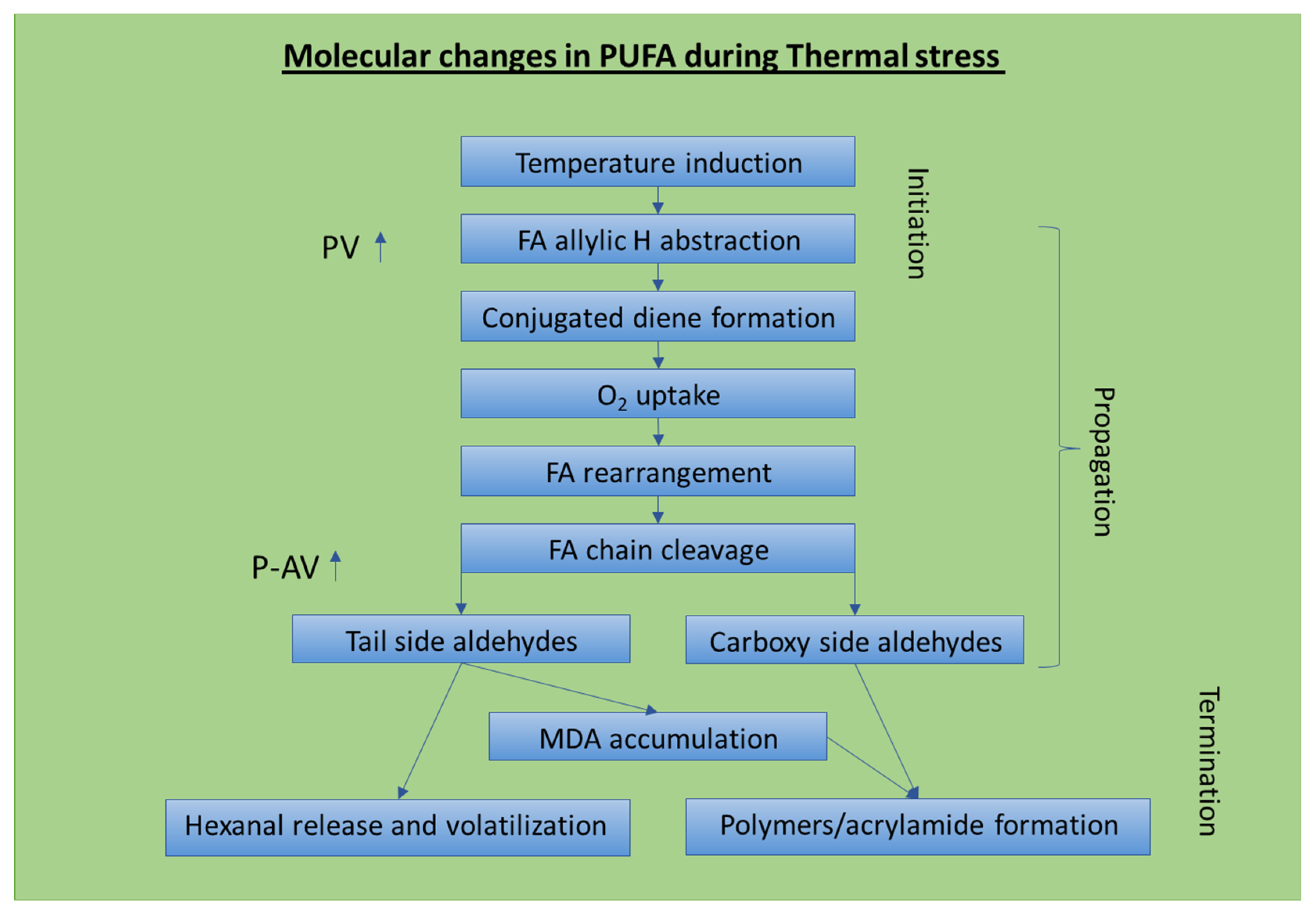
4. Chemical and Structural Changes during the Thermal Oxidation of Triacylglycerides and Resulting Unhealthy Components
5. TD NMR Sensor for the Compositional, Physicochemical and Textural Analysis of Food
5.1. Advanced TD NMR Applications for Food Quality Anaylsis
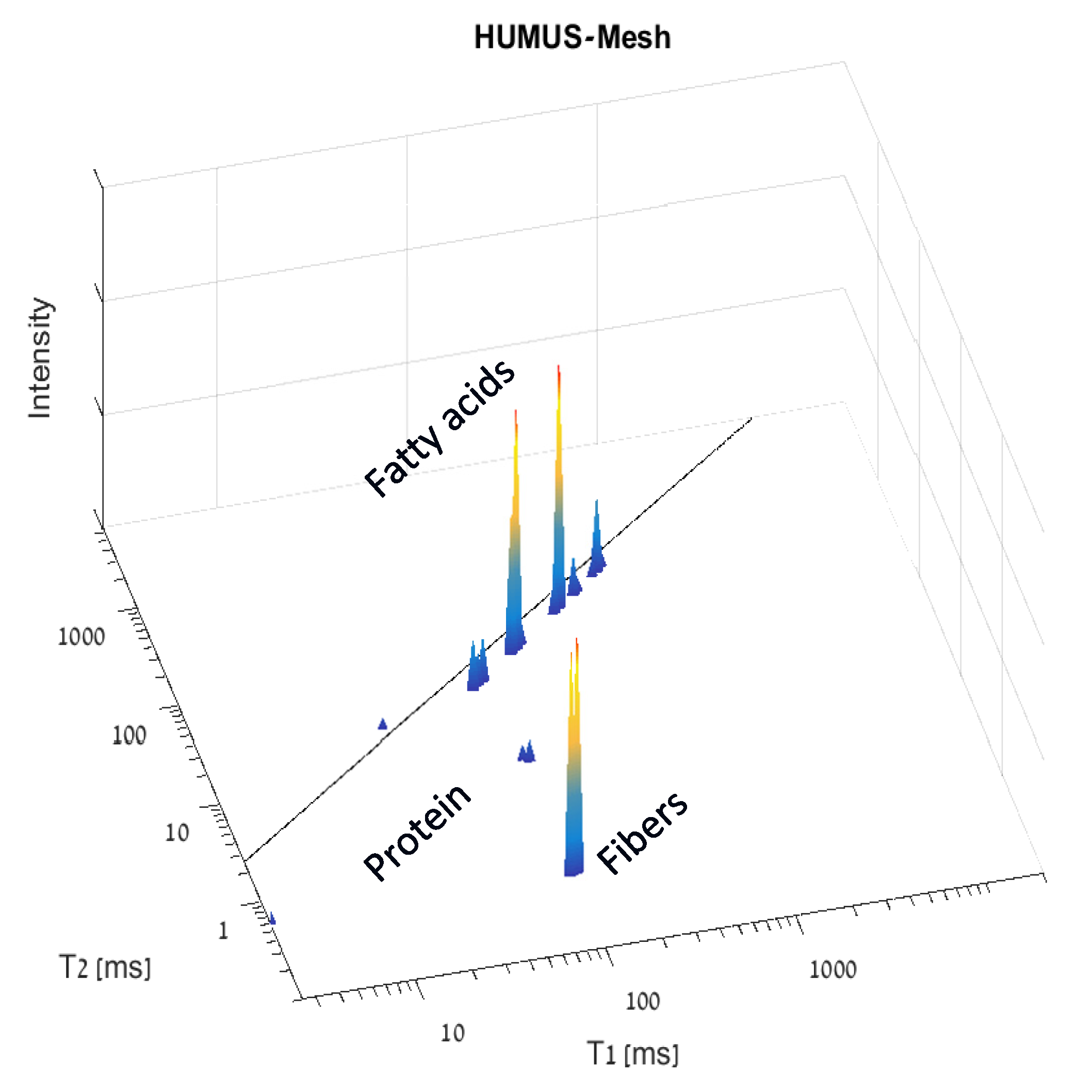
5.2. Demonstration of TD NMR Fingerprinting of Seeds under Oxidative Thermal Stress Conditions for Determining Their Food Safety
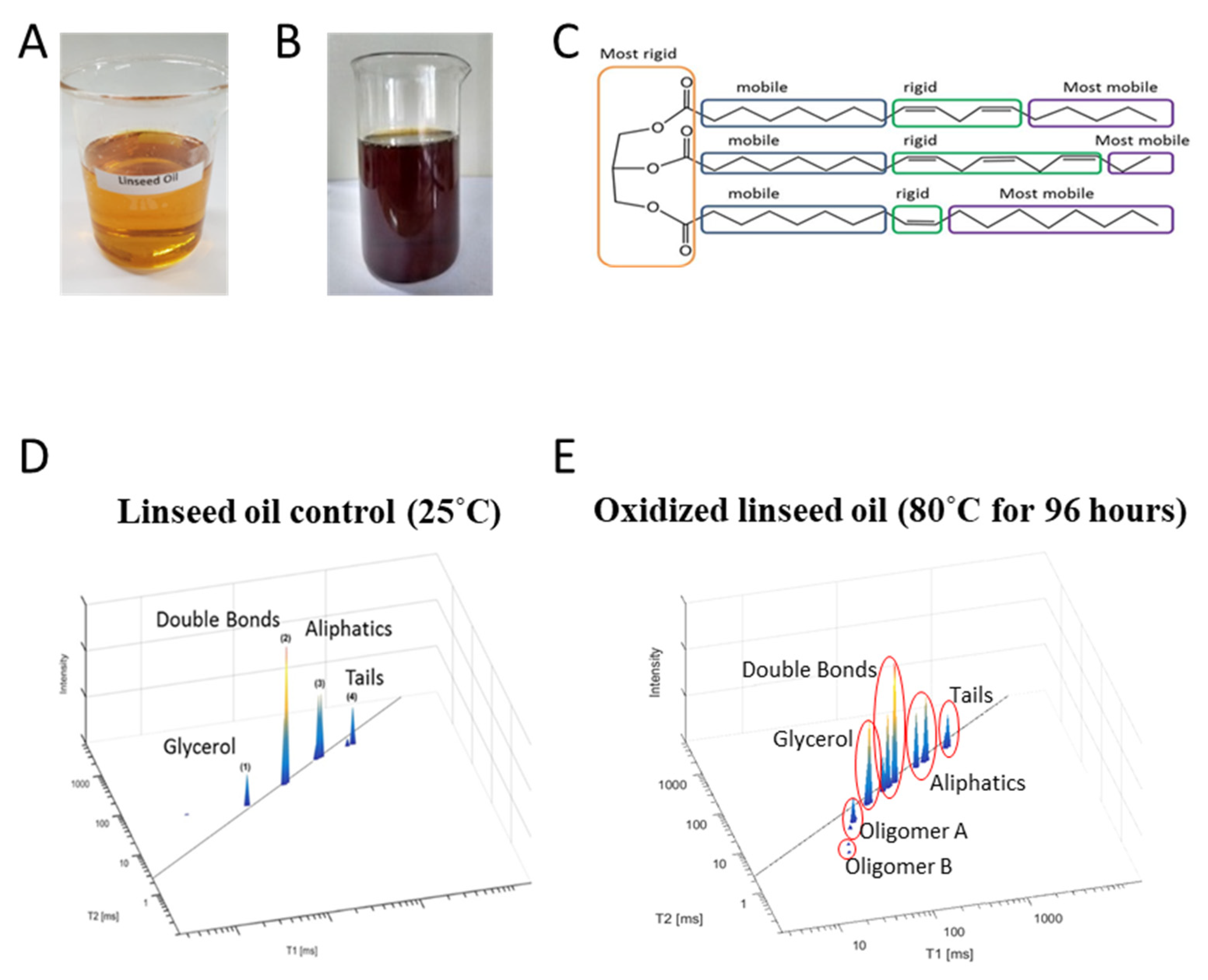
5.3. Demonstration of TD NMR Fingerprinting of Extracted Seed Oils and Their Oxidation
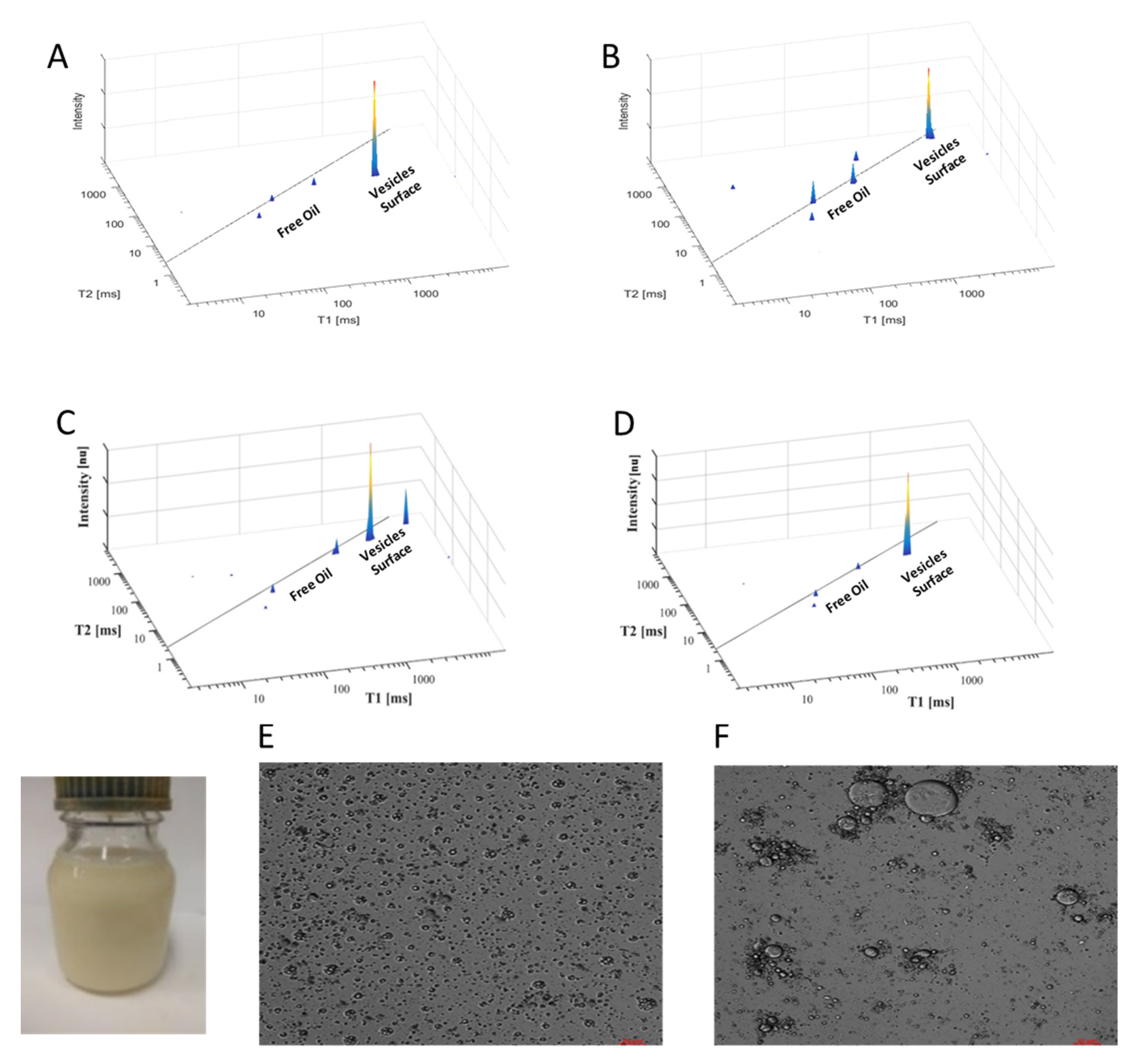
5.4. Demonstration of TD NMR Fingerprinting of Milk and Plant Milk Substitutes
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hackl, L.S.; Mehta, S.; Lambertini, E.; Nordhagen, S.; McClafferty, B. Literature Review Linking Food Safety and Nutrition; Global Alliance for Improved Nutrition: Geneva, Switzerland, 2020. [Google Scholar]
- Jacobsen, C.; García-Moreno, P.J.; Yesiltas, B.; Sørensen, A.-D.M. Lipid Oxidation and Traditional Methods for Evaluation. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Sørensen, A.-D.M., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 183–200. ISBN 978-0-12-821391-9. [Google Scholar]
- García Moreno, P.J.; Jacobsen, C.; Marcatili, P.; Gregersen, S.; Overgaard, M.T.; Andersen, M.L.; Sørensen, A.-D.M.; Hansen, E.B. Emulsifying Peptides from Potato Protein Predicted by Bioinformatics: Stabilization of Fish Oil-in-Water Emulsions. Food Hydrocoll. 2020, 101, 105529. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Sørensen, A.-D.M.; Jacobsen, C. High Fat (>50%) Oil-in-Water Emulsions as Omega-3 Delivery Systems. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Sørensen, A.-D.M., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 255–273. ISBN 978-0-12-821391-9. [Google Scholar]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.; Guadix, E.M. Development of Fish Oil-Loaded Microcapsules Containing Whey Protein Hydrolysate as Film-Forming Material for Fortification of Low-Fat Mayonnaise. Foods 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A Review of Thermo-Oxidative Degradation of Food Lipids Studied by 1H NMR Spectroscopy: Influence of Degradative Conditions and Food Lipid Nature. Compr. Rev. Food. Sci. Food. Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Bintsis, T. Microbial Pollution and Food Safety. AIMS Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Di, L.; Wu, X.; You, W.; Huang, B. Occurrence, Speciation, and Risks of Trace Metals in Soils of Greenhouse Vegetable Production from the Vicinity of Industrial Areas in the Yangtze River Delta, China. Environ. Sci. Pollut. Res. 2019, 26, 8696–8708. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety: Pesticide Residue. 2016. Available online: https://www.who.int/news-room/questions-and-answers/item/food-safety-pesticide-residue (accessed on 10 February 2022).
- Chen, Y.P.; Zou, M.; Qi, C.; Xie, M.X.; Wang, D.N.; Wang, Y.F.; Xue, Q.; Li, J.F.; Chen, Y. Immunosensor Based on Magnetic Relaxation Switch and Biotin–Streptavidin System for the Detection of Kanamycin in Milk. Biosens. Bioelectron. 2013, 39, 112–117. [Google Scholar] [CrossRef]
- World Health Organization. Food Additives. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/food-additives (accessed on 10 February 2022).
- Kader, A.A.; Rolle, R.S.; Food and Agriculture Organization of the United Nations. The Role of Post-Harvest Management in Assuring the Quality and Safety of Horticultural Produce; Food & Agriculture Organization: Rome, Italy, 2004; ISBN 978-92-5-105137-5. [Google Scholar]
- Johnson, D.R.; Decker, E.A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food. Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; ISBN 978-0-471-67849-6. [Google Scholar]
- Schaich, K.M. Challenges in Elucidating Lipid Oxidation Mechanisms: When, Where, and How Do Products Arise? In Lipid Oxidation; Logan, A., Nienaber, U., Pan, X., Eds.; AOCS Press: Urbana, IL, USA, 2013; pp. 1–52. ISBN 978-0-9830791-6-3. [Google Scholar]
- Capitani, D.; Sobolev, A.P.; Di Tullio, V.; Mannina, L.; Proietti, N. Portable NMR in Food Analysis. Chem. Biol. Technol. Agric. 2017, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Colnago, L.A.; Wiesman, Z.; Pages, G.; Musse, M.; Monaretto, T.; Windt, C.W.; Rondeau-Mouro, C. Low Field, Time Domain NMR in the Agriculture and Agrifood Sectors: An Overview of Applications in Plants, Foods and Biofuels. J. Magn. Reson. 2021, 323, 106899. [Google Scholar] [CrossRef]
- Rudszuck, T.; Förster, E.; Nirschl, H.; Guthausen, G. Low-Field NMR for Quality Control on Oils. Magn. Reson. Chem. 2019, 57, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I. Measurement of Lipid Oxidation: A Review. J. Am. Oil. Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Haywood, R.M.; Chander, C.L.; Atherton, M.; Lynch, E.J.; Grootveld, M.C. Generation of Lipid Peroxidation Products in Culinary Oils and Fats during Episodes of Thermal Stressing: A High Field 1H NMR Study. FEBS Lett. 1994, 355, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.-S. Application of NMR Spectroscopy for Foods and Lipids. In Advances in NMR Spectroscopy for Lipid Oxidation Assessment; Hwang, H.-S., Ed.; Springer Briefs in Food, Health, and Nutrition; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–13. ISBN 978-3-319-54196-9. [Google Scholar]
- Frankel, E.N. Methods to Determine Extent of Oxidation. In Lipid Oxidation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 99–127. ISBN 978-0-9531949-8-8. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid Oxidation: Measurement Methods. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; ISBN 978-0-471-67849-6. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- St. Angelo, A.J.; Ory, R.L.; Brown, L.E. Comparison of Methods for Determining Peroxidation in Processed Whole Peanut Products. J. Am. Oil. Chem. Soc. 1975, 52, 34–35. [Google Scholar] [CrossRef]
- List, G.R.; Evans, C.D.; Kwolek, W.F.; Warner, K.; Boundy, B.K.; Cowan, J.C. Oxidation and Quality of Soybean Oil: A Preliminary Study of the Anisidine Test. J. Am. Oil. Chem. Soc. 1974, 51, 17–21. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A Review of Analytical Methods Measuring Lipid Oxidation Status in Foods: A Challenging Task. Eur. Food. Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Chan, H.W.-S.; Levett, G. Autoxidation of Methyl Linoleate. Separation and Analysis of Isomeric Mixtures of Methyl Linoleate Hydroperoxides and Methyl Hydroxylinoleates. Lipids 1977, 12, 99–104. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring the Oxidation of Unsaturated Oils and Formation of Oxygenated Aldehydes by Proton NMR. Eur. J. Lipid Sci. Technol. 2005, 107, 36–47. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillen, M.D. Analysis of Hydroperoxides, Aldehydes and Epoxides by 1 H Nuclear Magnetic Resonance in Sunflower Oil Oxidized at 70 and 100 °C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef] [PubMed]
- Khor, Y.P.; Hew, K.S.; Abas, F.; Lai, O.M.; Cheong, L.Z.; Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M.; Tan, C.P. Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles. Foods 2019, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Qin, W.; Li, M.; Shen, Q.; Saleh, A.S.M. Application of Chromatographic Techniques in the Detection and Identification of Constituents Formed during Food Frying: A Review: Chromatographic Analysis of Frying Oil. Compr. Rev. Food. Sci. Food. Saf. 2015, 14, 601–633. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, S.; Krishna, A.G.G. Assessment of Oxidation in Heated Safflower Oil by Physical, Chemical and Spectroscopic Methods. J. Food Lipids 1998, 5, 247–267. [Google Scholar] [CrossRef]
- Kaufmann, A.; Ryser, B.; Suter, B. HPLC with Evaporative Light Scattering Detection for the Determination of Polar Compounds in Used Frying Oils. Eur. Food. Res. Technol. 2001, 213, 372–376. [Google Scholar] [CrossRef]
- Ajmal, M.; Nadeem, M.; Imran, M.; Junaid, M. Lipid Compositional Changes and Oxidation Status of Ultra-High Temperature Treated Milk. Lipids Health Dis. 2018, 17, 227. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R.; Moosavi, S.M.R. Evaluating the Performance of Peroxide and Conjugated Diene Values in Monitoring the Quality of Used Frying Oils. J. Agric. Sci. Technol. 2009, 11, 173–179. [Google Scholar]
- Blümich, B. Low-Field and Benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef]
- Resende, M.T.; Linder, C.; Wiesman, Z. 1H LF-NMR Energy Relaxation Time Characterization of the Chemical and Morphological Structure of PUFA-Rich Linseed Oil During Oxidation with and Without Antioxidants. Eur. J. Lipid Sci. Technol. 2019, 121, 1800339. [Google Scholar] [CrossRef]
- Resende, M.T.; Campisi-Pinto, S.; Linder, C.; Wiesman, Z. Multidimensional Proton Nuclear Magnetic Resonance Relaxation Morphological and Chemical Spectrum Graphics for Monitoring and Characterization of Polyunsaturated Fatty-Acid Oxidation. J. Am. Oil. Chem. Soc. 2019, 96, 125–135. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study by Proton Nuclear Magnetic Resonance of the Thermal Oxidation of Oils Rich in Oleic Acyl Groups. J. Am. Oil. Chem. Soc. 2005, 82, 349–355. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Formation of Hydroperoxy- and Hydroxyalkenals during Thermal Oxidative Degradation of Sesame Oil Monitored by Proton NMR. Eur. J. Lipid Sci. Technol. 2004, 106, 680–687. [Google Scholar] [CrossRef]
- Kirtil, E.; Cikrikci, S.; McCarthy, M.J.; Oztop, M.H. Recent Advances in Time Domain NMR & MRI Sensors and Their Food Applications. Curr. Opin. Food. Sci. 2017, 17, 9–15. [Google Scholar] [CrossRef]
- Mitchell, J.; Gladden, L.F.; Chandrasekera, T.C.; Fordham, E.J. Low-Field Permanent Magnets for Industrial Process and Quality Control. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 76, 1–60. [Google Scholar] [CrossRef]
- Luo, Y.; Alocilja, E.C. Portable Nuclear Magnetic Resonance Biosensor and Assay for a Highly Sensitive and Rapid Detection of Foodborne Bacteria in Complex Matrices. J. Biol. Eng. 2017, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Resende, M.T.; Linder, C.; Wiesman, Z. Low-Field Nuclear Magnetic Resonance Time Domain Characterization of Polyunsaturated Fatty Acid–Rich Linseed and Fish Oil Emulsions during Thermal Air Oxidation. J. Am. Oil. Chem. Soc. 2021, 98, 495–508. [Google Scholar] [CrossRef]
- Ghelichi, S.; Hajfathalian, M.; García-Moreno, P.J.; Yesiltas, B.; Moltke-Sørensen, A.-D.; Jacobsen, C. Food Enrichment with Omega-3 Polyunsaturated Fatty Acids. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Moltke Sørensen, A.-D., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 395–425. ISBN 978-0-12-821391-9. [Google Scholar]
- Wiesman, Z.; Linder, C.; Resende, M.T.; Ayalon, N.; Levi, O.; Bernardinelli, O.D.; Colnago, L.A.; Mitre, C.I.N.; Jackman, R. 2D and 3D Spectrum Graphics of the Chemical-Morphological Domains of Complex Biomass by Low Field Proton NMR Energy Relaxation Signal Analysis. Energy Fuels 2018, 32, 5090–5102. [Google Scholar] [CrossRef]
- Le, P.; Zhang, L.; Lim, V.; McCarthy, M.J.; Nitin, N. A Novel Approach for Measuring Resistance of Escherichia Coli and Listeria Monocytogenes to Hydrogen Peroxide Using Label-Free Magnetic Resonance Imaging and Relaxometry. Food Control 2015, 50, 560–567. [Google Scholar] [CrossRef]
- Gertz, C. Chemical and Physical Parameters as Quality Indicators of Used Frying Fats. Eur. J. Lipid Sci. Technol. 2000, 102, 566–572. [Google Scholar] [CrossRef]
- Conte, P.; Maccotta, A.; Pasquale, C.; Alonzo, G. Supramolecular Organization of Triglycerides in Extra-Virgin Olive Oils as Assessed by NMR Relaxometry. Fresenius Environ. Bull. 2010, 19, 2077–2082. [Google Scholar]
- Doll, K.M.; Hwang, H.-S. Thermal Modification of Vegetable Oils. Lipid Technol. 2013, 25, 83–85. [Google Scholar] [CrossRef]
- Barthel, G.; Grosch, W. Peroxide Value Determination—Comparison of Some Methods. J. Am. Oil. Chem. Soc. 1974, 51, 540–544. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Velasco, J. Analysis of Lipid Hydroperoxides. Eur. J. Lipid Sci. Technol. 2002, 104, 420–428. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation Process of Oils with High Content of Linoleic Acyl Groups and Formation of Toxic Hydroperoxy- and Hydroxyalkenals. A Study By1H Nuclear Magnetic Resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- White, P.J. Conjugated Diene, Anisidine Value, and Carbonyl Value Analyses. In Methods to Access Quality and Stability of Oils and Fat-Containing Foods; AOCS Publishing: Urbana, IL, USA, 1995; ISBN 978-1-00-304095-8. [Google Scholar]
- Guillén, M.D.; Goicoechea, E. Detection of Primary and Secondary Oxidation Products by Fourier Transform Infrared Spectroscopy (FTIR) and 1H Nuclear Magnetic Resonance (NMR) in Sunflower Oil during Storage. J. Agric. Food. Chem. 2007, 55, 10729–10736. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Neff, W.E.; Selke, E.; Weisleder, D. Photosensitized Oxidation of Methyl Linoleate: Secondary and Volatile Thermal Decomposition Products. Lipids 1982, 17, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.D.; Goicoechea, E. Oxidation of Corn Oil at Room Temperature: Primary and Secondary Oxidation Products and Determination of Their Concentration in the Oil Liquid Matrix from 1H Nuclear Magnetic Resonance Data. Food Chem. 2009, 116, 183–192. [Google Scholar] [CrossRef]
- Instituto de la Grasa (CSIC); Dobarganes, M.C. Formation of Volatiles and Short-Chain Bound Compounds; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- Zhang, D.; Haputhanthri, R.; Ansar, S.M.; Vangala, K.; De Silva, H.I.; Sygula, A.; Saebo, S.; Pittman, C.U. Ultrasensitive Detection of Malondialdehyde with Surface-Enhanced Raman Spectroscopy. Anal. Bioanal. Chem. 2010, 398, 3193–3201. [Google Scholar] [CrossRef]
- Reindl, B.; Stan, H.J. Determination of Volatile Aldehydes in Meat as 2,4-Dinitrophenylhydrazones Using Reversed-Phase High-Performance Liquid Chromatography. J. Agric. Food Chem. 1982, 30, 849–854. [Google Scholar] [CrossRef]
- Acrylamide. 2009. Available online: https://www.food.gov.uk/safety-hygiene/acrylamide (accessed on 10 February 2022).
- Firestone, D. Gel-Permeation Liquid Chromatographic Method. In AOAC Polymerized Triglycerides in Oils and Fats; AOAC International: Rockville, MD, USA, 1996. [Google Scholar]
- Patrikios, I.S.; Mavromoustakos, T.M. Monounsaturated Fatty Acid Ether Oligomers Formed during Heating of Virgin Olive Oil Show Agglutination Activity against Human Red Blood Cells. J. Agric. Food Chem. 2014, 62, 867–874. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Winkler-Moser, J.K.; Liu, S.X. Structural Effect of Lignans and Sesamol on Polymerization of Soybean Oil at Frying Temperature. J. Am. Oil. Chem. Soc. 2012, 89, 1067–1076. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Rennick, K.A.; Hwang, H.-S.; Berhow, M.A.; Vaughn, S.F. Effect of Tocopherols on the Anti-Polymerization Activity of Oryzanol and Corn Steryl Ferulates in Soybean Oil. J. Am. Oil. Chem. Soc. 2013, 90, 1351–1358. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.; Yi, B.; Kim, M.-J.; Kim, C.; Lee, J. Analysis of Trans Fat in Edible Oils with Cooking Process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Uriarte, P.S. Simultaneous Control of the Evolution of the Percentage in Weight of Polar Compounds, Iodine Value, Acyl Groups Proportions and Aldehydes Concentrations in Sunflower Oil Submitted to Frying Temperature in an Industrial Fryer. Food Control 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring of Heat-Induced Degradation of Edible Oils by Proton NMR. Eur. J. Lipid Sci. Technol. 2008, 110, 52–60. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Loureiro Mendes, A.C.; Jacobsen, C.; Ceylan, Z.; Chronakis, I.S.; Bean, S.R.; García Moreno, P.J. Development of Kafirin-Based Nanocapsules by Electrospraying for Encapsulation of Fish Oil. Lebensm. Wiss. Technol. 2021, 136, 110297. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.M.; Guadix, E.M. Development of Spray-Dried Microcapsules as Efficient Omega-3 Delivery Systems for Food Fortification Purposes. In Proceedings of the 2021 AOCS Annual Meeting & Expo, Atlanta, GA, USA, 3–14 May 2021; Volume 98, pp. 127–128. [Google Scholar]
- Yesiltas, B.; García Moreno, P.J.; Sørensen, A.-D.M.; Soria Caindec, A.M.; Hyldig, G.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Enrichment of Mayonnaise with a High Fat Fish Oil-in-Water Emulsion Stabilized with Modified DATEM C14 Enhances Oxidative Stability. Food Chem. 2021, 341, 128141. [Google Scholar] [CrossRef]
- Todt, H.; Guthausen, G.; Burk, W.; Schmalbein, D.; Kamlowski, A. Water/Moisture and Fat Analysis by Time-Domain NMR. Food Chem. 2006, 96, 436–440. [Google Scholar] [CrossRef]
- Tang, F.; Vasas, M.; Hatzakis, E.; Spyros, A. Magnetic Resonance Applications in Food Analysis. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 98, pp. 239–306. [Google Scholar]
- Hills, B.P. Applications of Low-Field NMR to Food Science. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2006; Volume 58, pp. 177–230. [Google Scholar]
- Tyl, C.E.; Brecker, L.; Wagner, K. 1 H NMR Spectroscopy as Tool to Follow Changes in the Fatty Acids of Fish Oils. Eur. J. Lipid Sci. Technol. 2008, 110, 141–148. [Google Scholar] [CrossRef]
- Blümich, B. Introduction to Compact NMR: A Review of Methods. Trends Anal. Chem. 2016, 83, 2–11. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M. Recent Developments in the Food Quality Detected by Non-Invasive Nuclear Magnetic Resonance Technology. Crit. Rev. Food. Sci. Nutr. 2019, 59, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Rondeau-Mouro, C. 2D TD-NMR Analysis of Complex Food Products. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1483–1502. ISBN 978-3-319-28388-3. [Google Scholar]
- van Duynhoven, J.; Voda, A.; Witek, M.; Van As, H. Time-Domain NMR Applied to Food Products. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2010; Volume 69, pp. 145–197. [Google Scholar]
- Campisi-Pinto, S.; Levi, O.; Benson, D.; Cohen, M.; Resende, M.T.; Saunders, M.; Linder, C.; Wiesman, Z. Analysis of the Regularization Parameters of Primal–Dual Interior Method for Convex Objectives Applied to 1H Low Field Nuclear Magnetic Resonance Data Processing. Appl. Magn. Reson. 2018, 49, 1129–1150. [Google Scholar] [CrossRef]
- Campisi-Pinto, S.; Levi, O.; Benson, D.; Resende, M.T.; Saunders, M.; Linder, C.; Wiesman, Z. Simulation-Based Sensitivity Analysis of Regularization Parameters for Robust Reconstruction of Complex Material’s T1-T21H LF-NMR Energy Relaxation Signals. Appl. Magn. Reson. 2020, 51, 41–58. [Google Scholar] [CrossRef]
- Berman, P.; Leshem, A.; Etziony, O.; Levi, O.; Parmet, Y.; Saunders, M.; Wiesman, Z. Novel 1H Low Field Nuclear Magnetic Resonance Applications for the Field of Biodiesel. Biotechnol. Biofuels 2013, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, P.; Levi, O.; Parmet, Y.; Saunders, M.; Wiesman, Z. Laplace Inversion of Low-Resolution NMR Relaxometry Data Using Sparse Representation Methods. Concepts Magn. Reson. A Bridg. Educ. Res. 2013, 42, 72–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, M.T.; Osheter, T.; Linder, C.; Wiesman, Z. Proton Low Field NMR Relaxation Time Domain Sensor for Monitoring of Oxidation Stability of PUFA-Rich Oils and Emulsion Products. Foods 2021, 10, 1385. [Google Scholar] [CrossRef]
- Resende, M.T.; Linder, C.; Wiesman, Z. Alkyl Tail Segments Mobility as a Marker for Omega-3 Polyunsaturated Fatty Acid-Rich Linseed Oil Oxidative Aging. J. Am. Oil. Chem. Soc. 2020, 97, 1283–1297. [Google Scholar] [CrossRef]
- Berman, P.; Meiri, N.; Colnago, L.A.; Moraes, T.B.; Linder, C.; Levi, O.; Parmet, Y.; Saunders, M.; Wiesman, Z. Study of Liquid-Phase Molecular Packing Interactions and Morphology of Fatty Acid Methyl Esters (Biodiesel). Biotechnol. Biofuels 2015, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Iwahashi, M.; Kasahara, Y. Dynamic Molecular Movements and Aggregation Structures of Lipids in a Liquid State. Curr. Opin. Colloid Interface Sci. 2011, 16, 359–366. [Google Scholar] [CrossRef]
- Fhaner, M.; Hwang, H.; Winkler-Moser, J.K.; Bakota, E.L.; Liu, S.X. Protection of Fish Oil from Oxidation with Sesamol. Eur. J. Lipid Sci. Technol. 2016, 118, 885–897. [Google Scholar] [CrossRef]
- Shorthouse, J. Intralipid®. In A Dictionary of Anaesthesia; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-182605-4. [Google Scholar]
- Fridjonsson, E.O.; Graham, B.F.; Akhfash, M.; May, E.F.; Johns, M.L. Optimized Droplet Sizing of Water-in-Crude Oil Emulsions Using Nuclear Magnetic Resonance. Energy Fuels 2014, 28, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Haiduc, A.M.; Trezza, E.E.; van Dusschoten, D.; Reszka, A.A.; van Duynhoven, J.P.M. Non-Invasive ‘through-Package’ Assessment of the Microstructural Quality of a Model Food Emulsion by the NMR MOUSE. LWT 2007, 40, 737–743. [Google Scholar] [CrossRef]
| Linseed Control | Linseed after Heating (180 °C for 20 min) | ||||||
|---|---|---|---|---|---|---|---|
| Peaks | T1 (ms) | T2 (ms) | Assignment | Peaks | T1 (ms) | T2 (ms) | Assignment |
| 1 | 13.651 | 0.606 | Lignoc * | 1 | 24.517 | 1.793 | Lignoc |
| 2 | 51.205 | 4.866 | Protein | 2 | 28.408 | 12.107 | Lignoc |
| 3 | 106.945 | 0.705 | Lignoc | 3 | 79.656 | 35.837 | Gly |
| 4 | 115.118 | 0.69 | Lignoc | 4 | 115.118 | 63.009 | Gly |
| 5 | 85.744 | 29.478 | Gly * | 5 | 192.769 | 2.228 | Protein |
| 6 | 92.298 | 34.315 | Gly | 6 | 179.082 | 150.116 | Gly |
| 7 | 133.387 | 103.798 | DB * | 7 | 192.769 | 163.73 | DB |
| 8 | 347.468 | 203.416 | DB | 8 | 502.156 | 335.099 | DB |
| 9 | 374.024 | 226.732 | Aliphatic * | 9 | 581.846 | 390.079 | Aliphatic |
| 10 | 674.183 | 474.223 | Tail * | 10 | 840.876 | 552.029 | Tail |
| 11 | 781.173 | 552.029 | Tail | 11 | 1631.526 | 1058.595 | Tail |
| 12 | 1048.784 | 781.217 | Tail | 12 | 1890.443 | 1232.279 | Tail |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osheter, T.; Linder, C.; Wiesman, Z. Time Domain (TD) Proton NMR Analysis of the Oxidative Safety and Quality of Lipid-Rich Foods. Biosensors 2022, 12, 230. https://doi.org/10.3390/bios12040230
Osheter T, Linder C, Wiesman Z. Time Domain (TD) Proton NMR Analysis of the Oxidative Safety and Quality of Lipid-Rich Foods. Biosensors. 2022; 12(4):230. https://doi.org/10.3390/bios12040230
Chicago/Turabian StyleOsheter, Tatiana, Charles Linder, and Zeev Wiesman. 2022. "Time Domain (TD) Proton NMR Analysis of the Oxidative Safety and Quality of Lipid-Rich Foods" Biosensors 12, no. 4: 230. https://doi.org/10.3390/bios12040230
APA StyleOsheter, T., Linder, C., & Wiesman, Z. (2022). Time Domain (TD) Proton NMR Analysis of the Oxidative Safety and Quality of Lipid-Rich Foods. Biosensors, 12(4), 230. https://doi.org/10.3390/bios12040230





