Recent Progress on Micro-Fabricated Alkali Metal Vapor Cells
Abstract
1. Introduction
2. Recent Progress on Micro-Fabricated Alkali Vapor Cells
2.1. Bonding Methods
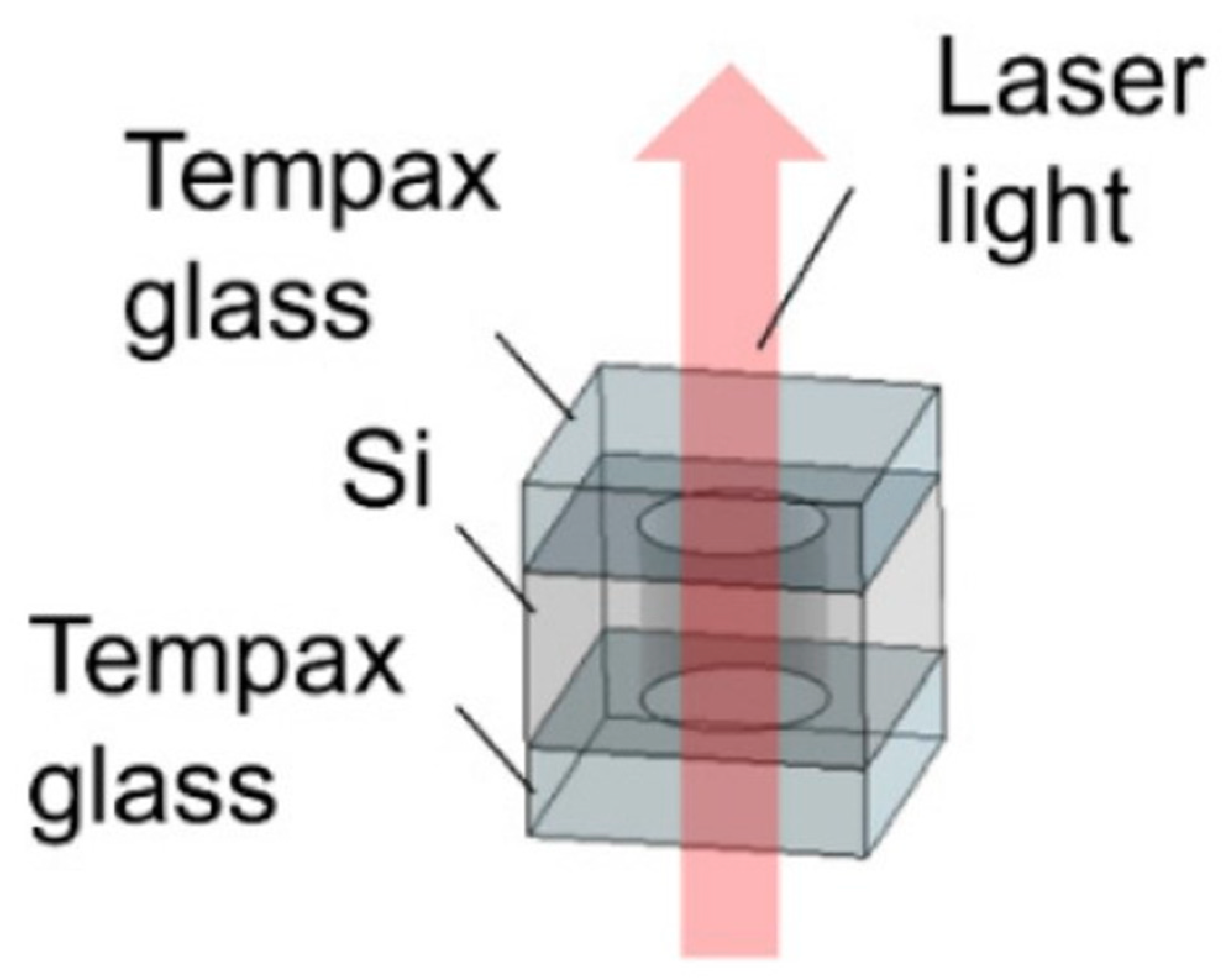
2.1.1. Glass–Silicon–Glass Anodic Bonding
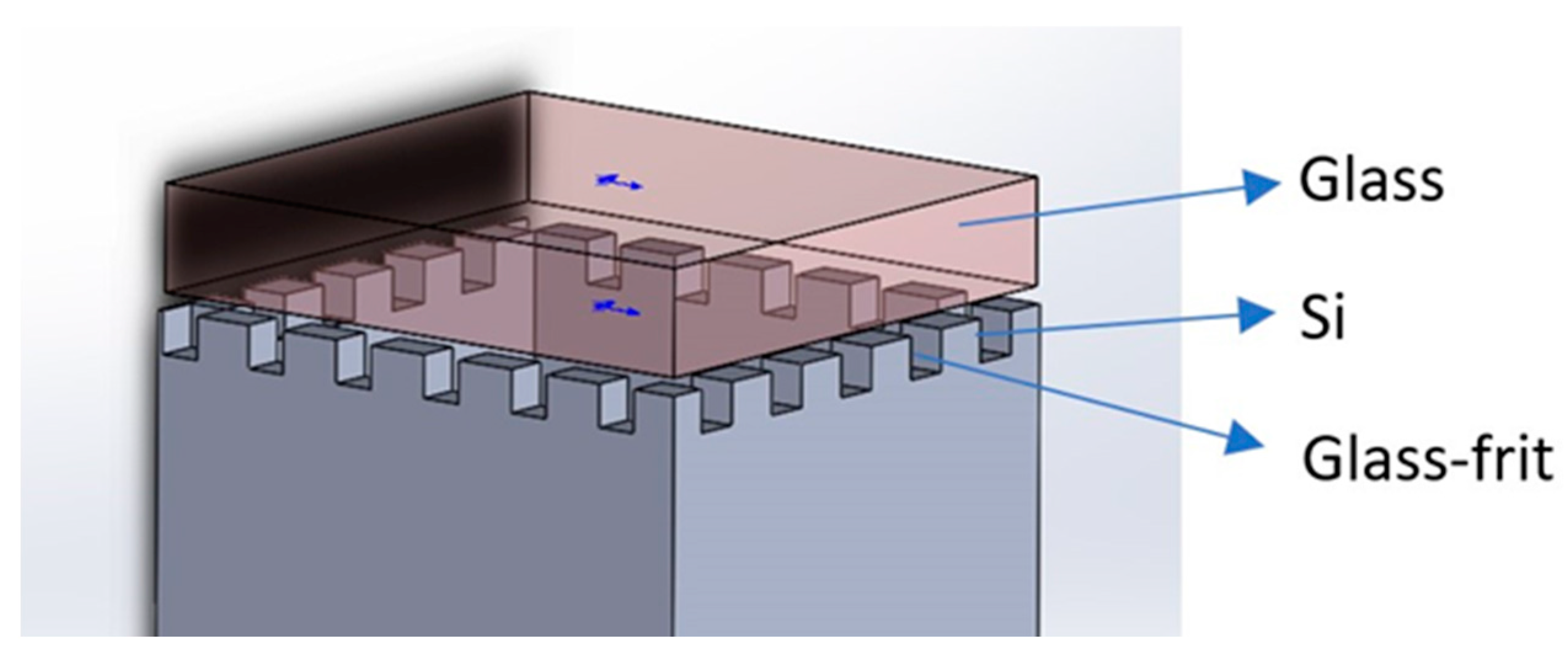
2.1.2. Sacrificial Micro-Channel Bonding
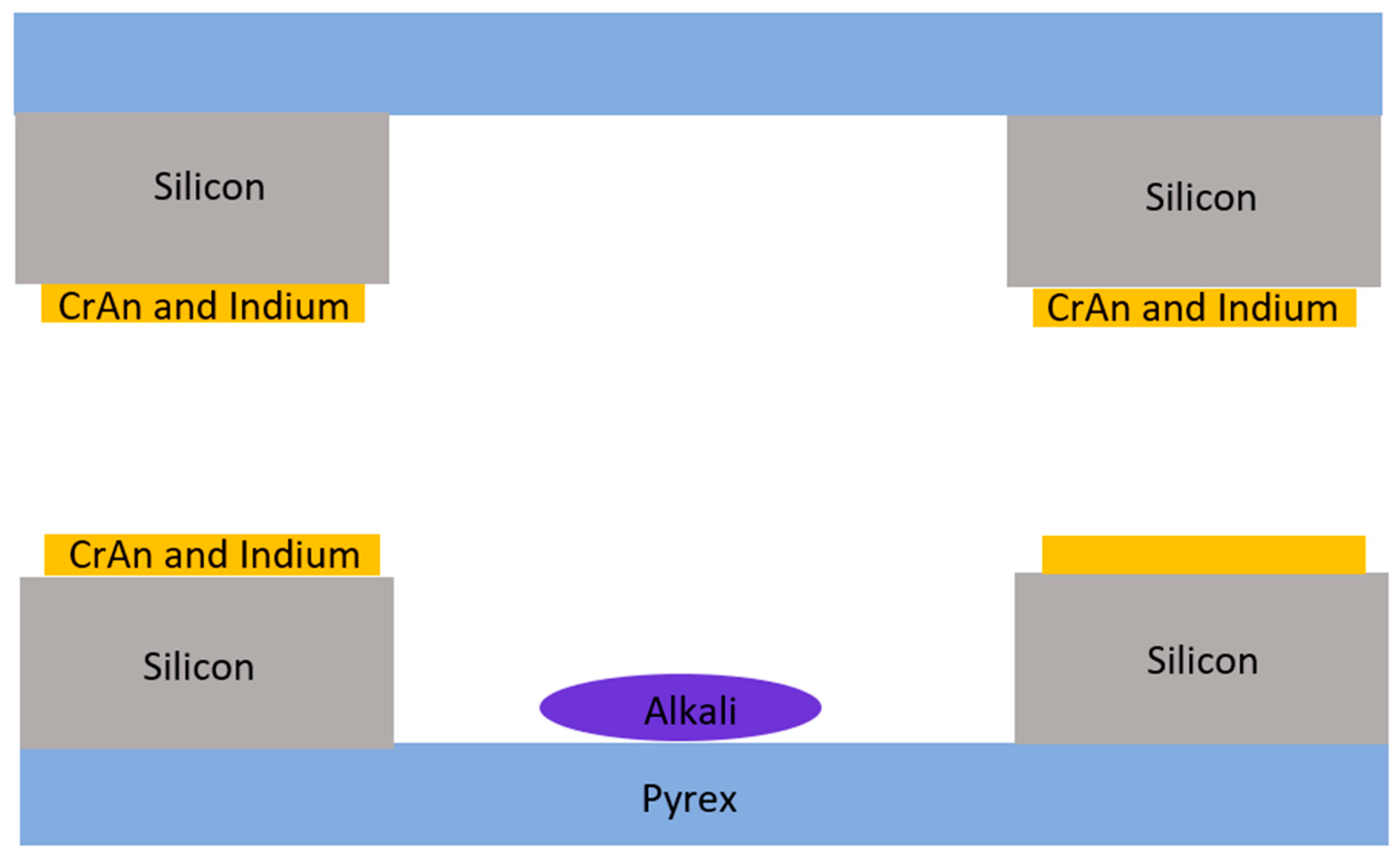
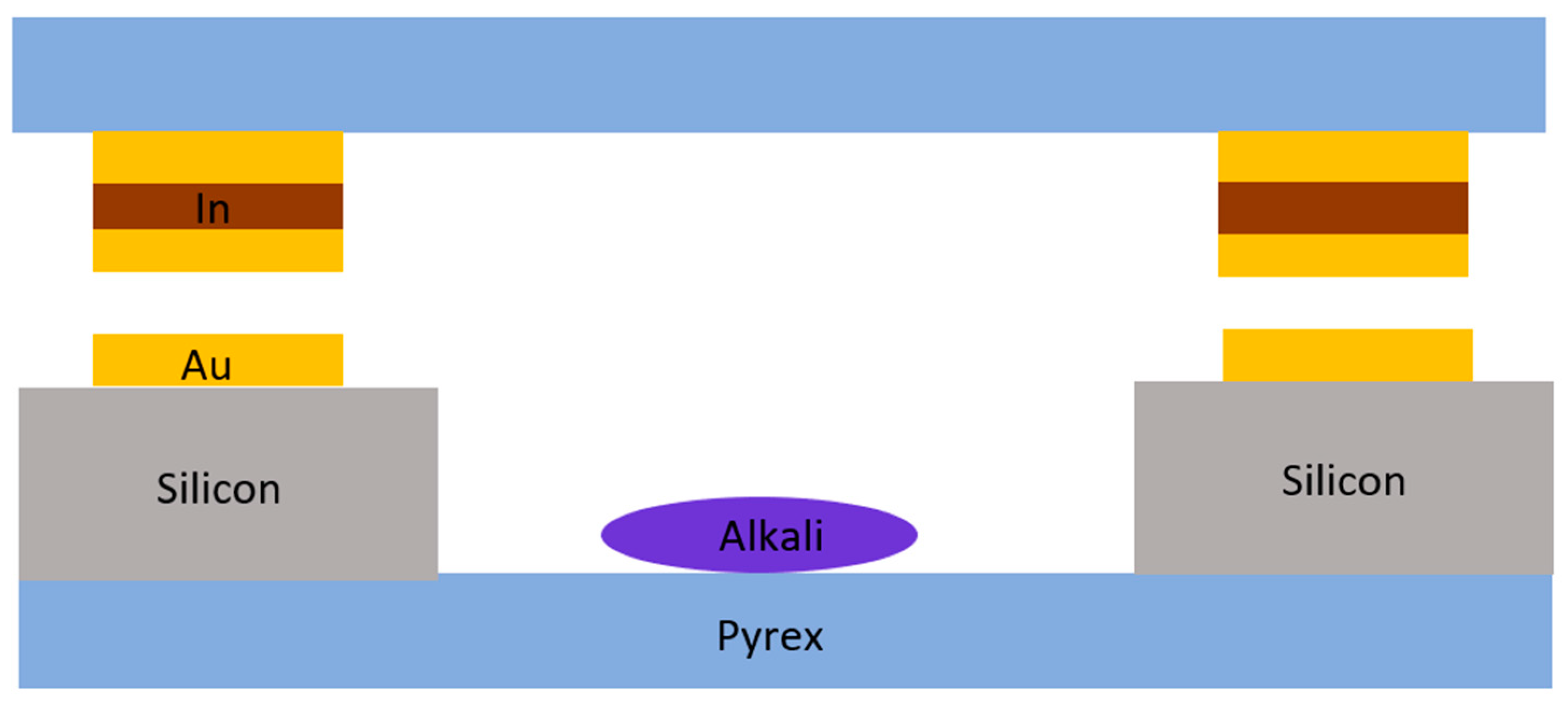
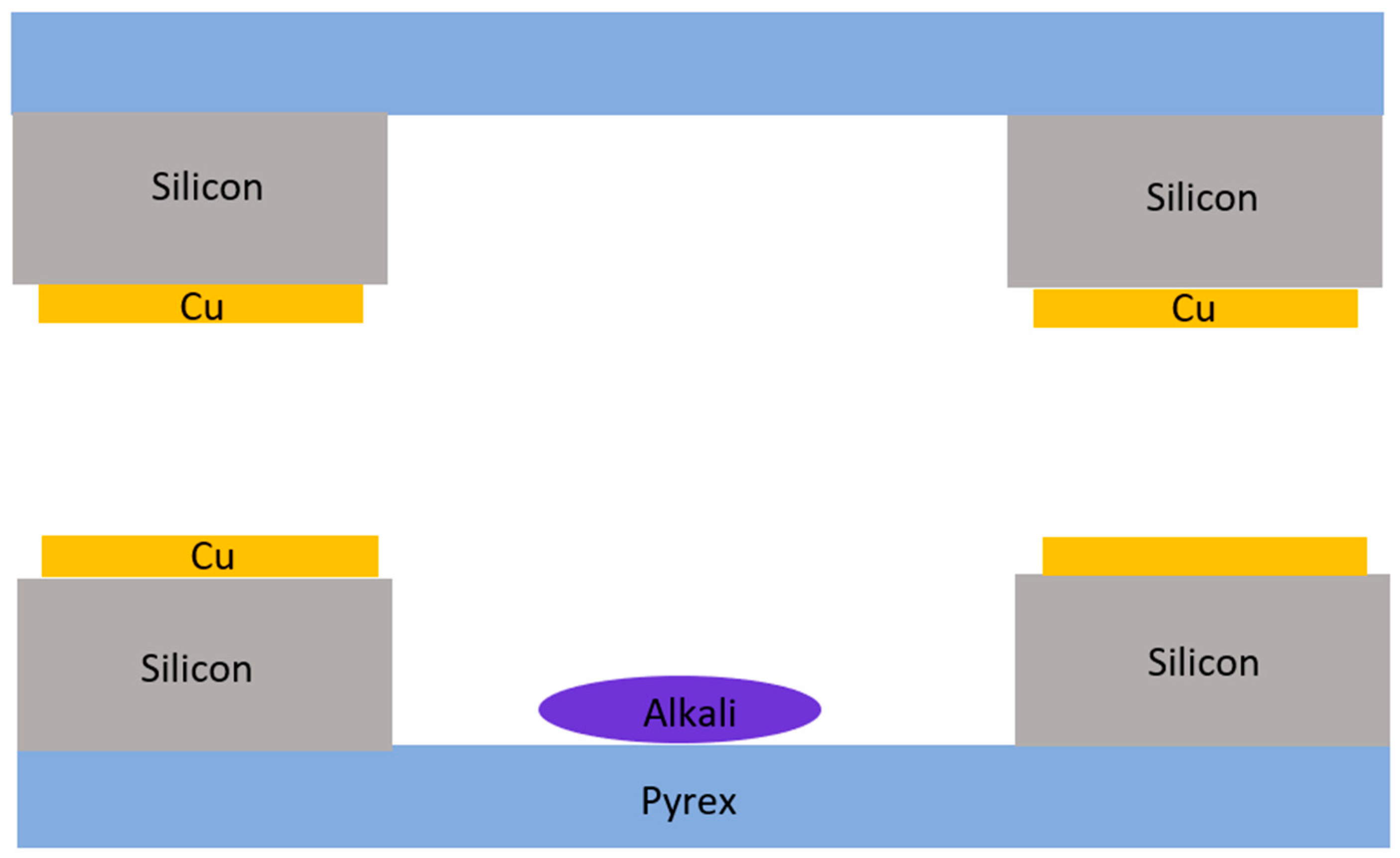
2.1.3. Metal Film Thermocompression Bonding
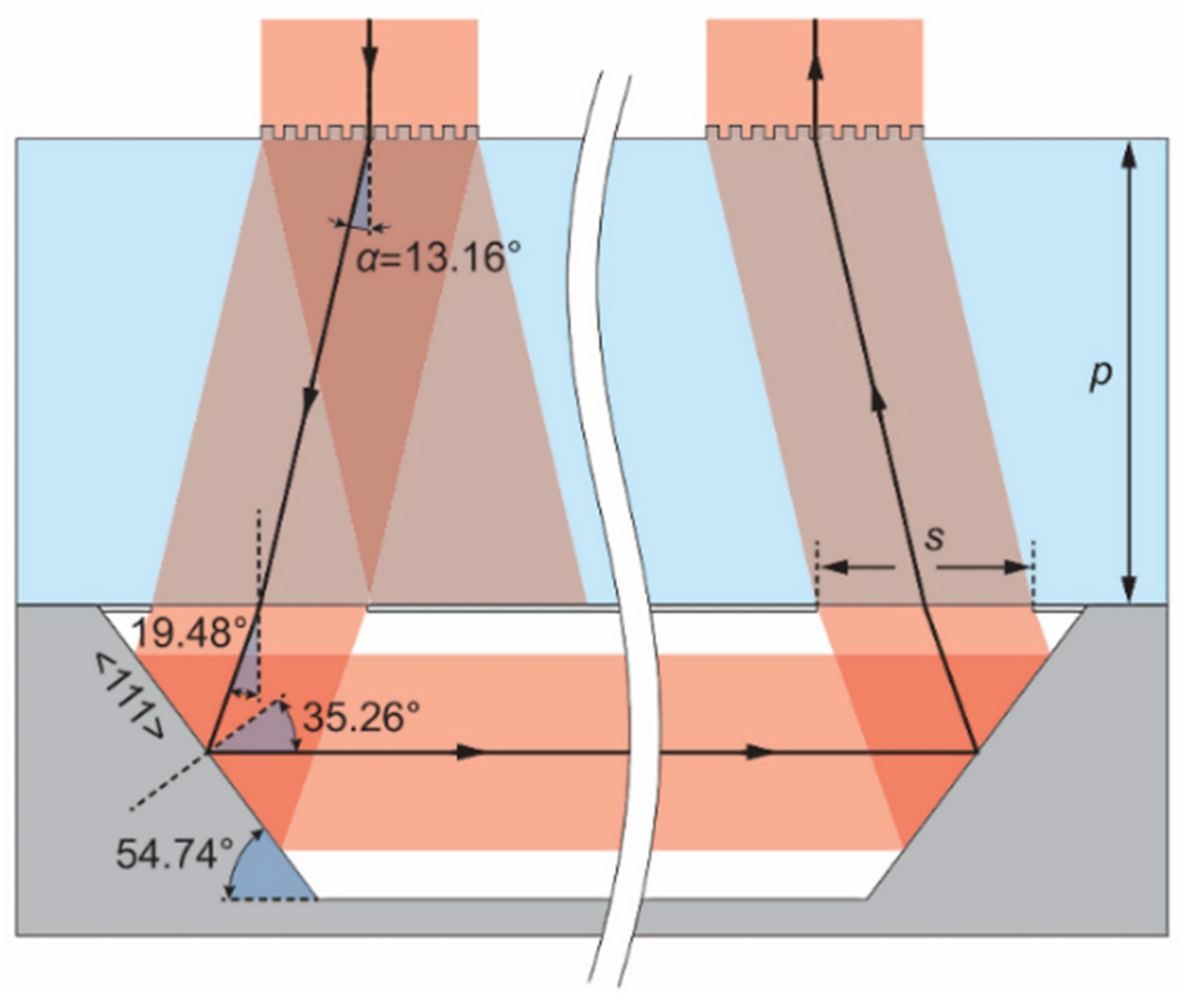
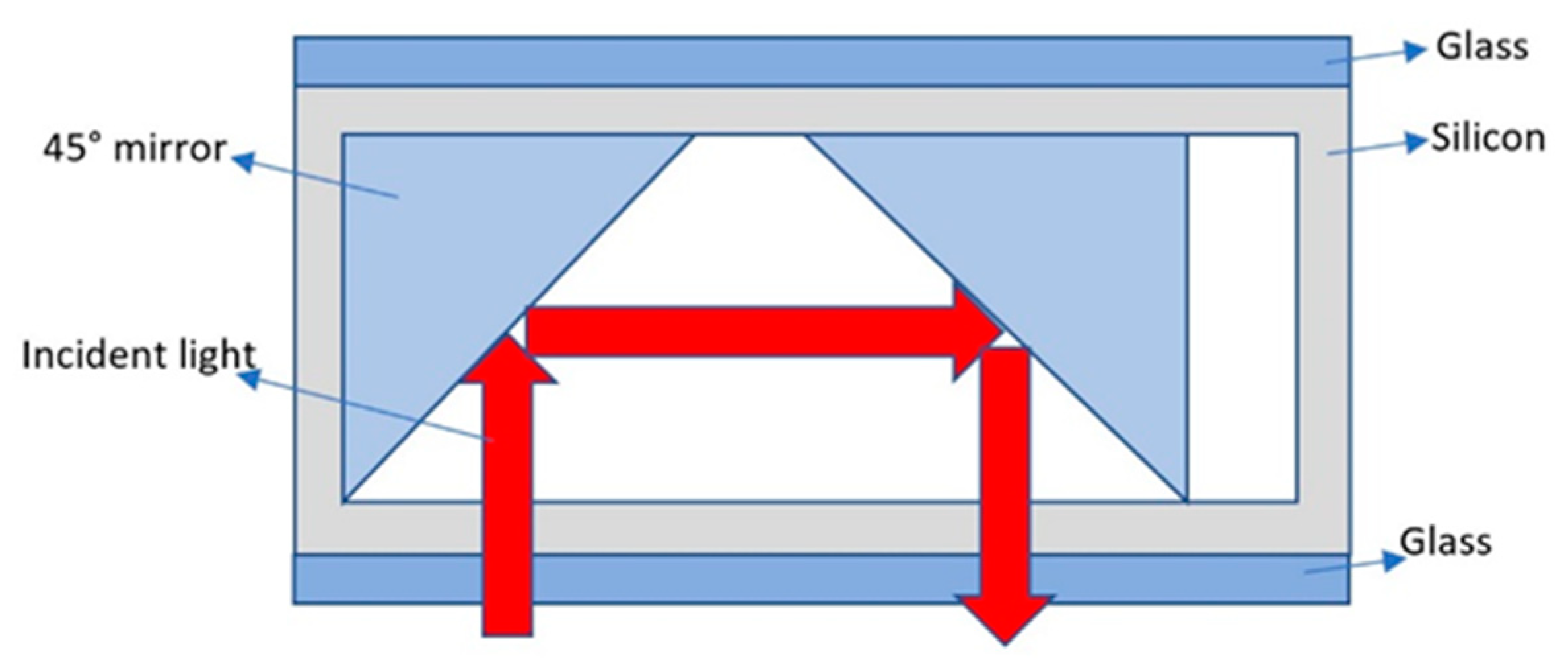
2.2. Design of Light-Passing Scheme for Alkali Vapor Cells
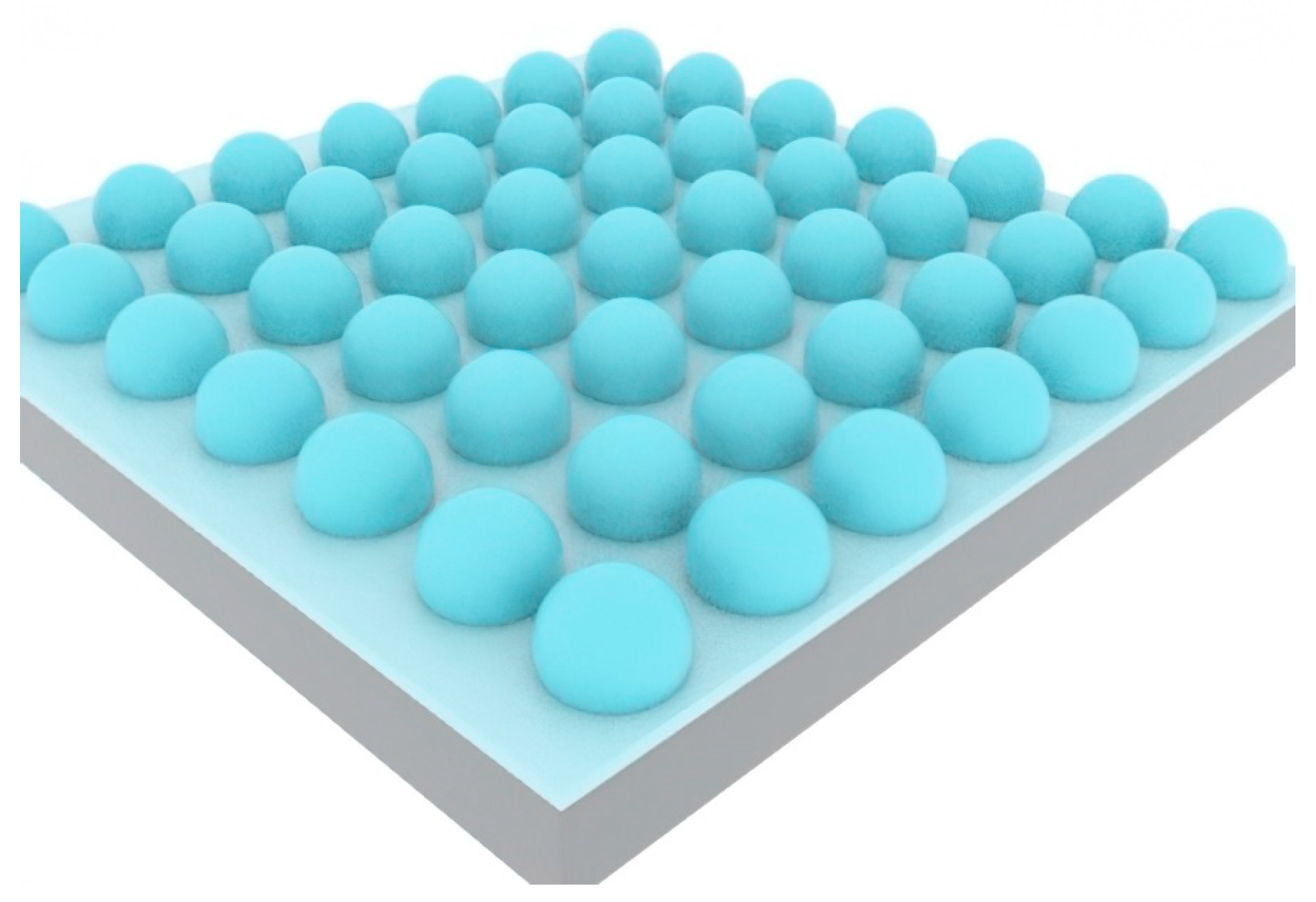
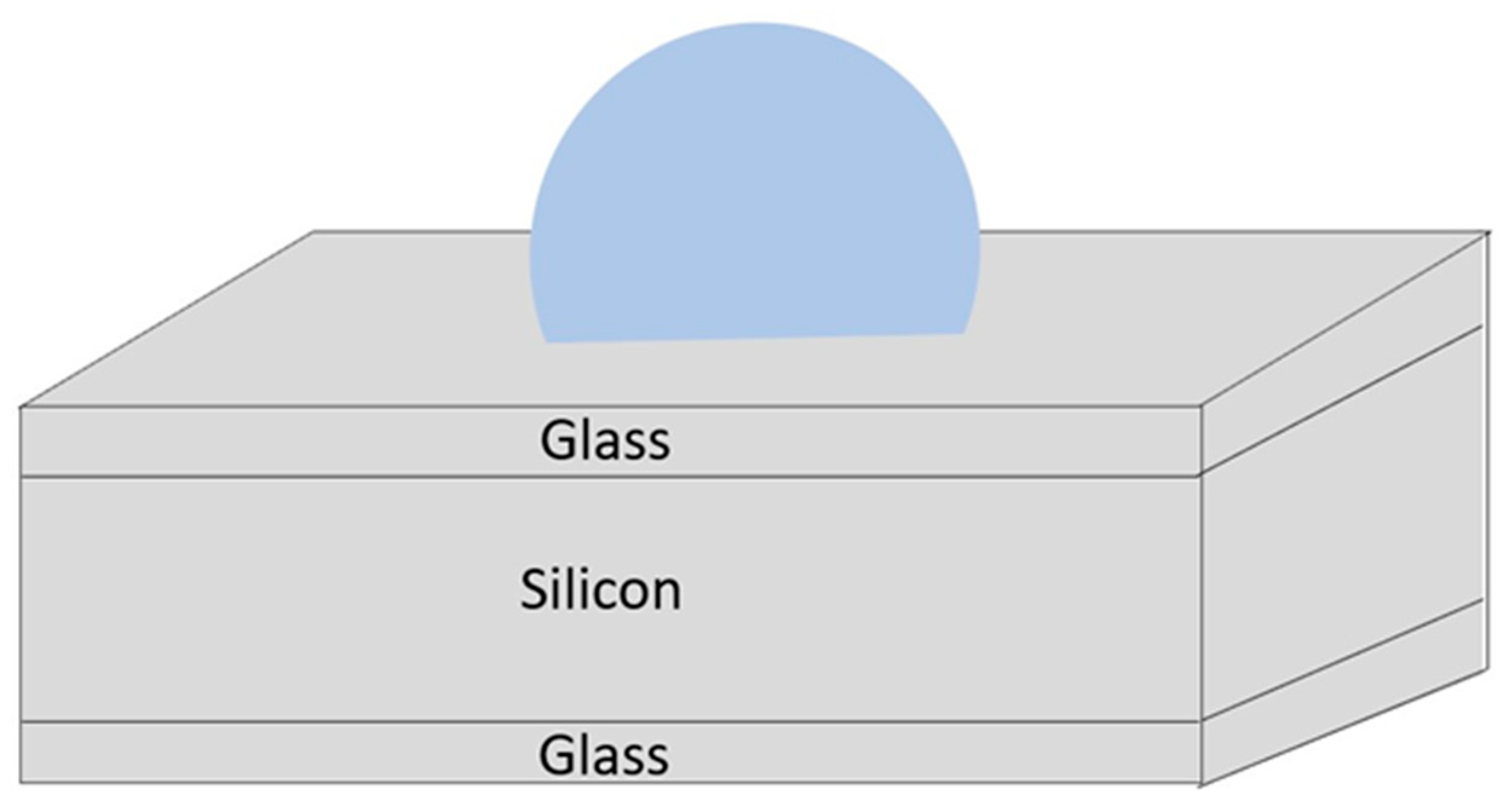
2.2.1. Spherical Alkali Vapor Cells through Wafer Blowing
2.2.2. Anodic Bonding Square Alkali Vapor Cell
2.3. Alkali Metal Packaging Methods in Micro-Fabricated Alkali Vapor Cells
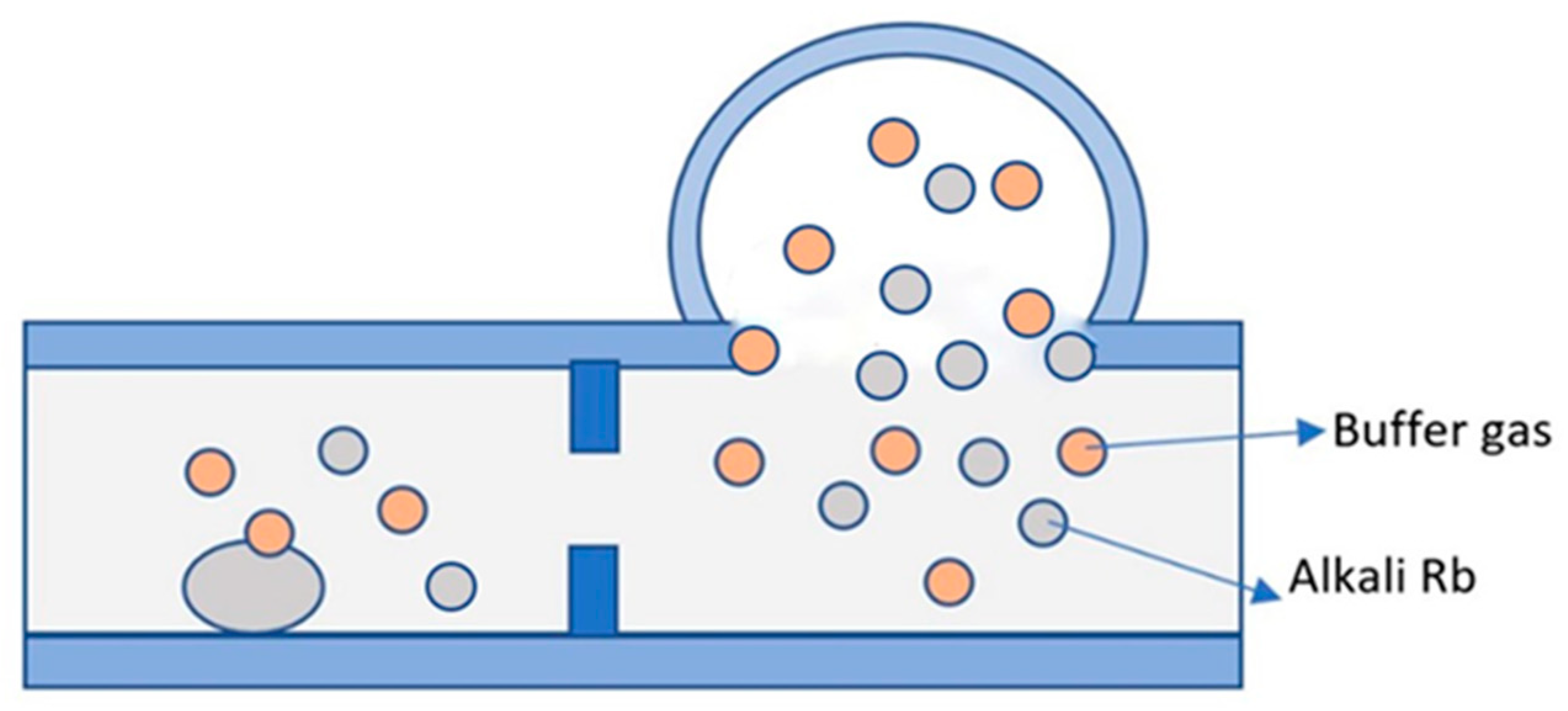
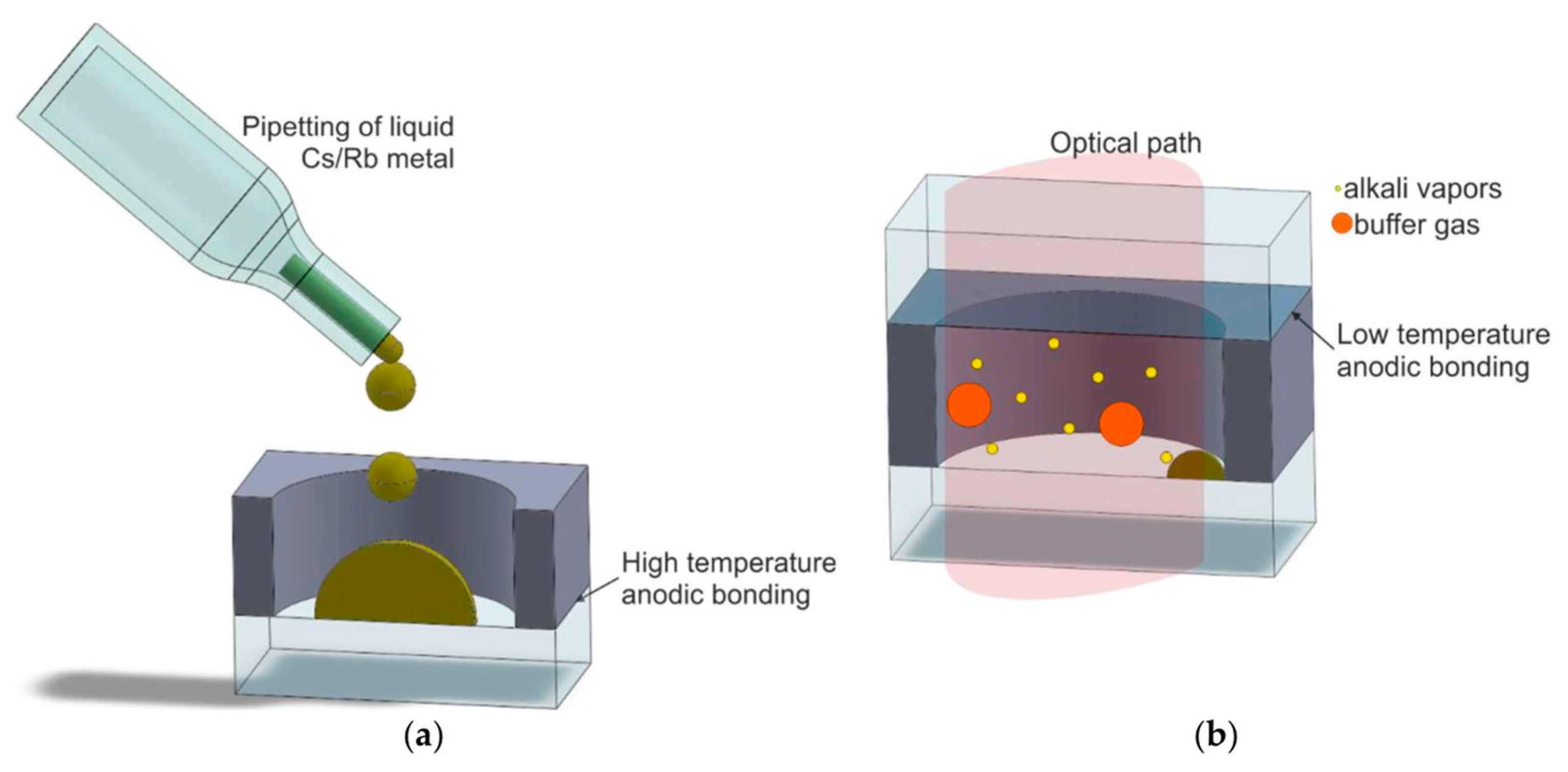
2.3.1. Direct Filling Method of Alkali Metal Elements in the Physical Method
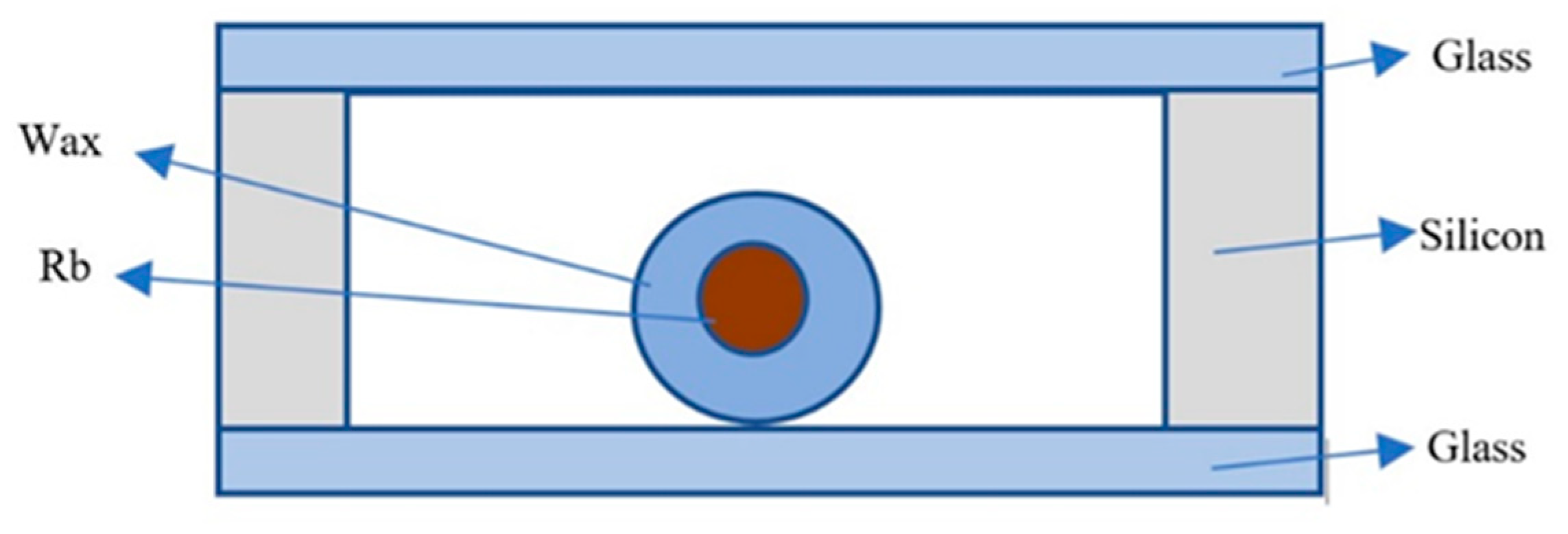
2.3.2. Alkali Metal Wax Bag Filling Method in the Physical Method
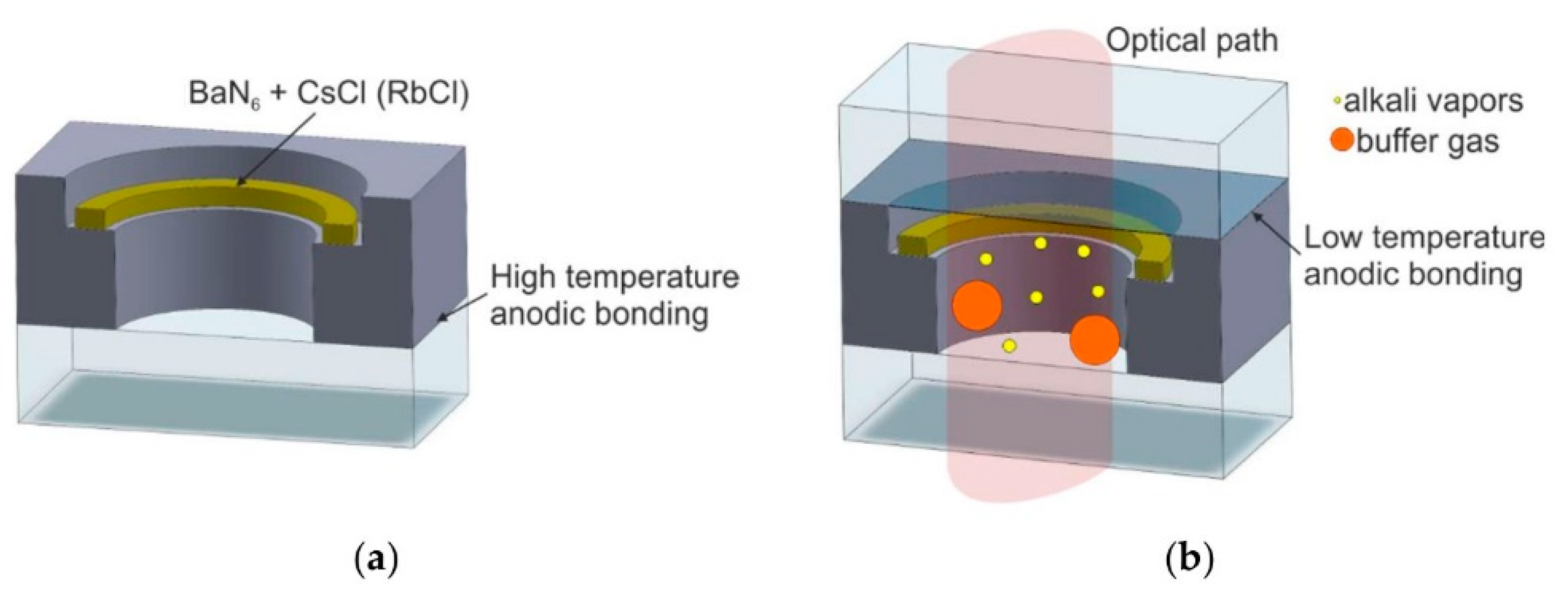
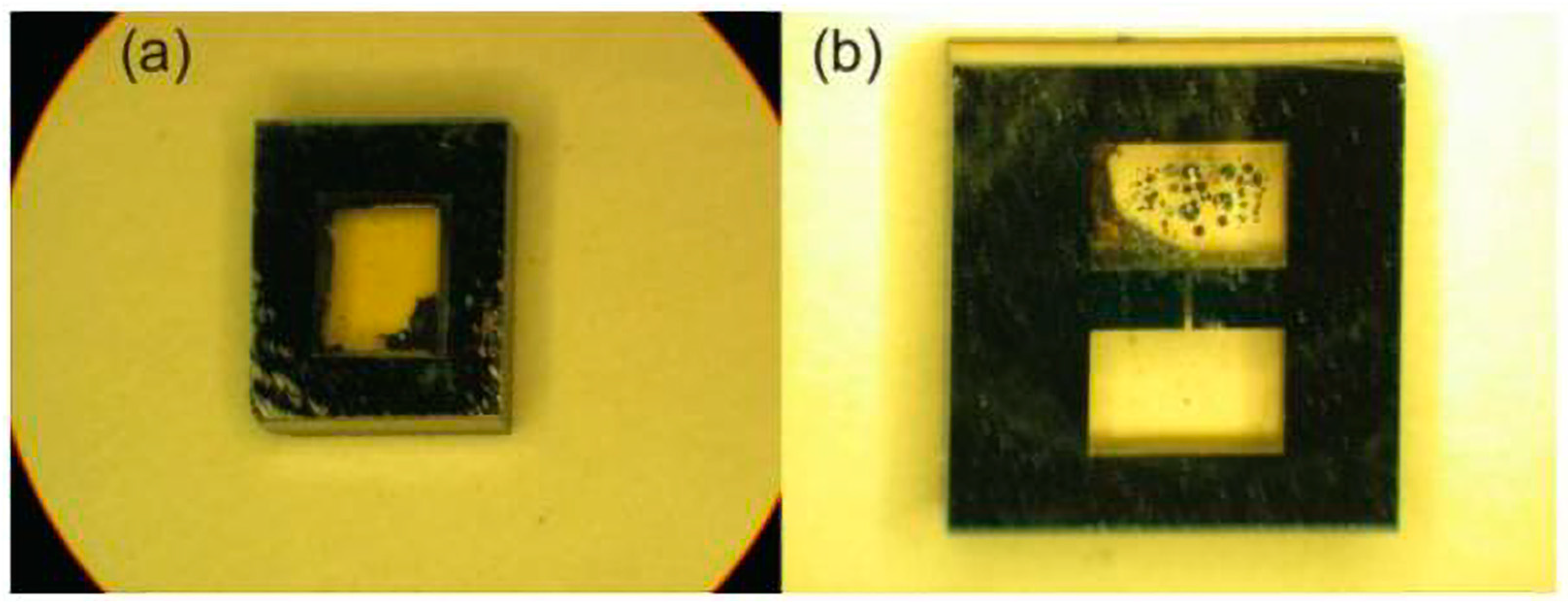
2.3.3. Chemical Reaction to Form Alkali Metal
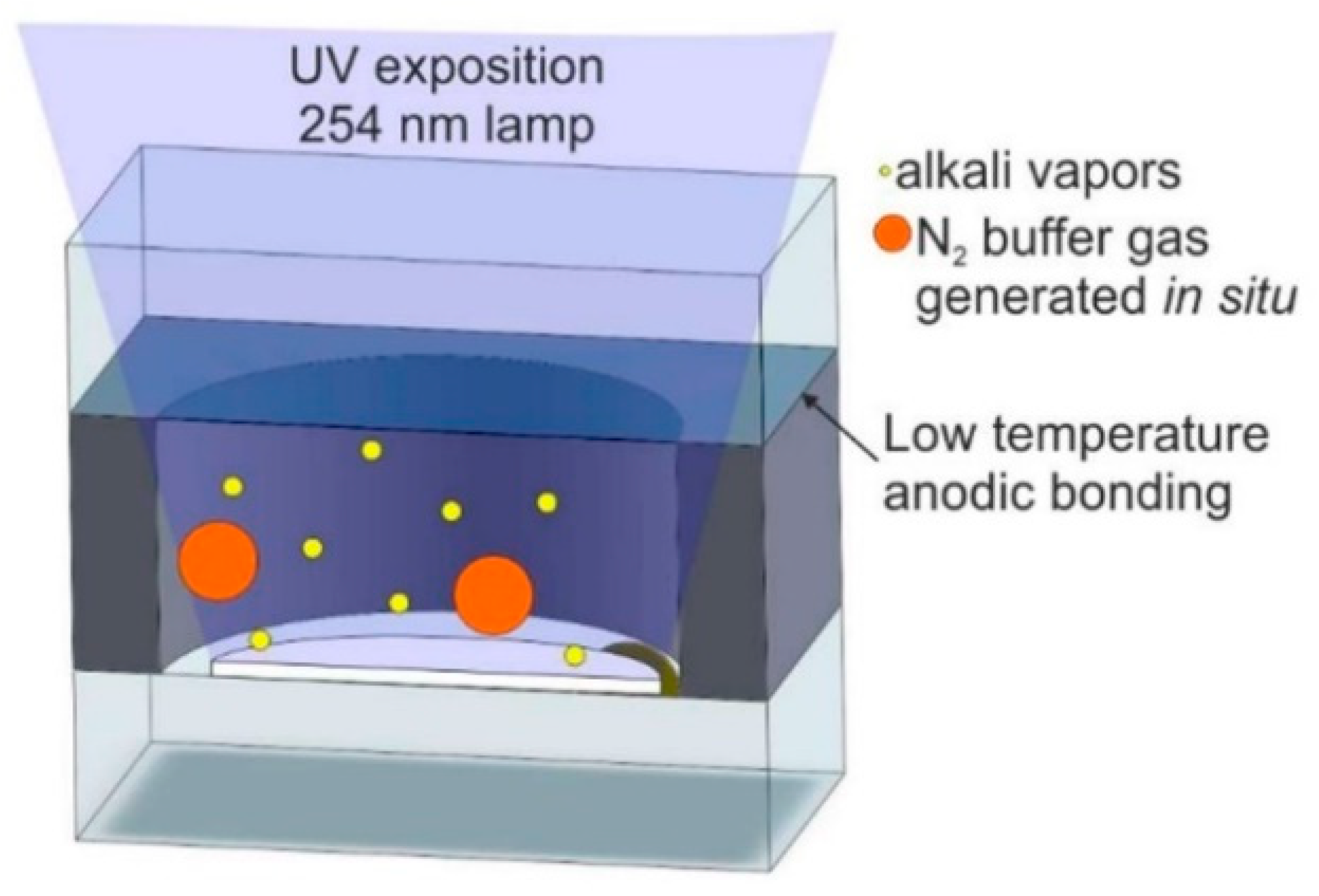
2.3.4. UV Decomposition Method
2.3.5. Electrochemical Decomposition
3. Applications and Outlooks of Alkali Vapor Cells
3.1. Applications and Recent Progress on Alkali Vapor Cells
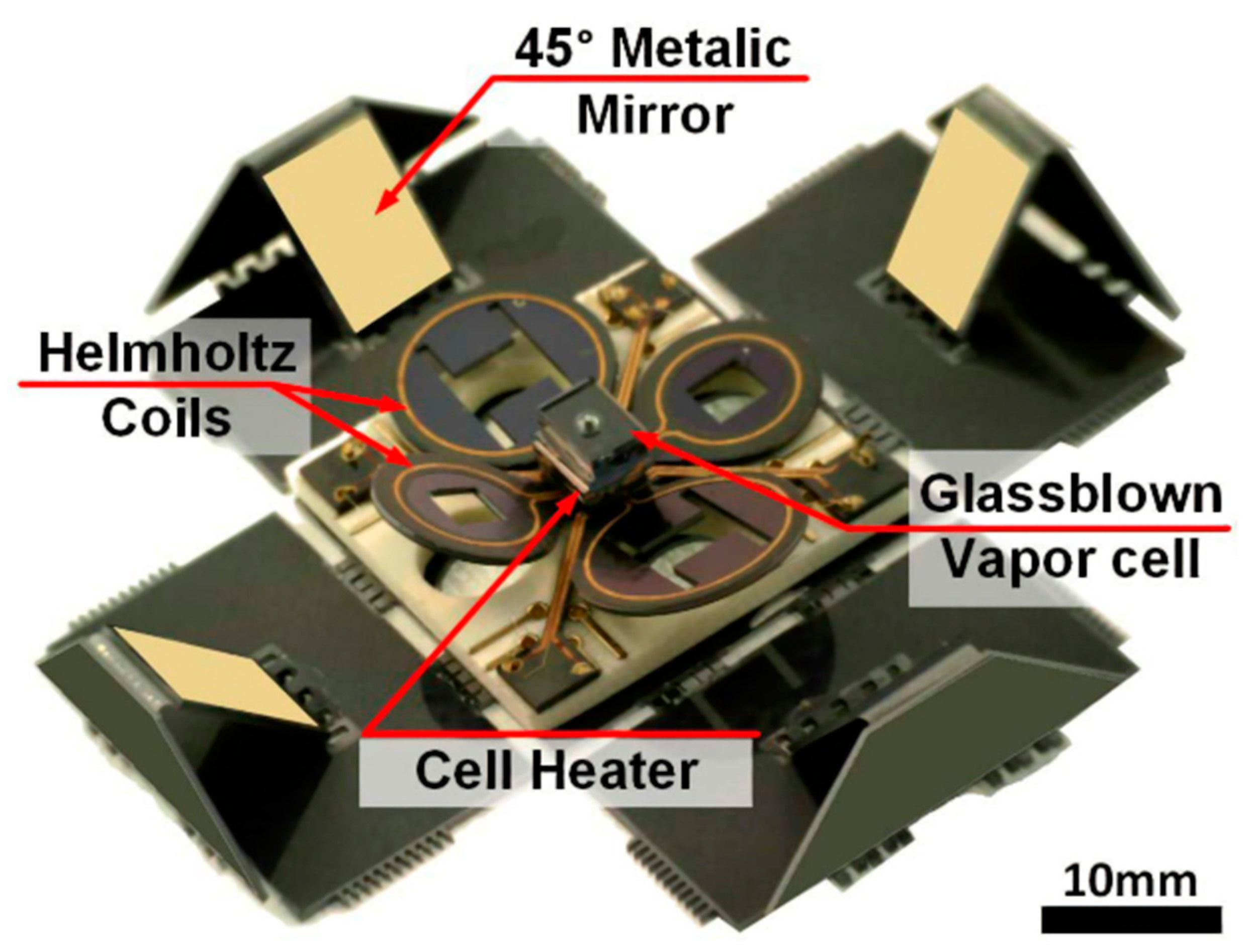
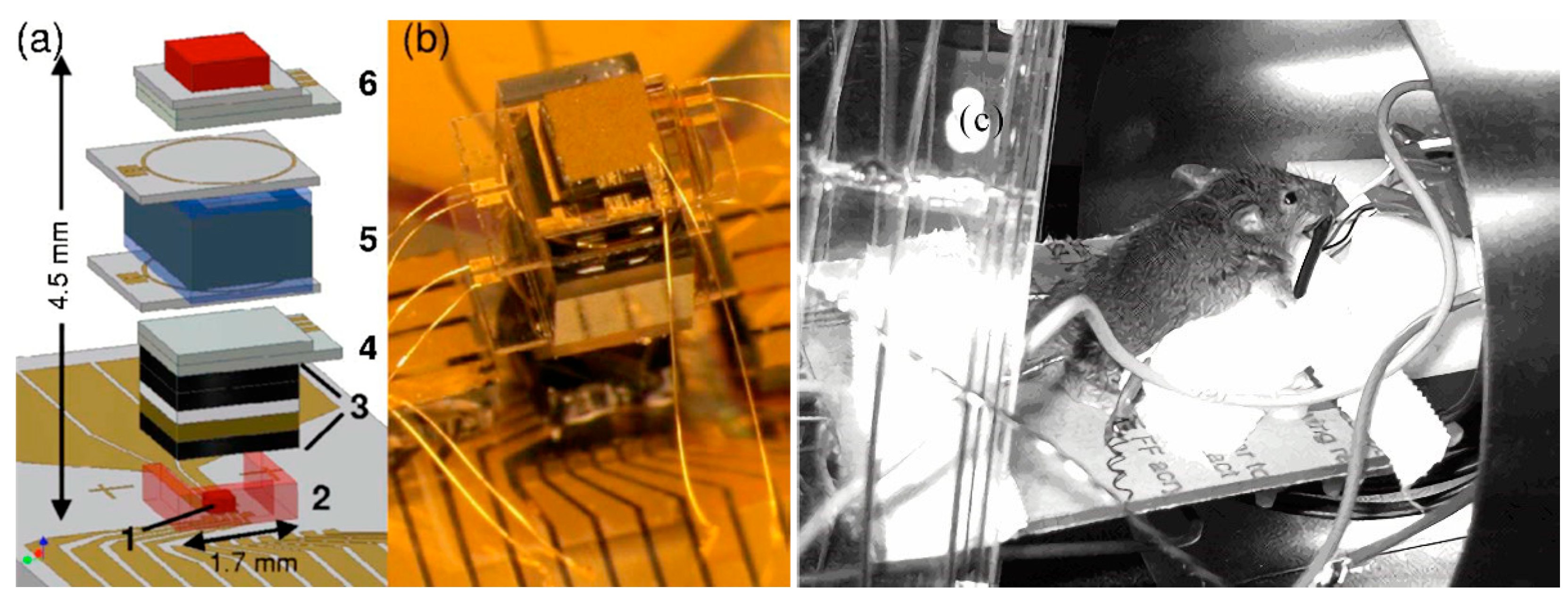
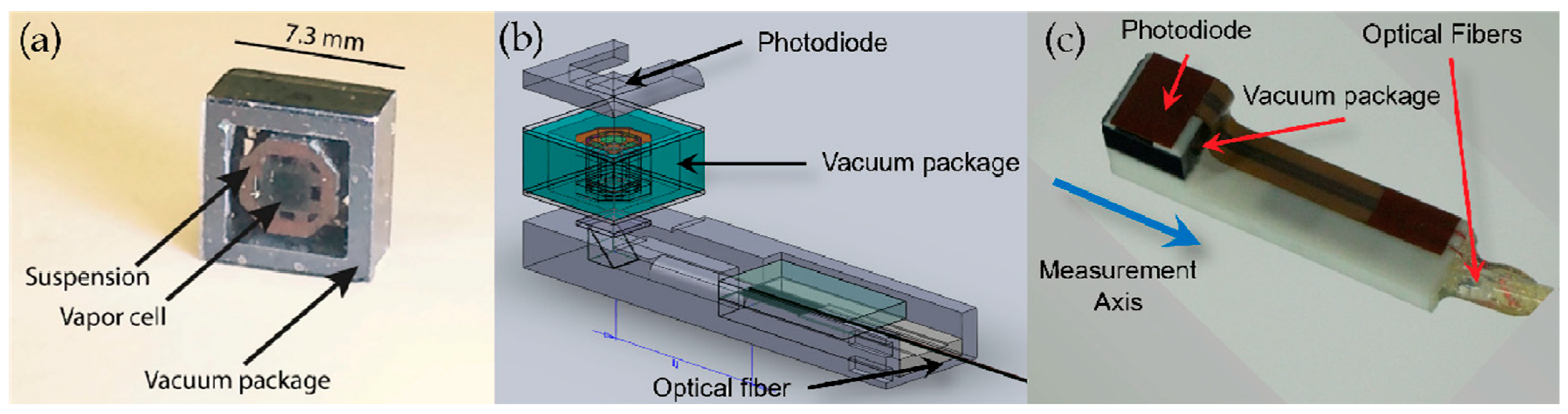
3.1.1. Application of Atomic Gyroscope to Micro-Fabricated Alkali Vapor Cells
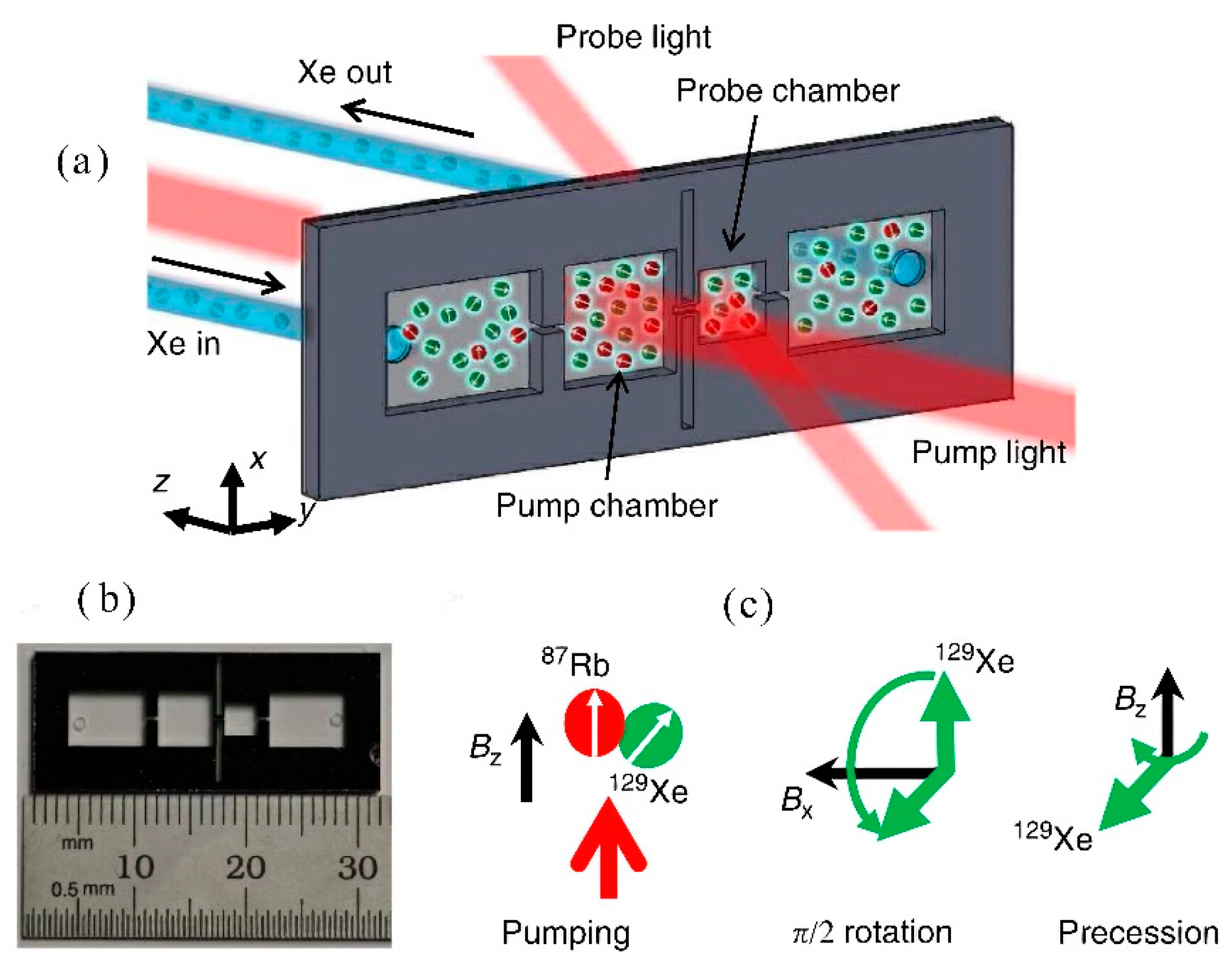
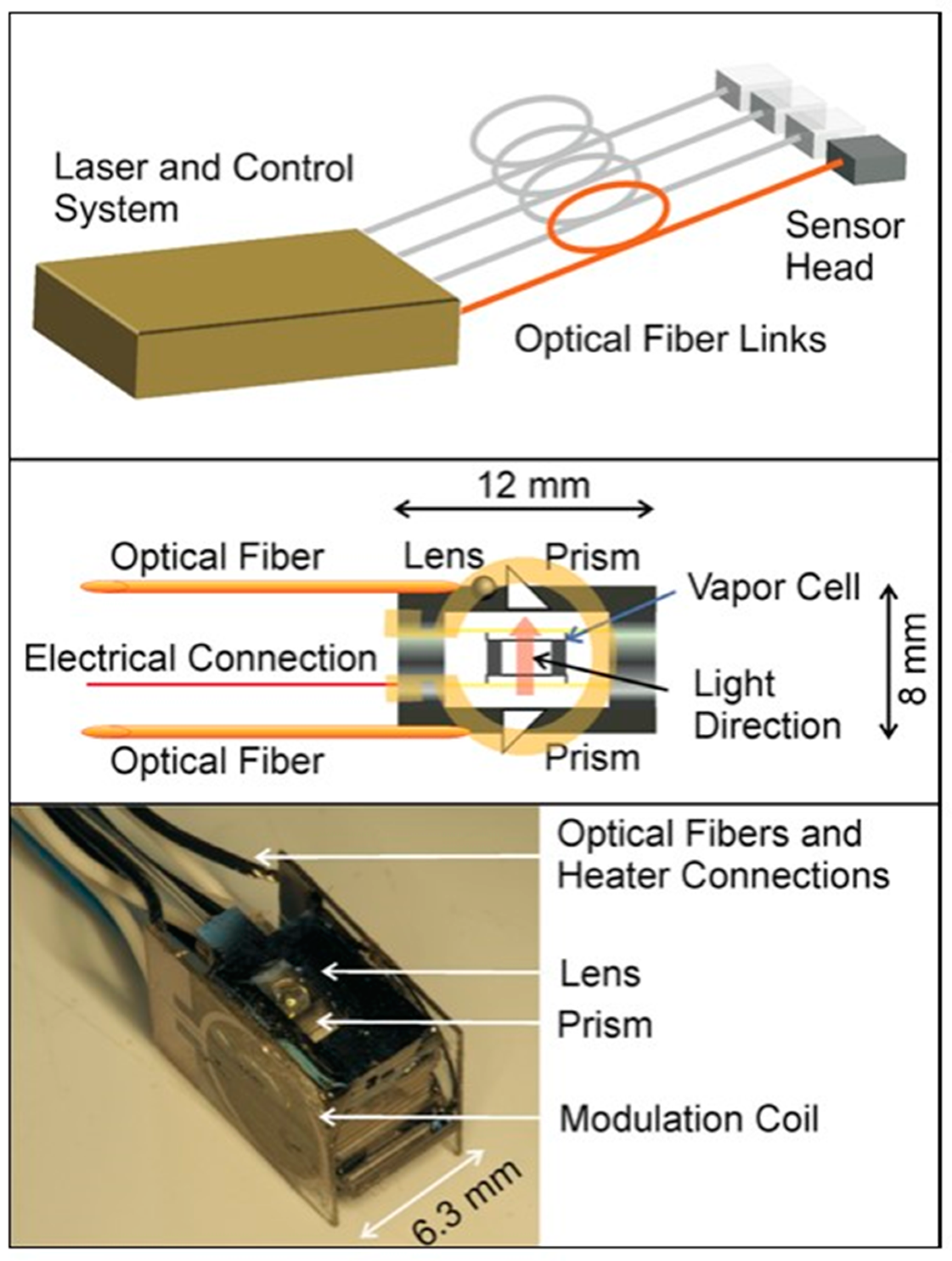
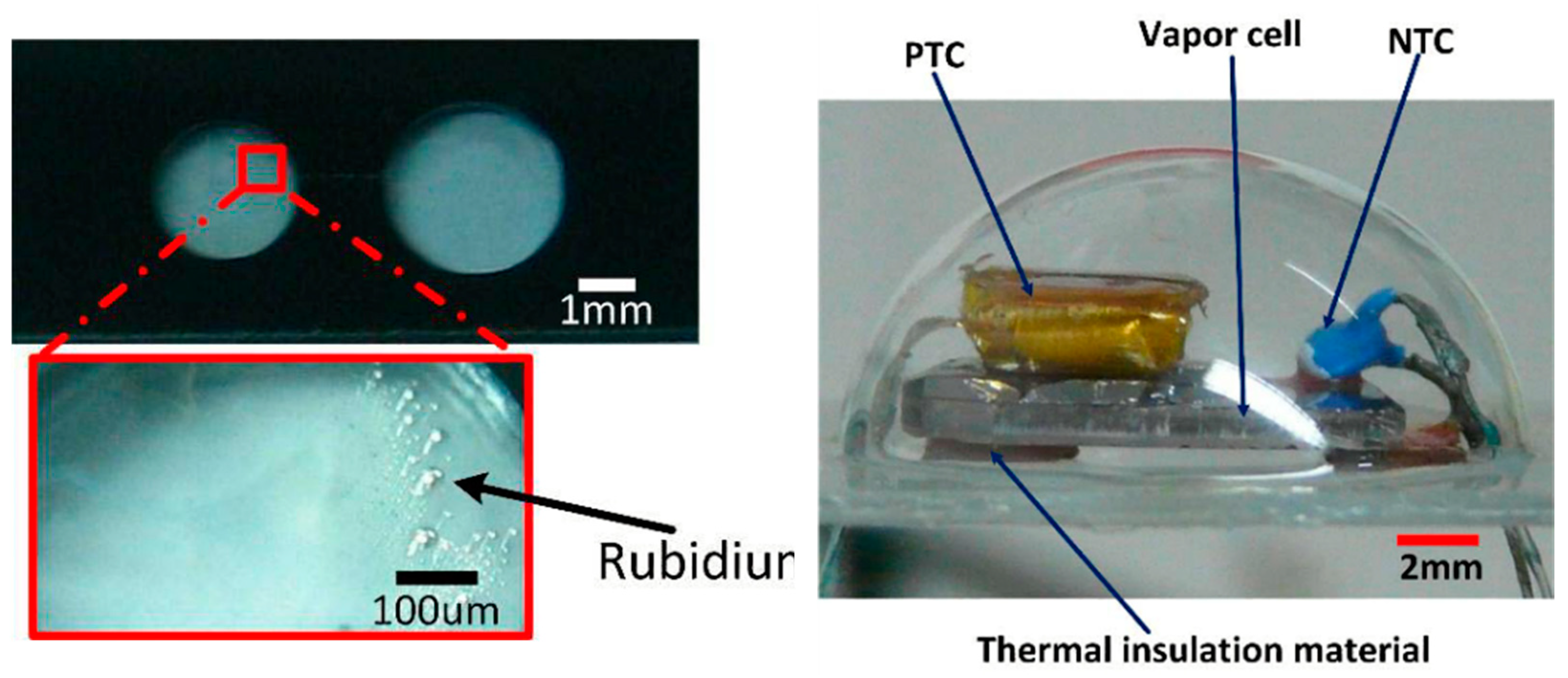
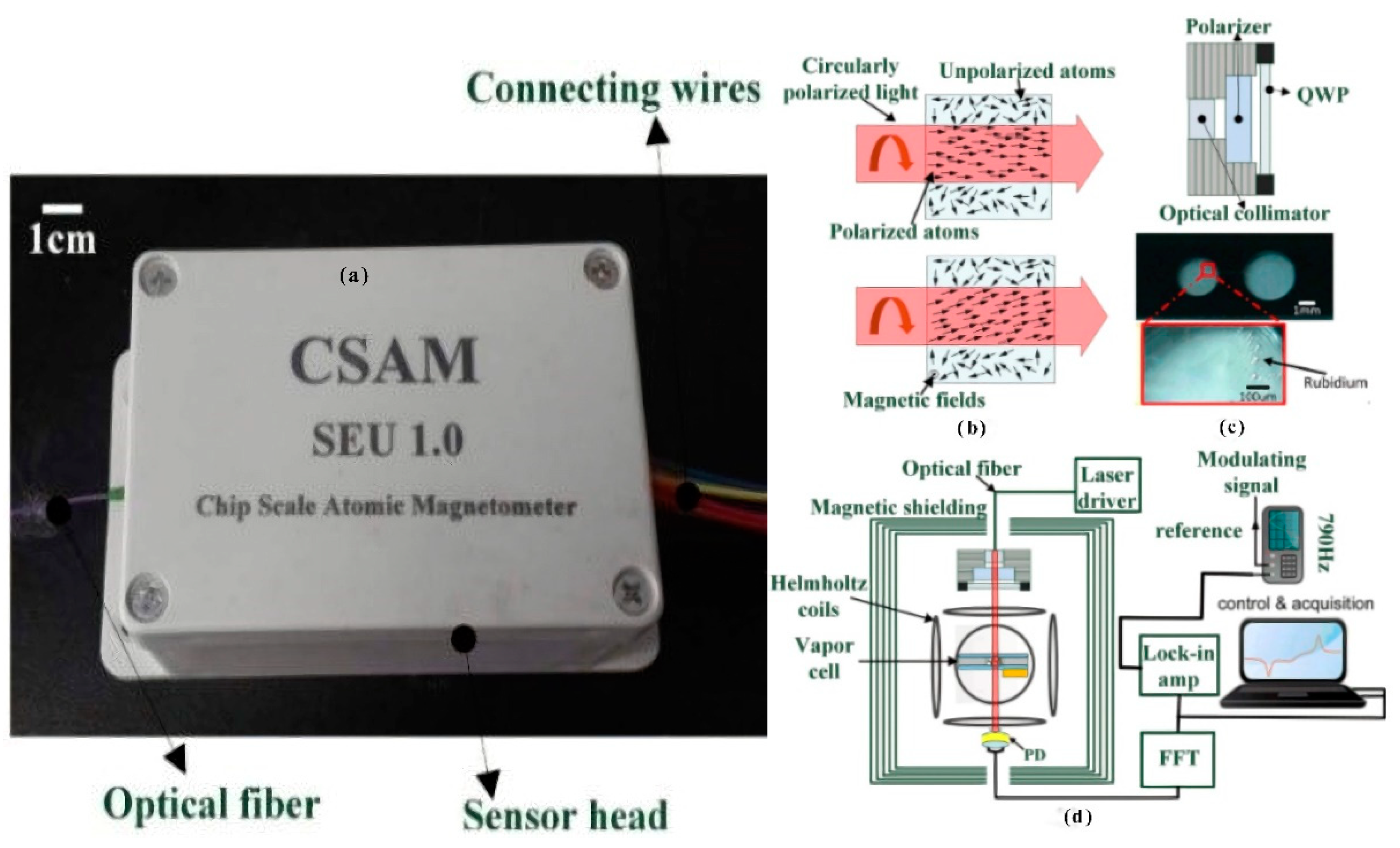
3.1.2. Application of Atomic Magnetometer to Micro-Fabricated Alkali Vapor Cells
3.2. Conclusions and Outlooks of Alkali Vapor Cells
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, D.A.; Paradis, E.G.; Raithel, G. A vapor-cell atomic sensor for radio-frequency field detection using a polarization-selective field enhancement resonator. Appl. Phys. Lett. 2018, 113, 1–6. [Google Scholar] [CrossRef]
- Jiménez-Martínez, R.; Kołodyński, J.; Troullinou, C.; Lucivero, V.G.; Kong, J.; Mitchell, M.W. Signal tracking beyond the time resolution of an atomic sensor by Kalman filtering. Phys. Rev. Lett. 2018, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gerginov, V.; da Silva, F.C.S.; Hati, A.; Nelson, C. An atomic sensor for direct detection of weak microwave signals. IEEE Trans. Microw. Theory Tech. 2019, 67, 3485–3493. [Google Scholar] [CrossRef]
- Godone, A.; Levi, F.; Calosso, C.E.; Micalizio, S. High-performing vapor-cell frequency standards. Riv. Nuovo Cim. 2015, 38, 133–171. [Google Scholar] [CrossRef]
- Kitching, J. Chip-scale atomic devices. Appl. Phys. Lett. 2018, 5, 031302. [Google Scholar] [CrossRef]
- Warneke, B.A.; Pister, K.S.J. MEMS for distributed wireless sensor networks. In Proceedings of the9th International Conference on Electronics, Circuits and Systems, Dubrovnik, Croatia, 15–18 September 2002; pp. 291–294. [Google Scholar] [CrossRef]
- Bogue, R. Recent developments in MEMS sensors: A review of applications. Sens. Rev. 2013, 33, 2260–2288. [Google Scholar] [CrossRef]
- Mishra, M.K.; Dubey, V.; Mishra, P.; Khan, I.J.J. MEMS technology: A review. J. Eng. Res. Rep. 2019, 4, 1–24. [Google Scholar] [CrossRef]
- Gundeti, V.M. Folded MEMS Approach to NMRG; University of California: Irvine, CA, USA, 2015; p. 1596231. [Google Scholar]
- Guo, Z.S.; Chen, F.C.; Li, B.Y.; Cao, L.; Song, K. Research development of silicon MEMS gyroscopes: A review. Microsyst. Technol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Noor, R.M.; Venu, G.; Andrei, M.S. A status on components development for folded micro NMR gyro. In Proceedings of the IEEE International Symposium on Inertial Sensors and Systems, Kauai, HI, USA, 27–30 March 2017; pp. 156–159. [Google Scholar]
- Noor, R.M. MEMS Components for NMR Atomic Sensors; University of California: Irvine, CA, USA, 2019. [Google Scholar]
- Noor, R.M.; Shkel, A.M. MEMS components for NMR atomic sensors. J. Microelectromech. Syst. 2018, 27, 1148–1159. [Google Scholar] [CrossRef]
- Gorecki, C.; Hasegawa, M.; Passilly, N.; Chutani, R.K.; Dziuban, P.; Gailliou, S.; Giordano, V. Towards the realization of the first European MEMS atomic clock. In Proceedings of the 2009 IEEE/LEOS International Conference on Optical MEMS and Nanophotonics, Clearwater, FL, USA, 17–20 August 2009; pp. 47–48. [Google Scholar] [CrossRef]
- Knapkiewicz, P.; Dziuban, J.; Walczak, R.; Mauri, L.; Dziuban, P.; Gorecki, C.J.P. MEMS caesium vapour cell for European micro-atomic-clock. Proc. Eng. 2010, 5, 721–724. [Google Scholar] [CrossRef]
- Hasegawa, M.; Chutani, R.K.; Gorecki, C.; Boudot, R.; Dziuban, P.; Giordano, V.; Clatot, S.; Mauri, L. Microfabrication of cesium vapor cells with buffer gas for MEMS atomic clocks. Sens. Actuators A Phys. 2011, 167, 594–601. [Google Scholar] [CrossRef]
- Al-Samaneh, A.; Sanayeh, M.B.; Renz, S.; Wahl, D.; Michalzik, R.J.I.P.T.L. Polarization control and dynamic properties of VCSELs for MEMS atomic clock applications. IEEE Photonics Technol. Lett. 2011, 15, 1049–1051. [Google Scholar] [CrossRef]
- Chutani, R.K.; Galliou, S.; Passilly, N.; Gorecki, C.; Sitomaniemi, A.; Herkkinen, M.; Kautio, K.; Keranen, A.; Jornod, A. Thermal management of fully LTCC-packaged Cs vapour cell for MEMS atomic clock. Sens. Actuators A 2012, 174, 58–68. [Google Scholar] [CrossRef]
- Song, X.; Dong, H.F.; Fang, J.C. Chip scale atomic magnetometer based on SERF. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 5–8. [Google Scholar] [CrossRef]
- Mhaskar, R.R.; Knappe, S.; Kitching, J. Low-frequency characterization of MEMS-based portable atomic magnetometer. In Proceedings of the 2010 IEEE International Frequency Control Symposium, Newport Beach, CA, USA, 1–4 June 2010; pp. 376–379. [Google Scholar] [CrossRef]
- Li, J.D.; Zhou, B.Q.; Wang, Z.; Lu, J.X.; Hu, Z.H.; Liu, G.; Fang, J.C. SERF atomic magnetometer–recent advances and applications: A review. IEEE Sens. J. 2018, 18, 8198–8207. [Google Scholar] [CrossRef]
- Zhang, R.; Dyer, T.; Brockie, N.; Parsa, R.; Mhaskar, R. Subpicotesla scalar atomic magnetometer with a microfabricated cell. AIP J. Appl. Phys. 2019, 126, 124503. [Google Scholar] [CrossRef]
- Kominis, I.; Kornack, T.; Allred, J.; Romalis, M.V.J.N. A subfemtotesla multichannel atomic magnetometer. Nature 2003, 422, 596–599. [Google Scholar] [CrossRef]
- Ono, Y.; Ishiyama, A.; Kasai, N.; Chinone, K.J.A. Development of biomagnetic measurement system for mice with high spatial resolution. Appl. Phys. Lett. 2004, 85, 332–334. [Google Scholar] [CrossRef]
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Li, Y.; Cima, M.J. A BioMEMS review: MEMS technology for physiologically integrated devices. IEEE J. Mag. 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Weitschies, W.; Kosch, O.; Monnikes, H.; Trahms, L. Magnetic marker monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv. Drug Deliv. Rev. 2005, 57, 1210–1222. [Google Scholar] [CrossRef]
- Khoshnoud, F.; de Silva, C.W.J.I.I.; Magazine, M. Recent advances in MEMS sensor technology–biomedical applications. IEEE Instrum. Meas. Mag. 2012, 15, 8–14. [Google Scholar] [CrossRef]
- Adachi, Y.; Oyama, D.; Terazono, Y.; Hayashi, T.; Shibuya, T.; Kawabata, S.J.I.T. Calibration of room temperature magnetic sensor array for biomagnetic measurement. IEEE Trans. Magn. 2019, 55, 1–6. [Google Scholar] [CrossRef]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartali, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, D.; Han, Y.C.; Cheng, W.; Sun, J.Y.; Wan, K.; Liu, H.; Greiser, A.; Zhou, X.Y.; Chen, Y.C. Age and gender impact the measurement of myocardial interstitial fibrosis in a healthy adult Chinese population: A cardiac magnetic resonance study. Clin. Translational Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Furuya, Y.; Ono, T. Micro-fabricated vapor cells with sealed Rb atoms by distillation at wafer level and two-step bonding for miniature atomic clocks. Opt. Express 2021, 29, 44316–44321. [Google Scholar] [CrossRef]
- Lv, Q.; He, L.; Xu, J. An experimental study of atomic vapor cell fabrication for atomic clock. In Proceedings of the 2011 International Conference on Electrical and Control Engineering, Yichang, China, 16–18 September 2011; pp. 552–554. [Google Scholar] [CrossRef]
- Petremand, Y.; Affolderbach, C.; Straessle, R.; Pellaton, M.; Briand, D.; Mileti, G.; de Rooij, N.F. Microfabricated rubidium vapour cell with a thick glass core for small-scale atomic clock applications. J. Micromech. Microeng. 2012, 22, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.D.; Zhang, S.G.; Yan, S.B. Micro-fabrication and hermeticity measurement of alkali-atom vapor cells based on anodic bonding. Chin. Opt. Lett. 2019, 17, 100201. [Google Scholar] [CrossRef]
- Boudot, R.; McGilligan, J.P.; Moore, K.R.; Maurice, V.; Martinez, G.D.; Hansen, A.; Clercq, E.; Kitching, J. Enhanced observation time of magneto-optical traps using micro-machined non-evaporable getter pumps. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Nishino, H.; Furuya, Y.; Ono, T. Micro vapor cells sealed by two-step bonding for miniature atomic clocks. Res. Sq. 2021, 1–21. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Hirai, Y.; Sugano, K.; Tsuchiya, T.; Tabata, O. Sacrificial microchannel sealing by glass-frit reflow for chip scale atomic magnetometer. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 251–257. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Hirai, Y.; Sugano, K.; Tsuchiya, T.; Tabata, O. Sacrificial microchannel sealing by glass-frit reflow for chip scale atomic magnetometer. Electron. Commun. Jpn. 2013, 96, 58–66. [Google Scholar] [CrossRef]
- Straessle, R.; Petremand, Y.; Briand, D.; de Rooij, N.F.; Pellaton, M.; Affolderbach, C.; Mileti, G. Towards wall-coated microfabricated cells: Alkali vapor-cells using indium thin-film low-temperature bonding. In Proceedings of the 2012 European Frequency and Time Forum, Gothenburg, Sweden, 23–27 April 2012; pp. 309–311. [Google Scholar] [CrossRef]
- Straessle, R.; Pellaton, M.; Affolderbach, C.; Petremand, Y.; Briand, D.; Mileti, G.; de Rooij, N.F. Low-temperature indium-bonded alkali vapor cell for chip-scale atomic clocks. J. Appl. Phys. 2013, 113, 064501. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wu, Y.N.; Xia, X.H.; Jiang, M.W.; Han, Y.; Zou, L.M.; Jin, P. Micro-fabricated alkali vapor cells sealed at low temperature using asymmetric Au–In transient liquid phase (TLP) bonding. Jpn. J. Appl. Phys. 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Karlen, S.; Haesler, J.; Overstolz, T.; Bergonzi, G.; Lecomte, S. Sealing of MEMS atomic vapor cells using Cu-Cu thermocompression bonding. J. Microelectromech. Syst. 2019, 29, 95–99. [Google Scholar] [CrossRef]
- Dziuban, J.; Gorecki, C.; Giordano, V.; Nieradko, L.; Maillotte, H.; Moraja, M. Procédé de Fabrication d’une Cellule à gaz Active Pour L’horloge Atomique à gaz Ainsi Obtenue. French Patent 06/09089, 17 October 2006. [Google Scholar]
- Eklund, E.J.; Shkel, A.M.; Knappe, S.; Donley, E.; Kitching, J.J.S. Glass-blown spherical microcells for chip-scale atomic devices. Sens. And. Actuators A: Physical. 2008, 143, 175–180. [Google Scholar] [CrossRef]
- Perez, M.A.; Nguyen, U.; Knappe, S.; Donley, E.; Kitching, J.; Shkel, A. Rubidium vapor cellwith integrated nonmetallic multilayer reflectors. In Proceedings of the 2008 IEEE 21st International Conference on Micro Electro Mechanical Systems, Tucson, AZ, USA, 13–17 January 2008; pp. 790–793. [Google Scholar] [CrossRef]
- Chen, Y.P.; Shang, J.T.; Ji, Y. Fabrication of low cost spherical alkali atom vapor cells by combining a low temperature anodic bonding and a Chemical Foaming Process (CFP). In Proceedings of the 2013 IEEE 15th Electronics Packaging Technology Conference (EPTC 2013), Singapore, 11–13 December 2013; pp. 809–812. [Google Scholar] [CrossRef]
- Ji, Y.; Shang, J.T.; Gan, Q.; Wu, L.; Wong, C.P. Micro-fabricated spherical rubidium vapor cell and its integration in 3-axis atomic magnetometer. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; pp. 946–949. [Google Scholar] [CrossRef]
- Ji, Y.; Gan, Q.; Wu, L.; Shang, J.T. Geometry influence of the micro alkali vapor cell on the sensitivity of the chip-scale atomic magnetometers. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 342–345. [Google Scholar] [CrossRef]
- Ji, Y.; Shang, J.T.; Gan, Q.; Wu, L.; Wong, C.P. Improvement of sensitivity by using microfabricated spherical alkali vapor cells for chip-scale atomic magnetometers. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1715–1722. [Google Scholar] [CrossRef]
- Noor, R.M.; Kulachenkov, N.; Asadian, M.H.; Shkel, A.M. Study on MEMS Glassblown cells for NMR sensors. In Proceedings of the 2019 IEEE International Symposium on Inertial Sensors and Systems (INERTIAL), Naples, FL, USA, 1–5 April 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Noor, R.M.; Asadian, M.H.; Shkel, A.M. Design Considerations for micro-glassblown atomic vapor cells. IEEE J. Microelectromech. Syst. 2019, 29, 25–35. [Google Scholar] [CrossRef]
- Knappe, S.; Velichansky, V.; Robinson, H.G.; Liew, L.; Moreland, J.; Kitching, J.; Hollberg, L. Atomic vapor cells for miniature frequency references. In Proceedings of the 2003 IEEE International Frequency Control Symposium and PDA Exhibition Jointly with the 17th European Frequency and Time Forum, Tampa, FL, USA, 4–8 May 2003; pp. 31–32. [Google Scholar] [CrossRef]
- Martínez, R.J.; Kennedy, D.J.; Rosenbluh, M.; Donley, A.D.; Knappe, S.; Seltzer, S.J.; Ring, H.L.; Bajaj, V.S.; Kitching, J. Optical hyperpolarization and NMR detection of 129 Xe on a microfluidic chip. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Chutani, R.; Maurice, V.; Passilly, N.; Gorecki, C.; Boudot, R.; Hafiz, M.A.; Abbe, P.; Galliou, S.; Rauch, J.Y.; de Clercq, E. Laser light routing in an elongated micromachined vapor cell with diffraction gratings for atomic clock applications. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Nishino, H.; Toda, M.; Yano, Y.; Kajita, M.; Ido, T.; Hara, M.; Ono, T. A Reflection type vapor cell based on local anodic bonding of 45° mirrors for micro atomic clocks. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII, Berlin, Germany, 23–27 June 2019; pp. 1530–1532. [Google Scholar] [CrossRef]
- Knapkiewicz, P. Technological assessment of MEMS alkali vapor cells for atomic references. Micromachines 2019, 10, 25. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Lal, A. Alkali metal-wax micropackets for chip-scale atomic clocks. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, Korea, 5–9 June 2005. [Google Scholar] [CrossRef]
- Knappe, S.; Gerginov, V.; Schwindt, P.D.D.; Shah, V.; Robinson, H.G.; Hollberg, L.; Kitching, J. Atomic vapor cells for chip-scale atomic clocks with improved long-term frequency stability. Opt. Lett. 2005, 30, 2351–2353. [Google Scholar] [CrossRef]
- Nieradko, L.; Gorecki, C.; Dziuban, J.; Douahi, A.; Giordano, V.; Beugnot, J.C.; Guerandel, S.; Moraja, M. From the implementation to the characterisation and assembling of microfabricated optical alcali vapor cell for MEMS atomic clocks. In Proceedings of the TRANSDUCERS 2007-2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; pp. 45–48. [Google Scholar] [CrossRef]
- Vecchio, F.; Venkatraman, V.; Shea, H.R.; Maeder, T.; Ryser, P. Dispensing and hermetic sealing Rb in a miniature reference cell for integrated atomic clocks. Sens. Actuators A Physical. 2011, 172, 330–335. [Google Scholar] [CrossRef]
- Liew, L.A.; Knappe, S.; Moreland, J.; Robinson, H.; Hollberg, L.; Kitching, J. Microfabricated alkali atom vapor cells. Appl. Phys. Lett. 2004, 84, 2694–2696. [Google Scholar] [CrossRef]
- Dong, H.F.; Fang, J.C.; Zhou, B.Q.; Qin, J.; Wan, S.A. Fabrication of atomic vapor cell chip for MEMS atomic spin-polarized gyroscope. Chin. J. Sci. Instrum. 2010, 11, 2592–2596. [Google Scholar]
- Ji, Y.; Shang, J.T.; Chen, Y.P.; Wong, C.P. Preparation of a Micro Rubidium vapor cell and its integration in a chip-scale atomic magnetometer. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; pp. 1488–1491. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, Y.; Ma, B. A new packaging method of alkali metal simple substrate and related key techniques. key engineering materials. Key Eng. Mater. 2013, 562, 1361–1366. [Google Scholar] [CrossRef]
- Knappe, S.; Shah, V.; Schwindt, P.D.D.; Hollberg, L.; Kitching, J. A microfabricated atomic clock. Appl. Phys. Lett. 2004, 85, 1460–1462. [Google Scholar] [CrossRef]
- Kitching, J.; Knappe, S.; Shah, V.; Schwindt, P.; Griffith, C.; Jimenez, R.; Preusser, J.; Liew, L.A.; Moreland, J. Microfabricated atomic magnetometers and applications. In Proceedings of the 2008 IEEE International Frequency Control Symposium, Honolulu, HI, USA, 19–21 May 2008; pp. 789–794. [Google Scholar] [CrossRef]
- Brannon, A.; Jankovic, M.; Breitbarth, J.; Popovic, Z.; Gerginov, V.; Shah, V.; Knappe, S.; Hollberg, L.; Kitching, J. Chip-Scale Atomic Clocks at NIST. NCSL Int. Workshop Symp. 2005, 49, 80–89. [Google Scholar]
- Ermak, S.V.; Semenov, V.V.; Piatyshev, E.N.; Kazakin, A.N.; Komarevtsev, I.M.; Velichko, E.N.; Davydov, V.V.; Petrenko, M.V. Microfabricated cells for chip-scale atomic clock based on coherent population trapping: Fabrication and investigation. Phys. Math. 2015, 1, 37–41. [Google Scholar] [CrossRef][Green Version]
- Liew, L.A.; Knappe, S.; Moreland, J.; Robinson, H.; Hollberg, L.; Kitching, J. Micromachined alkali atom vapor cells for chip-scale atomic clocks. In 17th IEEE International Conference on Micro Electro Mechanical Systems. Maastricht MEMS 2004 Technical Digest; IEEE: Maastricht, The Netherlands, 2004; pp. 113–116. [Google Scholar] [CrossRef]
- Ban, K.; Terao, A.; Mizutani, N.; Kobayashi, T.; Tabata, O. Characterization of alkali-metal vapor cells fabricated with an alkali-metal source tablet. J. Vac. Sci. Technol. A 2016, 34, 1–11. [Google Scholar] [CrossRef]
- Douahi, L.; Nieradko, J.C.; Beugnot, J.; Dziuban, H.; Maillote, R.; Boudot, S.; Guérandel, M.; Moraja, C.; Gorecki, V. New vapor cell technology for chip scale atomic clock. In Proceedings of the IEEE International Frequency Control Symposium 2007 Joint with the 21st European Frequency and Time Forum, Geneva, Switzerland, 29 May–1 June 2007; pp. 58–61. [Google Scholar]
- Douahi, L.; Nieradko, J.C.; Beugnot, J.; Dziuban, H.; Maillote, S.; Gue´randel, M.; Moraja, C.; Gorecki, V. Giordano, Vapour microcell for chip scale atomic frequency standard. Electron. Lett. 2007, 43, 33–34. [Google Scholar] [CrossRef]
- Knapkiewicz, J.A.; Dziuban, C.; Gorecki, P.; Dziuban, R.; Walczak, L. Komórka cezowa MEMS dla mikrozegara atomowego. Elektron. Konstr. Technol. Zastos. 2010, 51, 82–85. [Google Scholar]
- Chutani, R.K.; Boudot, R.; Mauri, L.; Gorecki, C.; Liu, X. Passilly, Effects of getters on hermetically sealed micromachined cesium–neon cells for atomic clocks. J. Micromech. Microeng. 2013, 23, 055022. [Google Scholar]
- Maurice, V.; Rutkowski, J.; Kroemer, E.; Bargiel, S.; Passilly, N.; Boudot, R.; Gorecki, C.; Mauri, L.; Moraja, M. Microfabricated vapor cells filled with a cesium dispensing paste for miniature atomic clocks. Appl. Phys. Lett. 2017, 110, 164103. [Google Scholar] [CrossRef]
- Han, R.; You, Z.; Zhang, F.; Xue, H.; Ruan, Y. Microfabricated vapor cells with reflective sidewalls for chip scale atomic sensors. Micromachines 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Wang, F.F.; Liang, D.C.; Jin, P.; Wang, Z.G. Fabrication of chip-scale alkali metal cells. Sci. China Press 2015, 45, 693–700. [Google Scholar] [CrossRef]
- Gong, F.; Jau, Y.Y.; Jensen, K.; Happer, W. Electrolytic fabrication of atomic clock cells. Rev. Sci. Instrum. 2006, 77, 076101. [Google Scholar] [CrossRef]
- Eklund, E.J.; Shkel, A.M. Glass blowing on a wafer level. IEEE J. Microelectromech. Syst. 2007, 16, 232–239. [Google Scholar] [CrossRef]
- Abbink, H.C.; Kanegsberg, E.; Marino, K.D.; Volk, C.H. Micro-cell for NMR Gyroscope. ed: Google Patents. US. Patent No. 7,292,031, 6 November 2007. [Google Scholar]
- Abbink, H.C.; Debley, W.P.; Geosling, C.E.; Sakaida, D.K.; Stewart, R.E. Middle layer of die structure that comprises a cavity that holds an alkali metal. ed: Google Patents. US. Patent No. 7,292,111, 6 November 2007. [Google Scholar]
- Aleksandrov, E.B.; Balabas, M.V.; Vershovskii, A.K.; Ivanov, A.E.; Yakobson, N.N.; Velichanskii, V.L.; Senkov, N.V. Laser pumping in the scheme of an Mx-magnetometer. Opt. Spectrosc. 1995, 78, 292–298. [Google Scholar]
- Budker, D.; Kimball, D.; Rochester, S.; Yashchuk, V.; Zolotorev, M. Sensitive magnetometry based on nonlinear magneto-optical rotation. Phys. Rev. A 2000, 62, 043403. [Google Scholar] [CrossRef]
- Affolderbach, C.; Stähler, M.; Knappe, S.; Wynands, R. An all-optical, high-sensitivity magnetic gradiometer. Appl. Phys. B 2002, 75, 605–612. [Google Scholar] [CrossRef]
- Tsuei, C.C.; Kirtley, J.R. Phase-sensitive evidence for d-wave pairing symmetry in electron-doped cuprate superconductors. Phys. Rev. Lett. 2000, 85, 1–4. [Google Scholar] [CrossRef]
- Harry, G.M.; Jin, I.; Paik, H.J.; Stevenson, T.R.; Wellstood, F.C. Two-stage superconducting-quantum-interference-device amplifier in a high-Q gravitational wave transducer. Appl. Phys. Lett. 2000, 76, 1446–1448. [Google Scholar] [CrossRef][Green Version]
- Greenberg, Y.S. Application of superconducting quantum interference devices to nuclear magnetic resonance. Rev. Mod. Phys. 1998, 70, 1–48. [Google Scholar] [CrossRef]
- McDermott, R.; Trabesinger, A.H.; Mück, M.; Hahn, E.L.; Pines, A.; Clarke, J. Liquid-state NMR and scalar couplings in microtesla magnetic fields. Science 2002, 295, 2247–2249. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Maine, A.T.; Vali, H. Paleomagnetic evidence of a low-temperature origin of carbonate in the Martian meteorite ALH84001. Science 1997, 275, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Tralshawala, N.; Claycomb, J.; Miller, J.H. Practical SQUID instrument for nondestructive testing. Appl. Phys. Lett. 1997, 71, 1573–1575. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—theory, instrumentation, and applications to noinvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 1–94. [Google Scholar] [CrossRef]
- Rodriguez, E.; George, N.; Lachaux, J.P.; Martinerie, J.; Renault, B.; Varela, F.J. Perception’s shadow: Long-distance synchronization of human brain activity. Lett. Nat. 1999, 397, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, J.; Yamagami, H.; Noguchi, T.; Morita, Y.; Tanaka, T.; Okuno, Y.; Yasuda, S.; Toyoda, K.; Gon, Y.; Toda, K.; et al. Detection of left ventricular thrombus by cardiac magnetic resonance in embolic stroke of undetermined source. Stroke 2017, 48, 2434–2440. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kobayashi, H.; Kobayashi, Y.; Yokoe, I.; Takei, M.; Kitamura, N. Association of cardiac magnetic resonance-detected myocardial abnormalities with disease characteristics and brain natriuretic peptide levels in systemic sclerosis without cardiac symptoms. Rheum. Dis. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Thiene, P.; Harding, J.T. Design and operation of stable rf-biased superconducting point-contact quantum devices, and a note on the properties of perfectly clean metal contacts. J. Appl. Phys. 1970, 41, 1572–1580. [Google Scholar] [CrossRef]
- Drung, D.; Bechstein, S.; Franke, K.P.; Scheiner, M.; Schurig, T. Improved direct-coupled dc SQUID read-out electronics with automatic bias voltage tuning. IEEE Trans. Appl. Supercond. 2001, 11, 880–883. [Google Scholar] [CrossRef]
- Oukhanski, N.; Stolz, R.; Zakosarenko, V.; Meyer, H.G. Low-drift broadband directly coupled dc SQUID read-out electronics. Phys. C Supercond. 2002, 368, 166–170. [Google Scholar] [CrossRef]
- Del Gratta, C.; Pizzella, V.; Tecchio, F.; Romani, G.L. Magnetoencephalography-a noninvasive brain imaging method with 1 ms time resolution. IOP Sci. 2001, 64, 1759. [Google Scholar] [CrossRef]
- Nenonen, J.; Montonen, J.; Katila, T. Thermal noise in biomagnetic measurements. Sci. Instrum. 1996, 67, 2397–2405. [Google Scholar] [CrossRef]
- Kandori, A.; Miyashita, T.; Tsukada, K. Cancellation technique of external noise inside a magnetically shielded room used for biomagnetic measurements. Rev. Sci. Instrum. 2000, 71, 2184–2190. [Google Scholar] [CrossRef]
- Lindseth, B.; Schwindt, P.; Kitching, J.; Fischer, D.; Shusterman, V. Non-contact measurement of cardiac electromagnetic field in mice by use of a microfabricated atomic magnetometer. In Proceedings of the 2007 Computers in Cardiology, Durham, NC, USA, 9 September– 3 October 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 443–446. [Google Scholar]
- Griffith, W.C.; Knappe, S.; Kitching, J. Femtotesla atomic magnetometry in a microfabricated vapor cell. Opt. Express 2010, 18, 27167–27172. [Google Scholar] [CrossRef]
- Sander, T.; Preusser, J.; Mhaskar, R.; Kitching, J.; Trahms, L.; Knappe, S. Magnetoencephalography with a chip-scale atomic magnetometer. Biomed. Opt. Express 2012, 3, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Alem, O.; Sander, T.H.; Mhaskar, R.; LeBlanc, J.; Eswaran, H.; Steinhoff, U.; Okada, Y.; Kitching, J.; Trahms, L.; Knappe, S. Fetal magnetocardiography measurements with an array of microfabricated optically pumped magnetometers. Phys. Med. Biol. 2015, 60, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Okuya, M.; Ito, N.; Shiozaki, K. ITO thin films prepared by a microwave heating. Thin Solid Film. 2007, 515, 8656–8659. [Google Scholar] [CrossRef]
- Alem, O.; Mhaskar, R.; Martinez, J.M.; Sheng, D.; Leblanc, J.; Trahms, L.; Sander, T.; Kitching, J.; Knappe, S. Magnetic field imaging with microfabricated optically-pumped magnetometers. Opt. Express 2017, 25, 7849–7858. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhang, X.; Qin, J.N.; Chen, C. Review of Bell—Bloom spin-exchange-relaxation free atomic magnetometer. Chin. J. Sci. Instrum. 2016, 37, 2648–2656. [Google Scholar]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.; Munoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.T.; Chen, B.; Lin, W.; Wong, C.P.; Zhang, D.; Xu, C.; Liu, J.W.; Huang, Q.A. Preparation of wafer-level glass cavities by a low-cost chemical foaming process (CFP). Lab Chip 2011, 11, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gan, Q.; Wu, L.; Shang, J.T.; Wong, C.P. Wafer-level hermetic all-glass packaging for microalkali vapor cells of chip-scale atomic devices. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 1551–1558. [Google Scholar] [CrossRef]
- Wu, L.; Shang, J.T.; Ji, Y.; Gan, Q.; Wong, C.P. Influence of buffer-gas pressure inside micro alkali vapor cells on the performance of chip-scale SERF magnetometers. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 8, 621–625. [Google Scholar] [CrossRef]
- Ji, Y.; Shang, J.T.; Li, G.; Zhang, J.; Zhang, J. Microfabricated shaped rubidium vapor cell for miniaturized atomic magnetometers. Magen. Sens. 2020, 4, 1–4. [Google Scholar] [CrossRef]



| Packaging Method | Advantages | Disadvantages | |
|---|---|---|---|
| Physical methods | Direct filling of alkali metal monomers method | Avoids the introduction of impurities during the filling process and improves the performance of the alkali vapor cell | Extremely demanding in terms of equipment and environment, increasing the cost and complexity of the operation process |
| Alkali metal wax package filling method | Oxidation during the filling process is avoided and the paraffin coating reduces the collision of alkali metal atoms with the inner wall of the vapor cell | The time control of the operating process of laser melting silicon nitride and paraffin wax is difficult, and the process is more complex; it cannot be batch-produced | |
| Chemical methods | Chemical reaction to produce elements method | No need to operate directly on the alkali metal, avoiding the problem that the alkali metal is prone to chemical reaction with the outside world during the transfer process, and the operation is simpler | May introduce residues of non-alkali metals, affecting light transmission |
| Ultraviolet photolysis method | Simple operation, no chemical impurities, can maintain the chemical purity of alkali metal for a long time, and can change the parameters to control the pressure of buffer gas | Longer time required for the photolysis of rubidium azide or cesium azide | |
| Electrochemical decomposition method | The amount of filling of base metal monomers can be well controlled, and wafer-level batch manufacturing can be achieved, enhancing the strength of anodic bonding | The operation is more tedious, and the process parameters have a large impact and high cost |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ye, M.; Lu, F.; Mao, Y.; Tian, H.; Li, J. Recent Progress on Micro-Fabricated Alkali Metal Vapor Cells. Biosensors 2022, 12, 165. https://doi.org/10.3390/bios12030165
Wang X, Ye M, Lu F, Mao Y, Tian H, Li J. Recent Progress on Micro-Fabricated Alkali Metal Vapor Cells. Biosensors. 2022; 12(3):165. https://doi.org/10.3390/bios12030165
Chicago/Turabian StyleWang, Xuelei, Mao Ye, Fei Lu, Yunkai Mao, Hao Tian, and Jianli Li. 2022. "Recent Progress on Micro-Fabricated Alkali Metal Vapor Cells" Biosensors 12, no. 3: 165. https://doi.org/10.3390/bios12030165
APA StyleWang, X., Ye, M., Lu, F., Mao, Y., Tian, H., & Li, J. (2022). Recent Progress on Micro-Fabricated Alkali Metal Vapor Cells. Biosensors, 12(3), 165. https://doi.org/10.3390/bios12030165






