Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement
Abstract
1. Introduction
2. Materials and Methods
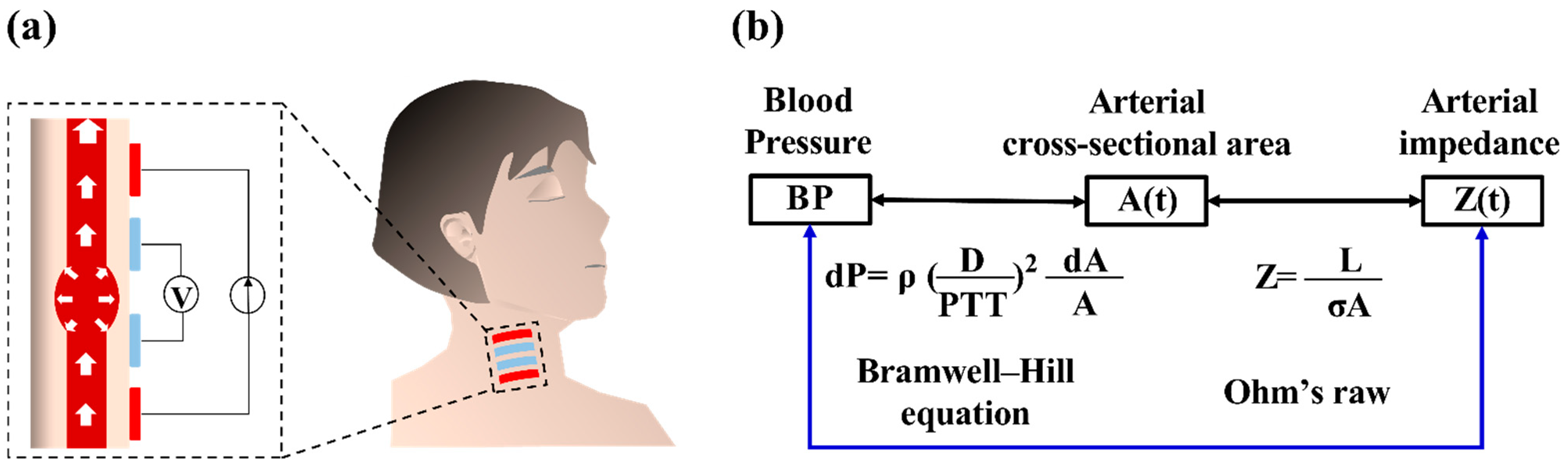
2.1. Physiological Correlation between IPG and BP
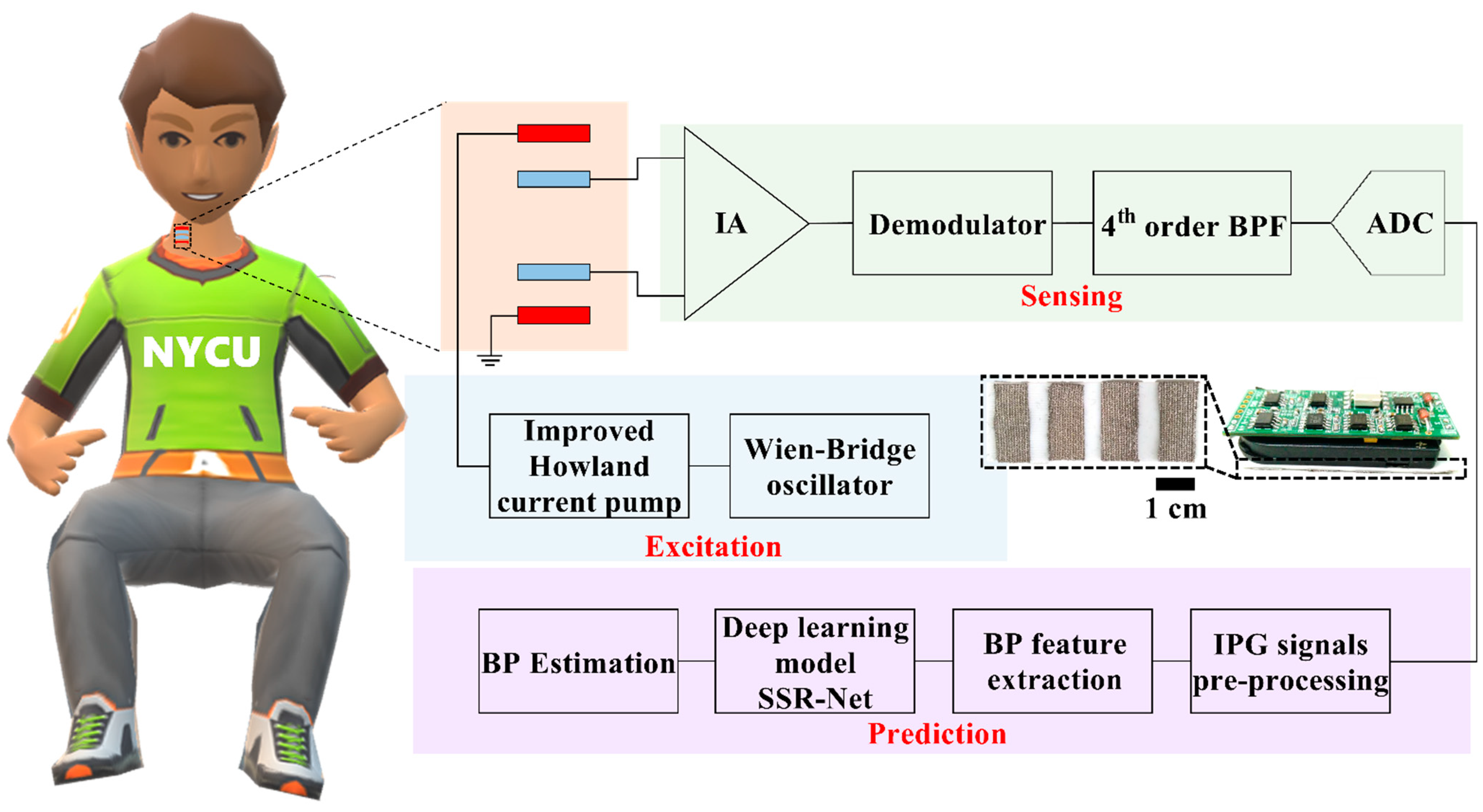
2.2. Wearable Intelligent BP System Design
2.2.1. IPG Sensing Device
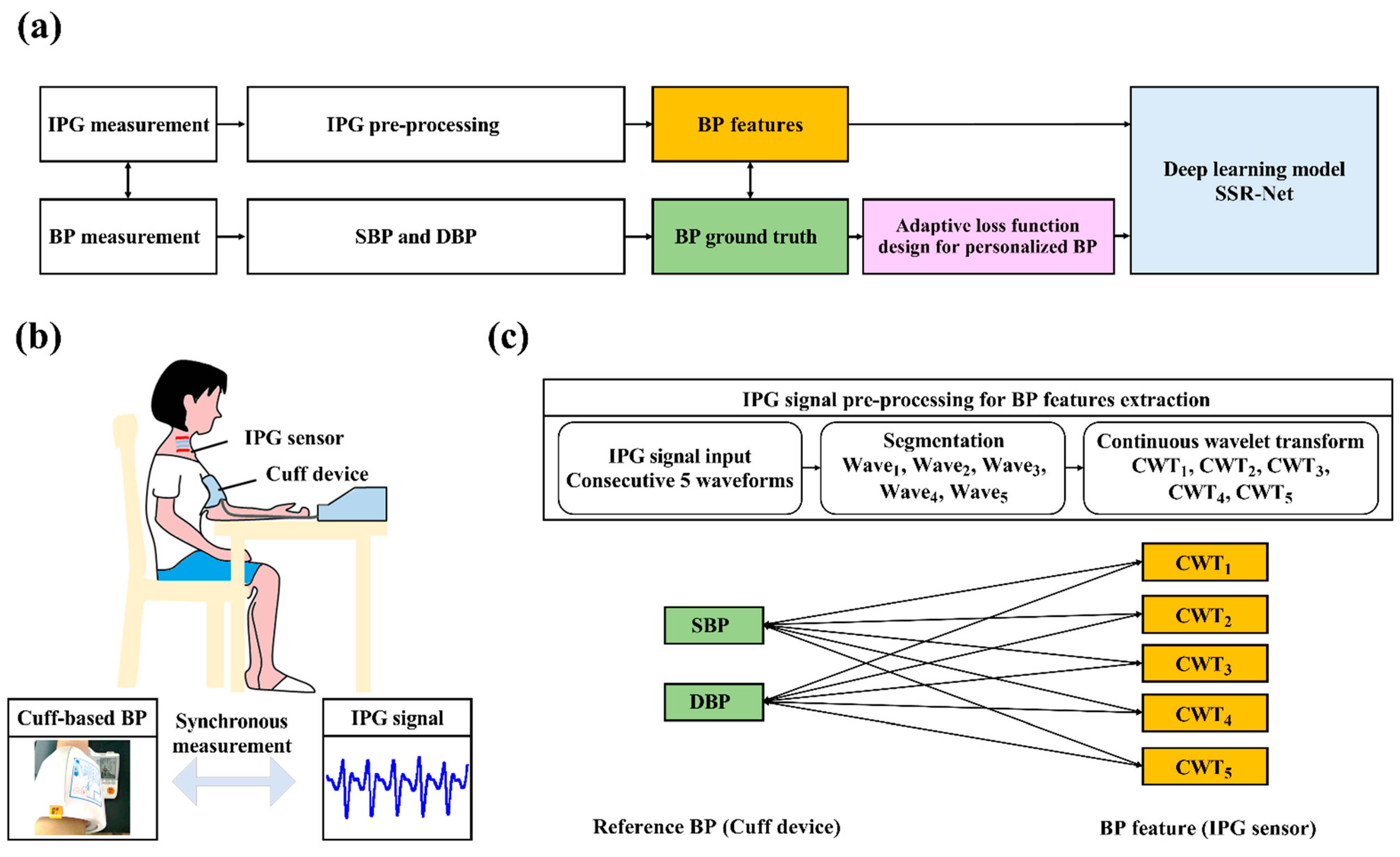
2.2.2. The AI-Based BP Estimation
- IPG Signals and Reference BP Acquisition:
- IPG Signal Pre-processing and BP Feature Extraction:
- Dataset Arrangements for Model Training and Testing:
- Loss Function Design for Personalized BP Monitoring:
- Environment Details:
2.2.3. Ethics Statement
3. Results
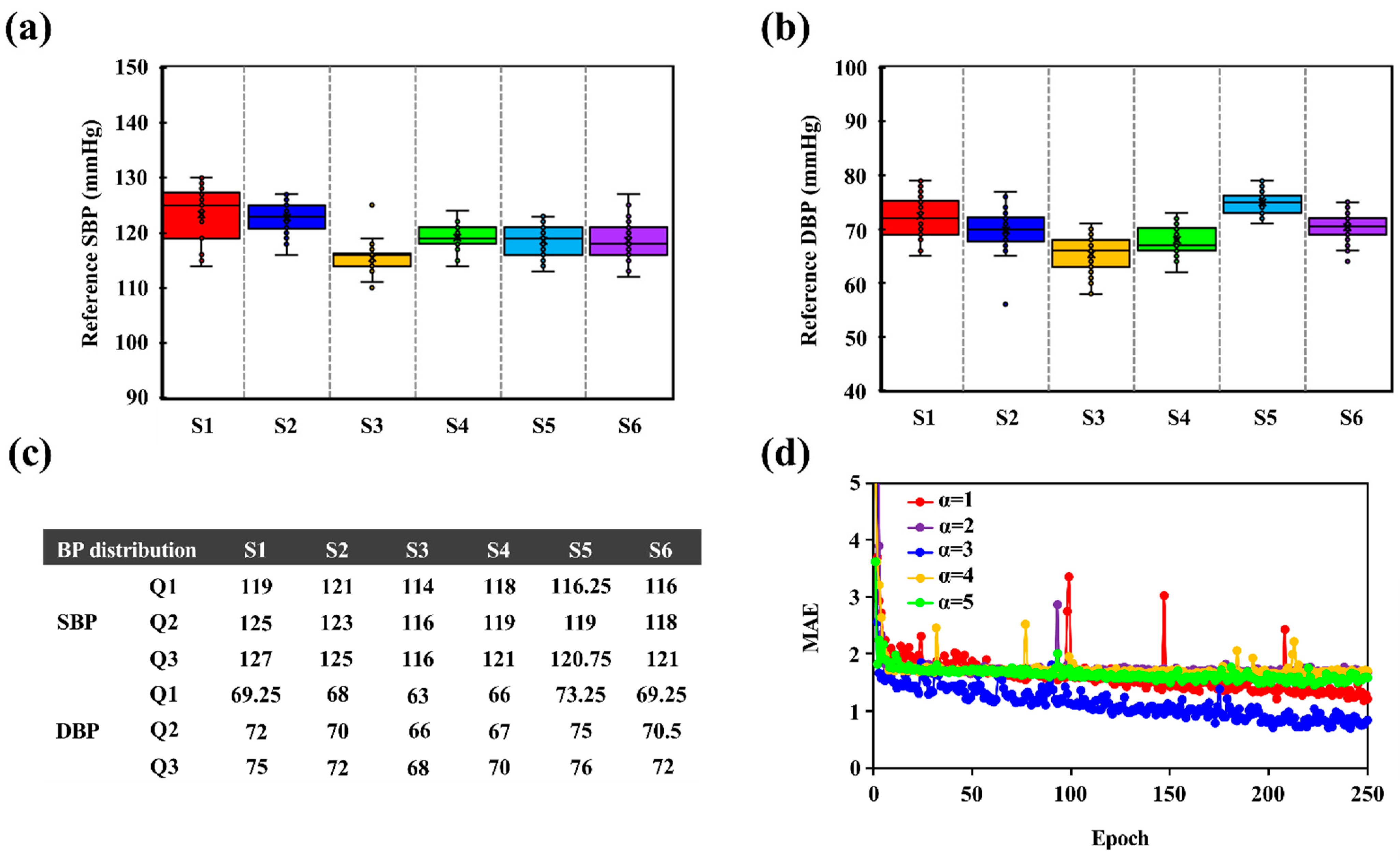
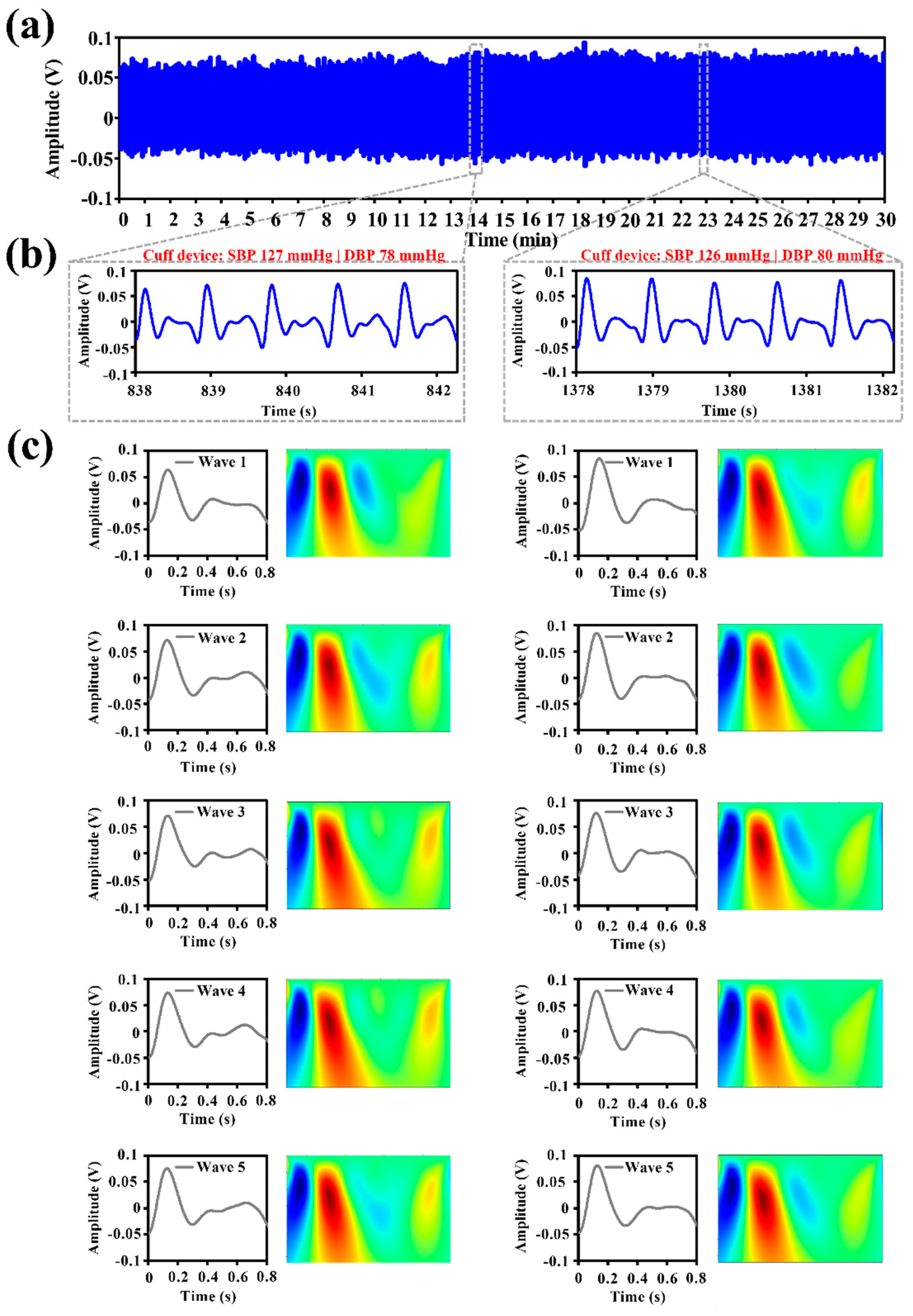
3.1. IPG Signal Measurement and Feature Extraction
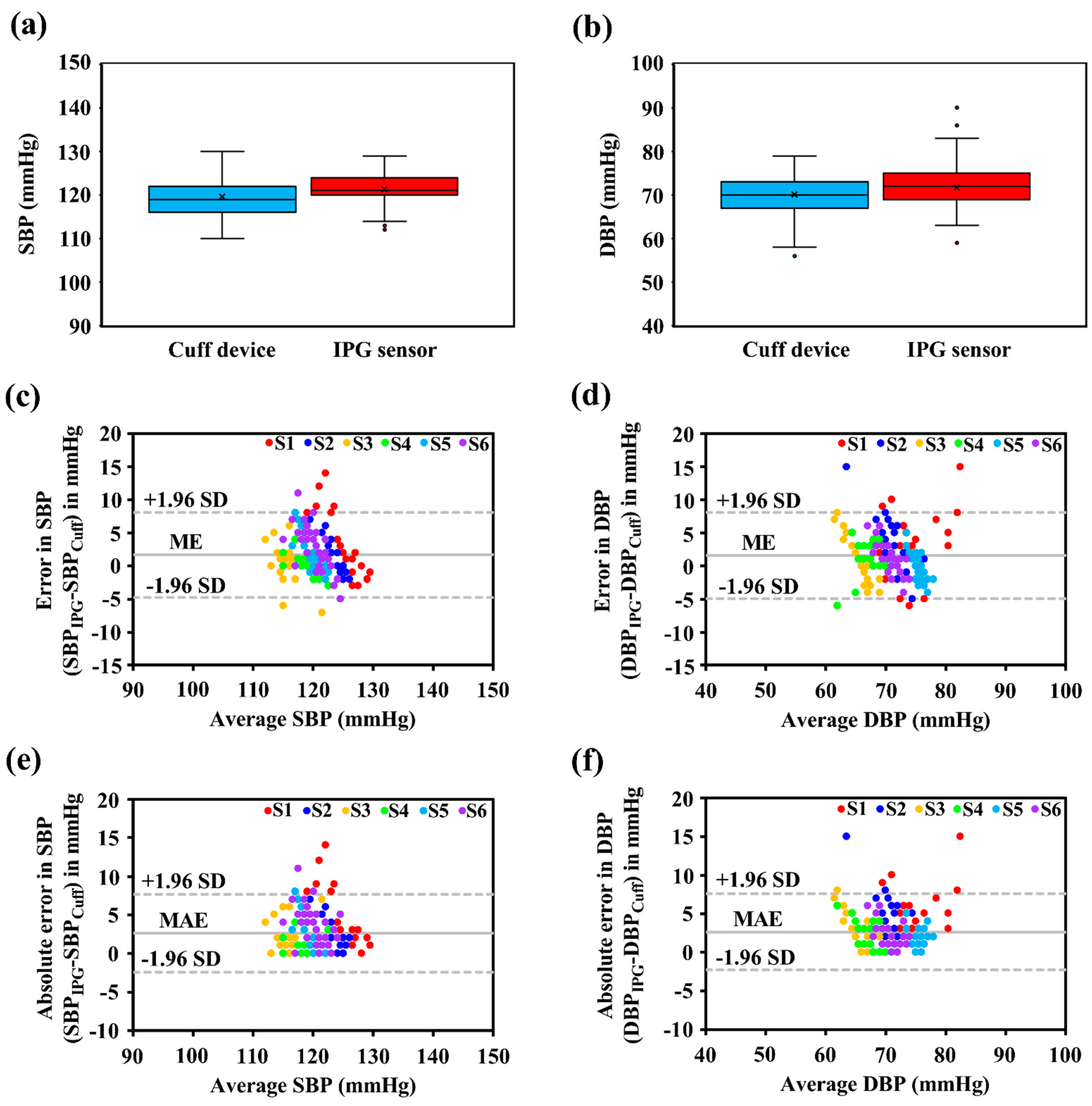
3.2. BP Accuracy Evaluation
4. Discussion
4.1. Innovation of Proposed Intelligent Bio-Impedance System
4.2. BP Measurement Performance
4.3. Comparisons with Previous Cuffless BP Works
4.4. Limitations and Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Georgi, N.; Kuchenbuch, M.; Corvol, A.; Jeannés, R.L.B. An Overview of Blood Pressure Measurement in Telemonitoring Context. IEEE Consum. Electron. Mag. 2020, 9, 42–50. [Google Scholar] [CrossRef]
- Yano, Y. Blood pressure management in an ecosystem context. Hypertens. Res. 2020, 43, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Rogge, D.E.; Fellner, C.; Flotzinger, D.; Grond, J.; Lerche, K.; Saugel, B. A novel art of continuous noninvasive blood pressure measurement. Nat. Commun. 2021, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Kario, K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension 2020, 76, 640–650. [Google Scholar] [CrossRef]
- Wang, T.-W.; Lin, S.-F. Wearable Piezoelectric-Based System for Continuous Beat-to-Beat Blood Pressure Measurement. Sensors 2020, 20, 851. [Google Scholar] [CrossRef] [PubMed]
- Tabei, F.; Gresham, J.M.; Askarian, B.; Jung, K.; Chong, J.W. Cuff-Less Blood Pressure Monitoring System Using Smartphones. IEEE Access 2020, 8, 11534–11545. [Google Scholar] [CrossRef]
- Ding, X.R.; Zhang, Y.T.; Liu, J.; Dai, W.X.; Tsang, H.K. Continuous Cuffless Blood Pressure Estimation Using Pulse Transit Time and Photoplethysmogram Intensity Ratio. IEEE Trans. Bio-Med. Eng. 2016, 63, 964–972. [Google Scholar] [CrossRef]
- Mateen, B.A.; Liley, J.; Denniston, A.K.; Holmes, C.C.; Vollmer, S.J. Improving the quality of machine learning in health applications and clinical research. Nat. Mach. Intell. 2020, 2, 554–556. [Google Scholar] [CrossRef]
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
- Li, R.C.; Asch, S.M.; Shah, N.H. Developing a delivery science for artificial intelligence in healthcare. NPJ Digit. Med. 2020, 3, 107. [Google Scholar] [CrossRef]
- Khalid, S.G.; Liu, H.; Zia, T.; Zhang, J.; Chen, F.; Zheng, D. Cuffless Blood Pressure Estimation Using Single Channel Photoplethysmography: A Two-Step Method. IEEE Access 2020, 8, 58146–58154. [Google Scholar] [CrossRef]
- Liu, D.; Görges, M.; Jenkins, S.A. University of Queensland vital signs dataset: Development of an accessible repository of anesthesia patient monitoring data for research. Anesth. Analg. 2012, 114, 584–589. [Google Scholar] [CrossRef]
- Saeed, M.; Villarroel, M.; Reisner, A.T.; Clifford, G.; Lehman, L.W.; Moody, G.; Heldt, T.; Kyaw, T.H.; Moody, B.; Mark, R.G. Multiparameter Intelligent Monitoring in Intensive Care II: A public-access intensive care unit database. Crit. Care Med. 2011, 39, 952–960. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, C.; Kyriacou, P.A. Deep learning models for cuffless blood pressure monitoring from PPG signals using attention mechanism. Biomed. Signal Process. Control 2021, 65, 102301. [Google Scholar] [CrossRef]
- Li, Y.-H.; Harfiya, L.N.; Purwandari, K.; Lin, Y.-D. Real-Time Cuffless Continuous Blood Pressure Estimation Using Deep Learning Model. Sensors 2020, 20, 5606. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.; Dey, S. Personalized Effect of Health Behavior on Blood Pressure: Machine Learning Based Prediction and Recommendation. In Proceedings of the 2018 IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018; pp. 1–6. [Google Scholar]
- Krittanawong, C.; Bomback, A.S.; Baber, U.; Bangalore, S.; Messerli, F.H.; Wilson Tang, W.H. Future Direction for Using Artificial Intelligence to Predict and Manage Hypertension. Curr. Hypertens. Rep. 2018, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Bhudia, R.P. Treatment of the hypertensive patient in 2030. J. Hum. Hypertens. 2020, 35, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.B. AI (Artificial Intelligence) and Hypertension Research. Curr. Hypertens. Rep. 2020, 22, 70. [Google Scholar] [CrossRef]
- Krittanawong, C.; Rogers, A.J.; Johnson, K.W.; Wang, Z.; Turakhia, M.P.; Halperin, J.L.; Narayan, S.M. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat. Rev. Cardiol. 2021, 18, 75–91. [Google Scholar] [CrossRef]
- Morra, L.; Mohanty, S.P.; Lamberti, F. Artificial Intelligence in Consumer Electronics. IEEE Consum. Electron. Mag. 2020, 9, 46–47. [Google Scholar] [CrossRef]
- Pirbhulal, S.; Wu, W.; Li, G.; Sangaiah, A.K. Medical Information Security for Wearable Body Sensor Networks in Smart Healthcare. IEEE Consum. Electron. Mag. 2019, 8, 37–41. [Google Scholar] [CrossRef]
- Kirk, S. The Wearables Revolution: Is Standardization a Help or a Hindrance?: Mainstream technology or just a passing phase? IEEE Consum. Electron. Mag. 2014, 3, 45–50. [Google Scholar] [CrossRef]
- Wang, T.-W.; Sung, Y.-L.; Chu, H.-W.; Lin, S.-F. IPG-based field potential measurement of cultured cardiomyocytes for optogenetic applications. Biosens. Bioelectron. 2021, 179, 113060. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Chu, H.-W.; Chou, L.; Sung, Y.-L.; Shih, Y.-T.; Hsu, P.-C.; Cheng, H.-M.; Lin, S.-F. Bio-Impedance Measurement Optimization for High-Resolution Carotid Pulse Sensing. Sensors 2021, 21, 1600. [Google Scholar] [CrossRef]
- Pesti, K.; Metshein, M.; Annus, P.; Kõiv, H.; Min, M. Electrode Placement Strategies for the Measurement of Radial Artery Bioimpedance: Simulations and Experiments. IEEE Trans. Instrum. Meas. 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Swanson, D.; Webster, J. Origin of the Electrical Impedance Pulse in the Limbs. In Proceedings of the 29th Annual Conference on Engineering in Medicine & Biology, Lyon, France, 22–26 August 2007; p. 324. [Google Scholar]
- Wang, T.W.; Chen, W.X.; Chu, H.W.; Lin, S.F. Single-Channel Bioimpedance Measurement for Wearable Continuous Blood Pressure Monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Wang, T.W.; Chu, H.W.; Chen, W.X.; Shih, Y.T.; Hsu, P.C.; Cheng, H.M.; Lin, S.F. Single-Channel Impedance Plethysmography Neck Patch Device for Unobtrusive Wearable Cardiovascular Monitoring. IEEE Access 2020, 8, 184909–184919. [Google Scholar] [CrossRef]
- Huynh, T.H.; Jafari, R.; Chung, W.Y. Noninvasive Cuffless Blood Pressure Estimation Using Pulse Transit Time and Impedance Plethysmography. IEEE Trans. Bio-Med. Eng. 2019, 66, 967–976. [Google Scholar] [CrossRef]
- Anand, G.; Lowe, A.; Al-Jumaily, A. Simulation of impedance measurements at human forearm within 1 kHz to 2 MHz. J. Electr. Bioimpedance 2016, 7, 20–27. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, Recent Advances, and Future Challenges in Bioimpedance Devices for Healthcare Applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef]
- Wang, J.-J.; Wei-Chih, H.; Kao, T.; Liu, C.-P.; Lin, S.-K. Development of forearm impedance plethysmography for the minimally invasive monitoring of cardiac pumping function. J. Biomed. Sci. Eng. 2011, 4, 122–129. [Google Scholar] [CrossRef][Green Version]
- Yang, T.-Y.; Huang, Y.-H.; Lin, Y.-Y.; Hsiu, P.-C.; Chuang, Y.-Y. SSR-Net: A Compact Soft Stagewise Regression Network for Age Estimation. In Proceedings of the IJCAI, Stockholm, Sweden, 13–19 July 2018; p. 7. [Google Scholar]
- Yang, T.-Y.; Hsu, J.-H.; Lin, Y.-Y.; Chuang, Y.-Y. Deepcd: Learning deep complementary descriptors for patch representations. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 3314–3322. [Google Scholar]
- Mallat, S. A Wavelet Tour of Signal Processing; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Thapliyal, H. Internet of Things-Based Consumer Electronics: Reviewing Existing Consumer Electronic Devices, Systems, and Platforms and Exploring New Research Paradigms. IEEE Consum. Electron. Mag. 2018, 7, 66–67. [Google Scholar] [CrossRef]
- Ibrahim, B.; Jafari, R. Cuffless Blood Pressure Monitoring from an Array of Wrist Bio-Impedance Sensors Using Subject-Specific Regression Models: Proof of Concept. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1723–1735. [Google Scholar] [CrossRef]
- Huynh, T.H.; Jafari, R.; Chung, W.-Y. A Robust Bioimpedance Structure for Smartwatch-Based Blood Pressure Monitoring. Sensors 2018, 18, 2095. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Liu, Z.D.; Liu, J.K.; Wen, B.; He, Q.Y.; Li, Y. Multi-Sensor Fusion Approach for Cuff-Less Blood Pressure Measurement. IEEE J. Biomed. Health Inform. 2020, 24, 79–91. [Google Scholar] [CrossRef]
- Marzorati, D.; Bovio, D.; Salito, C.; Mainardi, L.; Cerveri, P. Chest Wearable Apparatus for Cuffless Continuous Blood Pressure Measurements Based on PPG and PCG Signals. IEEE Access 2020, 8, 55424–55437. [Google Scholar] [CrossRef]
- Miao, F.; Wen, B.; Hu, Z.; Fortino, G.; Wang, X.-P.; Liu, Z.-D.; Tang, M.; Li, Y. Continuous blood pressure measurement from one-channel electrocardiogram signal using deep-learning techniques. Artif. Intell. Med. 2020, 108, 101919. [Google Scholar] [CrossRef]
| Model | SSR-Net | MobileNet-V2 | LSTM |
|---|---|---|---|
| Model size | 213 KB | 13,932 KB | 8744 KB |
| Model parameters | 0.04 M | 3.50 M | 215.99 M |
| Inference time on CPU | 0.17 s | 0.29 s | 0.25 s |
| Author | Physiological Signal | Deep Learning Model | Statistical Results | BP Estimation Error | |
| SBP | DBP | ||||
| Miao et al. [41] | ECG, 2-PPW | - | ME ± SD | 1.62 ± 7.76 | 1.49 ± 5.52 |
| Tabei et al. [6] | 2-PPG | - | MAE ± SD | 2.07 ± 2.06 | 2.12 ± 1.85 |
| Marzorati et al. [42] | PPG, PCG | - | ME ± SD | 1.47 ± 3.76 | 0.01 ± 7.55 |
| Miao et al. [43] | ECG | Res-LSTM | ME ± SD | −0.22 ± 5.82 | −0.75 ± 5.62 |
| El-Hajj et al. [14] | PPG | Attention based-RNN | ME ± SD | −0.52 ± 4.22 | −0.66 ± 2.07 |
| MAE ± SD | 2.58 ± 3.35 | 1.26 ± 1.63 | |||
| Our work | IPG | SSR-Net | ME ± SD | 1.69 ± 3.28 | 1.56 ± 3.32 |
| MAE ± SD | 2.63 ± 2.58 | 2.66 ± 2.52 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-W.; Syu, J.-Y.; Chu, H.-W.; Sung, Y.-L.; Chou, L.; Escott, E.; Escott, O.; Lin, T.-T.; Lin, S.-F. Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement. Biosensors 2022, 12, 150. https://doi.org/10.3390/bios12030150
Wang T-W, Syu J-Y, Chu H-W, Sung Y-L, Chou L, Escott E, Escott O, Lin T-T, Lin S-F. Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement. Biosensors. 2022; 12(3):150. https://doi.org/10.3390/bios12030150
Chicago/Turabian StyleWang, Ting-Wei, Jhen-Yang Syu, Hsiao-Wei Chu, Yen-Ling Sung, Lin Chou, Endian Escott, Olivia Escott, Ting-Tse Lin, and Shien-Fong Lin. 2022. "Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement" Biosensors 12, no. 3: 150. https://doi.org/10.3390/bios12030150
APA StyleWang, T.-W., Syu, J.-Y., Chu, H.-W., Sung, Y.-L., Chou, L., Escott, E., Escott, O., Lin, T.-T., & Lin, S.-F. (2022). Intelligent Bio-Impedance System for Personalized Continuous Blood Pressure Measurement. Biosensors, 12(3), 150. https://doi.org/10.3390/bios12030150





