Behavioral Effect of Terahertz Waves in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
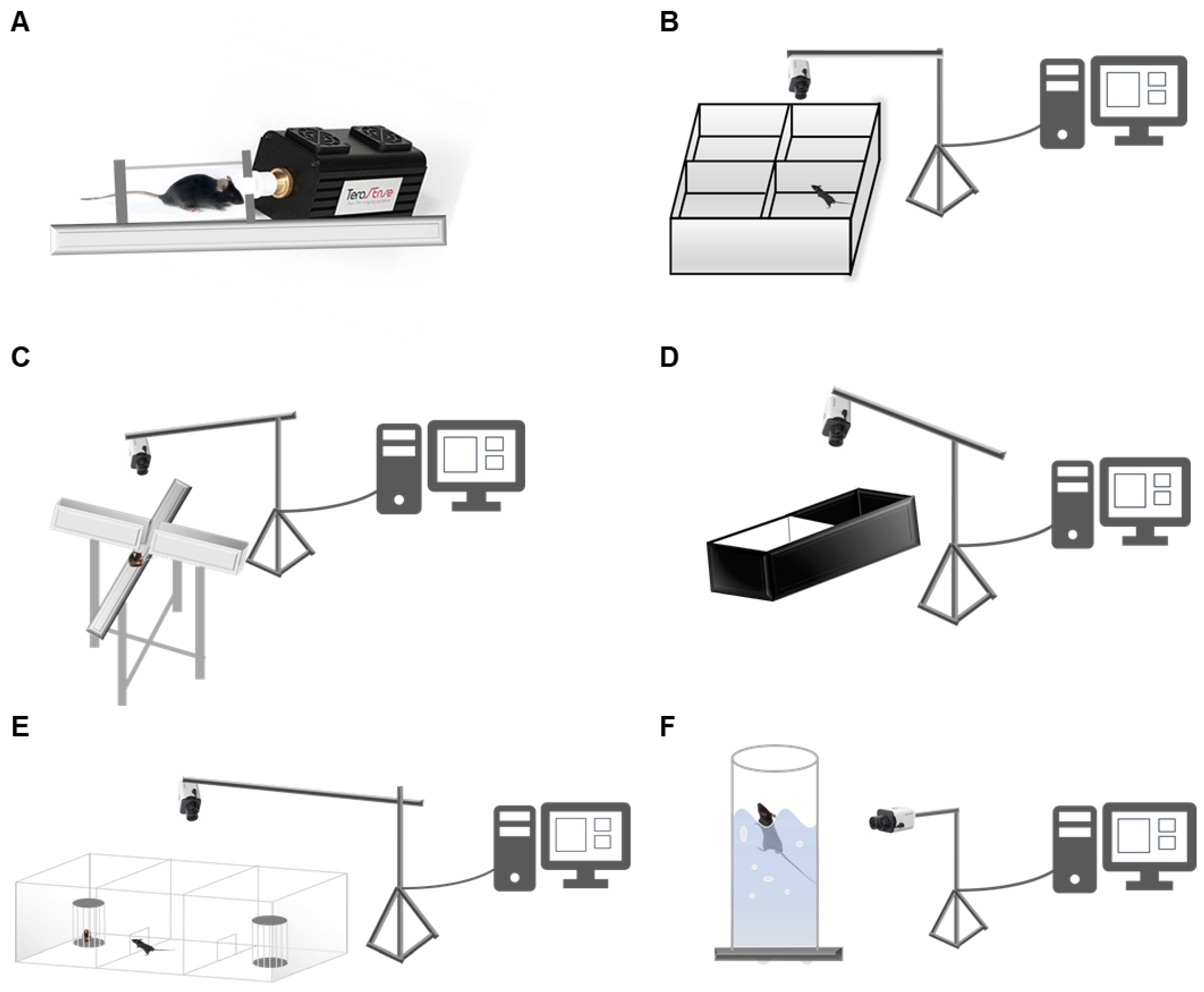
2.2. Terahertz Generator
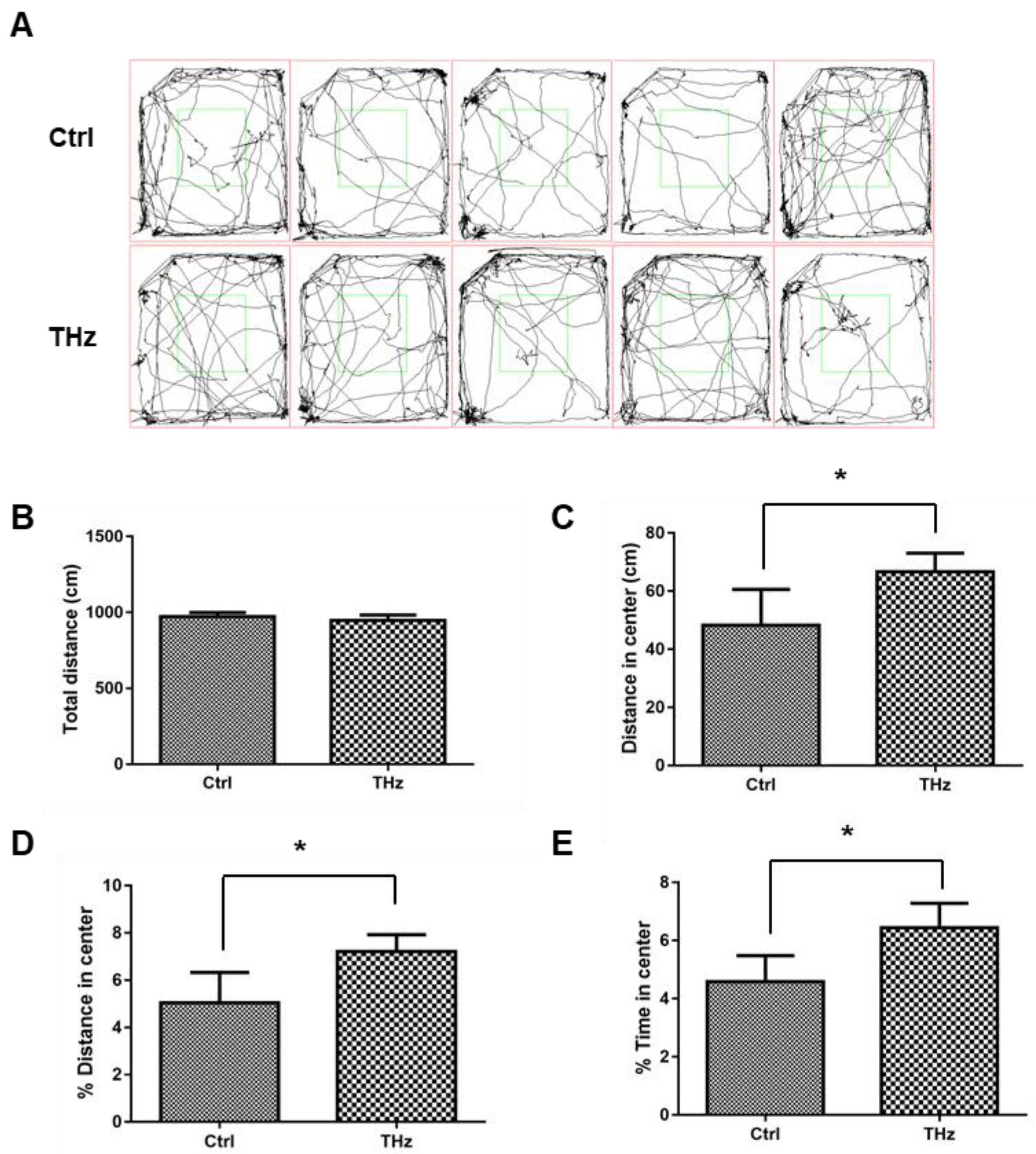
2.3. Open Field Test
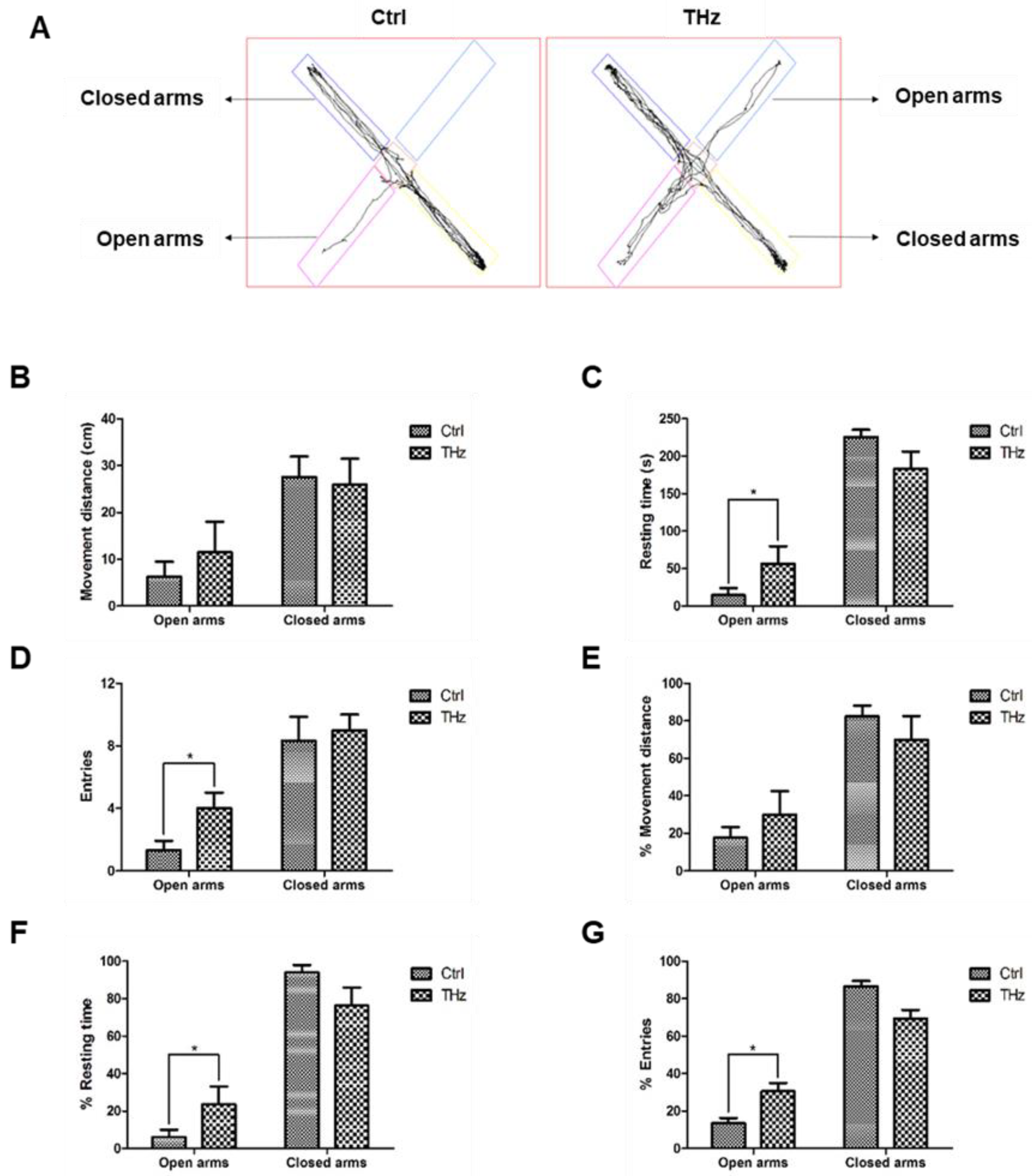
2.4. Elevated plus Maze
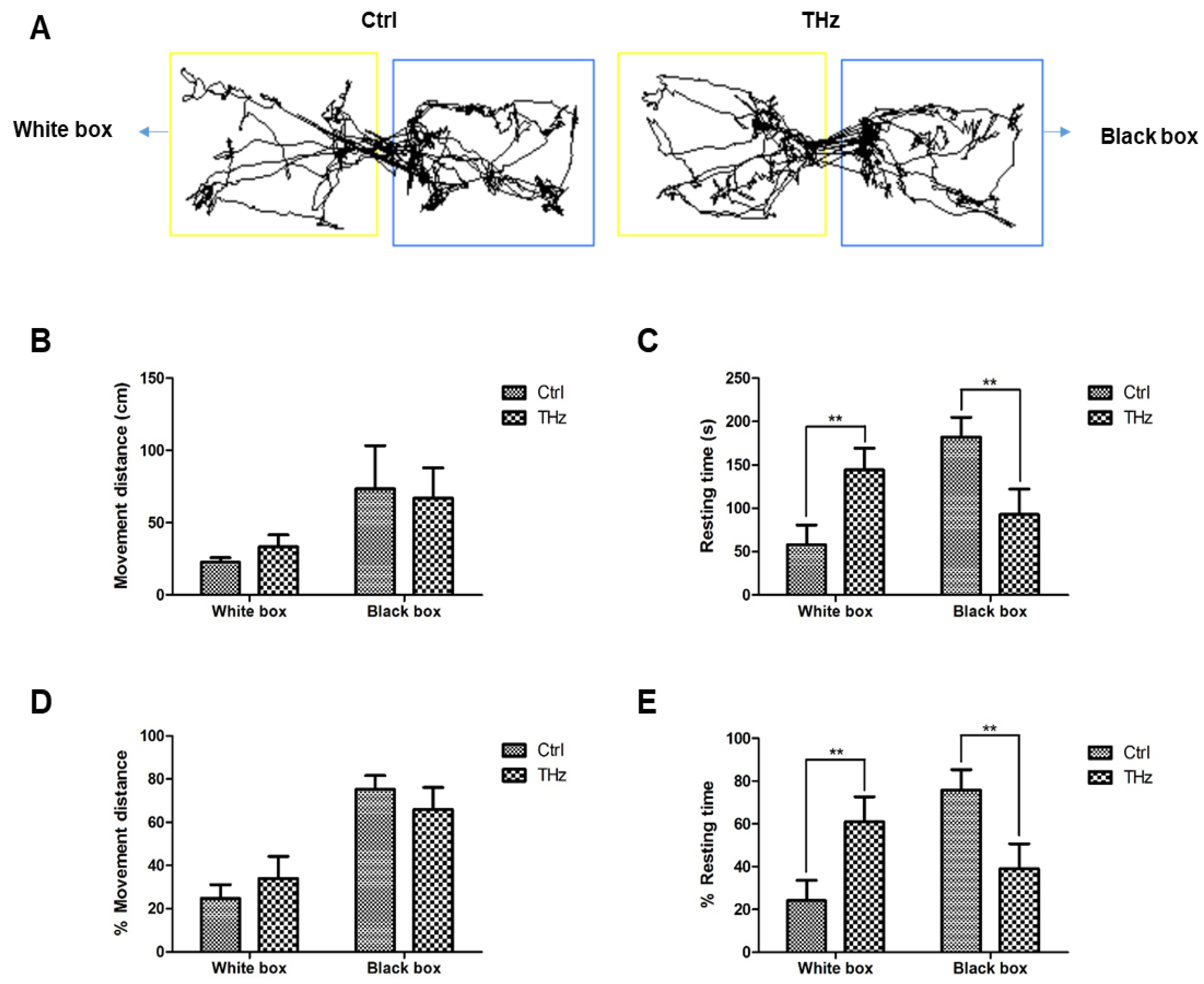
2.5. Light–Dark Box Test
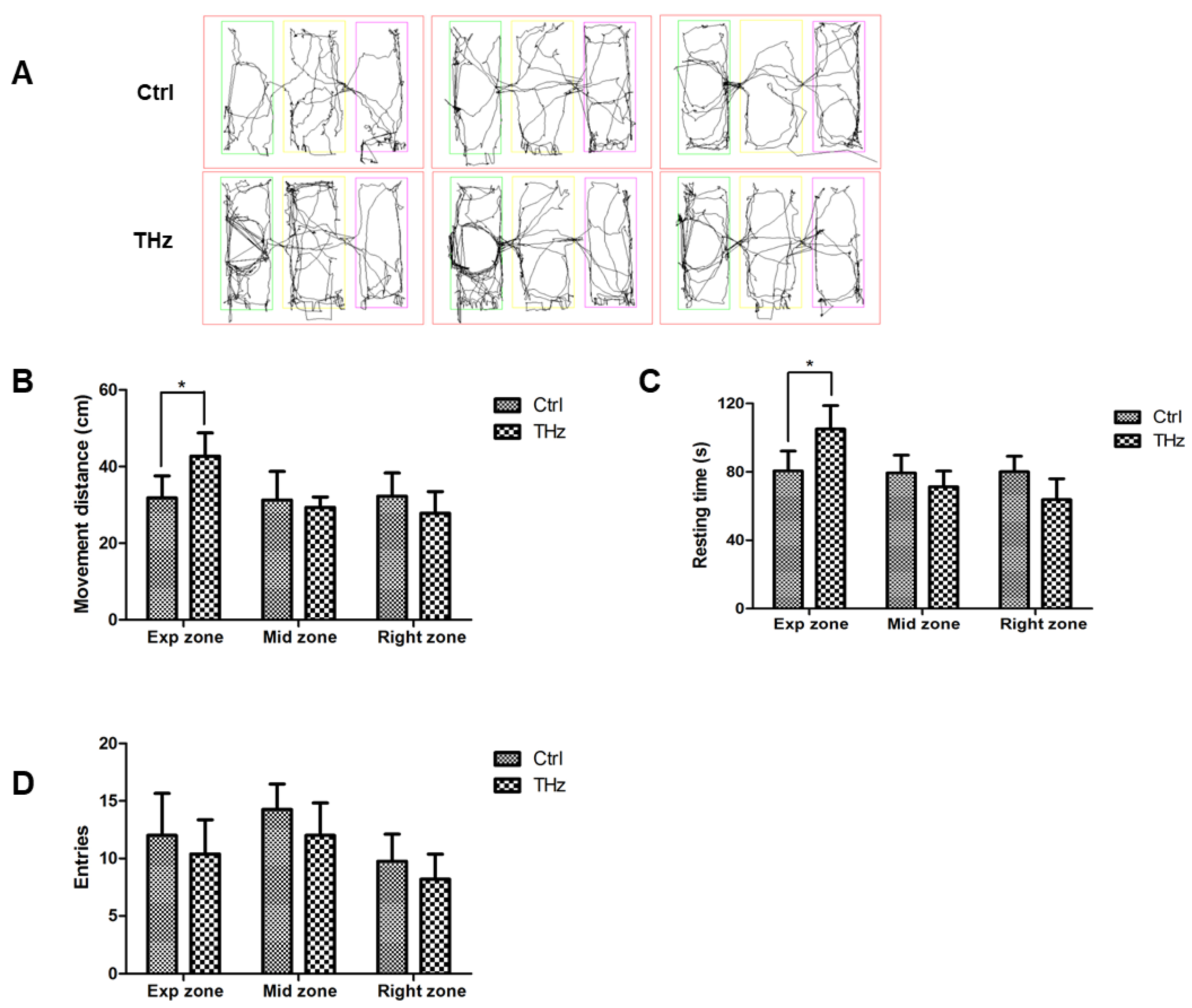
2.6. Three-Chambered Social Test
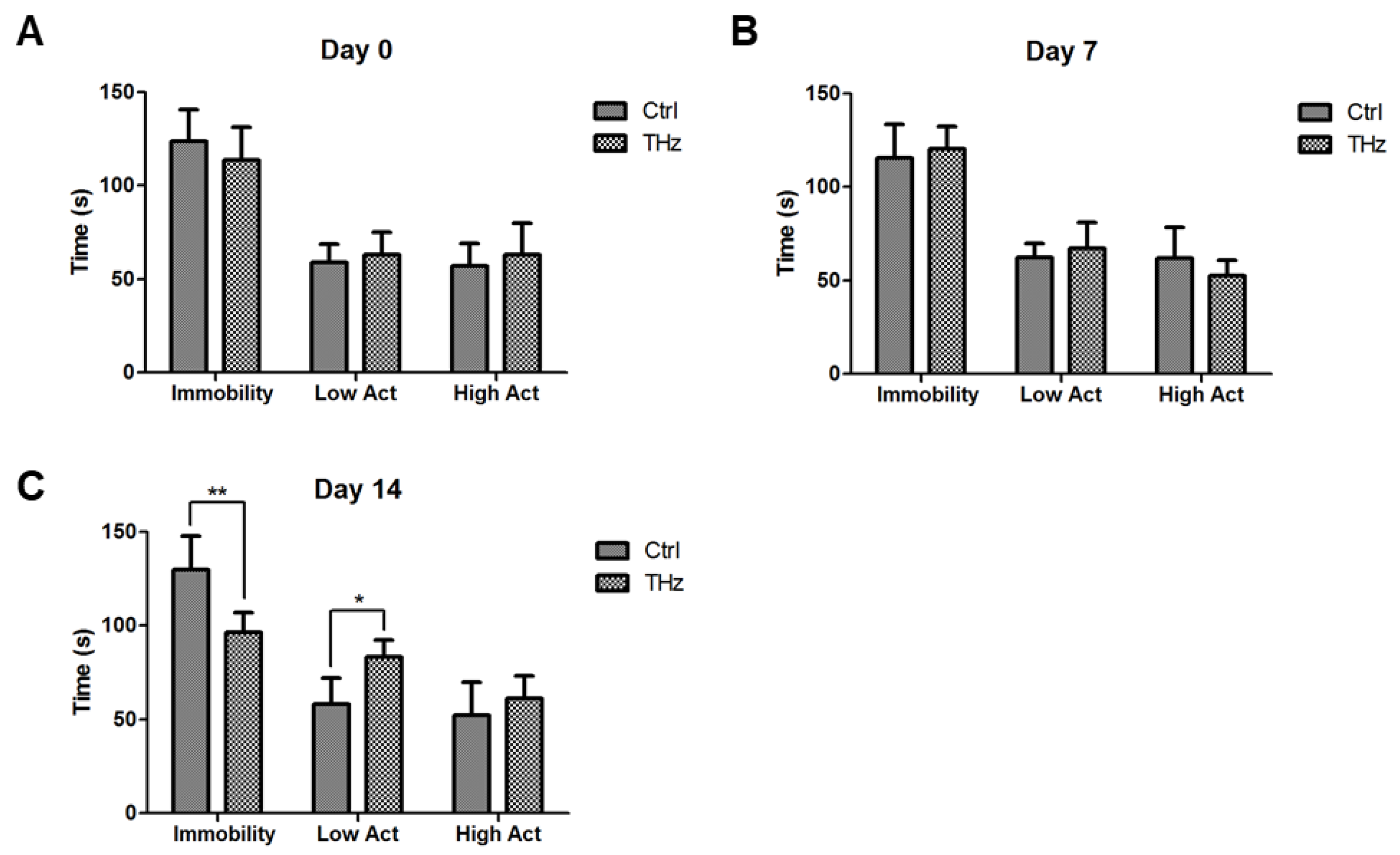
2.7. Forced Swim Test
3. Results
3.1. Analysis of the Open Field Test
3.2. Analysis of the Elevated plus Maze Test
3.3. Analysis of the Light–Dark Box Test
3.4. Analysis of the Three-Chambered Social Test
3.5. Analysis of the Forced Swim Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shumyatsky, P.; Alfano, R.R. Terahertz sources. J. Biomed. Opt. 2011, 16, 033001. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.H.; Fitzgerald, A.J.; Wallace, V.P. Non-Contact, Non-Destructive Testing in Various Industrial Sectors with Terahertz Technology. Sensors 2020, 20, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasteh, D.; Hoare, E.G.; Cherniakov, M.; Gashinova, M. Experimental Low-Terahertz Radar Image Analysis for Automotive Terrain Sensing. IEEE Geosci. Remote. Sens. Lett. 2016, 13, 490–494. [Google Scholar] [CrossRef]
- Bychanok, D.; Gorokhov, G.; Plyushch, A.; Ronca, A.; Lavorgna, M.; Xia, H.; Lamberti, P.; Kuzhir, P. Terahertz Optics of Materials with Spatially Harmonically Distributed Refractive Index. Materials 2020, 13, 5208. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Cordeiro, C.M.B.; Franco, M.A.R.; Sultana, J.; Cruz, A.L.S.; Abbott, D. Terahertz optical fibers [Invited]. Opt. Express 2020, 28, 16089–16117. [Google Scholar] [CrossRef]
- Ma, Z.T.; Geng, Z.X.; Fan, Z.Y.; Liu, J.; Chen, H.D. Modulators for Terahertz Communication: The Current State of the Art. Research 2019, 2019, 6482975. [Google Scholar] [CrossRef] [Green Version]
- Bai, P.; Zhang, Y.; Wang, T.; Fu, Z.; Shao, D.; Li, Z.; Wan, W.; Li, H.; Cao, J.; Guo, X.; et al. Broadband THz to NIR up-converter for photon-type THz imaging. Nat. Commun. 2019, 10, 3513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tang, Y.; Shi, A.; Bao, L.; Shen, Y.; Shen, R.; Ye, Y. Recent Developments in Spectroscopic Techniques for the Detection of Explosives. Materials 2018, 11, 1364. [Google Scholar] [CrossRef] [Green Version]
- Globus, T.R.; Woolard, D.L.; Khromova, T.; Crowe, T.W.; Bykhovskaia, M.; Gelmont, B.L.; Hesler, J.; Samuels, A.C. THz-Spectroscopy of Biological Molecules. J. Biol. Phys. 2003, 29, 89–100. [Google Scholar] [CrossRef]
- Wu, L.; Xu, D.; Wang, Y.; Zhang, Y.; Wang, H.; Liao, B.; Gong, S.; Chen, T.; Wu, N.; Feng, H.; et al. Horizontal-scanning attenuated total reflection terahertz imaging for biological tissues. Neurophotonics 2020, 7, 025005. [Google Scholar] [CrossRef]
- Laman, N.; Harsha, S.S.; Grischkowsky, D.; Melinger, J.S. High-resolution waveguide THz spectroscopy of biological molecules. Biophys. J. 2008, 94, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Qi, C.; Zhu, Z.; Wang, C.; Song, B.; Chang, C. Terahertz Wave Accelerates DNA Unwinding: A Molecular Dynamics Simulation Study. J. Phys. Chem. Lett. 2020, 11, 7002–7008. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Hoffmann, M.; Helm, H.; Wilk, R.; Rutz, F.; Kleine-Ostmann, T.; Koch, M.; Jepsen, P. Terahertz time-domain spectroscopy and imaging of artificial RNA. Opt. Express 2005, 13, 5205–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Yan, S.; Zang, Z.; Wei, D.; Cui, H.L.; Du, C. Label-free protein detection using terahertz time-domain spectroscopy. Biomed. Opt. Express 2018, 9, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elayan, H.; Eckford, A.W.; Adve, R.S. Information Rates of Controlled Protein Interactions Using Terahertz Communication. IEEE Trans. Nanobiosci. 2021, 20, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Shimakura, K.; Ohtake, H.; Takayanagi, J.; Tomoda, K.; Nakajima, T.; Terada, H.; Makino, K. Nondestructive analysis of structure and components of tablet coated with film by the usage of terahertz time-domain reflection spectroscopy. J. Pharm. Sci. 2014, 103, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Standaert, D.G. Ten Unsolved Questions About Neuroinflammation in Parkinson’s Disease. Mov. Disord. 2021, 36, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ol’shevskaia Iu, S.; Kozlov, A.S.; Petrov, A.K.; Zapara, T.A.; Ratushniak, A.S. Influence of terahertz (submillimeter) laser radiation on neurons in vitro. Zhurnal Vyss. Nervn. Deiatelnosti Im. I P Pavlov. 2009, 59, 353–359. [Google Scholar]
- Kirichuk, V.F.; Antipova, O.N.; Krylova, Y.A. Effect of continuous irradiation with terahertz electromagnetic waves of the NO frequency range on behavioral reactions of male albino rats under stress conditions. Bull. Exp. Biol. Med. 2014, 157, 184–189. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.K.; Kim, H.G.; Kim, K.B.; Kim, H.R. Possible Effects of Radiofrequency Electromagnetic Field Exposure on Central Nerve System. Biomol. Ther. 2019, 27, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bucan, M.; Abel, T. The mouse: Genetics meets behaviour. Nat. Rev. Genet. 2002, 3, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef]
- Wang, Q.; Timberlake, M.A., 2nd; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- Czeh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Prog. Neuro-Psychoph. 2016, 64, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Guell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Takumi, T.; Tamada, K.; Hatanaka, F.; Nakai, N.; Bolton, P.F. Behavioral neuroscience of autism. Neurosci. Biobehav. Rev. 2020, 110, 60–76. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.; Fone, K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Nadler, J.J.; Moy, S.S.; Dold, G.; Trang, D.; Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.P.; Piven, J.; Magnuson, T.R.; et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, e3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [PubMed]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. 2008, 22, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Toth, I.; Neumann, I.D. Animal models of social avoidance and social fear. Cell. Tissue Res. 2013, 354, 107–118. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019, 1916, 69–74. [Google Scholar]
- Bondar, N.P.; Kovalenko, I.L.; Avgustinovich, D.F.; Khamoyan, A.G.; Kudryavtseva, N.N. Behavioral Effect of Terahertz Waves in Male Mice. Bull. Exp. Biol. Med. 2008, 145, 401–405. [Google Scholar] [CrossRef]
- Kirichuk, V.F.; Efimova, N.V.; Andronov, E.V. Effect of High Power Terahertz Irradiation on Platelet Aggregation and Behavioral Reactions of Albino Rats. Bull. Exp. Biol. Med. 2009, 148, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Braz. J. Psychiatry 2013, 35, S101–S111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. Jove-J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011, 8, 26. [Google Scholar] [CrossRef]
- Miczek, K.A.; Maxson, S.C.; Fish, E.W.; Faccidomo, S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 2001, 125, 167–181. [Google Scholar] [CrossRef]
- Piccin, A.; Contarino, A. Long-lasting pseudo-social aggressive behavior in opiate-withdrawn mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 97, 109780. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Liu, R.; Li, B.; Wang, S.; Fan, R.; Zhao, X.; Xu, D. Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors 2022, 12, 79. https://doi.org/10.3390/bios12020079
Qi M, Liu R, Li B, Wang S, Fan R, Zhao X, Xu D. Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors. 2022; 12(2):79. https://doi.org/10.3390/bios12020079
Chicago/Turabian StyleQi, Miao, Rong Liu, Bing Li, Shuai Wang, Runze Fan, Xinyi Zhao, and Dehui Xu. 2022. "Behavioral Effect of Terahertz Waves in C57BL/6 Mice" Biosensors 12, no. 2: 79. https://doi.org/10.3390/bios12020079
APA StyleQi, M., Liu, R., Li, B., Wang, S., Fan, R., Zhao, X., & Xu, D. (2022). Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors, 12(2), 79. https://doi.org/10.3390/bios12020079




