A Novel Detachable, Reusable, and Versatile Acoustic Tweezer Manipulation Platform for Biochemical Analysis and Detection Systems
Abstract
:1. Introduction
2. Materials and Methods
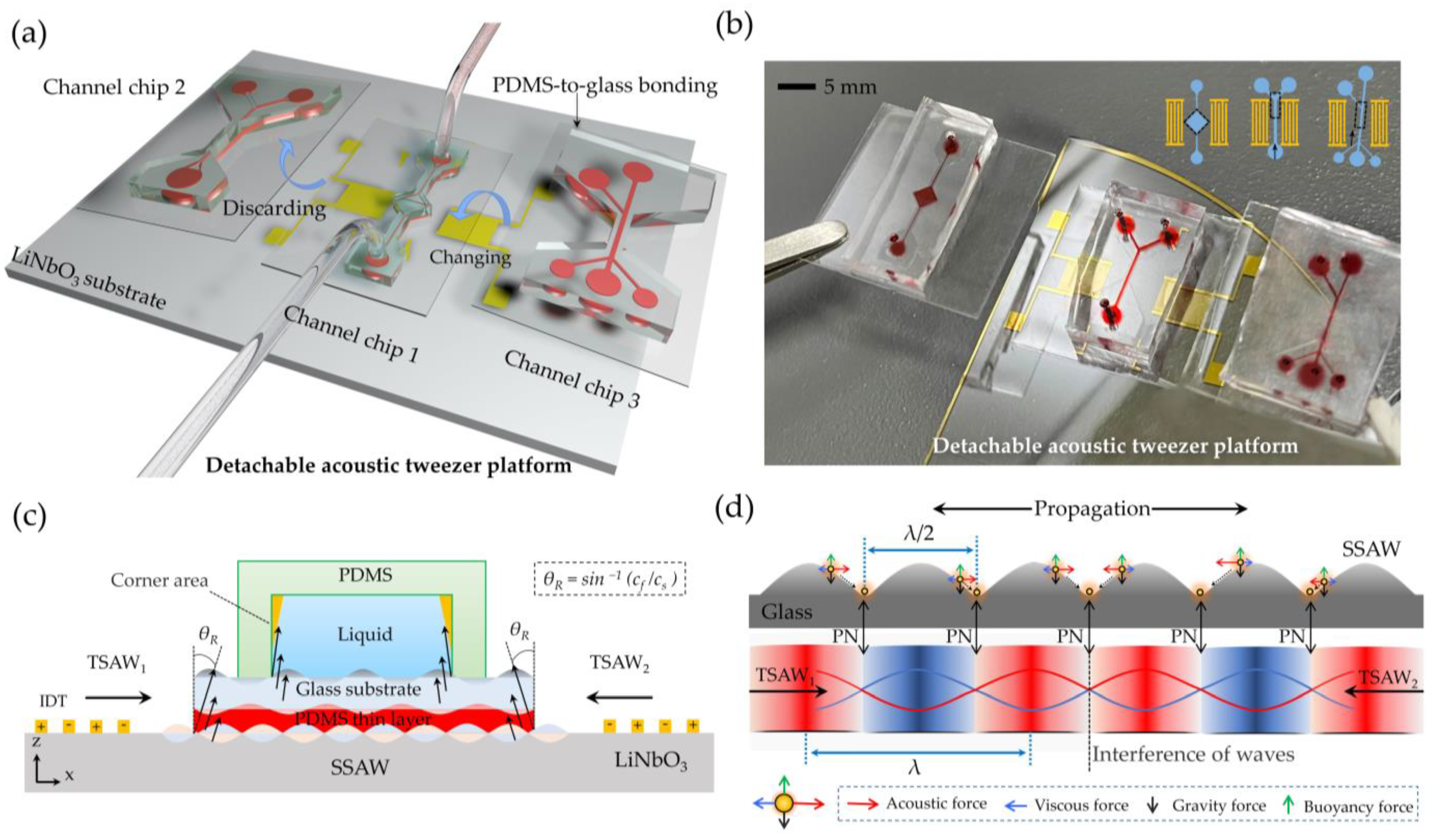
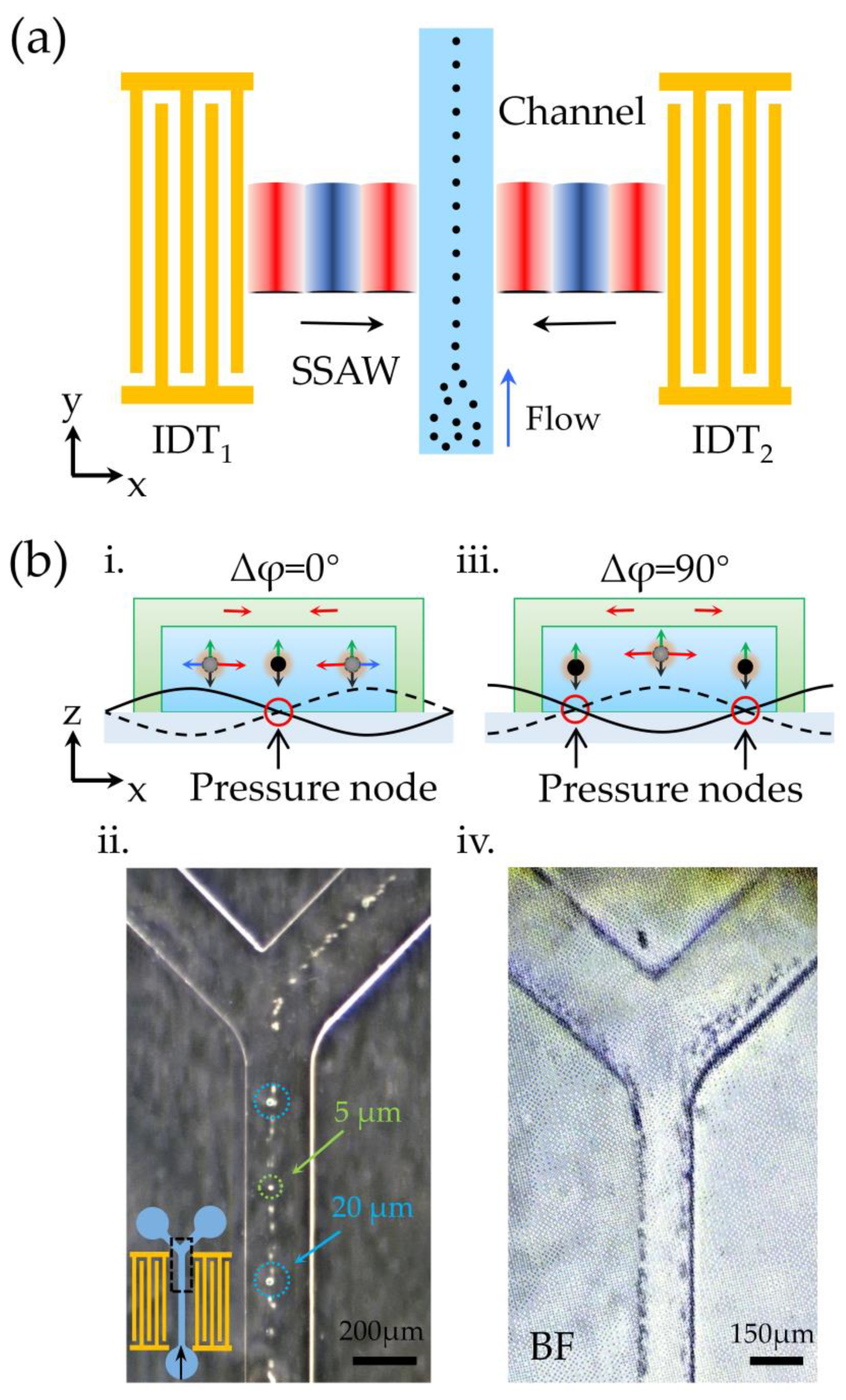
2.1. Working Mechanism
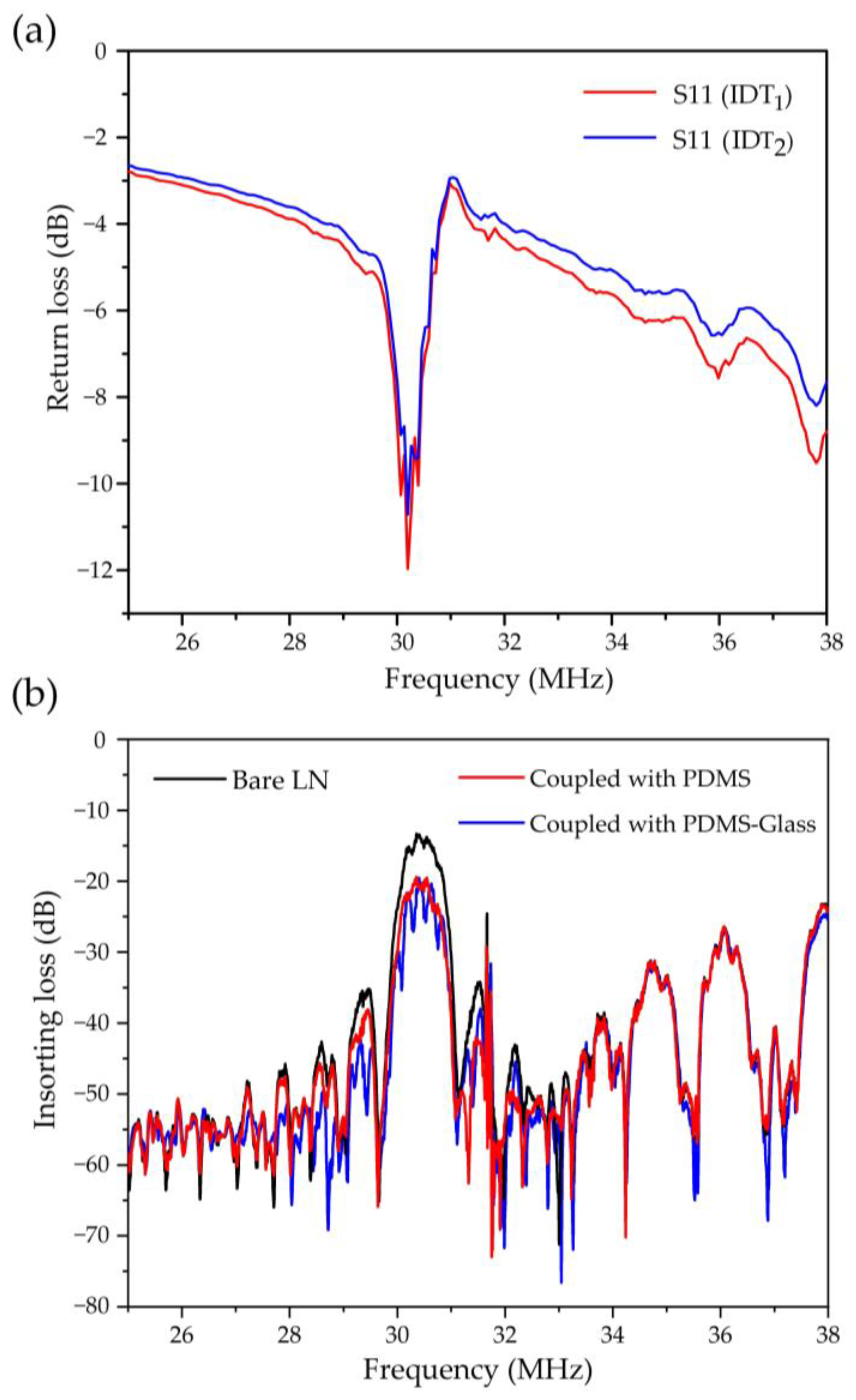
2.2. Device Fabrication
2.3. Sample Preparation and System Setup
3. Results and Discussion
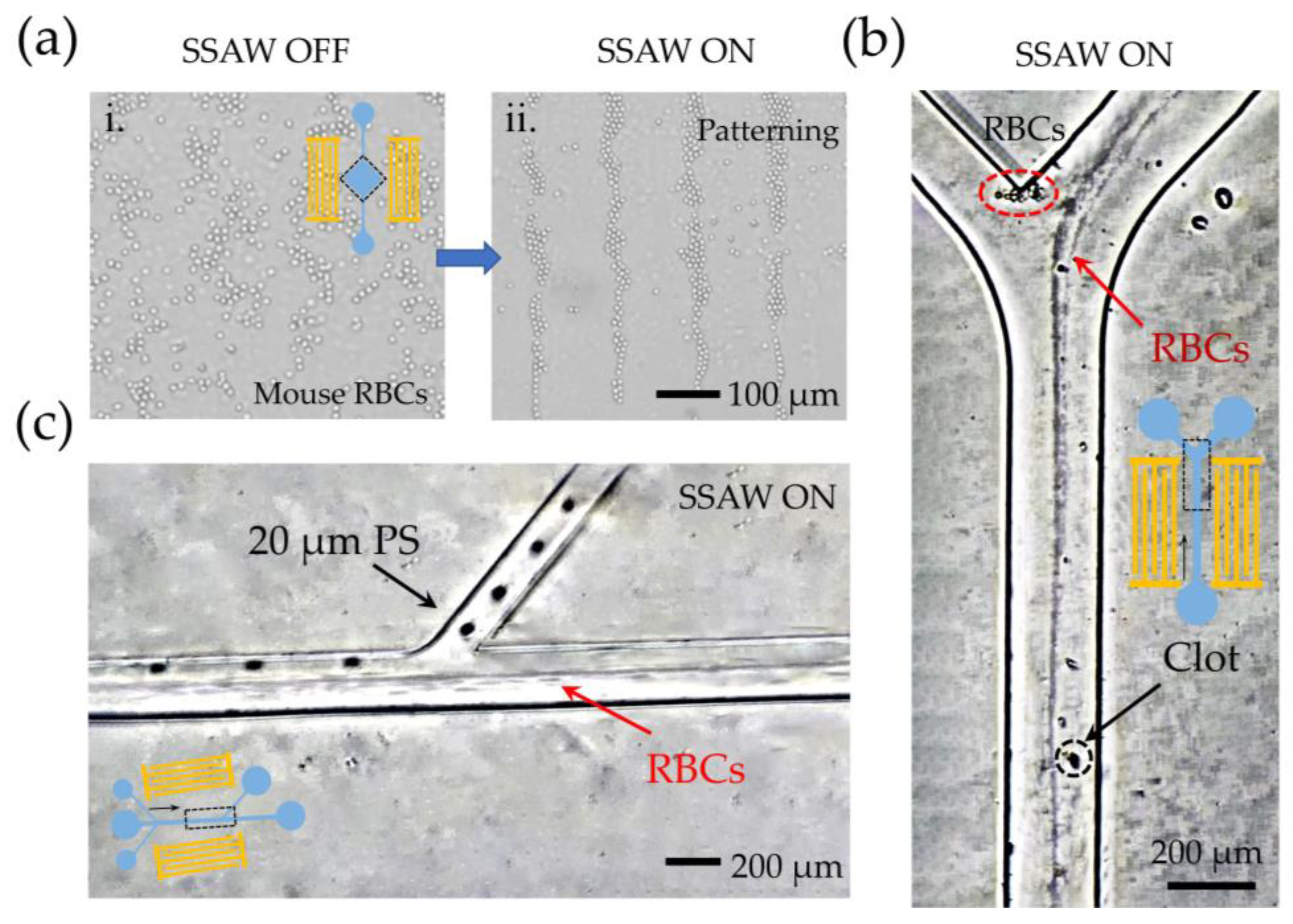
3.1. Demo I: Parallel Processing and Enrichment of the Sample
3.2. Demo II: Capability for Precise Cell Alignment
3.3. Demo III: Capability for Separation and Purification of Cells
3.4. The Actual Application Capability of the Manipulation Platform
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Lin, X.; Huang, H.; Li, P.; Wu, J.; Huang, X.; Cai, H.; Han, H.; Zheng, J.; Zhou, H. Au@ Ag@ ZIF-8 multifunction probe with internally o-phenylenediamine encoding for the colorimetric, fluorescence, and SERS multi-mode optical detection of reactive sulfur species. Sensor Actuat. B-Chem. 2022, 361, 131762. [Google Scholar] [CrossRef]
- Borodaenko, Y.; Syubaev, S.; Gurbatov, S.; Zhizhchenko, A.; Porfirev, A.; Khonina, S.; Mitsai, E.; Gerasimenko, A.V.; Shevlyagin, A.; Modin, E.; et al. Deep Subwavelength Laser-Induced Periodic Surface Structures on Silicon as a Novel Multifunctional Biosensing Platform. ACS Appl. Mater. Inter. 2021, 13, 54551–54560. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Jiang, Y.; Fan, J.; An, C.; Li, J.; Chen, T.; Li, X. High-Efficiency Capture of Cells by Softening Cell Membrane. Small 2022, 18, 2106547. [Google Scholar] [CrossRef]
- Huang, Z.; Kong, W.; Kuang, D. Arrangement of Micro Dielectric Particles with Vector Vortex Beam Generated by Dual-Helical Dielectric Cone. IEEE J. Sel. Top. Quant. 2021, 27, 3400808. [Google Scholar] [CrossRef]
- Emmerich, M.E.; Sinnigen, A.S.; Neubauer, P.; Birkholz, M. Dielectrophoretic separation of blood cells. Biomed. Microdevices 2022, 24, 30. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Niu, J.; Jin, H.; Lin, S.; Cui, D. Geometric structure design of passive label-free microfluidic systems for biological micro-object separation. Microsyst. Nanoeng. 2022, 8, 62. [Google Scholar] [CrossRef]
- Xiao, Z.; Sun, L.; Yang, Y.; Feng, Z.; Dai, S.; Yang, H.; Zhang, X.; Sheu, C.; Guo, W. High-performance passive plasma separation on OSTE pillar forest. Biosensors 2021, 11, 355. [Google Scholar] [CrossRef]
- Ji, M.; Duan, J.; Zang, W.; Luo, Z.; Qu, Z.; Li, X.; Zhang, B. Ultra-high precision passive particle sorting chip coupling inertial microfluidics and single row micropillar arrays. J. Micromech. Microeng. 2022, 32, 045004. [Google Scholar] [CrossRef]
- Mao, Z.; Yoshida, K.; Kim, J.W. Active sorting of droplets by using an ECF (Electro-conjugate Fluid) micropump. Sens. Actuat. A-Phys. 2020, 303, 111702. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active microfluidic systems for cell sorting and separation. Curr. Opin. Biomed. Eng. 2020, 13, 60–68. [Google Scholar] [CrossRef]
- Wei, S.; Earl, S.K.; Lin, J.; Kou, S.S.; Yuan, X.C. Active sorting of orbital angular momentum states of light with a cascaded tunable resonator. Light Sci. Appl. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, F.; Rubinsky, B. A correlation between electric fields that target the cell membrane potential and dividing hela cancer cell growth inhibition. IEEE T Bio-Med. Eng. 2020, 68, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Kim, S.H.; Park, J.K.; Park, S. Dielectrophoresis in a slanted microchannel for separation of microparticles and bacteria. J. Nanosci. Nanotechno. 2013, 13, 7993–7997. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, S.; Chen, X.; Zhang, P.; Gu, G.; Wang, Y.; Zhang, H.; Zhang, Y.; Yang, H. Extraction of small extracellular vesicles by label-free and biocompatible on-chip magnetic separation. Lab. Chip. 2022, 22, 2476. [Google Scholar] [CrossRef] [PubMed]
- Hillion, A.; Hallali, N.; Clerc, P.; Lopez, S.; Lalatonne, Y.; Noûs, C.; Motte, L.; Gigoux, V.; Carrey, J. Real-Time Observation and Analysis of Magnetomechanical Actuation of Magnetic Nanoparticles in Cells. Nano Lett. 2022, 22, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Chikaarashi, T.; Watanabe, S.; Miyamoto, Y.; Omata, D.; Maruyama, K.; Suzuki, R.; Masuda, K. Experimental study of ultrasound retention of bubble-surrounded cells under various conditions of acoustic field and flow velocity. Jpn. J. Appl. Phys. 2022, 61, 1071. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F. Nonlinear large deformation of a spherical red blood cell induced by ultrasonic standing wave. Biomech Model. Mechan. 2022, 21, 589–604. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Duan, J.; Zang, W.; Wang, Y.; Qu, Z.; Zhang, B. A Novel Perturbed Spiral Sheathless Chip for Particle Separation Based on Traveling Surface Acoustic Waves (TSAW). Biosensors 2022, 12, 325. [Google Scholar] [CrossRef]
- Duan, J.; Ji, M.; Zhang, B. A Perturbed Asymmetrical Y-Type Sheathless Chip for Particle Manipulation Based on Adjustable Tilted-Angle Traveling Surface Acoustic Waves (ataTSAWs). Biosensors 2022, 12, 611. [Google Scholar] [CrossRef]
- Chen, X.; Lv, H.; Zhang, Y. A novel study on separation of particles driven in two steps based on standing surface acoustic waves. Chaos Soliton Fract. 2022, 162, 112419. [Google Scholar] [CrossRef]
- Sriphutkiat, Y.; Zhou, Y. The effect of phase modulation in dual-frequency excited standing surface acoustic wave (SSAW) on microparticle manipulation. Sens. Actuat. A-Phys. 2021, 332, 113072. [Google Scholar] [CrossRef]
- Nair, M.P.; Teo, A.J.; Li, K.H.H. Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, I.; Reichert, P.; Dual, J. Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab. Chip. 2015, 15, 2896–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, S.; Zhang, X.; He, F.; Liu, Z.; Hao, P. Acoustic particle migration and focusing in a tilted acoustic field. Phys. Fluids. 2021, 33, 122006. [Google Scholar] [CrossRef]
- Ren, L.; Yang, S.; Zhang, P.; Qu, Z.; Mao, Z.; Huang, P.H.; Chen, Y.; Wu, M.; Wang, L.; Li, P.; et al. Standing surface acoustic wave (SSAW)-based fluorescence-activated cell sorter. Small 2018, 14, 1801996. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Collins, D.J.; Ai, Y. Detachable acoustofluidic system for particle separation via a traveling surface acoustic wave. Anal. Chem. 2016, 88, 5316–5323. [Google Scholar] [CrossRef]
- Qian, J.; Ren, J.; Liu, Y.; Lam, R.H.; Lee, J.E.Y. A two-chip acoustofluidic particle manipulation platform with a detachable and reusable surface acoustic wave device. Analyst 2020, 145, 7752–7758. [Google Scholar] [CrossRef]
- Connacher, W.; Zhang, N.; Huang, A.; Mei, J.; Zhang, S.; Gopesh, T.; Friend, J. Micro/nano acoustofluidics: Materials, phenomena, design, devices, and applications. Lab. Chip. 2018, 18, 1952–1996. [Google Scholar] [CrossRef]
- Li, H.; Friend, J.R.; Yeo, L.Y. Surface acoustic wave concentration of particle and bioparticle suspensions. Biomed. Microdevices 2007, 9, 647–656. [Google Scholar] [CrossRef]
- Ning, S.; Liu, S.; Xiao, Y.; Zhang, G.; Cui, W.; Reed, M. A microfluidic chip with a serpentine channel enabling high-throughput cell separation using surface acoustic waves. Lab. Chip. 2021, 21, 4608–4617. [Google Scholar] [CrossRef]
- Wang, T.; Green, R.; Nair, R.R.; Howell, M.; Mohapatra, S.; Guldiken, R.; Mohapatra, S.S. Surface Acoustic Waves (SAW)-Based Biosensing for Quantification of Cell Growth in 2D and 3D Cultures. Sensors 2015, 15, 32045–32055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darzynkiewicz, Z.; Smolewski, P.; Bedner, E. Use of flow and laser scanning cytometry to study mechanisms regulating cell cycle and controlling cell death. Clin. Lab. Med. 2001, 21, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Court, J.; Navabi, H.; Adams, M.; Mason, M.D.; Hobot, J.A.; Newman, G.R.; Jasani, B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 2001, 247, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huck, W.T.S.; Balabani, S. Deformation of double emulsions under conditions of flow cytometry hydrodynamic focusing. Lab. Chip. 2015, 15, 4291–4301. [Google Scholar] [CrossRef]
- Patel, Y.M.; Jain, S.; Singh, A.K.; Khare, K.; Ahlawat, S.; Bahga, S.S. An inexpensive microfluidic device for three-dimensional hydrodynamic focusing in imaging flow cytometry. Biomicrofluidics 2020, 14, 064110. [Google Scholar] [CrossRef]
- Rajawat, A.; Tripathi, S. Disease diagnostics using hydrodynamic flow focusing in microfluidic devices: Beyond flow cytometry. Biomed. Eng. Lett. 2020, 10, 241–257. [Google Scholar] [CrossRef]
- Wu, M.; Huang, P.H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131. [Google Scholar] [CrossRef]
- Abdulla, A.; Zhang, T.; Ahmad, K.Z.; Li, S.; Lou, J.; Ding, X. Label-free Separation of Circulating Tumor Cells Using a Self-Amplified Inertial Focusing (SAIF) Microfluidic Chip. Anal. Chem. 2020, 92, 16170–16179. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, J.; Gao, K.; Hu, X.; Guo, S.S.; Zhao, X.Z.; Liao, F.; Yang, Y.; Liu, W. Noninvasive Optical Isolation and Identification of Circulating Tumor Cells Engineered by Fluorescent Microspheres. ACS Appl. Bio Mater. 2022, 5, 2768–2776. [Google Scholar] [CrossRef]
- Chiu, T.K.; Chao, A.C.; Chou, W.P.; Liao, C.J.; Wang, H.M.; Chang, J.H.; Chen, P.H.; Wu, M.H. Optically-induced-dielectrophoresis (ODEP)-based cell manipulation in a microfluidic system for high-purity isolation of integral circulating tumor cell (CTC) clusters based on their size characteristics. Sens. Actuat. B-Chem. 2018, 258, 1161–1173. [Google Scholar] [CrossRef]
- Lv, P.; Tang, Z.; Liang, X.; Guo, M.; Han, R. Spatially gradated segregation and recovery of circulating tumor cells from peripheral blood of cancer patients. Biomicrofluidics 2013, 7, 180–204. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ji, M.; Zhang, Y.; Qiao, X.; Yu, N.; Ding, C.; Yang, L.; Feng, R.; Chou, X.; Geng, W. A Novel Detachable, Reusable, and Versatile Acoustic Tweezer Manipulation Platform for Biochemical Analysis and Detection Systems. Biosensors 2022, 12, 1179. https://doi.org/10.3390/bios12121179
Liu Y, Ji M, Zhang Y, Qiao X, Yu N, Ding C, Yang L, Feng R, Chou X, Geng W. A Novel Detachable, Reusable, and Versatile Acoustic Tweezer Manipulation Platform for Biochemical Analysis and Detection Systems. Biosensors. 2022; 12(12):1179. https://doi.org/10.3390/bios12121179
Chicago/Turabian StyleLiu, Yukai, Miaomiao Ji, Yichi Zhang, Xiaojun Qiao, Nanxin Yu, Chenxi Ding, Lingxiao Yang, Rui Feng, Xiujian Chou, and Wenping Geng. 2022. "A Novel Detachable, Reusable, and Versatile Acoustic Tweezer Manipulation Platform for Biochemical Analysis and Detection Systems" Biosensors 12, no. 12: 1179. https://doi.org/10.3390/bios12121179
APA StyleLiu, Y., Ji, M., Zhang, Y., Qiao, X., Yu, N., Ding, C., Yang, L., Feng, R., Chou, X., & Geng, W. (2022). A Novel Detachable, Reusable, and Versatile Acoustic Tweezer Manipulation Platform for Biochemical Analysis and Detection Systems. Biosensors, 12(12), 1179. https://doi.org/10.3390/bios12121179





