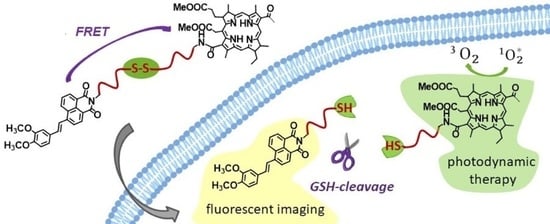
A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Optical Measurements and Singlet Oxygen Quantum Yield Determination
2.3. GSH-Responsive Fluorescence Emission Studies in Solution
2.4. Confocal Fluorescent Imaging In Vitro
2.5. Photoinduced Toxicity Studies
3. Results and Discussion
3.1. Synthesis
3.2. Spectroscopic and Photophysical Properties
3.3. Studies of the GSH-Responsive Behavior
3.4. In Vitro Confocal Laser Scanning Microscopy Studies of Conjugate BChl–S–S–NI
3.5. Photoinduced Activity at Tumor Cells of Murine Sarcoma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Xing, J.; Gong, Q.; Akakuru, O.U.; Liu, C.; Zoua, R.; Wu, A. Research advances in integrated theranostic probes for tumor fluorescence visualization and treatment. Nanoscale 2020, 12, 24311–24330. [Google Scholar] [CrossRef] [PubMed]
- Feofanov, A.; Sharonov, G.; Grichine, A.; Karmakova, T.; Pljutinskaya, A.; Lebedeva, V.; Ruziyev, R.; Yakubovskaya, R.; Mironov, A.; Refregier, M.; et al. Comparative study of photodynamic properties of 13,15-N-cycloimide derivatives of chlorin p6. Photochem. Photobiol. 2004, 79, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshia, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 342. [Google Scholar] [CrossRef] [PubMed]
- Göl, M.; Malkoç, M.; Yeşilot, S.; Durmuş, M. Novel zinc(II) phthalocyanine conjugates bearing different numbers of BODIPY and iodine groups as substituents on the periphery. Dyes Pigm. 2014, 111, 81–90. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Makarov, D.; Derkacheva, V.; Savvina, L.; Alerseeva, V.; Marinina, L.; Slivka, L.; Kaliya, O.; Lukyanets, E. Intramolecular energy transfer in rhodamine–phthalocyanine conjugates. J. Photochem. Photobiol. A 2008, 200, 161–168. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Grin, M.A.; Fedorova, O.A.; Zakharko, M.A.; Pritmov, D.A.; Mironov, A.F.; Arkhipova, A.N.; Fedorov, Y.V.; Jonusauskas, G.; Yakubovskaya, R.I.; et al. Novel bacteriochlorin–styrylnaphthalimide conjugate for simultaneous photodynamic therapy and fluorescence imaging. Phys. Chem. Chem. Phys. 2017, 19, 30195–30206. [Google Scholar] [CrossRef] [Green Version]
- Zakharko, M.A.; Panchenko, P.A.; Zarezin, D.P.; Nenajdenko, V.G.; Pritmov, D.A.; Grin, M.A.; Mironov, A.F.; Fedorova, O.A. Conjugates of 3,4-dimethoxy-4-styrylnaphthalimide and bacteriochlorin for theranostics in photodynamic therapy. Russ. Chem. Bull. 2020, 69, 1169–1178. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Zakharko, M.A.; Grin, M.A.; Mironov, A.F.; Pritmov, D.A.; Jonusauskas, G.; Fedorov Yu, V.; Fedorova, O.A. Effect of linker length on the spectroscopic properties of bacteriochlorin-1,8-naphthalimide conjugates for fluorescence-guided photodynamic therapy. J. Photochem. Photobiol. A 2020, 390, 112338. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Sergeeva, A.N.; Fedorova, O.A.; Fedorov, Y.V.; Reshetnikov, R.I.; Schelkunova, A.E.; Grin, M.A.; Mironov, A.F.; Jonusauskas, G. Spectroscopical study of bacteriopurpurinimide-naphthalimide conjugates for fluorescent diagnostics and photodynamic therapy. J. Photochem. Photobiol. B 2014, 133, 140–144. [Google Scholar] [CrossRef]
- Williams, M.P.A.; Ethirajan, M.; Ohkubo, K.; Chen, P.; Pera, P.; Morgan, J.; White, W.H., III; Shibata, M.; Fukuzumi, S.; Kadish, K.M.; et al. Synthesis, photophysical, electrochemical, tumor-imaging and phototherapeutic properties of purpurinimide-N-substituted cyanine dyes joined with variable lengths of linkers. Bioconjug. Chem. 2011, 22, 2283–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perricone, C.; Carolis, C.D.; Perricone, R. Glutathione: A key player in autoimmunity. Autoimmun. Rev. 2009, 8, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Wang, Q.; Guana, J.; Wana, J.; Li, Z. Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10, 24397–24409. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Sessler, J.L.; Kim, J.S. Disulfide-based multifunctional conjugates for targeted theranostic drug delivery. Acc. Chem. Res. 2015, 48, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, X.; Guo, Z.; Tang, J.; Shen, Y.; James, T.D.; Tian, H.; Zhu, W. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588. [Google Scholar] [CrossRef]
- Chow, S.Y.S.; Wong, R.C.H.; Zhao, S.; Lo, P.-C.; Ng, D.K.P. Disulfide-linked dendritic oligomeric phthalocyanines as glutathione-responsive photosensitizers for photodynamic therapy. Chem. Eur. J. 2018, 24, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-J.; Lo, P.-C.; Zhao, S.; Wong, R.C.H.; Wang, Q.; Fong, W.-P.; Ng, D.P. A biotin-conjugated glutathione-responsive FRET-based fluorescent probe with a ferrocenyl BODIPY as the dark quencher. Dalton Trans. 2016, 45, 17798–17806. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Wang, Y.; Wu, H.; Bao, K.; Sheng, R.; Li, X. Microenvironment-triggered dual-activation of a photosensitizer-fluorophore conjugate for tumor specific imaging and photodynamic therapy. Sci. Rep. 2020, 10, 12127. [Google Scholar] [CrossRef]
- Ruggiero, E.; Alonso-de Castro, S.; Habtemariam, A.; Salassa, L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: New opportunities and challenges in medicinal inorganic photochemistry. Dalton Trans. 2016, 45, 13012–13020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Sułek, A.; Dabrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Morozova, N.B.; Pavlova, M.A.; Plyutinskaya, A.D.; Pankratov, A.A.; Efendiev, K.T.; Semkina, A.S.; Pritmov, D.A.; Mironov, A.F.; Panchenko, P.A.; Fedorova, O.A. Photodiagnosis and photodynamic effects of bacteriochlorin-naphthalimide conjugates on tumor cells and mouse model. J. Photochem. Photobiol. B 2021, 223, 112294. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Adair, L.D.; Trinh, N.; Verite, P.M.; Jacquemin, D.; Jolliffe, K.A. Synthesis of nitro-aryl functionalised 4-amino-1,8-naphthalimides and their evaluation as fluorescent hypoxia sensors. J. Fluoresc. 2016, 26, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liu, K.; Chen, L.; Cao, X.; Yi, T. A programmed DNA marker based on bis(4-ethynyl-1,8-naphthalimide) and three-methane-bridged thiazole orange. Chem. Eur. J. 2015, 21, 16623–16630. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; Xu, Y.; Qian, X. Naphthalimides for labeling and sensing applications. Pure Appl. Chem. 2014, 86, 1237–1246. [Google Scholar] [CrossRef]
- Aderinto, S.O.; Imhanria, S. Fluorescent and colourimetric 1,8-naphthalimide-appended chemosensors for the tracking of metal ions: Selected examples from the year 2010 to 2017. Chem. Pap. 2018, 72, 1823–1851. [Google Scholar] [CrossRef]
- Sherts, A.; Salomon, J.; Brehndis, A.; Sheer, K. Palladium-Substituted Derivatives of Bacteriochlorophyll and Their. Application. Patent WO00/33833, 20 August 2004. [Google Scholar]
- Zheng, G.; Chance, B.; Glickson, J.D. Lipoprotein. Nanoplatforms. Patent WO2006/073419, 13 July 2006. [Google Scholar]
- Renschler, C.L.; Harrah, L.A. Determination of quantum yields of fluorescence by optimizing the fluorescence intensity. Anal. Chem. 1983, 55, 798–800. [Google Scholar] [CrossRef]
- Nad, S.; Kumbhakar, M.; Pal, H. Photophysical properties of coumarin-152 and coumarin-481 dyes: Unusual behavior in nonpolar and in higher polarity solvents. J. Phys. Chem. A 2003, 107, 4808–4816. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescent Spectroscopy; Kluwer Academic/Plenum Publishers: New York, USA, 1999; 725p. [Google Scholar]
- Krasnovsky, A.A., Jr.; Kozlov, A.S.; Roumbal, Y.V. Photochemical investigation of the IR absorption bands of molecular oxygen in organic and aqueous environment. Photochem. Photobiol. Sci. 2012, 11, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef] [Green Version]
- Hong, R.; Han, G.; Fernandez, J.M.; Kim, B.-J.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Yakubovskaya, R.I.; Kazachkina, N.I.; Karmakova, T.A.; Morozova, N.B.; Pankratov, A.A.; Plyutinskaya, A.D.; Feofanov, A.V.; Chissov, V.I.; Zebrev, A.I.; Tikhomirova, A.V. Recommendations for studying photo-induced antitumor properties of drugs. In Guidelines for Preclinical Study of Drugs; Mironov, A.N., Bunyatyan, N.D., Vasiliev, A.N., Eds.; Gref&K: Moscow, Russia, 2012; pp. 657–671. ISBN 978-5-8125-1466-3. [Google Scholar]
- Volman, D.H.; Hammond, G.S.; Gollnick, K. Spin-statistical factors in diffusion-controlled reactions. Adv. Photochem. 1988, 14, 1–90. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G. Singlet oxygen quantum yields of different photosensitizers in polar solvents and micellar solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Merkel, P.B.; Kearns, D.R. Radiationless decay of singlet molecular oxygen in solution. Experimental and theoretical study of electronic-to-vibrational energy transfer. J. Am. Chem. Soc. 1972, 94, 7244–7253. [Google Scholar] [CrossRef]
- Amat-Guerri, F.; Lempe, E.; Lissi, E.A.; Rodriguez, F.J.; Trull, F.R. Water-soluble 1,3-diphenylisobenzofuran derivatives. Synthesis and evaluation as singlet molecular oxygen acceptors for biological systems. J. Photochem. Photobiol. A 1996, 93, 49–56. [Google Scholar] [CrossRef]
- Chow, S.Y.S.; Wong, R.C.H.; Zhao, S.; Lo, P.-C.; Ng, D.K.P. A dual activatable photosensitizer toward targeted photodynamic therapy. J. Med. Chem. 2014, 57, 4088–4097. [Google Scholar] [CrossRef]
- Ottaviano, F.G.; Handy, D.E.; Loscalzo, J. Redox regulation in the extracellular environment. Circ. J. 2008, 72, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mironov, A.F.; Ostroverkhov, P.V.; Tikhonov, S.I.; Pogorilyy, V.A.; Kirin, N.S.; Chudakova, O.O.; Tsygankov, A.A.; Grin, V.A. Amino acid derivatives of natural chlorins as a platform for the creation of targeted photosensitizers in oncology. Fine Chem. Technol. 2020, 15, 16–33. [Google Scholar] [CrossRef]





| Compound | |||||
|---|---|---|---|---|---|
| NI–OH * | 414 | 622 (420) | 0.27 | – | – |
| BChl | 355; 515; 747 | 760 (515) | 0.03 | – | 0.79 (510) |
| BChl–S–S–NI | 357; 515; 752 | 607, 759 (420) | 0.04 | 0.94 | 0.65 (510) |
| BChl+NI–OH | 355, 515, 747 | 607, 770 (420) | - | - | - |
| Compound | Light Exposure | Control (without Light Exposure) | |||
|---|---|---|---|---|---|
| (a) | (b) | ||||
| RG-19 Filter (695–1000 nm) | BGG-15 Filter (360–600 nm) | RG-19 Filter (695–1000 nm | BGG-15 Filter (360–600 nm) | ||
| IC50/nM | |||||
| BChl | 296 ± 35 | 342 ± 28 | 381 ± 30 | 427 ± 33 | 1303 ± 130 |
| BChl–S–S–NI | 226 ± 33 | 251 ± 25 | 277 ± 27 | 353 ± 23 | 3895 ± 115 |
| NI–OH | 11,352 ± 125 | 10,504 ± 112 | 15,792 ± 132 | 14,017 ± 129 | 10,006 ± 152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, M.A.; Panchenko, P.A.; Alekhina, E.A.; Ignatova, A.A.; Plyutinskaya, A.D.; Pankratov, A.A.; Pritmov, D.A.; Grin, M.A.; Feofanov, A.V.; Fedorova, O.A. A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives. Biosensors 2022, 12, 1149. https://doi.org/10.3390/bios12121149
Pavlova MA, Panchenko PA, Alekhina EA, Ignatova AA, Plyutinskaya AD, Pankratov AA, Pritmov DA, Grin MA, Feofanov AV, Fedorova OA. A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives. Biosensors. 2022; 12(12):1149. https://doi.org/10.3390/bios12121149
Chicago/Turabian StylePavlova, Marina A., Pavel A. Panchenko, Ekaterina A. Alekhina, Anastasia A. Ignatova, Anna D. Plyutinskaya, Andrey A. Pankratov, Dmitriy A. Pritmov, Mikhail A. Grin, Alexey V. Feofanov, and Olga A. Fedorova. 2022. "A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives" Biosensors 12, no. 12: 1149. https://doi.org/10.3390/bios12121149
APA StylePavlova, M. A., Panchenko, P. A., Alekhina, E. A., Ignatova, A. A., Plyutinskaya, A. D., Pankratov, A. A., Pritmov, D. A., Grin, M. A., Feofanov, A. V., & Fedorova, O. A. (2022). A New Glutathione-Cleavable Theranostic for Photodynamic Therapy Based on Bacteriochlorin e and Styrylnaphthalimide Derivatives. Biosensors, 12(12), 1149. https://doi.org/10.3390/bios12121149