Electrochemiluminescence Aptasensor Based on Gd(OH)3 Nanocrystalline for Ochratoxin A Detection in Food Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthesis of Gd(OH)3
2.4. Fabrication of ECL Aptasensors
2.5. Determination of OTA in Real Samples
3. Results
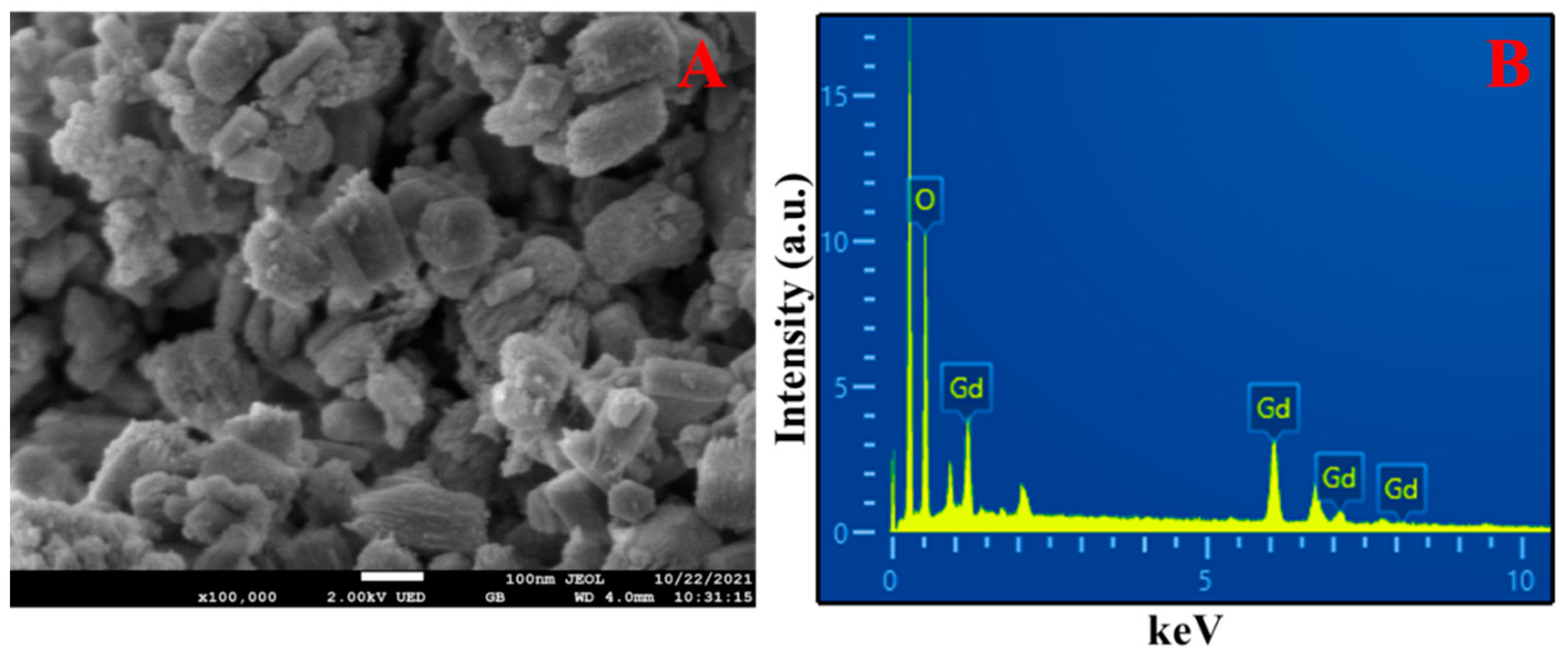
3.1. Characterization of Gd(OH)3
3.2. Optimizations of Conditions
3.3. Mechanism of ECL Aptasensor
3.4. Performance of ECL Aptasensor for OTA Detection
3.5. Stability, Reproducibility, and Selectivity of ECL Aptasensor
3.6. Detection of OTA in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, A.; Ferrara, M.; Perrone, G. Recent advances on the molecular aspects of ochratoxin A biosynthesis. Curr. Opin. Food Sci. 2017, 17, 49–56. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Dietrich, D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Toxicol. 2005, 35, 33–60. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Mirhosseini, H. Ochratoxin A quantification: Newly developed HPLC conditions. Food Control 2012, 23, 113–119. [Google Scholar] [CrossRef]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Assay of Ochratoxin A in wine and beer by high pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J. Agric. Food Chem. 2001, 49, 2733–2740. [Google Scholar] [CrossRef]
- Flajs, D.; Domijan, A.M.; Ivić, D.; Cvjetkovic´, B.; Peraica, M. ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Food Control 2009, 20, 590–592. [Google Scholar] [CrossRef]
- Yu, F.Y.; Chi, T.F.; Liu, B.H.; Su, C.C. Development of a sensitive enzyme-linked immunosorbent assay for the determination of ochratoxin A. J. Agric. Food Chem. 2005, 53, 6947–6953. [Google Scholar] [CrossRef]
- Wang, C.K.; Tan, R.; Chen, D. Fluorescence method for quickly detecting ochratoxin A in flour and beer using nitrogen doped carbon dots and silver nanoparticles. Talanta 2018, 182, 363–370. [Google Scholar] [CrossRef]
- Todescato, F.; Antognoli, A.; Meneghello, A.; Cretaio, E.; Signorini, R.; Bozio, R. Sensitive detection of Ochratoxin A in food and drinks using metal-enhanced fluorescence. Biosens. Bioelectron. 2014, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Qian, Y.R.; Cheng, Q.; Ma, Y.M.; Wu, D.; Ma, H.M.; Ren, X.; Wang, X.Y.; Wei, Q. A signal amplification of pDNA@Ag2S based photoelectrochemical competitive sensor for the sensitive detection of OTA in microfluidic devices. Biosens. Bioelectron. 2020, 168, 112503. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.X.; Liao, X.F.; Fang, L.; Jia, B.Y.; Liu, M.; Li, D.H.; Zhou, L.D.; Kong, W.J. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC Trend. Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Li, X.Y.; Falcone, N.; Hossain, M.N.; Kraatz, H.B.; Chen, X.J.; Huang, H. Development of a novel label-free impedimetric electrochemical sensor based on hydrogel/chitosan for the detection of ochratoxin A. Talanta 2021, 226, 122183. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.X.; Li, G.K.; Hu, Y.F. Progress on the application of electrochemiluminescence biosensor based on nanomaterials. Chin. Chem. Lett. 2019, 9, 1600–1606. [Google Scholar] [CrossRef]
- Sun, C.N.; Liao, X.F.; Huang, P.X.; Shan, G.Z.; Ma, X.; Fu, L.Z.; Zhou, L.D.; Kong, W.J. A self-assembled electrochemical immunosensor for ultra-sensitive detection of ochratoxin A in medicinal and edible malt. Food Chem. 2020, 315, 126289. [Google Scholar] [CrossRef]
- Yang, E.R.; Zhang, Y.J.; Shen, Y.F. Quantum dots for electrochemiluminescence bioanalysis A review. Anal. Chim. Acta. 2022, 1209, 339140. [Google Scholar] [CrossRef]
- Gao, W.Y.; Saqib, M.; Qi, L.M.; Zhang, W.; Xu, G.B. Recent advances in electrochemiluminescence devices for point-of-care testing. Curr. Opin. Electrochem. 2017, 3, 4–10. [Google Scholar] [CrossRef]
- Hyun, J.L.; Eun, H.L.; Sang, D.L.; Yeomin, Y.; Ahjeong, S. Quantitative screening for endocrine-disrupting bisphenol A in consumer and household products using NanoAptamer assay. Chemosphere 2018, 211, 72–80. [Google Scholar]
- Maugi, R.; Gamble, B.; Bunka, D.; Platt, M. A simple displacement aptamer assay on resistive pulse sensor for small molecule detection. Talanta 2021, 225, 122068. [Google Scholar] [CrossRef]
- Ni, S.J.; Zhuo, Z.J.; Pan, Y.F.; Yu, Y.Y.; Li, F.F.; Liu, J.; Wang, L.Y.; Wu, X.Q.; Li, D.J.; Wan, Y.Y.; et al. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS. Appl. Mater. Inter. 2021, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Ștefan, G.; Hosu, O.; Wael, K.D.; Lobo-Castanon, M.J.; Cristea, C. Aptamers in biomedicine: Selection strategies and recent advances. Electrochim. Acta 2021, 376, 137994. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.M.; Yu, D.P.; Zou, B.S.; Li, Y.D. Rare earth compound nanotubes. Adv. Mater. 2003, 15, 1442–1445. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Rare-earth-compound nanowires, nanotubes, and fullerene-like nanoparticles: Synthesis, characterization, and properties. Chem. Eur. J. 2003, 9, 5627–5635. [Google Scholar] [CrossRef]
- Cheraghali, R.; Aghazadeh, M. A simple and facile electrochemical route to synthesis of metal hydroxides and oxides ultrafine nanoparticles (M = La, Gd, Ni and Co). Anal. Bioanal. Chem. 2016, 8, 64–77. [Google Scholar]
- Li, G.G.; Liang, Y.J.; Zhang, M.F.; Yu, D.Y. Size-tunable synthesis and luminescent properties of Gd(OH)3:Eu3+ and Gd2O3:Eu3+ hexagonal nano-/microprisms. CrystEngComm 2014, 16, 6670–6679. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Liu, Y.; Peng, J.J.; Wu, Y.Q.; Zhang, Y.J.; Feng, W.; Li, F.Y. Long-term in vivo biodistribution and toxicity of Gd(OH)3 nanorods. Biomaterials 2013, 34, 508–515. [Google Scholar] [CrossRef]
- Xing, G.J.; Guo, Q.Y.; Liu, Q.J.; Li, Y.L.; Wang, Y.; Wu, Z.L.; Wu, G.M. Highly uniform Gd(OH)3 and Gd2O3:Eu3+ hexagram-like microcrystals: Glucose-assisted hydrothermal synthesis, growth mechanism and luminescence property. Ceram. Int. 2014, 40, 6569–6577. [Google Scholar] [CrossRef]
- Almeida, M.S.; Santos, M.A.B.; Goncalves, R.D.; Santos, M.R.D.; Marques, A.P.D.; Longo, E.; La Porta, F.D.; Pinatti, I.M.; Silva, M.D.P.; Godinho, M.J. Novel Gd(OH)3, GdOOH and Gd2O3 nanorods: Microwave-assisted hydrothermal synthesis and optical properties. Mat. Res. 2016, 19, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Wang, Y. A simple method to prepare Ln(OH)3 (Ln¼La, Sm, Tb, Eu, and Gd) nanorods using CTAB micelle solution and their room temperature photoluminescence properties. J. Alloys Compd. 2011, 509, 2060–2065. [Google Scholar] [CrossRef]
- Kang, J.G.; Jung, Y.; Min, B.K.; Sohn, Y. Full characterization of Eu(OH)3 and Eu2O3 nanorods. Appl. Surf. Sci. 2014, 314, 158–165. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.K.; Chen, M.; Wu, D.Z.; Cai, S.X.; Liu, M.M.; He, W.H.; Chen, J.H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(II)-ion signal-enhancement for simultaneous detection of OTA and AFB1. Sensor. Actuat. B-Chem. 2016, 235, 79–85. [Google Scholar] [CrossRef]
- Barthelmebs, L.; Hayat, A.; Limiadi, A.W.; Marty, J.L.; Noguer, T. Electrochemical DNA aptamer-based biosensor for OTA detection, using superparamagnetic nanoparticles. Sensor. Actuat. B-Chem. 2021, 156, 932–937. [Google Scholar] [CrossRef]
- Zhu, C.X.; Liu, D.; Li, Y.Y.; Ma, S.; Wang, M.; You, T.Y. Hairpin DNA assisted dual-ratiometric electrochemical aptasensor with high reliability and anti-interference ability for simultaneous detection of aflatoxin B1 and ochratoxin A. Biosens. Bioelectron. 2021, 175, 112654. [Google Scholar] [CrossRef] [PubMed]





| Samples | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 10 | 10.2 | 102.1 | 1.8 | |
| Grape | 1.0 | 1.01 | 101.4 | 2.1 |
| 0.1 | 0.099 | 99.52 | 1.7 | |
| 10 | 10.1 | 100.7 | 3.6 | |
| Corn | 1.0 | 0.98 | 98.56 | 2.8 |
| 0.1 | 0.097 | 97.76 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Wei, M.; Wang, X.; Hua, Q.; Tang, F.; Zhao, L.; Zhuang, X.; Luan, F. Electrochemiluminescence Aptasensor Based on Gd(OH)3 Nanocrystalline for Ochratoxin A Detection in Food Samples. Biosensors 2022, 12, 1141. https://doi.org/10.3390/bios12121141
Tian C, Wei M, Wang X, Hua Q, Tang F, Zhao L, Zhuang X, Luan F. Electrochemiluminescence Aptasensor Based on Gd(OH)3 Nanocrystalline for Ochratoxin A Detection in Food Samples. Biosensors. 2022; 12(12):1141. https://doi.org/10.3390/bios12121141
Chicago/Turabian StyleTian, Chunyuan, Minggang Wei, Xiaobin Wang, Qing Hua, Feiyan Tang, Lijun Zhao, Xuming Zhuang, and Feng Luan. 2022. "Electrochemiluminescence Aptasensor Based on Gd(OH)3 Nanocrystalline for Ochratoxin A Detection in Food Samples" Biosensors 12, no. 12: 1141. https://doi.org/10.3390/bios12121141
APA StyleTian, C., Wei, M., Wang, X., Hua, Q., Tang, F., Zhao, L., Zhuang, X., & Luan, F. (2022). Electrochemiluminescence Aptasensor Based on Gd(OH)3 Nanocrystalline for Ochratoxin A Detection in Food Samples. Biosensors, 12(12), 1141. https://doi.org/10.3390/bios12121141






