1. Introduction
Infectious diseases are caused by viruses and bacteria, and the number of outbreaks has increased [
1,
2]. Recently, human beings have been affected by the coronavirus pandemic since 2019 (COVID-19) and have changed their way of life owing to the lack of sufficient countermeasures, such as vaccines and antivirus drugs for COVID-19. In bacterial infections, drug-resistant bacteria are a major issue [
3]. To approach this issue, new mechanisms of action of antibiotics are required, which can be found in specific biological activities in bacteria [
4]. Therefore, finding new viral and bacterial specific nanoscaled structures and their behaviors may lead to the development of antiviral and antibacterial action mechanism drugs. Although bright-field and florescent microscopy has found many viral and bacterial specific microstructures and their behaviors [
5], fewer biological activities have been found recently due to the deflection limit. Thus, to find new viral- and bacterial-specific nanoscaled structures and their behaviors, a higher resolution is required to visualize viruses and bacteria in liquid conditions.
High-resolution imaging techniques often require fine probes, such as electron beam and sharp tip, for visualization. To visualize viruses and bacteria in liquid, the probes should be applicable in liquid and with less damage to viruses and bacteria. In situ imaging techniques for active viruses and living bacteria are preferred to remove pretreatments, such as fixation, dehydration, and staining, which inactivate viruses and kill bacteria, and affect the morphology, although the techniques usually need the pretreatments for high resolution imaging. In addition, resolution and frame rate are important. For the material analysis, the analysis and mapping capabilities of both organic and inorganic elements are important, because organic materials correspond to biological function and inorganic materials are essential for bacterial cultures. Furthermore, typical biological experiments require liquid control for biochemical reactions. These requirements are important for the visualization of viruses and bacteria in liquid.
High-resolution methods for visualizing in-liquid samples include atmospheric scanning electron microscopy (ASEM) [
6], liquid cell transmission electron microscopy (TEM) [
7], atomic force microscopy (AFM) in liquid [
8], and super-resolution fluorescence microscopy [
9]. ASEM has an electron-transparent membrane (ETM) between air for in-liquid samples and vacuum for electron beams. The ETM, which is usually silicon nitride (Si
3N
4), should be sufficiently thin for the transmission of the electron beam. With this ETM, in-liquid samples were visualized without pretreatment and mapped in terms of elemental and molecular distribution at a resolution of a few tens of nanometers. Furthermore, it sometimes combines with a fluorescent microscope for the distribution of specific organic materials [
10]. Obtaining one image requires a few minutes. The combination of ETM and the microfluidic device enables the culture of biological samples and the performance of biological experiments with precise chemical control [
11]. Liquid cell TEM also utilizes two ETMs, which sandwiches nanoscaled samples in-liquid. With high acceleration voltage, electron beams can transmit through the sandwiched in-liquid samples, resulting in in situ visualization and elemental mapping at sub-nm resolution. This high acceleration voltage can damage biological samples. Sometimes, it utilizes graphene, a single atomic layer of carbon, as the ETM and achieved sub-nm resolution at 3.85 fps [
12], but there is low brightness due to its conductivity. However, to achieve the liquid cell TEM, the biological samples should be pre-treated, sliced, and thinned down to less than 1 μm, and therefore the experiments in such a tiny space are difficult. AFM can visualize the surface topology and force mapping of samples at the nanoscale, which also works in liquids [
13]. It measures the frequency modulation of an AFM cantilever and scans the surface of the samples at a resolution of a few nanometers. With this, AFM does not need any pretreatments. The scan speed was increased, resulting in an image being acquired within a few seconds [
14]. However, the fluid control sometimes affects the motion of the cantilever by fluid flow. A super-resolution fluorescent microscope can visualize the fluorescent molecular distribution at a higher resolution than the diffraction limit using data analysis or optical processes. There are different types of super-resolution fluorescent microscopy, such as stochastic optical reconstruction microscopy (STORM) [
15] and stimulated emission depletion microscopy (STED) [
16] which can achieve a resolution of a few tens of nanometers. They use fluorescence, which requires staining with fluorescent dyes. Obtaining one image requires a few minutes to complete. Similar to fluorescent microscopy, they can perform biological experiments.
Considering that the imaging techniques of living biological samples require high resolution, frame rate, less damage, no pretreatments, material analysis, and biological experiment, the ASEM is preferable. Although the ASEM fulfils all the requirements and resolution of a few tens of nanometers, the image resolution was not sufficiently high in the case of viruses and microstructures of bacteria. This is because the electron beam was scattered in the ETM. Thin ETM is better in terms of resolution. On the other hand, the ETM should be sufficiently strong not to be broken by the application of atmospheric pressure. The ETM should be thick in terms of strength. This trade off makes high resolution ASEM imaging difficult, resulting in a thick and narrow membrane structure (low resolution and narrow viewing area); for example, 100 nm in thickness and 250 μm × 250 μm in size for ASEM [
17].
To improve the resolution of the ASEM and microfluidic liquid cell, the thickness of the ETM must be reduced while maintaining the viewing area. In this study, a microfluidic liquid cell with a super-thin ETM (~10 nm in thickness) of 100 μm × 100 μm in size was developed. Using this super-thin ETM, viruses in vacuum and nanostructures on bacteria in liquid can be visualized.
2. Microfluidic Liquid cell with a Super-Thin ETM
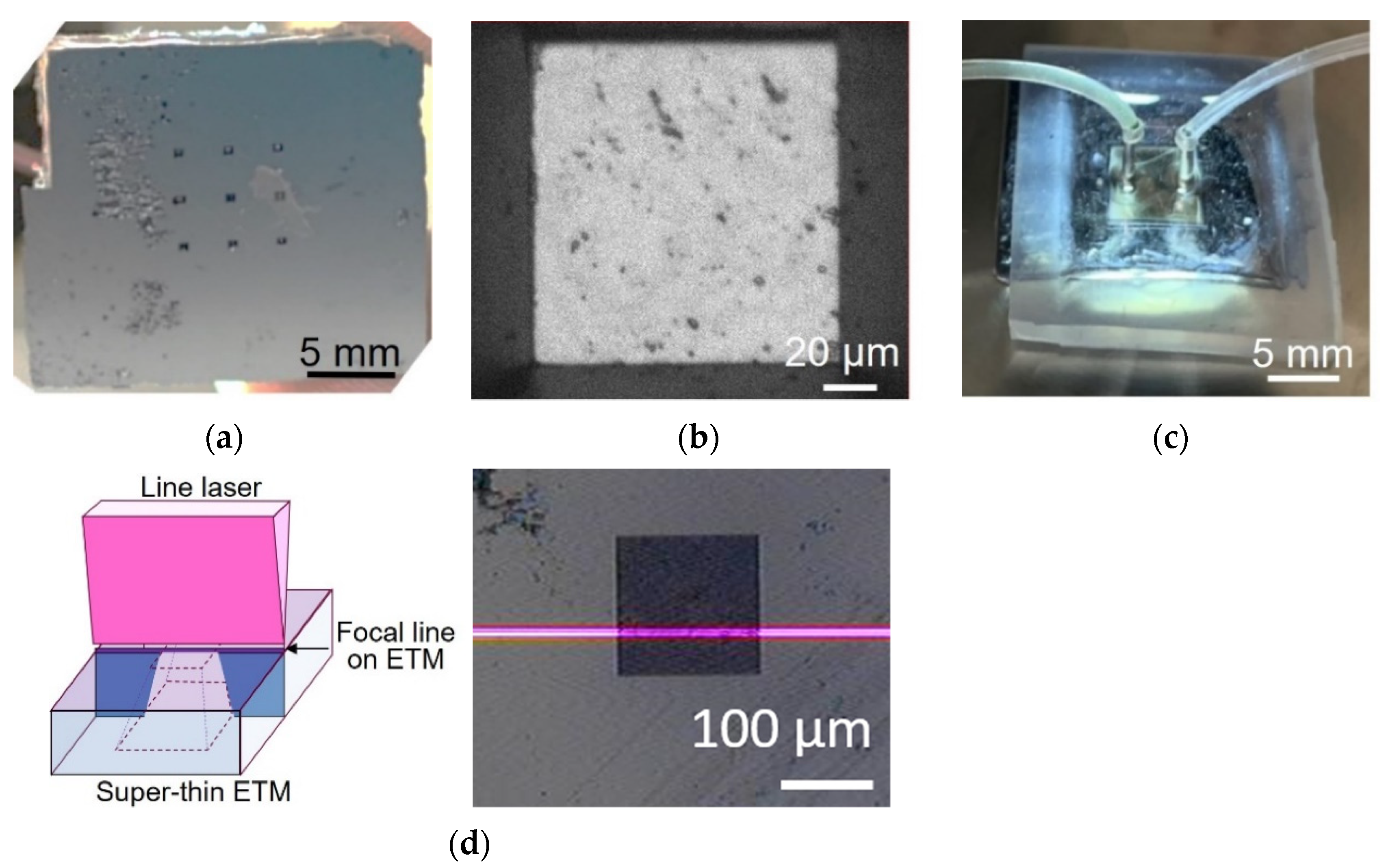
The microfluidic liquid cell with a super-thin ETM is shown in
Figure 1. It consisted of a substrate with a super-thin ETM and a microchannel. The super-thin ETM covered the observation windows and suppressed the scattering of the electron beam within itself as much as possible; therefore, the probe size for SEM imaging did not expand significantly. The super-thin ETM was Si
3N
4 of 10 nm deposited by low-pressure chemical vapor deposition (LPCVD), which was thinner than the guaranteed foundry thickness (30 nm). The microchannel (SI) contained an inlet and outlet to introduce a solution for bacterial culture or cellular response to chemicals. The microchannel was composed of polydimethylsiloxane (PDMS). Owing to the gas permeability and optical transparency of PDMS, gas and visible light can be fed to the bacteria inside for bacterial culture. The bottom of the PDMS microchannel was locally coated with a Ti metal film to reduce charging during SEM imaging.
The PDMS microchannel and substrate with a super-thin ETM were bonded before wet etching of Si. The PDMS microchannel was chemically inert and worked as a protection of ETM against long-term wet etching even at ~10 Å/h etching rate [
18]. It also protected stress caused by the flow of etchant in the step of ETM fabrication.
2.1. Design of Super Thin ETM
A super-thin ETM was designed in terms of electron beam scattering and strength. The scattering of the electron beam expands the probe size, which corresponds to the resolution. Smaller scattering is better. Consequently, strength was determined based on the size of the ETM. Sufficient strength was required to resist atmospheric pressure. Furthermore, a larger viewing area is preferable because more samples are visible through the ETM.
To consider electron beam scattering, the electron beam trajectories were calculated using a Monte Carlo simulation. An electron beam of 17 keV was irradiated onto the ETM, with the ideal point spot as an input. Because the electron beams were scattered inside the ETM, the output spot size increased.
Figure 2a,b show the scattering of the electron beam in the ETM of 10 nm and 80 nm, respectively. The calculated output spot size of the electron beam in the case of the 10-nm-thick Si
3N
4 membrane was 0.07 times compared to that in the case of the 80-nm-thick membrane (
Figure 2c). The Monte Carlo simulation stochastically calculated 100 trajectories of the electron beam, and therefore, the averaged spot size and standard deviation were employed as error bars (N = 5 in trial). The probe size of SEM imaging was proportional to square of thickness of Si
3N
4. Therefore, the resolution dramatically increases when the ETM becomes thinner. Single Scattering Monte Carlo Simulation V 3.05 was used, with 22.46, 11.21, and 3.17 g/cm
3 as effective atomic weight, effective atomic number, and density of Si
3N
4, respectively.
The maximum mechanical strength of the 10-nm-thick Si
3N
4 membrane was not high, while the maximum stress generated in the Si
3N
4 membrane dramatically increased in that range of thickness (
SI and Figure S1). The strength is related to Young’s modulus, ETM thickness, shape, and size. Young’s modulus and ETM thickness and shape are decided due to the material, fabrication, and visualization point of view. To avoid breakage of the 10-nm-thick Si
3N
4 membrane owing to the application of atmospheric pressure, the size of the Si
3N
4 membrane should be adjusted. The maximum mechanical stress on a 10-nm-thick Si
3N
4 membrane was calculated by changing its size using the finite element method (FEM) (
Figure 3). The fracture strength of Si
3N
4 has been reported to be in the range from 6 to 13 GPa [
19,
20]. The maximum stress must be lower than the fracture strength. In this case, to increase the fabrication yield of the super-thin ETM and to prevent the damage to SEM in case of the breakage of ETM, a safety ratio of 2 was set. The dimensions of the membrane were 100 μm × 100 μm. For the finite element method, COMSOL Multiphysics ver. 5.2a (COMSOL, Inc.) was used. Considering the symmetric shape of the model, the quarter FEM model was used for the simplification of the calculation. The model with two completely fixed boundaries and two boundaries fixed only in-plane motion and rotation. An equally distributed load was atmospheric pressure of 100 kPa, the density was 3100 kg/m
3, Young’s modulus was 250 × 10
9 Pa, and Poisson’s ratio was 0.23.
The observation window should be a tapered or shallow or large area to prevent the blockage of electrons. For the tapered shape, the anisotropic etching of silicon should be considered when designing the pattern of the observation windows. In the anisotropic etching of silicon, (111) is a crystalline facet that dramatically reduces the etching rate compared to (100) and (110) [
21]. The angle between the (100) and (111) planes was 54.7°. When the length of one side of the observation window, α, is 100 μm, the length of the pattern on the opposite side of the silicon substrate, β, should be designed according to Equation (1), where t is the thickness of the silicon substrate.
2.2. Fabrication Process
The ETM was fabricated by etching Si with tetramethylammonium hydroxide (TMAH), which also etched and damaged Si
3N
4 at ~10 Å/h [
18]. The damages to the ETM were on the several nm scale due to long etching time, and strongly affected the strength of the super-thin ETM. This is because its thickness was only 10 nm, which is not large in comparison to the damage. To avoid this damage, the pre-bonding of a silicon substrate with Si
3N
4 layers and a microchannel before the etching of silicon were proposed in this study. The bonded microchannel functioned as a protective layer of Si
3N
4 against etching and damages caused by the flow of liquid and mechanical contacts.
Figure 4 shows the fabrication process of a microfluidic liquid cell with a super-thin ETM. A Cr layer with a thickness of 150 nm was sputtered on a silicon substrate ((100), 300 nm thickness) with a Si
3N
4 thickness of 10 nm deposited by LPCVD (
Figure 4a). The Cr layer was patterned as a mask for etching (
Figure 4b). Reactive ion etching of SF
6 was performed to locally etch the Si
3N
4 membrane to etch Si (
Figure 4c). A PDMS microchannel was prepared by soft lithography, an SU-8 mold was fabricated by photolithography and should be treated by hexamethyldisilazane (HMDS) vapor treatment for the detachment of PDMS replica (10 min exposure to HMDS vapor and 1 min baking at 95 °C) (
Figure 4a’). PDMS (base to agent was 10:1, cure at 100 °C for 60 min) was cast on the SU-8 mold. A PDMS replica was fabricated (
Figure 4b’). Ti with a thickness of 100 nm was locally sputtered onto the PDMS microchannel through a shadow mask hole (
Figure 4c’). Further, the patterned substrate and PDMS microchannel were bonded by irradiation with vacuum ultraviolet light (
Figure 4d). The bonded structure was anisotropically etched with TMAH at 90 °C until a freestanding super-thin ETM was formed (about 4 h 20 min) (
Figure 4e). The inlet and outlet of the microfluidic liquid cell were formed using a biopsy punch (
Figure 4f). Without any additional processes, microfluidic liquid cell is available.
2.3. Experimental Setup
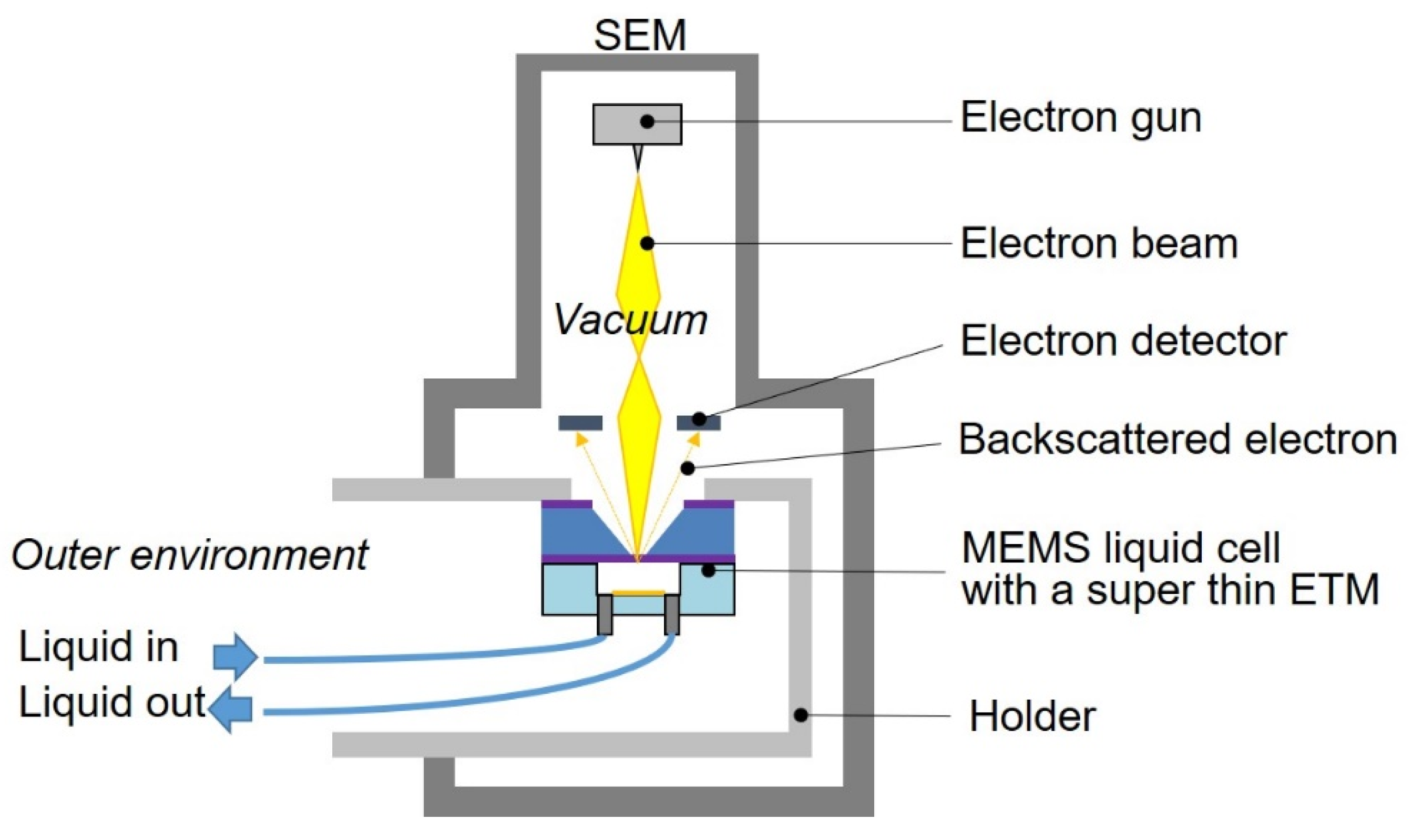
Figure 5 shows the experimental setup using the microfluidic liquid cell. The microfluidic liquid cell with the super-thin ETM was placed and glued onto a special specimen holder with an epoxy resin. The specimen holder was installed in a SEM (BiSEM-10, Technex Lab Co., Ltd., Tokyo, Japan) and connected to the outer environment. Thereafter, the super-thin ETM was separated into air and vacuum, where almost 100 kPa was applied. The microchannel was located on the side of the air and was connected to a syringe pump for the introduction of cyanobacterial suspensions and the perfusion of the culture medium. To bring cyanobacteria close to the super-thin ETM, a cyanobacterial suspension was introduced with a flow rate of 2 μL/min, stopped, and then the substrate was placed down for 30 min.
When only a super-thin ETM without a microchannel was used for the characterization, the suspension containing gold nanoparticles or T4 bacteriophages was dropped and dried on the super-thin ETM. The dried samples prepared on the super-thin ETM were introduced into the SEM specimen chamber for SEM imaging. On the side of the vacuum, it was 10−3 Pa for the generation and irradiation of the electron beam. The acceleration voltage was 17 kV and the spatial resolution was 10 nm. In addition, backscattered electrons were used for visualization rather than secondary electrons in terms of the electron energy to pass through the super-thin ETM.