Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Construction of the Immunosensor
2.4. Immunoassay Procedure
3. Results
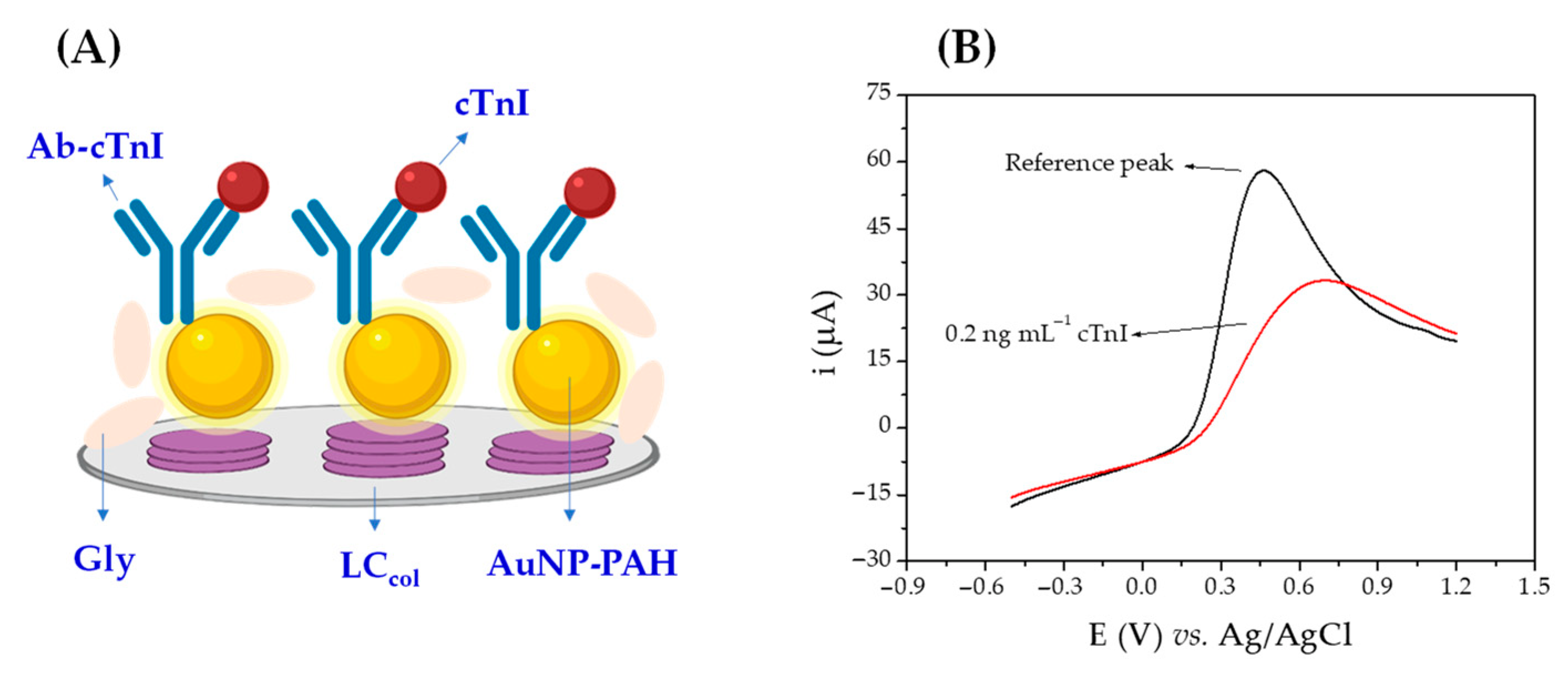
3.1. Fabrication and Sensing Principle of the Immunosensor
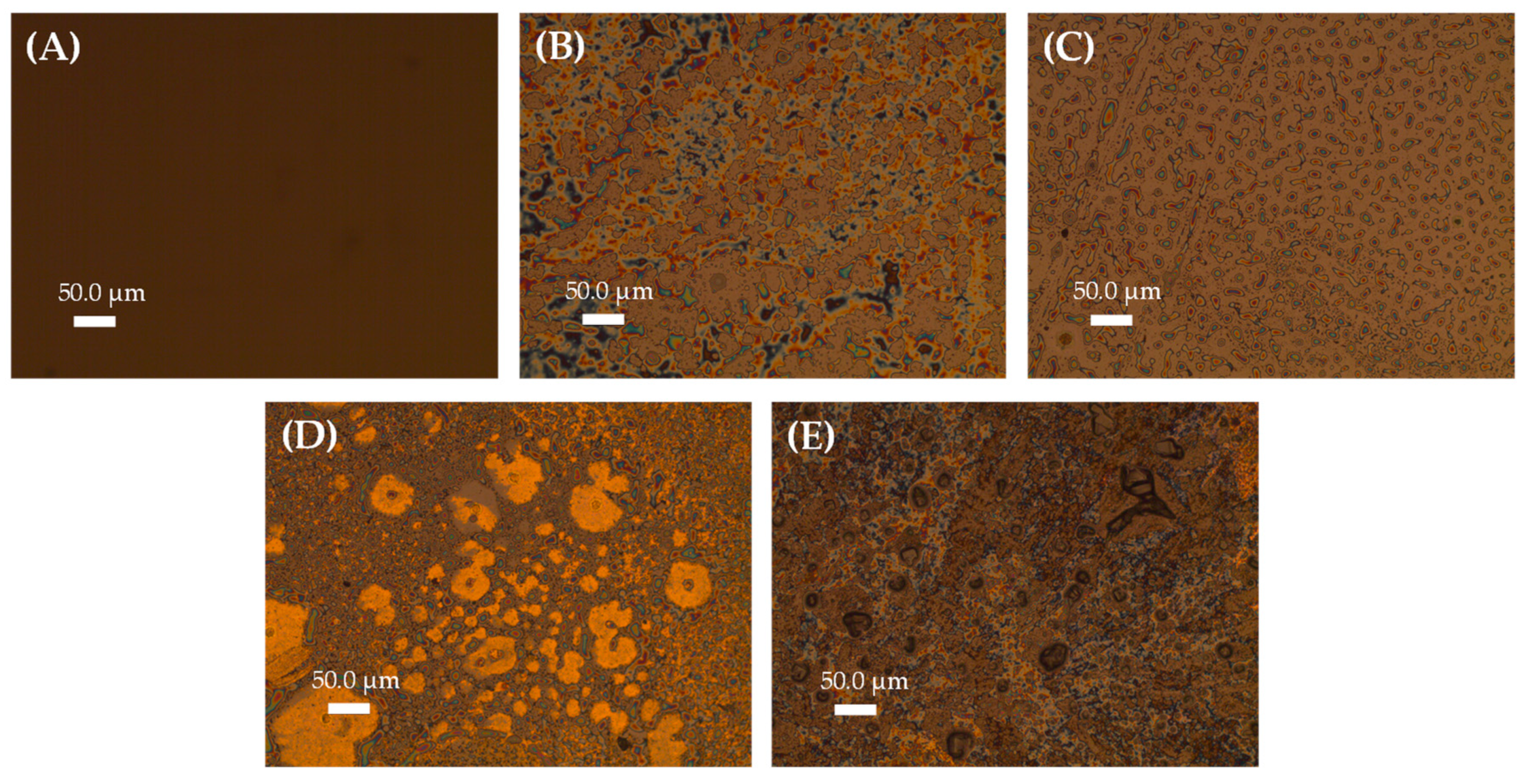
3.2. Morphological Characterization
3.3. Electrochemical Studies
3.4. Immunosensor Analytical Performance
3.4.1. Selection of the Electrochemical Technique
3.4.2. Platform Redox Response Optimization
3.4.3. Incubation Time and Association Constant
3.4.4. Calibration Curve for cTnI
3.5. Reproducibility, Study of Possible Interfering Compounds, and Application of the Immunosensor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanzadeh, M.; Shadjou, N.; Eskandani, M.; de la Guardia, M.; Omidinia, E. Electrochemical Nano-Immunosensing of Effective Cardiac Biomarkers for Acute Myocardial Infarction. TrAC Trends Anal. Chem. 2013, 49, 20–30. [Google Scholar] [CrossRef]
- Kim, S.-J. Global Awareness of Myocardial Infarction Symptoms in General Population. Korean Circ. J. 2021, 51, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Shoushtari, A.M.; Rabiee, M.; Uzun, L.; Mak, W.C.; Turner, A.P.F. An Electrochemical Immunosensor for Cardiac Troponin I Using Electrospun Carboxylated Multi-Walled Carbon Nanotube-Whiskered Nanofibres. Talanta 2018, 182, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Thoker, Z.A.; Khan, K.A.; Rashid, I. Zafar Correlation of Cardiac Troponin I Levels with Infective Endocarditis & Its Adverse Clinical Outcomes. Int. J. Cardiol. 2016, 222, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Abdorahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Alavi, S.H.; Tayebi, L. Nanomaterials-Based Electrochemical Immunosensors for Cardiac Troponin Recognition: An Illustrated Review. TrAC Trends Anal. Chem. 2016, 82, 337–347. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Electrochemical Biosensors for the Determination of Cardiovascular Markers: A Review. Electroanalysis 2014, 26, 1132–1153. [Google Scholar] [CrossRef]
- Singal, S.; Srivastava, A.K.; Biradar, A.M.; Mulchandani, A. Rajesh Pt Nanoparticles-Chemical Vapor Deposited Graphene Composite Based Immunosensor for the Detection of Human Cardiac Troponin I. Sens. Actuators B Chem. 2014, 205, 363–370. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Bratashov, D.N.; Byzova, N.A.; Dzantiev, B.B.; Khlebtsov, N.G. SERS-Based Lateral Flow Immunoassay of Troponin I by Using Gap-Enhanced Raman Tags. Nano Res. 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Chen, F.; Wu, Q.; Song, D.; Wang, X.; Ma, P.; Sun, Y. Fe3O4@PDA Immune Probe-Based Signal Amplification in Surface Plasmon Resonance (SPR) Biosensing of Human Cardiac Troponin I. Colloids Surf. B Biointerfaces 2019, 177, 105–111. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.Y.; Zhu, W.; Liu, S.P.; Zhang, J.X.; Zhuang, H.; Zhang, J.L.; Li, Y.S.; Gao, F. Highly Sensitive Lanthanide-Doped Nanoparticles-Based Point-of-Care Diagnosis of Human Cardiac Troponin I. Int. J. Nanomed. 2022, 17, 635–646. [Google Scholar] [CrossRef]
- Zhan, T.; Su, Y.; Lai, W.; Chen, Z.; Zhang, C. A Dry Chemistry-Based Ultrasensitive Electrochemiluminescence Immunosensor for Sample-to-Answer Detection of Cardiac Troponin I. Biosens. Bioelectron. 2022, 214, 114494. [Google Scholar] [CrossRef]
- Olkowicz, M.; Rybakowska, I.; Chlopicki, S.; Smolenski, R.T. Development and Analytical Comparison of Microflow and Nanoflow Liquid Chromatography/Mass Spectrometry Procedures for Quantification of Cardiac Troponin T in Mouse Hearts. Talanta 2015, 131, 510–520. [Google Scholar] [CrossRef]
- Cenci, L.; Anesi, A.; Busato, M.; Guella, G.; Bossi, A.M. Molecularly Imprinted Polymers Coupled to Matrix Assisted Laser Desorption Ionization Mass Spectrometry for Femtomoles Detection of Cardiac Troponin I Peptides. J. Mol. Recognit. 2016, 29, 41–50. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for Cardiac Biomarkers Detection: A Review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Sethuraman, S.; Rajan, K.S.; Krishnan, U.M.; Rayappan, J.B.B. Functionalized Graphene Quantum Dot Interfaced Electrochemical Detection of Cardiac Troponin I: An Antibody Free Approach. Sci. Rep. 2019, 9, 17348. [Google Scholar] [CrossRef]
- Liu, G.; Qi, M.; Zhang, Y.; Cao, C.; Goldys, E.M. Nanocomposites of Gold Nanoparticles and Graphene Oxide towards an Stable Label-Free Electrochemical Immunosensor for Detection of Cardiac Marker Troponin-I. Anal. Chim. Acta 2016, 909, 1–8. [Google Scholar] [CrossRef]
- Mohammed, M.-I.; Desmulliez, M.P.Y. Lab-on-a-Chip Based Immunosensor Principles and Technologies for the Detection of Cardiac Biomarkers: A Review. Lab Chip 2011, 11, 569–595. [Google Scholar] [CrossRef]
- Szunerits, S.; Mishyn, V.; Grabowska, I.; Boukherroub, R. Electrochemical Cardiovascular Platforms: Current State of the Art and Beyond. Biosens. Bioelectron. 2019, 131, 287–298. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene Quantum Dots-Based Electrochemical Biosensing Platform for Early Detection of Acute Myocardial Infarction. Biosensors 2022, 12, 77. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Ghodsi, E.; Abdollahi, S.; Nadri, S. Porous Graphene Oxide Nanostructure as an Excellent Scaffold for Label-Free Electrochemical Biosensor: Detection of Cardiac Troponin I. Mater. Sci. Eng. C 2016, 69, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhao, X.; Ju, X.; Qiu, S.; Hu, W.; Fan, T.; Zhang, J. A New Molecularly Imprinted Polymer (MIP)-Based Electrochemical Sensor for Monitoring Cardiac Troponin I (CTnI) in the Serum. Electroanalysis 2016, 28, 2044–2049. [Google Scholar] [CrossRef]
- Huo, X.; Liu, X.; Liu, J.; Sukumaran, P.; Alwarappan, S.; Wong, D.K.Y. Strategic Applications of Nanomaterials as Sensing Platforms and Signal Amplification Markers at Electrochemical Immunosensors. Electroanalysis 2016, 28, 1730–1749. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-Based Electrochemical Immunosensors. Micromachines 2019, 10, E397. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Imamura, A.H.; Gomes, N.O.; Almeida, M.B.; Scheidt, D.T.; Raymundo-Pereira, P.A.; Oliveira, O.N.; Janegitz, B.C.; Machado, S.A.S.; Carrilho, E. Electrochemical Immunosensors Using Electrodeposited Gold Nanostructures for Detecting the S Proteins from SARS-CoV and SARS-CoV-2. Anal. Bioanal. Chem. 2022, 414, 5507–5517. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. “Gold Rush” in Modern Science: Fabrication Strategies and Typical Advanced Applications of Gold Nanoparticles in Sensing. Coord. Chem. Rev. 2018, 359, 1–31. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Liu, X.; Liu, H.; Bao, H.; Wang, J.; Zeng, H. A Label-Free Immunosensor Based on Gold Nanoparticles/Thionine for Sensitive Detection of PAT Protein in Genetically Modified Crops. Front. Chem. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. What Are the Reasons for Low Use of Graphene Quantum Dots in Immunosensing of Cancer Biomarkers? Mater. Sci. Eng. C 2017, 71, 1313–1326. [Google Scholar] [CrossRef]
- Wei, T.; Dai, Z.; Lin, Y.; Du, D. Electrochemical Immunoassays Based on Graphene: A Review. Electroanalysis 2016, 28, 4–12. [Google Scholar] [CrossRef]
- Khalil, I.; Rahmati, S.; Muhd Julkapli, N.; Yehye, W.A. Graphene Metal Nanocomposites—Recent Progress in Electrochemical Biosensing Applications. J. Ind. Eng. Chem. 2018, 59, 425–439. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. A New Era for Liquid Crystal Research: Applications of Liquid Crystals in Soft Matter Nano-, Bio- and Microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Oladepo, S.A. Development and Application of Liquid Crystals as Stimuli-Responsive Sensors. Molecules 2022, 27, 1453. [Google Scholar] [CrossRef]
- Qu, R.; Li, G. Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications. Biosensors 2022, 12, 205. [Google Scholar] [CrossRef]
- Hussain, Z.; Qazi, F.; Ahmed, M.I.; Usman, A.; Riaz, A.; Abbasi, A.D. Liquid Crystals Based Sensing Platform-Technological Aspects. Biosens. Bioelectron. 2016, 85, 110–127. [Google Scholar] [CrossRef]
- Kim, H.; An, Z.; Jang, C.-H. Label-Free Optical Detection of Thrombin Using a Liquid Crystal-Based Aptasensor. Microchem. J. 2018, 141, 71–79. [Google Scholar] [CrossRef]
- Munir, S.; Kang, I.-K.; Park, S.-Y. Polyelectrolytes Functionalized Nematic Liquid Crystal-Based Biosensors: An Overview. TrAC Trends Anal. Chem. 2016, 83, 80–94. [Google Scholar] [CrossRef]
- Omer, M.; Park, S.-Y. Preparation of QP4VP-b-LCP Liquid Crystal Block Copolymer and Its Application as a Biosensor. Anal. Bioanal. Chem. 2014, 406, 5369–5378. [Google Scholar] [CrossRef]
- Talamini, L.; Zanato, N.; Zapp, E.; Brondani, D.; Westphal, E.; Gallardo, H.; Vieira, I.C. Heparin-Gold Nanoparticles and Liquid Crystal Applied in Label-Free Electrochemical Immunosensor for Prostate-Specific Antigen. Electroanalysis 2018, 30, 353–360. [Google Scholar] [CrossRef]
- Zapp, E.; da Silva, P.S.; Westphal, E.; Gallardo, H.; Spinelli, A.; Vieira, I.C. Troponin T Immunosensor Based on Liquid Crystal and Silsesquioxane-Supported Gold Nanoparticles. Bioconjug. Chem. 2014, 25, 1638–1643. [Google Scholar] [CrossRef]
- Zapp, E.; Westphal, E.; Gallardo, H.; de Souza, B.; Cruz Vieira, I. Liquid Crystal and Gold Nanoparticles Applied to Electrochemical Immunosensor for Cardiac Biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Silva, T.R.; Brondani, D.; Zapp, E.; Cruz Vieira, I. Electrochemical Sensor Based on Gold Nanoparticles Stabilized in Poly(Allylamine Hydrochloride) for Determination of Vanillin. Electroanalysis 2015, 27, 465–472. [Google Scholar] [CrossRef]
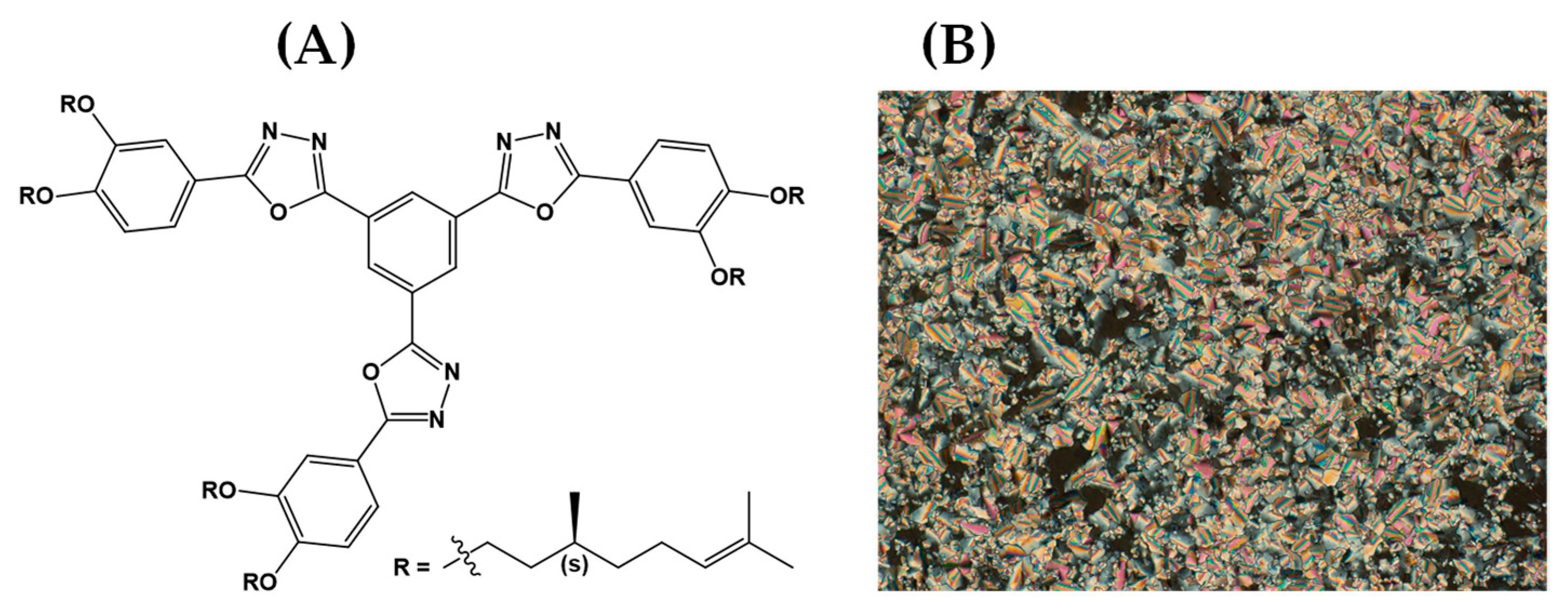
- Girotto, E.; Eccher, J.; Vieira, A.A.; Bechtold, I.H.; Gallardo, H. Luminescent Columnar Liquid Crystals Based on 1,3,4-Oxadiazole. Tetrahedron 2014, 70, 3355–3360. [Google Scholar] [CrossRef]
- Mendes, R.K.; Ferreira, D.C.M.; Carvalhal, R.F.; Peroni, L.A.; Kubota, L.T. Development of an Electrochemical Immunosensor For. J. Braz. Chem. Soc. 2009, 20, 795–801. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A Review on Impedimetric Biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- Ferreira, A.A.P. Preparation and Characterization of Imunosensors for Disease Diagnosis; Fugivara, C.S., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 8; pp. 183–214. [Google Scholar]
- Szymańska, I.; Radecka, H.; Radecki, J.; Kaliszan, R. Electrochemical Impedance Spectroscopy for Study of Amyloid β-Peptide Interactions with (−) Nicotine Ditartrate and (−) Cotinine. Biosens. Bioelectron. 2007, 22, 1955–1960. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Liu, S.G.; Liu, H.; Echegoyen, L. Ion Recognition at the Interface of Self-Assembled Monolayers (SAMs) of Bis-Thioctic Ester Derivatives of Oligo(Ethyleneglycols). Chemistry 2000, 6, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Sandil, D.; Srivastava, S.; Malhotra, B.D.; Sharma, S.C.; Puri, N.K. Biofunctionalized Tungsten Trioxide-Reduced Graphene Oxide Nanocomposites for Sensitive Electrochemical Immunosensing of Cardiac Biomarker. J. Alloys Compd. 2018, 763, 102–110. [Google Scholar] [CrossRef]
- Cen, S.-Y.; Ge, X.-Y.; Chen, Y.; Wang, A.-J.; Feng, J.-J. Label-Free Electrochemical Immunosensor for Ultrasensitive Determination of Cardiac Troponin I Based on Porous Fluffy-like AuPtPd Trimetallic Alloyed Nanodendrites. Microchem. J. 2021, 169, 106568. [Google Scholar] [CrossRef]
- Niu, P.; Jiang, J.; Liu, K.; Wang, S.; Xu, T.; Wang, Z.; Wang, T.; Zhang, X.; Ding, Z.; Liu, Y.; et al. Prefab Hollow Glass Microsphere-Based Immunosensor with Liquid Crystal Sensitization for Acute Myocardial Infarction Biomarker Detection. Biosensors 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Goldmann, B.U.; Heeschen, C.; Kreymann, G.; Berger, J.; Meinertz, T. Emergency Room Triage of Patients with Acute Chest Pain by Means of Rapid Testing for Cardiac Troponin T or Troponin I. N. Engl. J. Med. 1997, 337, 1648–1653. [Google Scholar] [CrossRef]




| Sample | [cTnI] Detected (ng mL−1) * | Relative Error (%) |
|---|---|---|
| Plasma (0.05 ng mL−1 of cTnI) | 0.056 ± 0.007 | 12.0 |
| Plasma (0.05 ng mL−1 of cTnI) | 0.049 ± 0.002 | −2.0 |
| Plasma (0.1 ng mL−1 of cTnI) | 0.095 ± 0.019 | −5.0 |
| Method * | Material | Linear Range (ng mL−1) | LOD (ng mL−1) | Incubation Time (min) | Reference |
|---|---|---|---|---|---|
| Electrochemical | WNFs/GCE | 0.5–100 | 0.04 | 110 | [3] |
| Electrochemical | APTES/WO3-RGO/ITO | 0.01–250 | 0.01 | 10 | [49] |
| Electrochemical | AuPtPd FNDs/GCE | 0.01–100 | 0.003 | 80 | [50] |
| WGM | LC | 0–40 | 1.103 | 30 | [51] |
| Electrochemical | AuNPs–PAH/LCcol/GCE | 0.01–0.3 | 0.005 (LSV)MILOS0.01 (EIS) | 10 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapp, E.; Brondani, D.; Silva, T.R.; Girotto, E.; Gallardo, H.; Vieira, I.C. Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection. Biosensors 2022, 12, 1113. https://doi.org/10.3390/bios12121113
Zapp E, Brondani D, Silva TR, Girotto E, Gallardo H, Vieira IC. Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection. Biosensors. 2022; 12(12):1113. https://doi.org/10.3390/bios12121113
Chicago/Turabian StyleZapp, Eduardo, Daniela Brondani, Tânia Regina Silva, Edivandro Girotto, Hugo Gallardo, and Iolanda Cruz Vieira. 2022. "Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection" Biosensors 12, no. 12: 1113. https://doi.org/10.3390/bios12121113
APA StyleZapp, E., Brondani, D., Silva, T. R., Girotto, E., Gallardo, H., & Vieira, I. C. (2022). Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection. Biosensors, 12(12), 1113. https://doi.org/10.3390/bios12121113








