Detection of Trace Amounts of Water in Organic Solvents by DNA-Based Nanomechanical Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
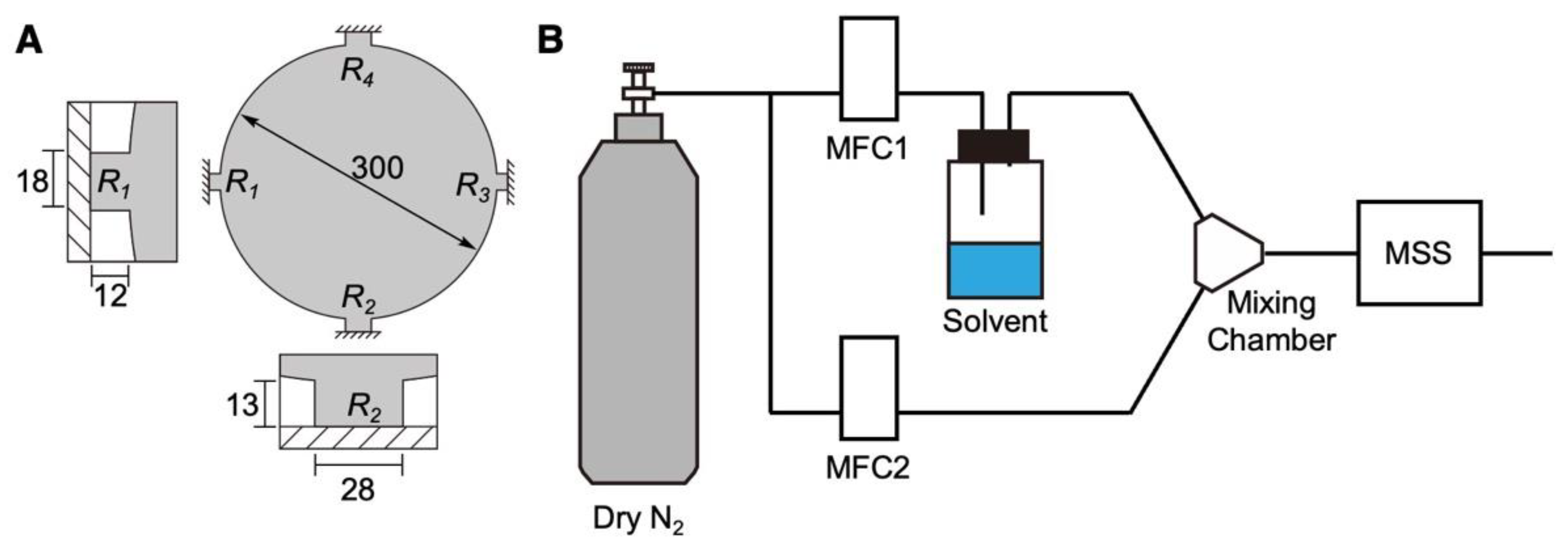
2.2. Fabrication of DNA-Coated MSS
2.3. Characterization of the DNA Film
2.4. Preparation of Dehydrated Organic Solvents and Water-Contaminated Solvents
2.5. Vapor Sensing
2.6. Measurement of the Humidity Dependence
2.7. Numerical Simulation
3. Results and Discussion
3.1. Fabrication of the DNA-Coated MSS
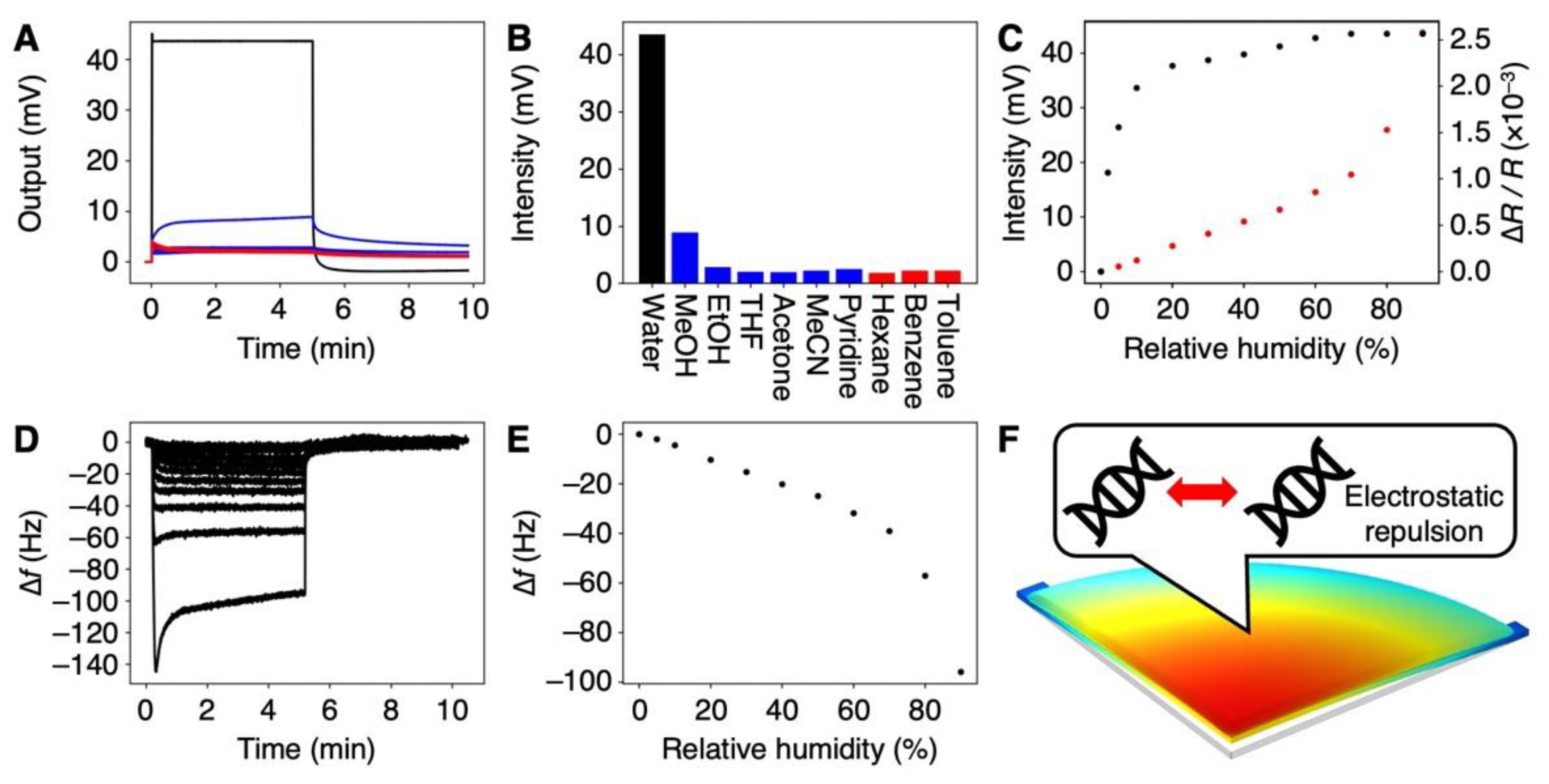
3.2. Selectivity of the DNA-Coated MSS
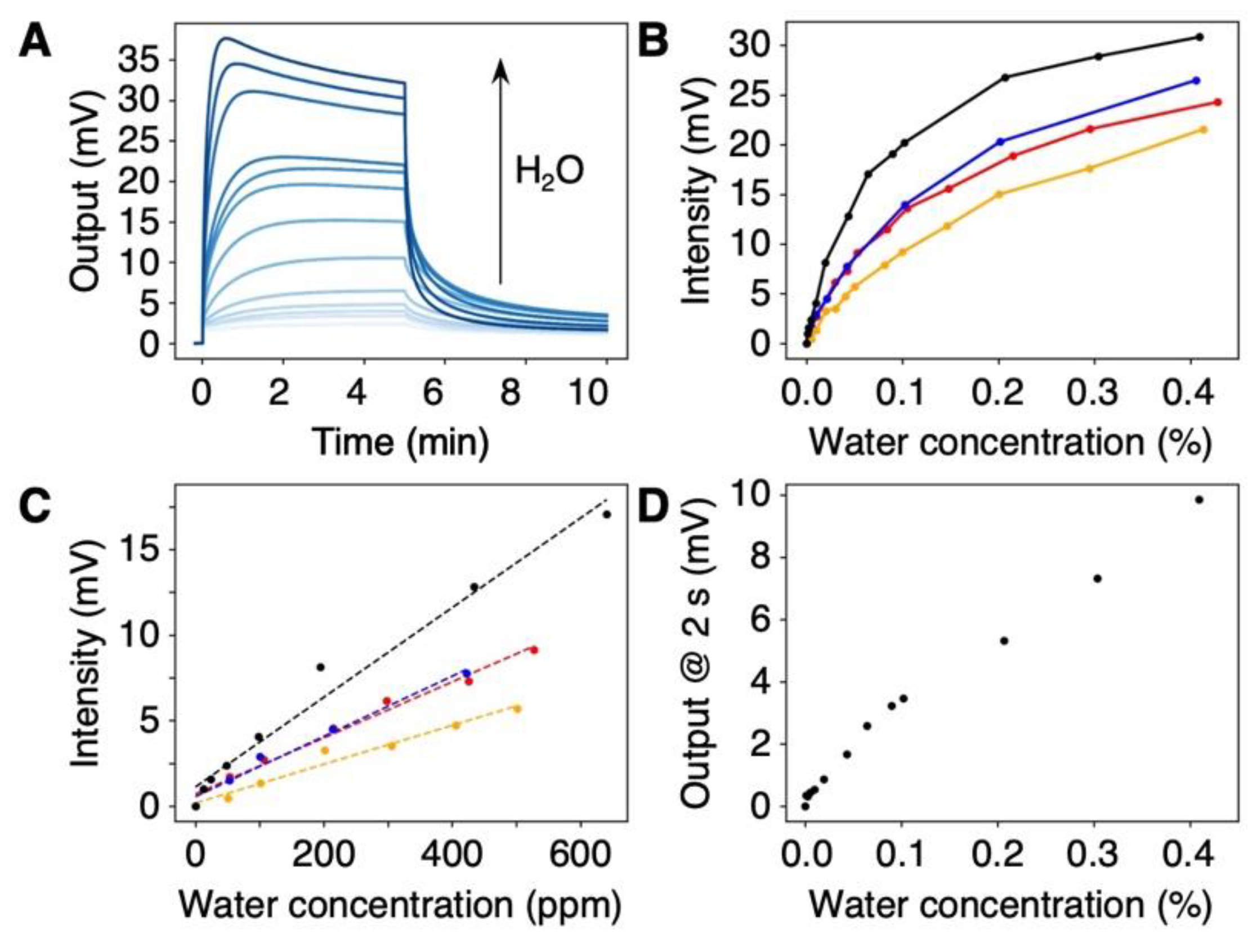
3.3. Detection of Trace Amounts of Water in Organic Solvents
3.4. Reproducibility of the DNA-Based MSS
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, Y.; Cervantes-Salguero, K.; Minami, K.; Kawamata, I.; Nakanishi, W.; Hill, J.P.; Murata, S.; Ariga, K. Supramolecular 1-D polymerization of DNA origami through a dynamic process at the 2-dimensionally confined air-water interface. Phys. Chem. Chem. Phys. 2016, 18, 12576–12581. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, Y.; Cervantes-Salguero, K.; Nakanishi, W.; Kawamata, I.; Minami, K.; Komatsu, H.; Murata, S.; Hill, J.P.; Ariga, K. In situ 2D-extraction of DNA wheels by 3D through-solution transport. Phys. Chem. Chem. Phys. 2015, 17, 32122–32125. [Google Scholar] [CrossRef]
- Skeete, Z.; Cheng, H.; Crew, E.; Lin, L.; Zhao, W.; Joseph, P.; Shan, S.; Cronk, H.; Luo, J.; Li, Y.; et al. Design of functional nanoparticles and assemblies for theranostic applications. ACS Appl. Mater. Interfaces 2014, 6, 21752–21768. [Google Scholar] [CrossRef]
- Ji, Q.; Yamazaki, T.; Sun, J.; Górecka, Ż.; Huang, N.-C.; Hsu, S.-h.; Shrestha, L.K.; Hill, J.P.; Ariga, K. Spongelike Porous Silica Nanosheets: From “Soft” Molecular Trapping to DNA Delivery. ACS Appl. Mater. Interfaces 2017, 9, 4509–4518. [Google Scholar] [CrossRef]
- Li, Y.; Cu, Y.T.; Luo, D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nat. Biotechnol. 2005, 23, 885–889. [Google Scholar] [CrossRef]
- Lee, J.B.; Roh, Y.H.; Um, S.H.; Funabashi, H.; Cheng, W.; Cha, J.J.; Kiatwuthinon, P.; Muller, D.A.; Luo, D. Multifunctional nanoarchitectures from DNA-based ABC monomers. Nat. Nanotechnol. 2009, 4, 430–436. [Google Scholar] [CrossRef]
- Tay, C.Y.; Yuan, L.; Leong, D.T. Nature-inspired DNA nanosensor for real-time in situ detection of mRNA in living cells. ACS Nano 2015, 9, 5609–5617. [Google Scholar] [CrossRef]
- Teles, F.; Fonseca, L. Trends in DNA biosensors. Talanta 2008, 77, 606–623. [Google Scholar] [CrossRef]
- Kavita, V. DNA Biosensors-A Review. J. Bioeng. Biomed. Sci. 2017, 7, 222. [Google Scholar] [CrossRef]
- Hamed, K.-K.; Vahideh, R.; Ali, E.; Fatemeh, S. DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A mini-review. J. Cell Dev. Biol. 2020, 3, 28–35. [Google Scholar] [CrossRef]
- Debnath, M.; Prasad, G.B.K.S.; Bisen, P.S. DNA Biosensors. In Molecular Diagnostics: Promises and Possibilities; Springer: Dordrecht, The Netherlands, 2010; pp. 209–225. [Google Scholar] [CrossRef]
- Schneider, B.; Patel, K.; Berman, H.M. Hydration of the Phosphate Group in Double-Helical DNA. Biophys. J. 1998, 75, 2422–2434. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.; Wennerstrom, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar] [CrossRef]
- Zhan, J.; Matsuno, H.; Masunaga, H.; Ogawa, H.; Tanaka, K. Green solid films with tunable mechanical properties made from deoxyribonucleic acid. NPG Asia Mater. 2014, 6, e92. [Google Scholar] [CrossRef]
- Nakano, M.; Tateishi-Karimata, H.; Tanaka, S.; Tama, F.; Miyashita, O.; Nakano, S.; Sugimoto, N. Thermodynamic properties of water molecules in the presence of cosolute depend on DNA structure: A study using grid inhomogeneous solvation theory. Nucleic Acids Res. 2015, 43, 10114–10125. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Kosaka, P.M.; Mokry, G.; Pini, V.; Malvar, O.; del Rey, M.; Ramos, D.; San Paulo, A.; Tamayo, J.; Calleja, M. Hydration induced stress on DNA monolayers grafted on microcantilevers. Langmuir 2014, 30, 10962–10969. [Google Scholar] [CrossRef]
- Mertens, J.; Rogero, C.; Calleja, M.; Ramos, D.; Martin-Gago, J.A.; Briones, C.; Tamayo, J. Label-free detection of DNA hybridization based on hydration-induced tension in nucleic acid films. Nat. Nanotechnol. 2008, 3, 301–307. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Ramos, D.; Mendieta-Moreno, J.I.; Fierro, J.L.G.; Mendieta, J.; Tamayo, J.; Calleja, M. Effect of water-DNA interactions on elastic properties of DNA self-assembled monolayers. Sci. Rep. 2017, 7, 536. [Google Scholar] [CrossRef]
- Cagliani, A.; Kosaka, P.; Tamayo, J.; Davis, Z.J. Monitoring the hydration of DNA self-assembled monolayers using an extensional nanomechanical resonator. Lab Chip 2012, 12, 2069–2073. [Google Scholar] [CrossRef]
- Zhang, J.; Lang, H.P.; Yoshikawa, G.; Gerber, C. Optimization of DNA hybridization efficiency by pH-driven nanomechanical bending. Langmuir 2012, 28, 6494–6501. [Google Scholar] [CrossRef]
- Fritz, J.; Baller, M.K.; Lang, H.P.; Rothuizen, H.; Vettiger, P.; Meyer, E.; Guntherodt, H.; Gerber, C.; Gimzewski, J.K. Translating biomolecular recognition into nanomechanics. Science 2000, 288, 316–318. [Google Scholar] [CrossRef]
- McKendry, R.; Zhang, J.; Arntz, Y.; Strunz, T.; Hegner, M.; Lang, H.P.; Baller, M.K.; Certa, U.; Meyer, E.; Guntherodt, H.J.; et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc. Natl. Acad. Sci. USA 2002, 99, 9783–9788. [Google Scholar] [CrossRef]
- Waggoner, P.S.; Craighead, H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef]
- Ruz, J.J.; Malvar, O.; Gil-Santos, E.; Ramos, D.; Calleja, M.; Tamayo, J. A Review on Theory and Modelling of Nanomechanical Sensors for Biological Applications. Processes 2021, 9, 164. [Google Scholar] [CrossRef]
- Minami, K.; Imamura, G.; Tamura, R.; Shiba, K.; Yoshikawa, G. Recent Advances in Nanomechanical Membrane-Type Surface Stress Sensors towards Artificial Olfaction. Biosensors 2022, 12, 762. [Google Scholar] [CrossRef]
- Margolis, S.A. Sources of systematic bias in the measurement of water by the coulometric and volumetric Karl Fischer methods. Anal. Chem. 1997, 69, 4864–4871. [Google Scholar] [CrossRef]
- Liang, Y.Y. Automation of Karl Fischer water titration by flow injection sampling. Anal. Chem. 2002, 62, 2504–2506. [Google Scholar] [CrossRef]
- Larsson, W.; Jalbert, J.; Gilbert, R.; Cedergren, A. Efficiency of methods for Karl Fischer determination of water in oils based on oven evaporation and azeotropic distillation. Anal. Chem. 2003, 75, 1227–1232. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Kim, W.Y.; Kim, J.S. Fluorescent and colorimetric sensors for the detection of humidity or water content. Chem. Soc. Rev. 2016, 45, 1242–1256. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors for determination of water in the organic solvents: A review. J. Iran. Chem. Soc. 2021, 19, 1–22. [Google Scholar] [CrossRef]
- Qi, H.; Liu, J.; Deng, Y.; Gao, S.; Mäder, E. Cellulose fibres with carbon nanotube networks for water sensing. J. Mater. Chem. A 2014, 2, 5541–5547. [Google Scholar] [CrossRef]
- Kang, E.; Park, H.R.; Yoon, J.; Yu, H.-Y.; Chang, S.-K.; Kim, B.; Choi, K.; Ahn, S. A simple method to determine the water content in organic solvents using the 1H NMR chemical shifts differences between water and solvent. Microchem. J. 2018, 138, 395–400. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Rohrer, H. Nanomechanical membrane-type surface stress sensor. Nano Lett. 2011, 11, 1044–1048. [Google Scholar] [CrossRef]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-reactive chemical sensor arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Akiyama, T.; Loizeau, F.; Shiba, K.; Gautsch, S.; Nakayama, T.; Vettiger, P.; de Rooij, N.F.; Aono, M. Two dimensional array of piezoresistive nanomechanical Membrane-type Surface Stress Sensor (MSS) with improved sensitivity. Sensors 2012, 12, 15873–15887. [Google Scholar] [CrossRef]
- Minami, K.; Yoshikawa, G. Finite Element Analysis of Interface Dependence on Nanomechanical Sensing. Sensors 2020, 20, 1518. [Google Scholar] [CrossRef]
- Minami, K.; Yoshikawa, G. Effects of partial attachment at the interface between receptor and substrate on nanomechanical cantilever sensing. Sens. Actuators A Phys. 2021, 319, 112533. [Google Scholar] [CrossRef]
- Yoshikawa, G. Mechanical analysis and optimization of a microcantilever sensor coated with a solid receptor film. Appl. Phys. Lett. 2011, 98, 173502. [Google Scholar] [CrossRef]
- Loizeau, F.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Yoshikawa, G.; de Rooij, N.F. Comparing membrane- and cantilever-based surface stress sensors for reproducibility. Sens. Actuators A Phys. 2015, 228, 9–15. [Google Scholar] [CrossRef]
- MacDougall, D.; Crummett, W.B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 2002, 52, 2242–2249. [Google Scholar] [CrossRef]
- Mistry, K.; Nguyen, V.H.; Arabi, M.; Ibrahim, K.H.; Asgarimoghaddam, H.; Yavuz, M.; Munoz-Rojas, D.; Abdel-Rahman, E.; Musselman, K.P. Highly Sensitive Self-Actuated Zinc Oxide Resonant Microcantilever Humidity Sensor. Nano Lett. 2022, 22, 3196–3203. [Google Scholar] [CrossRef]
- Buttry, D.A.; Ward, M.D. Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance. Chem. Rev. 1992, 92, 1355–1379. [Google Scholar] [CrossRef]
- Ray, S.G.; Cohen, H.; Naaman, R.; Rabin, Y. Where is the sodium in self-assembled monolayers of single-stranded DNA? J. Am. Chem. Soc. 2005, 127, 17138–17139. [Google Scholar] [CrossRef]
- Chai, D.I.; Lautens, M. Tandem Pd-catalyzed double C-C bond formation: Effect of water. J. Org. Chem. 2009, 74, 3054–3061. [Google Scholar] [CrossRef]
- Grünke, S. Main and side reactions in the Karl Fischer solution. Food Control 2001, 12, 419–426. [Google Scholar] [CrossRef]
- Saraullo, A.; Martos, P.A.; Pawliszyn, J. Water analysis by solid phase microextraction based on physical chemical properties of the coating. Anal. Chem. 1997, 69, 1992–1998. [Google Scholar] [CrossRef]
- Sheng, L.; Dajing, C.; Yuquan, C. A surface acoustic wave humidity sensor with high sensitivity based on electrospun MWCNT/Nafion nanofiber films. Nanotechnology 2011, 22, 265504. [Google Scholar] [CrossRef]
- Sun, C.; Shi, Q.; Yazici, M.S.; Lee, C.; Liu, Y. Development of a Highly Sensitive Humidity Sensor Based on a Piezoelectric Micromachined Ultrasonic Transducer Array Functionalized with Graphene Oxide Thin Film. Sensors 2018, 18, 4352. [Google Scholar] [CrossRef]
- Wang, X.-H.; Ding, Y.-F.; Zhang, J.; Zhu, Z.-Q.; You, S.-Z.; Chen, S.-Q.; Zhu, J. Humidity sensitive properties of ZnO nanotetrapods investigated by a quartz crystal microbalance. Sens. Actuators B Chem. 2006, 115, 421–427. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three-dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sens. Actuators B Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Qiu, X.; Tang, R.; Zhu, J.; Oiler, J.; Yu, C.; Wang, Z.; Yu, H. Experiment and theoretical analysis of relative humidity sensor based on film bulk acoustic-wave resonator. Sens. Actuators B Chem. 2010, 147, 381–384. [Google Scholar] [CrossRef]
- Loizeau, F.; Akiyama, T.; Gautsch, S.; Vettiger, P.; Yoshikawa, G.; de Rooij, N. Membrane-Type Surface Stress Sensor with Piezoresistive Readout. Proc. Eng. 2012, 47, 1085–1088. [Google Scholar] [CrossRef]
- Addabbo, T.; Fort, A.; Mugnaini, M.; Vignoli, V.; Baldi, A.; Bruzzi, M. Quartz-Crystal Microbalance Gas Sensors Based on TiO2Nanoparticles. IEEE Trans. Instrum. Meas. 2018, 67, 722–730. [Google Scholar] [CrossRef]
- Xu, J.; Bertke, M.; Li, X.; Mu, H.; Zhou, H.; Yu, F.; Hamdana, G.; Schmidt, A.; Bremers, H.; Peiner, E. Fabrication of ZnO nanorods and Chitosan@ZnO nanorods on MEMS piezoresistive self-actuating silicon microcantilever for humidity sensing. Sens. Actuators B Chem. 2018, 273, 276–287. [Google Scholar] [CrossRef]
- Karabacak, D.M.; Brongersma, S.H.; Crego-Calama, M. Enhanced sensitivity volatile detection with low power integrated micromechanical resonators. Lab. Chip 2010, 10, 1976–1982. [Google Scholar] [CrossRef]
- Imamura, G.; Minami, K.; Shiba, K.; Mistry, K.; Musselman, K.; Yavuz, M.; Yoshikawa, G.; Saiki, K.; Obata, S. Graphene Oxide as a Sensing Material for Gas Detection Based on Nanomechanical Sensors in the Static Mode. Chemosensors 2020, 8, 82. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, X.-h.; Zhang, B.-y.; Zhang, Z.; Hou, D.; Zhou, Z.-k. Facile fabrication of high sensitivity cellulose nanocrystals based QCM humidity sensors with asymmetric electrode structure. Sens. Actuators B Chem. 2020, 302, 127192. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Ye, Z.; Liu, C.; Xie, G.; Du, X.; Jiang, Y. Novel highly sensitive QCM humidity sensor with low hysteresis based on graphene oxide (GO)/poly(ethyleneimine) layered film. Sens. Actuators B Chem. 2016, 234, 145–154. [Google Scholar] [CrossRef]
- Verd, J.; Sansa, M.; Uranga, A.; Perez-Murano, F.; Segura, J.; Barniol, N. Metal microelectromechanical oscillator exhibiting ultra-high water vapor resolution. Lab Chip 2011, 11, 2670–2672. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Melosh, N.A.; Khuri-Yakub, B.T. Mesoporous thin-film on highly-sensitive resonant chemical sensor for relative humidity and CO2 detection. Anal. Chem. 2012, 84, 3063–3066. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, T.; Minami, K.; Yamazaki, T.; Yoshikawa, G.; Ariga, K. Detection of Trace Amounts of Water in Organic Solvents by DNA-Based Nanomechanical Sensors. Biosensors 2022, 12, 1103. https://doi.org/10.3390/bios12121103
Murata T, Minami K, Yamazaki T, Yoshikawa G, Ariga K. Detection of Trace Amounts of Water in Organic Solvents by DNA-Based Nanomechanical Sensors. Biosensors. 2022; 12(12):1103. https://doi.org/10.3390/bios12121103
Chicago/Turabian StyleMurata, Tomohiro, Kosuke Minami, Tomohiko Yamazaki, Genki Yoshikawa, and Katsuhiko Ariga. 2022. "Detection of Trace Amounts of Water in Organic Solvents by DNA-Based Nanomechanical Sensors" Biosensors 12, no. 12: 1103. https://doi.org/10.3390/bios12121103
APA StyleMurata, T., Minami, K., Yamazaki, T., Yoshikawa, G., & Ariga, K. (2022). Detection of Trace Amounts of Water in Organic Solvents by DNA-Based Nanomechanical Sensors. Biosensors, 12(12), 1103. https://doi.org/10.3390/bios12121103








