Ir(III) Complexes with AIE Characteristics for Biological Applications
Abstract
:1. Introduction
2. Improving Photophysical Property of Ir(III) Complexes with AIE Activity
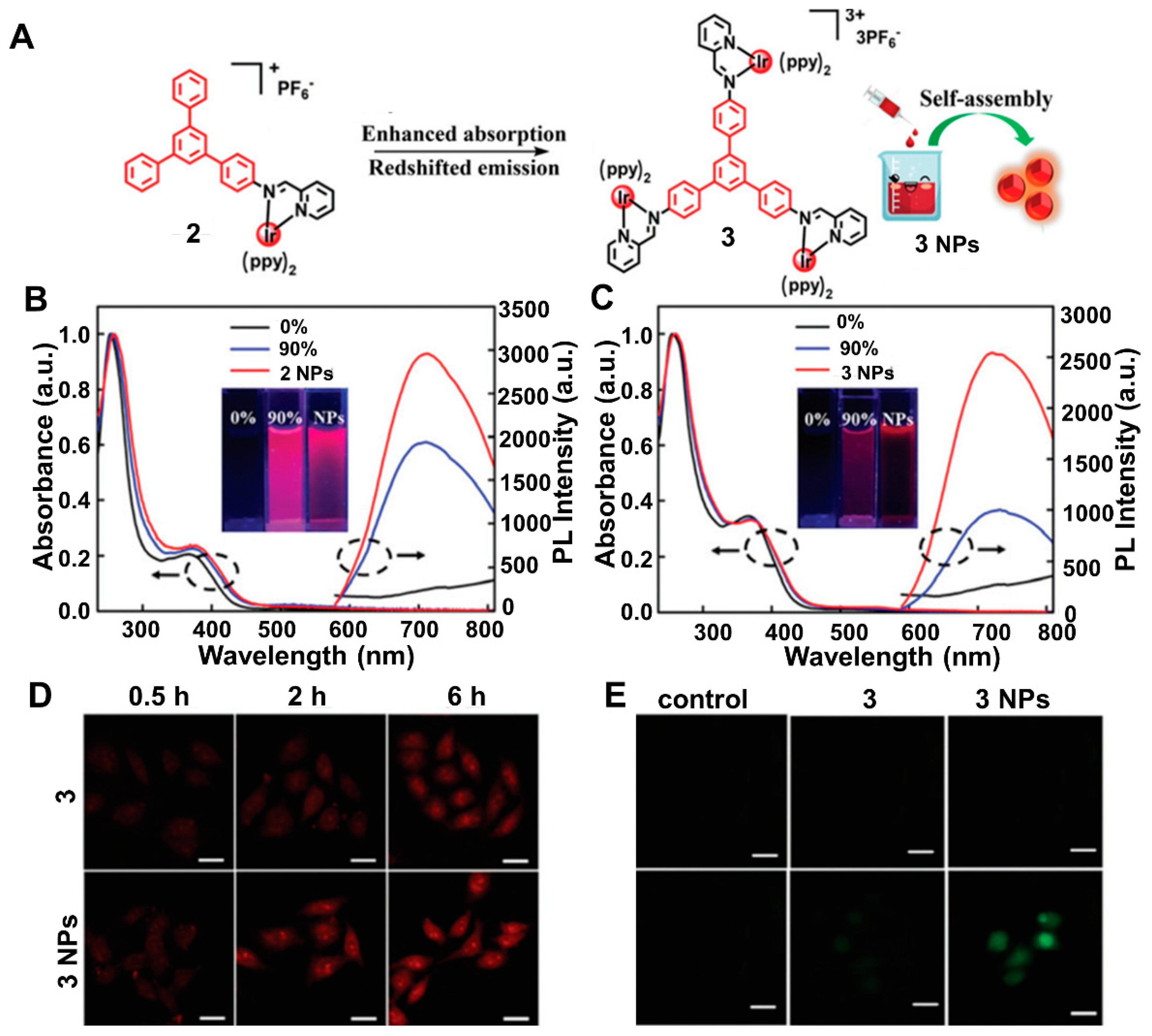
2.1. Red-Shift Emission
2.2. Enhancing Molar Absorption Coefficient
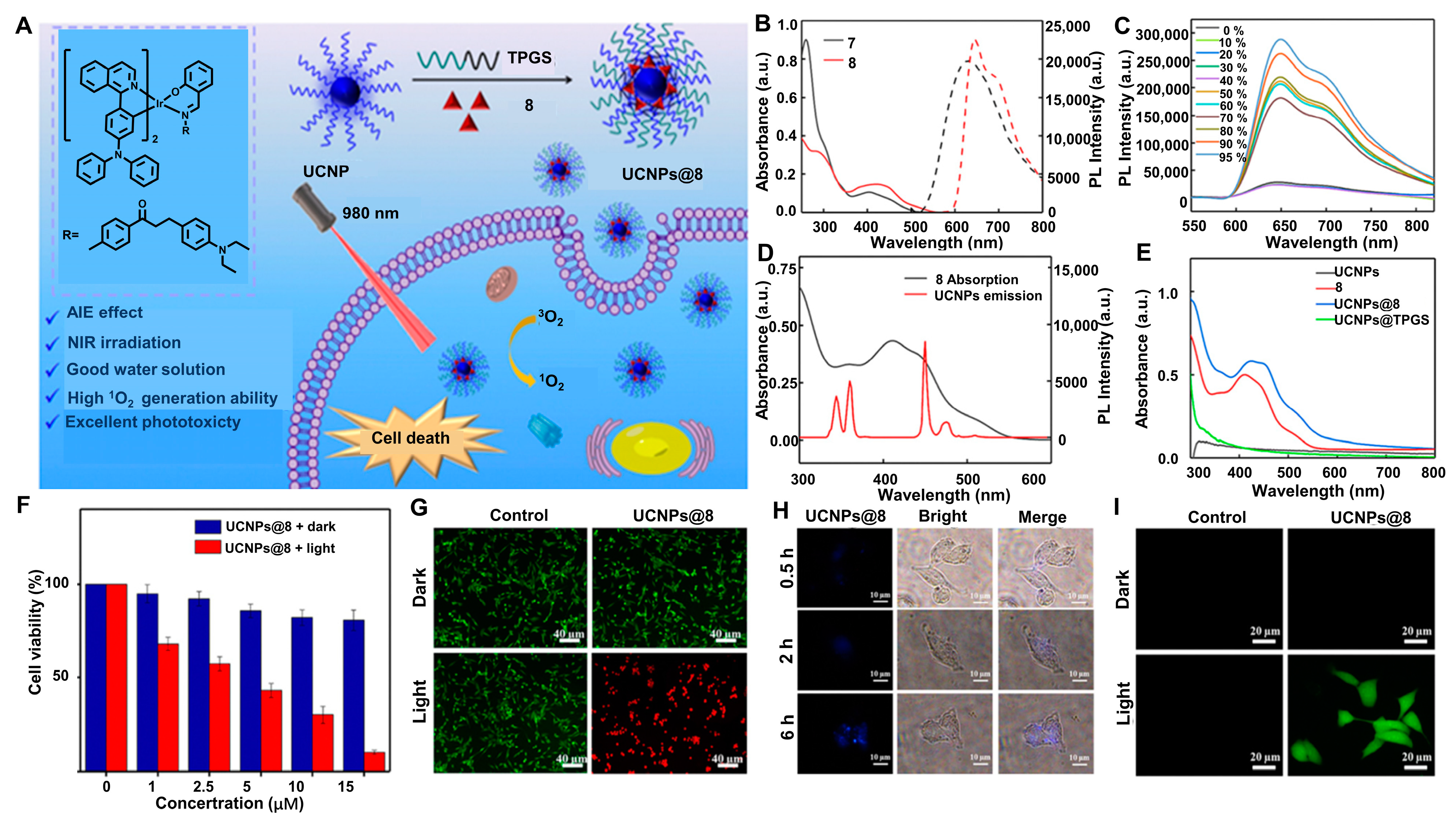
2.3. With the UCNPs
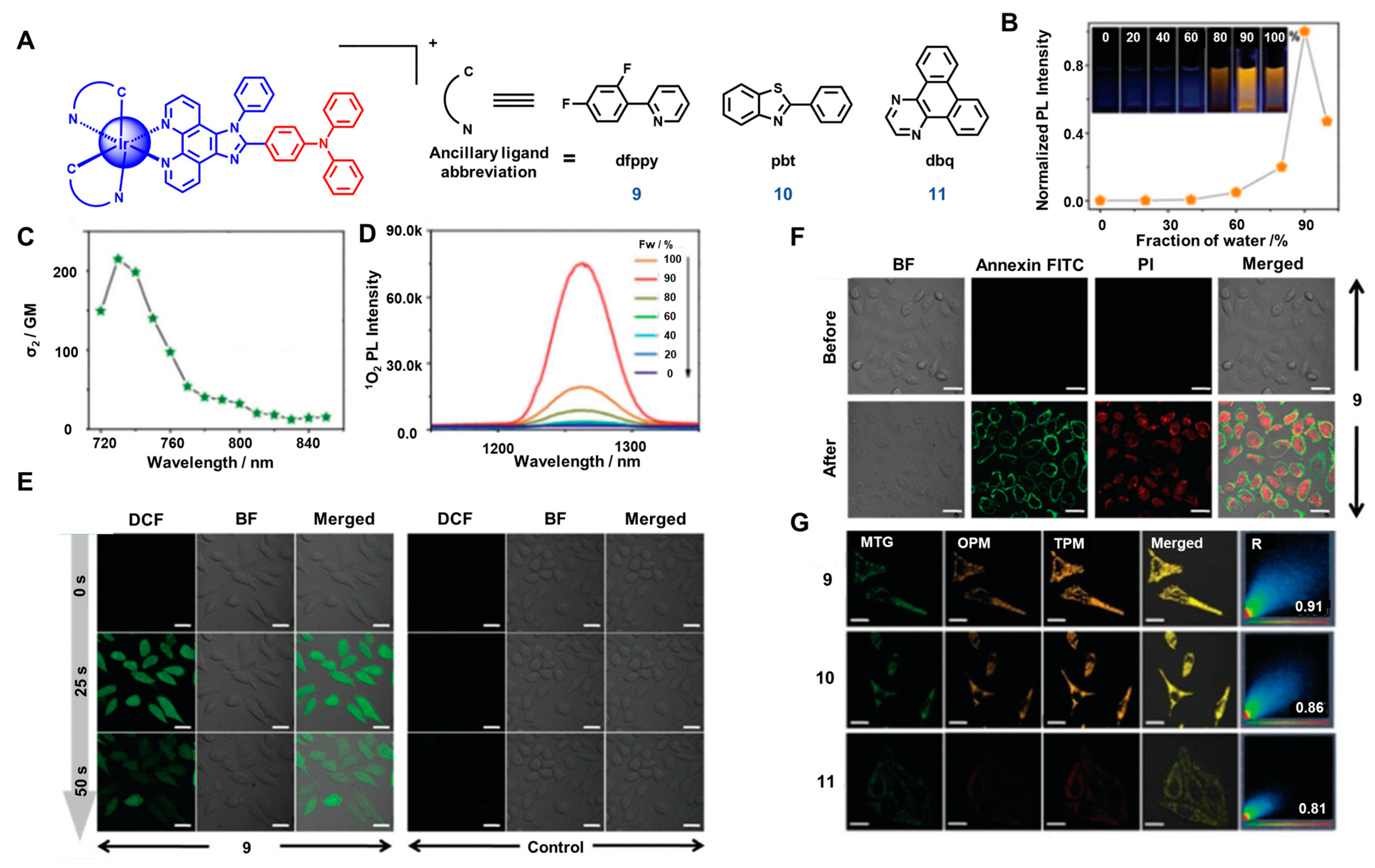
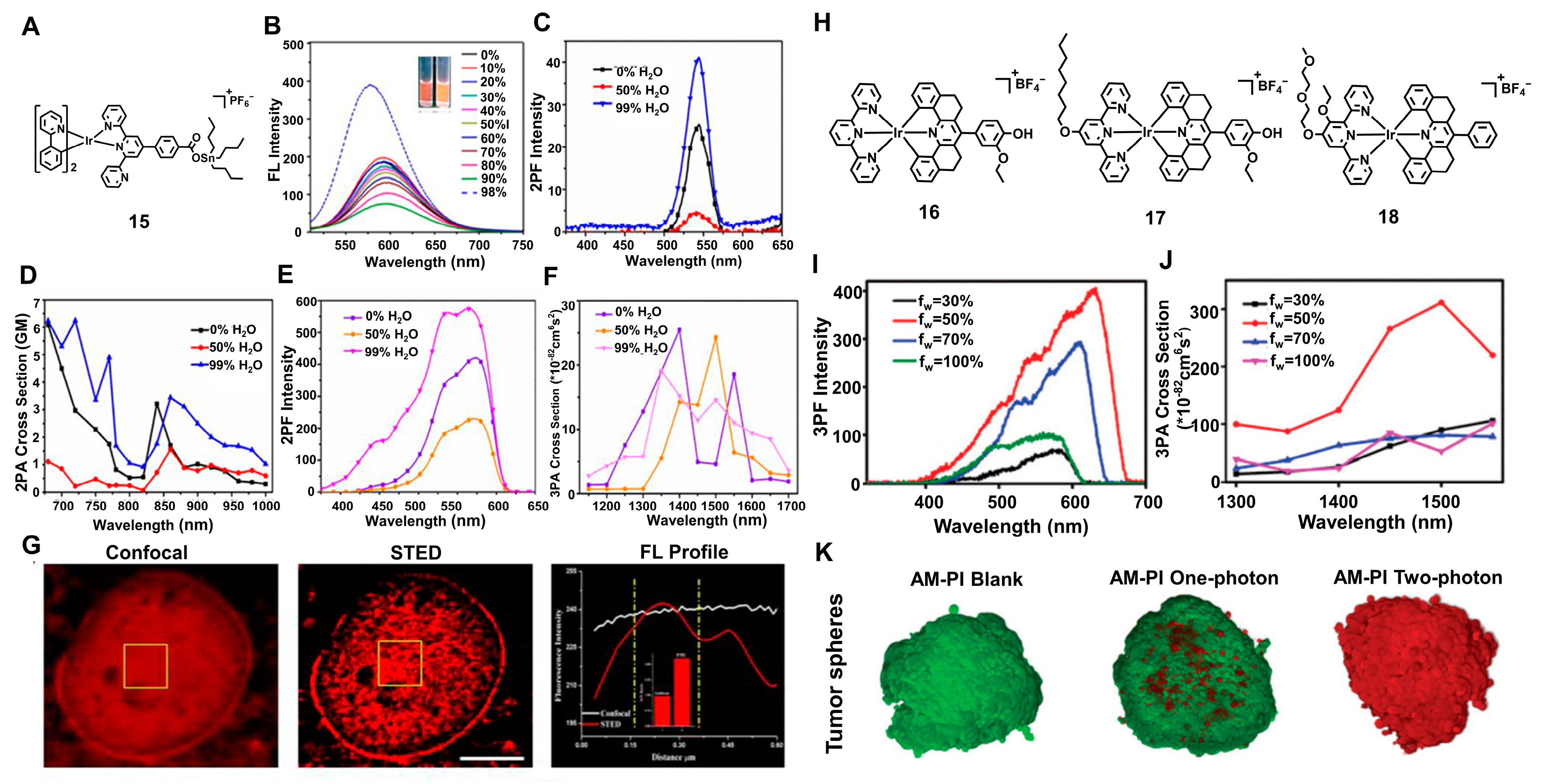
2.4. Two-Photon Absorption (TPA)
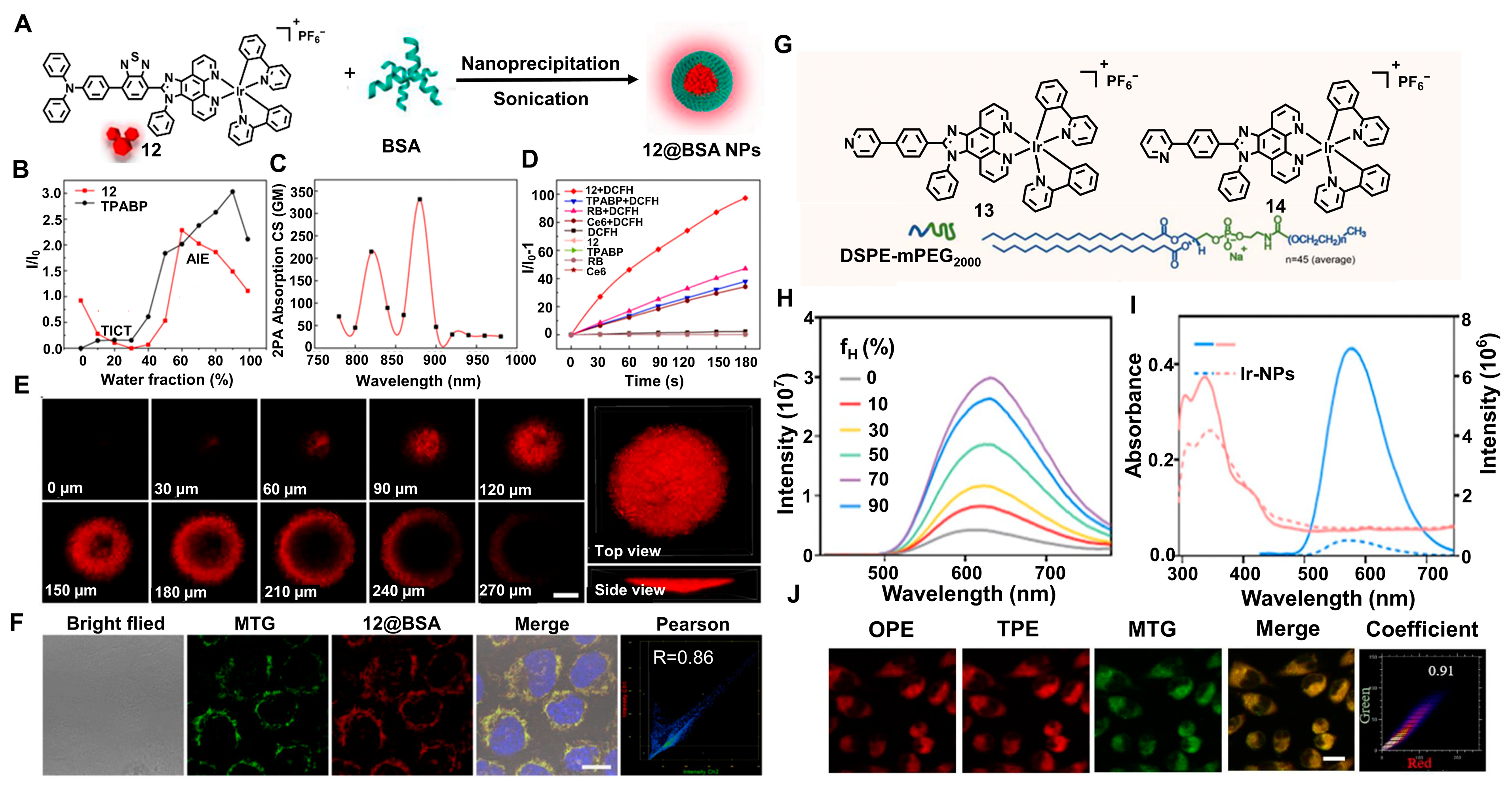
2.5. Multiphoton Absorption (MPA)
3. Accurate Targeting of Ir(III) Complexes with AIE Activity
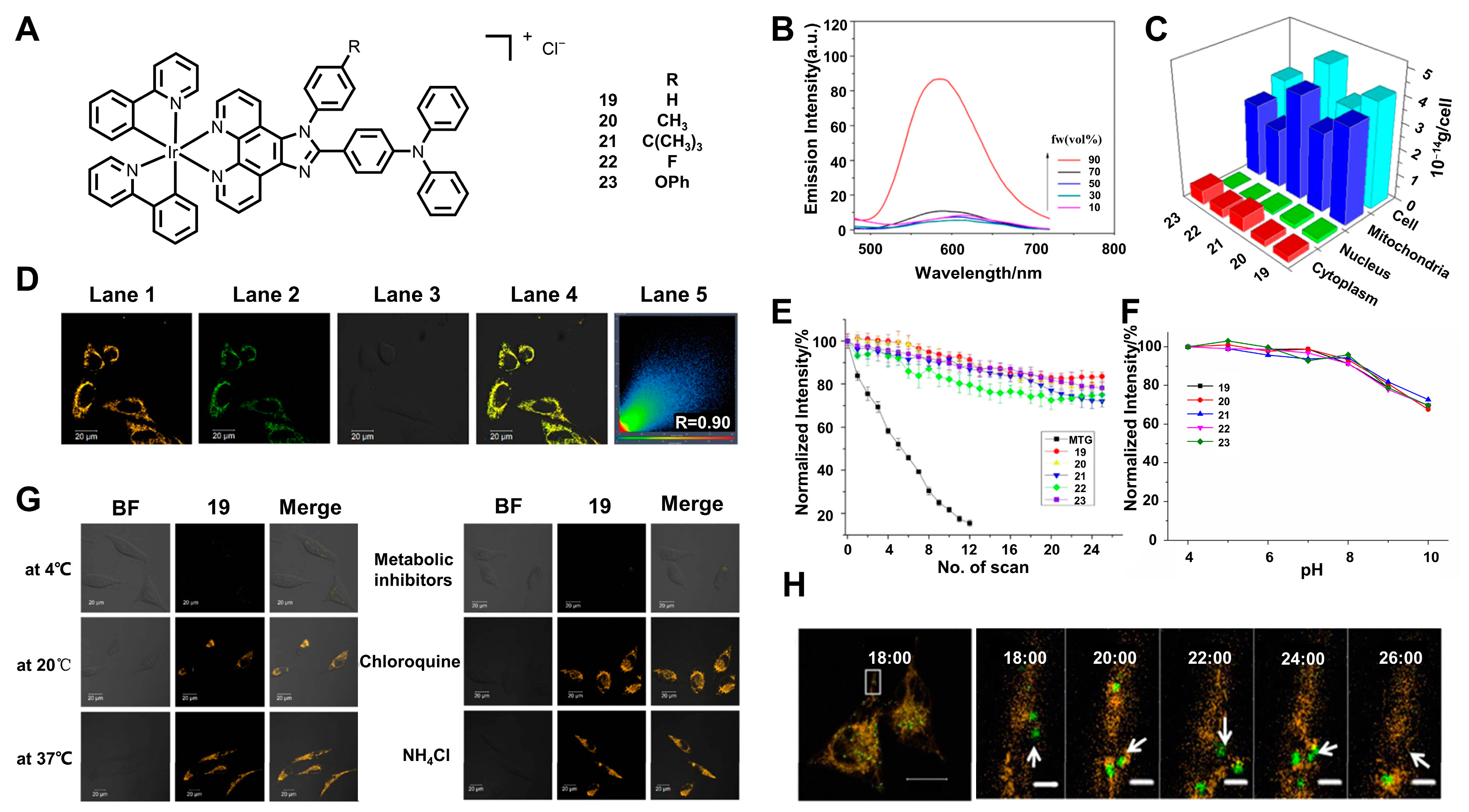
3.1. Targeting Mitochondria
3.2. Targeting Nucleolus
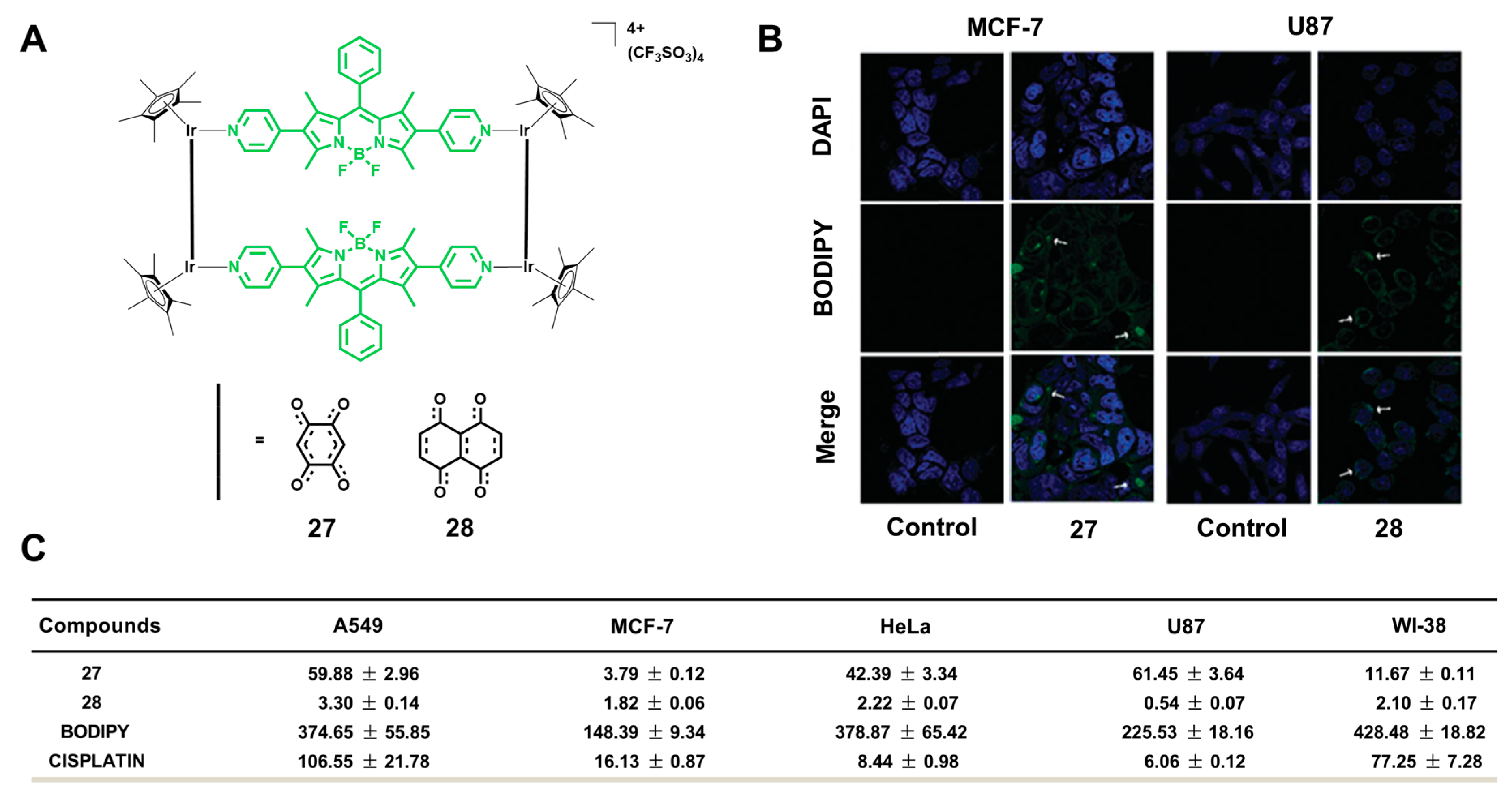
3.3. Selective Toxicity of Cells
4. Specific Response of Ir(III) Complexes with AIE Activity
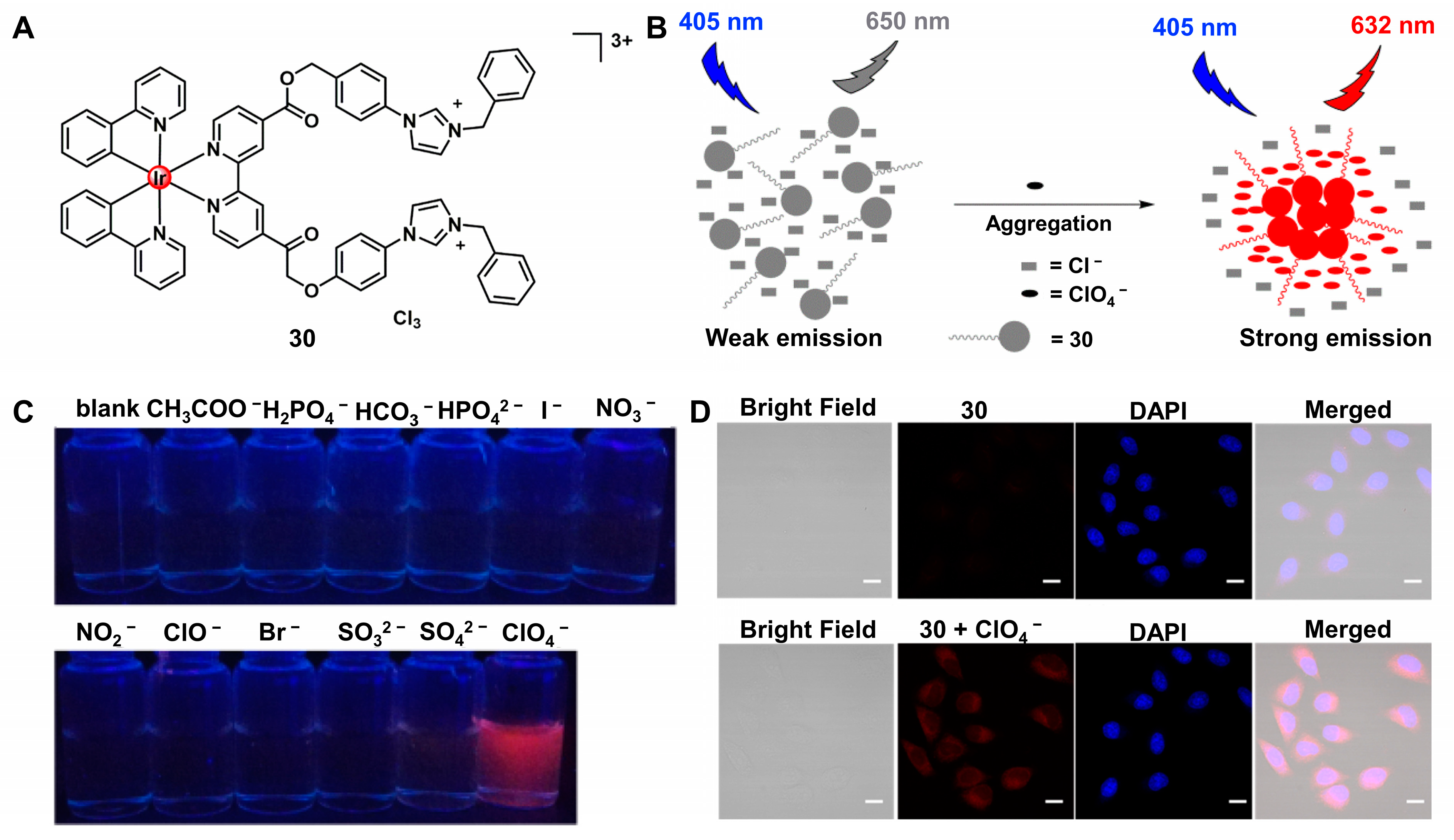
4.1. Perchlorate (ClO4−)
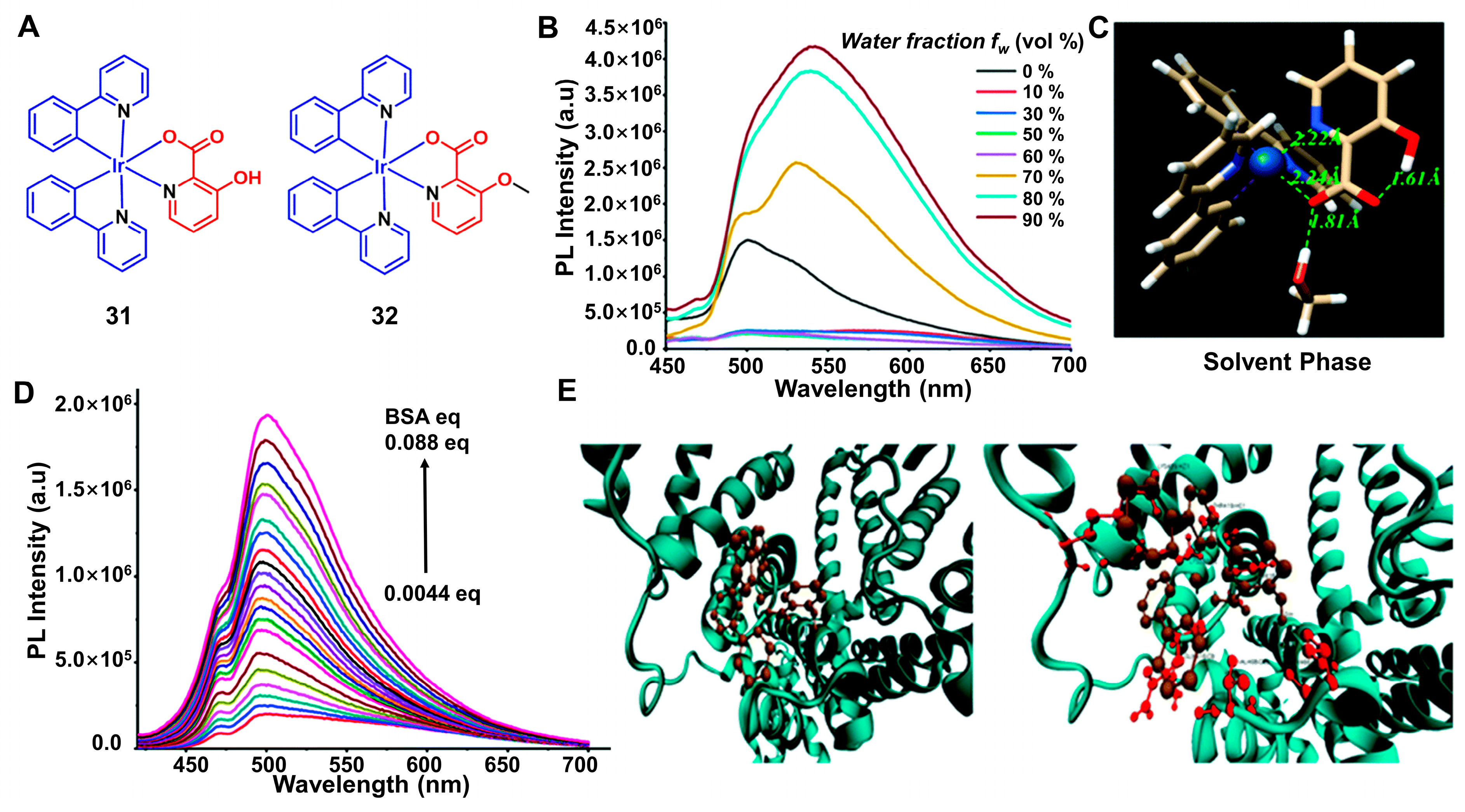
4.2. BSA
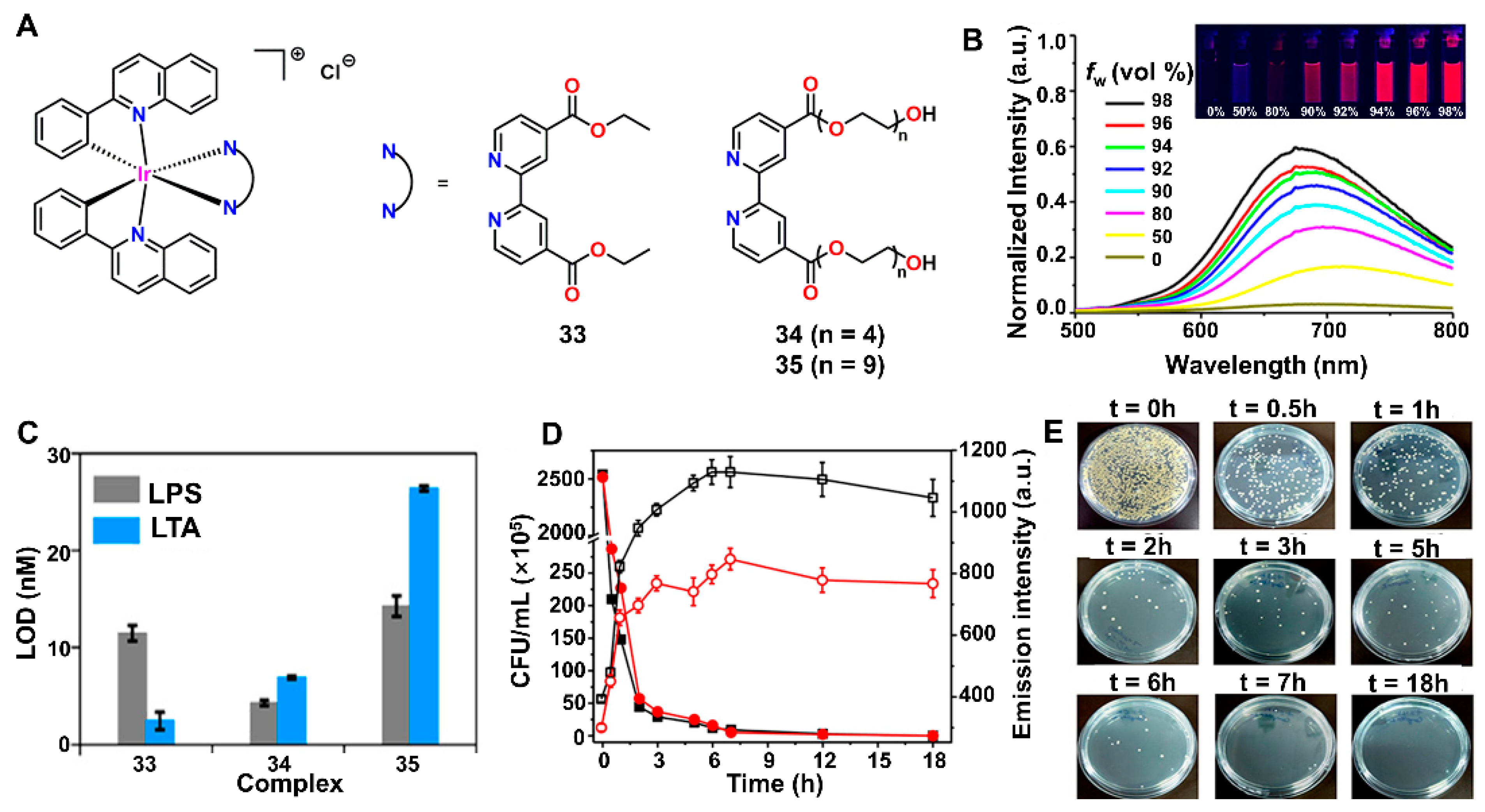
4.3. Drug-Resistant Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kober, E.M.; Meyer, T.J. Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2′-bipyridine). Inorg. Chem. 1982, 21, 3967–3977. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and photophysics of coordination compounds: Iridium. Top. Curr. Chem. 2007, 281, 143–203. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Climent, C.; Alemany, P.; Laskar, I.R. “Aggregation-induced emission” of transition metal compounds: Design, mechanistic insights, and applications. J. Photoch. Photobio. C Photoch. Rev. 2019, 41. [Google Scholar] [CrossRef]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent developments in the application of phosphorescent iridium(III) complex systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Lee, L.C.-C.; Lo, K.K.-W. Luminescent and photofunctional transition metal complexes: From molecular design to diagnostic and therapeutic applications. J. Am. Chem. Soc. 2022, 144, 14420–14440. [Google Scholar] [CrossRef]
- Guan, R.; Xie, L.; Ji, L.; Chao, H. Phosphorescent iridium(III) complexes for anticancer applications. Eur. J. Inorg. Chem. 2020, 2020, 3978–3986. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coordin. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-targeted delivery of photosensitizers for cancer treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Qiu, K.; Zhang, P.; Blacque, O.; Malcomson, T.; Paterson, M.J.; Clarkson, G.J.; Staniforth, M.; Stavros, V.G.; et al. Targeted photoredox catalysis in cancer cells. Nat. Chem. 2019, 11, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Rees, T.W.; Ji, L.; Chao, H. Recent advances in ruthenium(II) and iridium(III) complexes containing nanosystems for cancer treatment and bioimaging. Coordi. Chem. Rev. 2021, 443, 214016. [Google Scholar] [CrossRef]
- Liu, Y.; Song, N.; Chen, L.; Xie, Z.-G. BODIPY@Ir(III) complexes assembling organic nanoparticles for enhanced photodynamic therapy. Chinese J. Polym. Sci. 2018, 36, 417–424. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, S.; Cheng, X.; Cai, X.; Liu, B. Bioorthogonal turn-on probe based on aggregation-induced emission characteristics for cancer cell imaging and ablation. Angew. Chem. Inter. Ed. 2016, 55, 6457–6461. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, light emission, nanoaggregation, and restricted intramolecular rotation of 1,1-substituted 2,3,4,5-tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Cancer immunotherapy: Massively evoking immunogenic cell death by focused mitochondrial oxidative stress using an AIE luminogen with a twisted molecular structure. Adv. Mater. 2019, 31, 1970372. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Tang, B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, M.; Tan, H.; Song, N.; Li, M.; Xiao, P.; Yan, D.; Zhang, L.; Wang, D.; Tang, B.Z. The fast-growing field of photo-driven theranostics based on aggregation-induced emission. Chem. Soc. Rev. 2022, 51, 1983–2030. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, Z.; Liu, P.; Yang, Y.W.; Wang, L.; Wang, D.; Tang, B.Z. Nanomaterials with supramolecular assembly based on AIE luminogens for theranostic applications. Adv. Mater. 2020, 32, e2004208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, L.; Li, F.; Yu, M.; Liu, Z.; Yi, T.; Huang, C. Aggregation-induced phosphorescent emission (AIPE) of iridium(III) complexes. Chem. Commun. 2008, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Xie, J.; Cui, D.; Liu, S.; Li, G.; Zhu, D.; Su, Z. A mechanochromic cyclemetalated cationic Ir(III) complex with AIE activity by strategic modification of ligands. Dalton Trans. 2020, 49, 13066–13071. [Google Scholar] [CrossRef]
- Li, D.; Li, G.; Xie, J.; Zhu, D.; Su, Z.; Bryce, M.R. Strategic modification of ligands for remarkable piezochromic luminescence (PCL) based on a neutral Ir(III) phosphor. J. Mater. Chem. C 2019, 7, 10876–10880. [Google Scholar] [CrossRef]
- Di, L.; Xing, Y.; Yang, Z.; Qiao, C.; Xia, Z. Photostable aggregation-induced emission of iridium(III) complex realizing robust and high-resolution imaging of latent fingerprints. Sens. Actuat. B Chem. 2023, 375, 132898. [Google Scholar] [CrossRef]
- Li, G.; Ren, X.; Shan, G.; Che, W.; Zhu, D.; Yan, L.; Su, Z.; Bryce, M.R. New AIE-active dinuclear Ir(III) complexes with reversible piezochromic phosphorescence behaviour. Chem. Commun. 2015, 51, 13036–13039. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhao, L.; Zhang, Q.; Chang, M.; Li, C.; Zhang, H.; Lu, Y.; Chen, Y. An acceptor–donor–acceptor structured small molecule for effective NIR triggered dual phototherapy of cancer. Adv. Funct. Mater. 2020, 30, 1910301. [Google Scholar] [CrossRef]
- Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.S.; Baigildin, V.A.; Tunik, S.P. Phosphorescent NIR emitters for biomedicine: Applications, advances and challenges. Dalton Trans. 2022, 51, 1257–1280. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, G.; Zhao, C.; Zhou, L.; Wang, X.; Wei, S. Magnetic stomatocyte-like nanomotor as photosensitizer carrier for photodynamic therapy based cancer treatment. Colloid. Surface. B Biointerfaces 2020, 194, 111204. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Wang, H.; Zha, Y.; Zheng, P.; Yang, T.; Ma, D.; Qiu, L.; Xu, X.; Hu, Y.; et al. Mitochondria-targeted magnetic gold nanoheterostructure for multi-modal imaging guided photothermal and photodynamic therapy of triple-negative breast cancer. Chem. Eng. J. 2021, 403, 126364. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Hartley, R.C.; Murphy, M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011, 15, 3021–3038. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.K.; Sazanovich, I.V.; Baggaley, E.; Bonneau, M.; Guerchais, V.; Williams, J.A.G.; Weinstein, J.A.; Bryant, H.E. Metal complexes for two-photon photodynamic therapy: A cyclometallated iridium complex induces two-photon photosensitization of cancer cells under near-IR light. Chem. Eur. J. 2017, 23, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, Y.; Zhang, M.; Luo, L.; Wu, J.; Zhou, H.; Guan, L.; Battaglia, G.; Tian, Y. Localization matters: A nuclear targeting two-photon absorption iridium complex in photodynamic therapy. Chem. Commun. 2017, 53, 3303–3306. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; You, Y.; Nam, W. Lysosome-specific one-photon fluorescence staining and two-photon singlet oxygen generation by molecular dyad. RSC Adv. 2014, 4, 16913–16916. [Google Scholar] [CrossRef]
- Kuang, S.; Sun, L.; Zhang, X.; Liao, X.; Rees, T.W.; Zeng, L.; Chen, Y.; Zhang, X.; Ji, L.; Chao, H. A mitochondrion-localized two-photon photosensitizer generating carbon radicals against hypoxic tumors. Angew. Chem. Inter. Ed. 2020, 59, 20697–20703. [Google Scholar] [CrossRef]
- Cao, R.; Jia, J.; Ma, X.; Zhou, M.; Fei, H. Membrane localized iridium(III) complex induces endoplasmic reticulum stress and mitochondria-mediated apoptosis in human cancer cells. J. Med. Chem. 2013, 56, 3636–3644. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-targeted triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Murase, T.; Yoshihara, T.; Tobita, S. Mitochondria-specific oxygen probe based on iridium complexes bearing triphenylphosphonium cation. Chem. Lett. 2012, 41, 262–263. [Google Scholar] [CrossRef]
- Chen, Y.; Rees, T.W.; Ji, L.; Chao, H. Mitochondrial dynamics tracking with iridium(III) complexes. Curr. Opin. Chem. Biol. 2018, 43, 51–57. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Wang, X.; Su, Z.; Bryce, M.R. Dinuclear metal complexes: Multifunctional properties and applications. Chem. Soc. Rev. 2020, 49, 765–838. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lu, Y.; McGoldrick, N.; Zhang, C.; Yang, W.; Zhao, J.; Draper, S.M. Dual phosphorescent dinuclear transition metal complexes, and their application as triplet photosensitizers for TTA upconversion and photodynamic therapy. J. Mater. Chem. C 2016, 4, 6131–6139. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhang, F.; Xu, B.; Tian, W.; Xie, Z. Supramolecular hybrids of AIEgen with carbon dots for noninvasive long-term bioimaging. Chem. Mater. 2016, 28, 8825–8833. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, Y.; Wang, Y.; Zhao, S.; Wu, X.; Peng, X.; Huang, L.; Jiang, L.; Lan, M.; Yi, X.-Y. An iridium complex as an AIE-active photosensitizer for image-guided photodynamic therapy. Chem. Asian J. 2021, 16, 1780–1785. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient near-infrared photosensitizer with aggregation-induced emission for imaging-guided photodynamic therapy in multiple xenograft tumor models. ACS Nano. 2020, 14, 854–866. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Shan, G.; Leung, A.C.S.; Lee, M.M.S.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; Tang, B.Z. Facile synthesis of red/NIR AIE luminogens with simple structures, bright emissions, and high photostabilities, and their applications for specific imaging of lipid droplets and image-guided photodynamic therapy. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Tong, X.; Li, Y.; Yang, Z.; Zhu, D.; Su, Z.; Xie, Z. Near-infrared-emitting AIE multinuclear cationic Ir(III) complex-assembled nanoparticles for photodynamic therapy. Dalton Trans. 2020, 49, 15332–15338. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Liu, S.; Zhang, C.; Chuah, C.; Tang, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Ou, H.; Ding, D.; et al. Enlarging the reservoir: High absorption coefficient dyes enable synergetic near infrared-II fluorescence imaging and near infrared-I photothermal therapy. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Che, W.; Zhu, D.; Li, G.; Xie, Z.; Song, N.; Liu, S.; Tang, B.Z.; Liu, X.; et al. AIE multinuclear Ir(III) complexes for biocompatible organic nanoparticles with highly enhanced photodynamic performance. Adv. Sci. 2019, 6, 1802050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Liu, B.; Sun, Q.; Feng, L.; He, F.; Yang, P.; Gai, S.; Quan, Z.; Lin, J. Upconverted metal-organic framework janus architecture for near-infrared and ultrasound co-enhanced high performance tumor therapy. ACS Nano. 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, Z.; Wang, L.; Wang, D.; Tang, B.Z. Triple-jump photodynamic theranostics: MnO2 combined upconversion nanoplatforms involving a type-I photosensitizer with aggregation-induced emission characteristics for potent cancer treatment. Adv. Mater. 2021, 33, e2103748. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, J.; Chang, Y.; Wang, W.; Wang, R.; Wang, Z.; Li, G.; Zhu, D.; Bryce, M.R. AIE-active iridium(III) complex integrated with upconversion nanoparticles for NIR-irradiated photodynamic therapy. Chem. Commun. 2022, 58, 10056–10059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, S.; Jia, Q.; Huang, L.; Lan, M.; Wang, P.; Zhang, W. Lysosome-targetable carbon dots for highly efficient photothermal/photodynamic synergistic cancer therapy and photoacoustic/two-photon excited fluorescence imaging. Chem. Eng. J. 2020, 388. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Hong, Y.; Lin, R.; Liu, Z.; Chen, M.; Lam, J.W.Y.; Ning, G.H.; Zheng, X.; Qin, A.; et al. Click synthesis enabled sulfur atom strategy for polymerization-enhanced and two-photon photosensitization. Angew. Chem. Int. Ed. 2022, 61, e202202005. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, F.; Zhang, Q.; Eda, G.; Ji, W. Layered hybrid perovskites for highly efficient three-photon absorbers: Theory and experimental observation. Adv. Sci. 2019, 6, 1801626. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Lee, M.M.S.; Nie, J.J.; Zhang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Xu, F.J.; Wang, D.; Tang, B.Z. Three-pronged attack by homologous far-red/NIR AIEgens to achieve 1+1+1>3 synergistic enhanced photodynamic therapy. Angew. Chem. Int. Ed. 2020, 59, 9610–9616. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, C.; Meng, H.; Li, R.; Mao, L.; Hu, D.; Tian, M.; Yuan, J.; Wei, Y. Multifunctional organic fluorescent probe with aggregation-induced emission characteristics: Ultrafast tumor monitoring, two-photon imaging, and image-guide photodynamic therapy. ACS Appl. Mater. Interfaces 2021, 13, 7987–7996. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coordi. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef] [Green Version]
- Porrès, L.; Mongin, O.; Katan, C.; Charlot, M.; Pons, T.; Mertz, J.; Blanchard-Desce, M. Enhanced two-photon absorption with novel octupolar propeller-shaped fluorophores derived from triphenylamine. Org. Lett. 2004, 6, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, C.; Yuan, B.; Liu, X.; Chen, Y.; Ji, L.; Chao, H. Selectively lighting up two-photon photodynamic activity in mitochondria with AIE-active iridium(Ⅲ) complexes. Chem. Commun. 2017, 53, 2052–2055. [Google Scholar] [CrossRef]
- Fang, F.; Yuan, Y.; Wan, Y.; Li, J.; Song, Y.; Chen, W.C.; Zhao, D.; Chi, Y.; Li, M.; Lee, C.S.; et al. Near-infrared thermally activated delayed fluorescence nanoparticle: A metal-free photosensitizer for two-photon-activated photodynamic therapy at the cell and small animal levels. Small 2022, 18, e2106215. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, W.; Xu, Z.; Zhang, Z.; Tang, S.; Fan, M.; Li, Z.; Zhang, J.; Yang, C.; Law, W.-C.; et al. A mitochondrion-targeting two-photon photosensitizer with aggregation-induced emission characteristics for hypoxia-tolerant photodynamic therapy. Chem. Eng. J. 2022, 448. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.N.; Ma, W.; Yang, Y.; Chen, G.; Fu, H.; Cui, C.; Yu, Z.; Wang, X. Multifunctional AIE iridium (III) photosensitizer nanoparticles for two-photon-activated imaging and mitochondria targeting photodynamic therapy. J. Nanobiotechnol. 2021, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, X.; Cao, H.; Wang, H.; Zhu, T.; Tian, X.; Li, D.; Zhou, H.; Wu, J.; Tian, Y. Multiphoton absorption iridium(III)-organotin(IV) dimetal complex with AIE behavior for both sensitive detection of tyrosine and antibacterial activity. ACS Appl. Bio. Mater. 2020, 3, 8105–8112. [Google Scholar] [CrossRef]
- Lu, X.; Xiong, C.; Li, B.; Du, W.; Li, D.; Ma, W.; Tian, X.; Tian, Y.; Zhang, Q. Three-photon absorption iridium(Ⅲ) photosensitizers featuring aggregation induced emission. Inorg. Chem. Front. 2022, 9, 1890–1896. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Zhang, T.; Xing, D. Mitochondria-specific agents for photodynamic cancer therapy: A key determinant to boost the efficacy. Adv. Healthc. Mater. 2021, 10, e2001240. [Google Scholar] [CrossRef]
- Wang, K.; Klionsky, D.J. Mitochondria removal by autophagy. Autophagy 2011, 7, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Liu, J.; Chen, Y.; Guan, R.; Ouyang, C.; Zhu, Y.; Ji, L.; Chao, H. Cyclometalated iridium(III) complexes as AIE phosphorescent probes for real-time monitoring of mitophagy in living cells. Sci. Rep. 2016, 6, 22039. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Dash, S.; Climent, C.; Kaur, G.; Choudhury, A.R.; Casanova, D.; Alemany, P.; Chowdhury, R.; Laskar, I.R. ‘Aggregation induced emission’ active iridium(Ⅲ) complexes with applications in mitochondrial staining. RSC Adv. 2017, 7, 5642–5648. [Google Scholar] [CrossRef]
- Sheet, S.K.; Sen, B.; Aguan, K.; Khatua, S. A cationic organoiridium(Ⅲ) complex-based AIEgen for selective light-up detection of rRNA and nucleolar staining. Dalton Trans. 2018, 47, 11477–11490. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Das, A.; Ghate, N.B.; Kim, T.; Ryu, J.Y.; Lee, J.; Mandal, N.; Lee, C.Y. Novel BODIPY-based Ru(II) and Ir(III) metalla-rectangles: Cellular localization of compounds and their antiproliferative activities. Chem. Commun. 2016, 52, 4274–4277. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, K.H.; Crawford, R.L.; Cosens, B.; Hess, T.F. Development of drinking water standards for perchlorate in the United States. J Environ. Manage. 2009, 91, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Capen, C.C. Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol. Pathol. 1997, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Ni, S. Highly selective sensing of ClO4− in water with a simple cationic iridium(III) complex and its application in bioimaging. J. Photoch. Photobio. A Chem. 2016, 324, 1–7. [Google Scholar] [CrossRef]
- Lu, F.; Yamamura, M.; Nabeshima, T. A highly selective and sensitive ratiometric chemodosimeter for Hg2+ ions based on an iridium(Ⅲ) complex via thioacetal deprotection reaction. Dalton Trans. 2013, 42, 12093–12100. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhang, Y. A water–soluble cationic Ir(III) complex for turn–on sensing of ClO4− based on aggregation–induced emission. Sensor. Actuat. B Chem. 2017, 245, 599–604. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Setua, P.; Sarkar, N. Photoinduced electron transfer in a protein−surfactant complex: Probing the interaction of SDS with BSA. J. Phys. Chem. B 2006, 110, 16607–16617. [Google Scholar] [CrossRef]
- Bayraktutan, T.; Onganer, Y. Spectral-luminescent study of coumarin 35 as fluorescent “light-up” probe for BSA and DNA monitoring. Dyes Pigments 2017, 142, 62–68. [Google Scholar] [CrossRef]
- Kachwal, V.; Sharma, P.K.; Sarmah, A.; Chowdhury, S.; Laskar, I.R. A multistimuli responsive heteroleptic iridium(Ⅲ) complex: Role of hydrogen bonding in probing solvent, pH and bovine serum albumin (BSA). J. Mater. Chem. C 2020, 8, 6605–6614. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, PMC-S14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Castillo, F.Y.; Loera-Muro, A.; Jacques, M.; Garneau, P.; Avelar-González, F.J.; Harel, J.; Guerrero-Barrera, A.L. Waterborne pathogens: Detection methods and challenges. Pathogens 2015, 4, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2017, 42. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Prasad, P.; Gupta, S.; Sasmal, P.K. Simultaneous ultrasensitive detection and elimination of drug-resistant bacteria by cyclometalated iridium(III) complexes. ACS Appl. Mater. Interfaces 2020, 12, 35967–35976. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Sun, Y.; Huang, M.; Zhang, Z.; Yan, D.; Cui, J.; Zhu, D.; Zeng, Z.; Wang, D.; Tang, B. Ir(III) Complexes with AIE Characteristics for Biological Applications. Biosensors 2022, 12, 1104. https://doi.org/10.3390/bios12121104
Pei Y, Sun Y, Huang M, Zhang Z, Yan D, Cui J, Zhu D, Zeng Z, Wang D, Tang B. Ir(III) Complexes with AIE Characteristics for Biological Applications. Biosensors. 2022; 12(12):1104. https://doi.org/10.3390/bios12121104
Chicago/Turabian StylePei, Yu, Yan Sun, Meijia Huang, Zhijun Zhang, Dingyuan Yan, Jie Cui, Dongxia Zhu, Zebing Zeng, Dong Wang, and Benzhong Tang. 2022. "Ir(III) Complexes with AIE Characteristics for Biological Applications" Biosensors 12, no. 12: 1104. https://doi.org/10.3390/bios12121104
APA StylePei, Y., Sun, Y., Huang, M., Zhang, Z., Yan, D., Cui, J., Zhu, D., Zeng, Z., Wang, D., & Tang, B. (2022). Ir(III) Complexes with AIE Characteristics for Biological Applications. Biosensors, 12(12), 1104. https://doi.org/10.3390/bios12121104








