Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases
Abstract
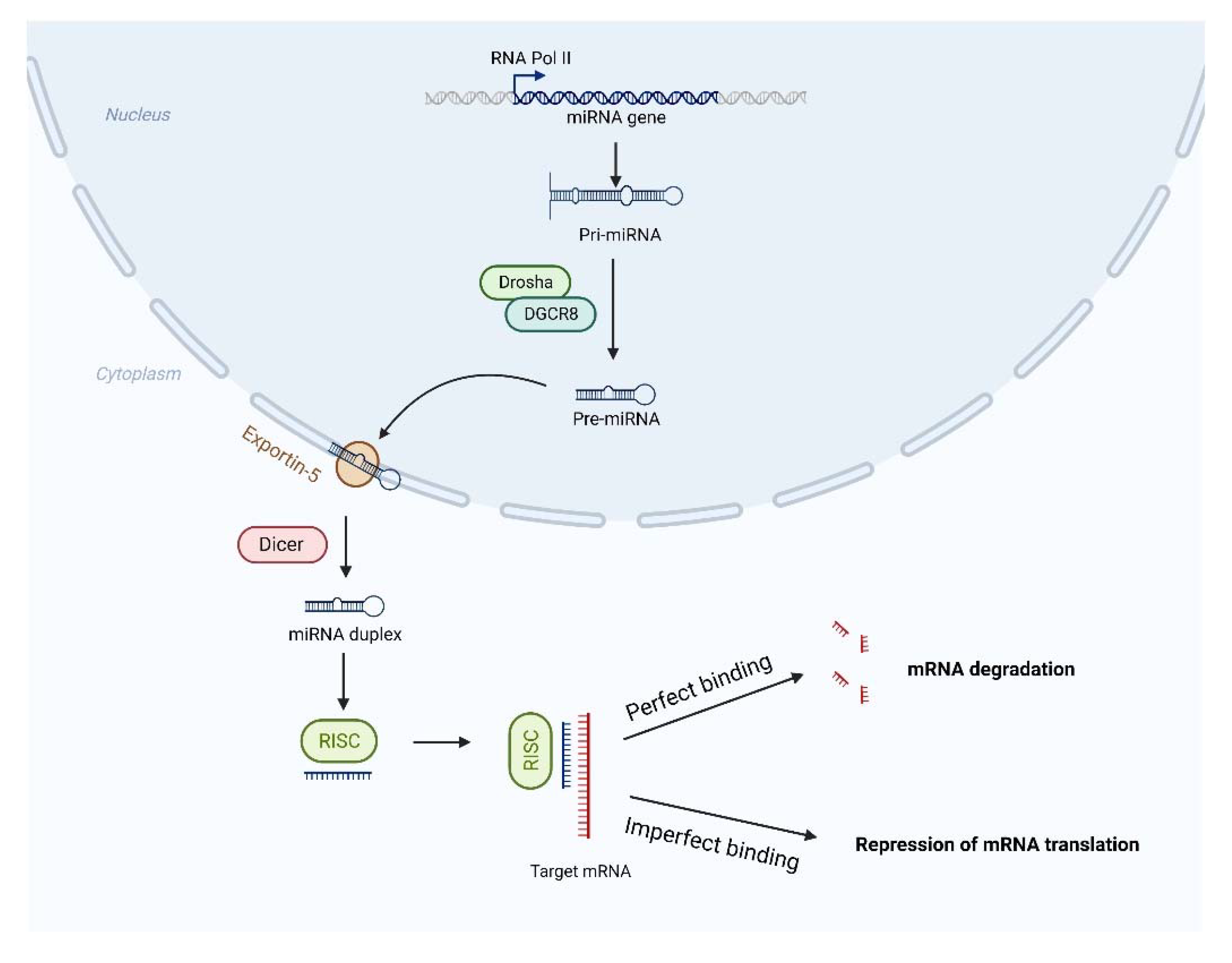
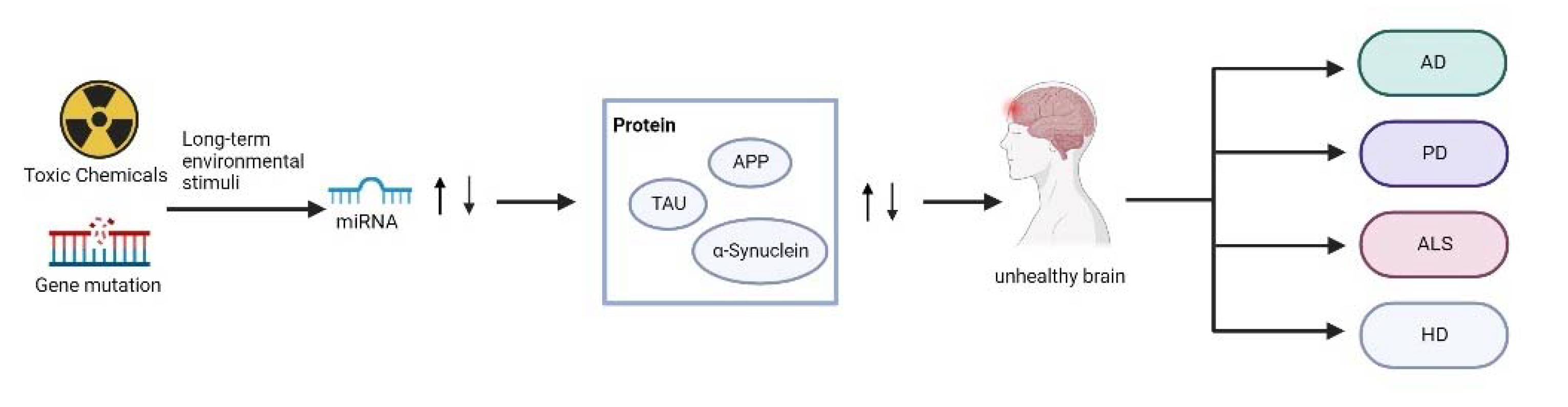
:1. Introduction
2. Roles of miRNAs in Neurodegenerative Diseases
2.1. miRNA in AD
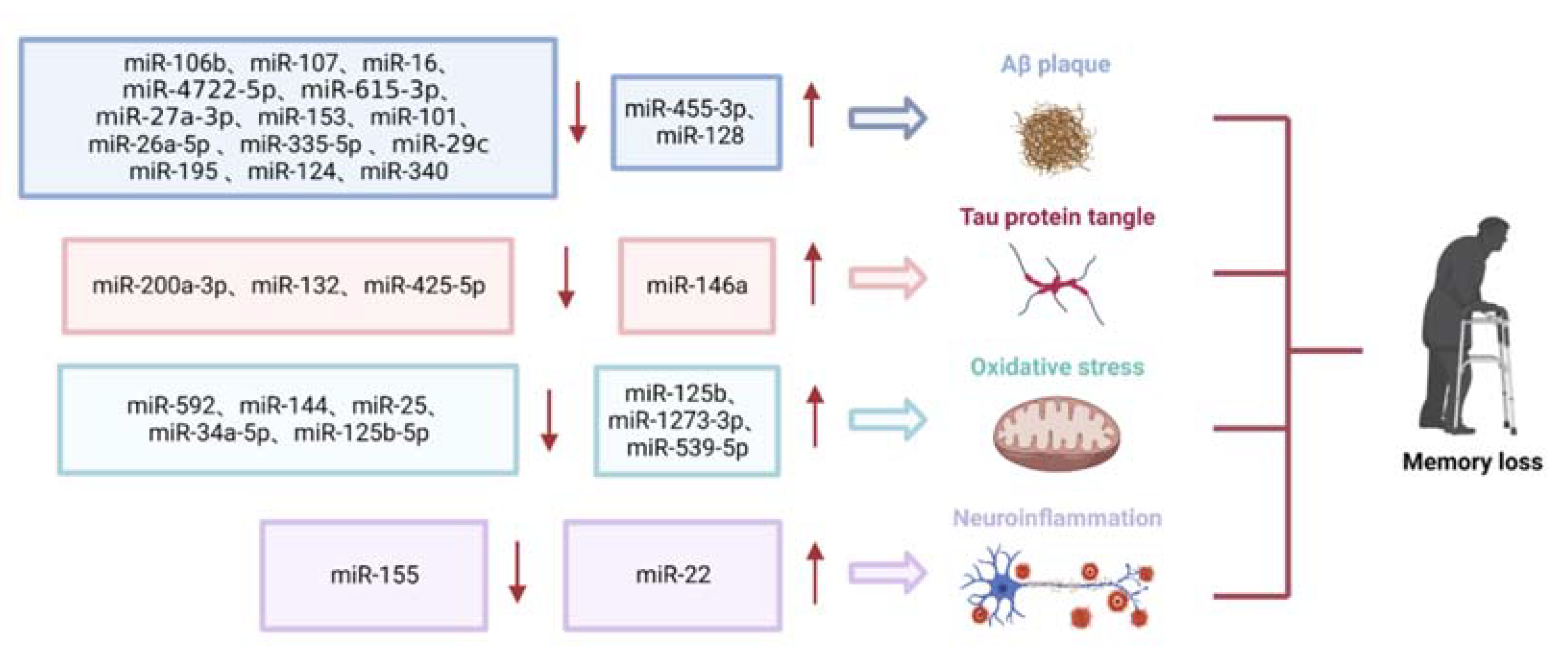
2.1.1. Role of miRNAs in Aβ Deposition
2.1.2. Role of miRNAs in Tau Phosphorylation
2.1.3. Role of miRNAs in Oxidative Stress
2.1.4. Role of miRNAs in Neuroinflammation
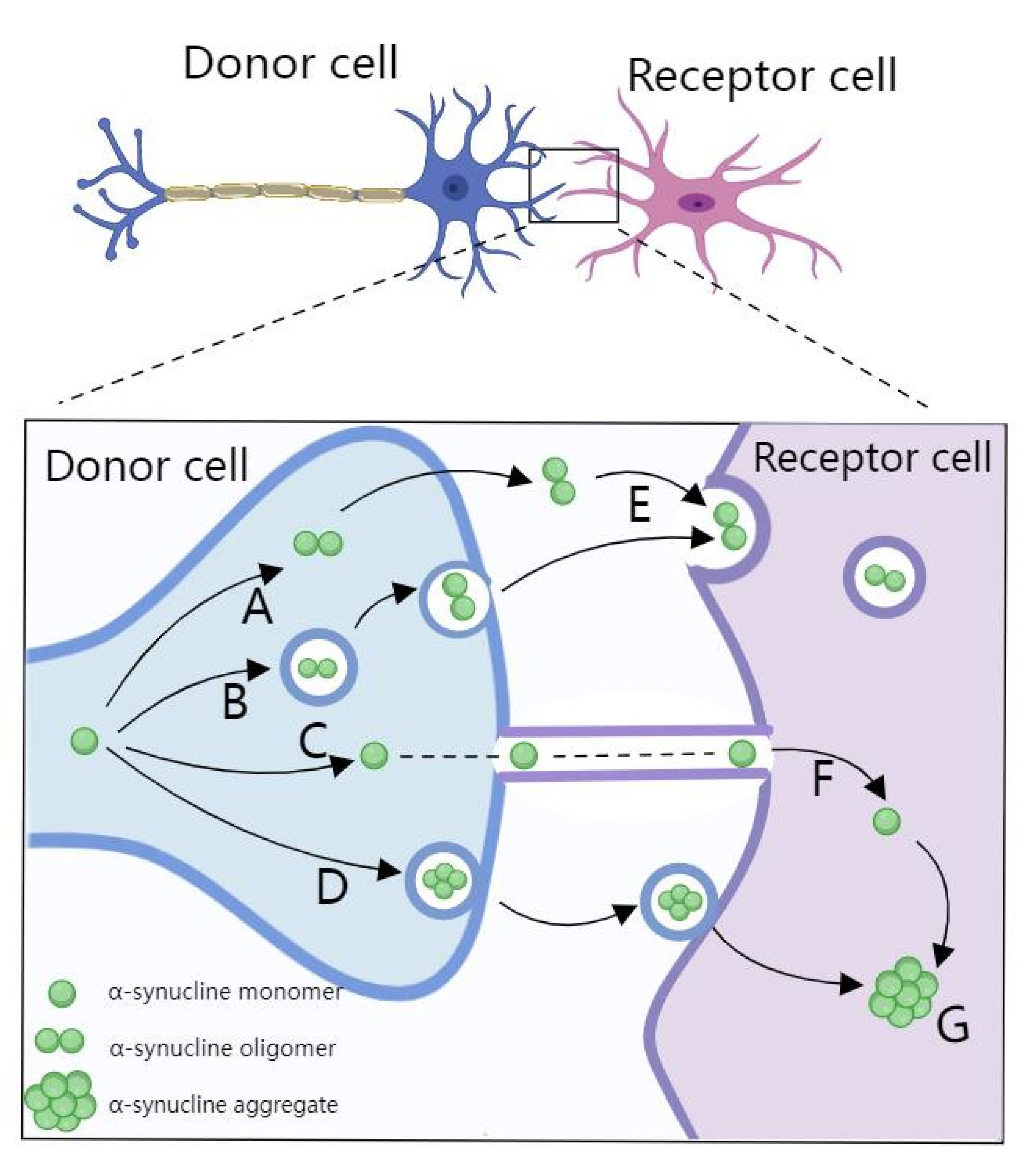
2.2. miRNA in PD
2.3. miRNA in ALS
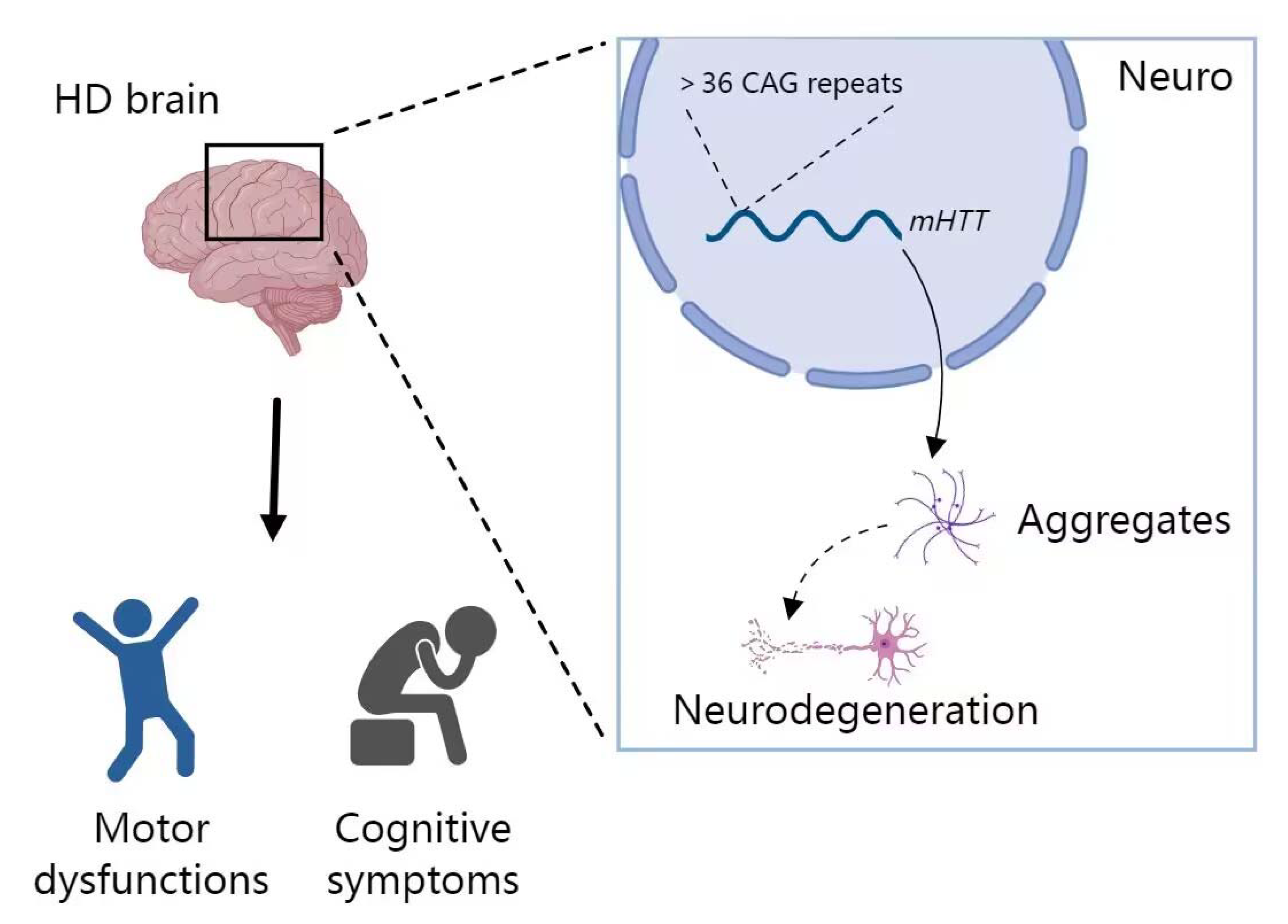
2.4. miRNA in HD
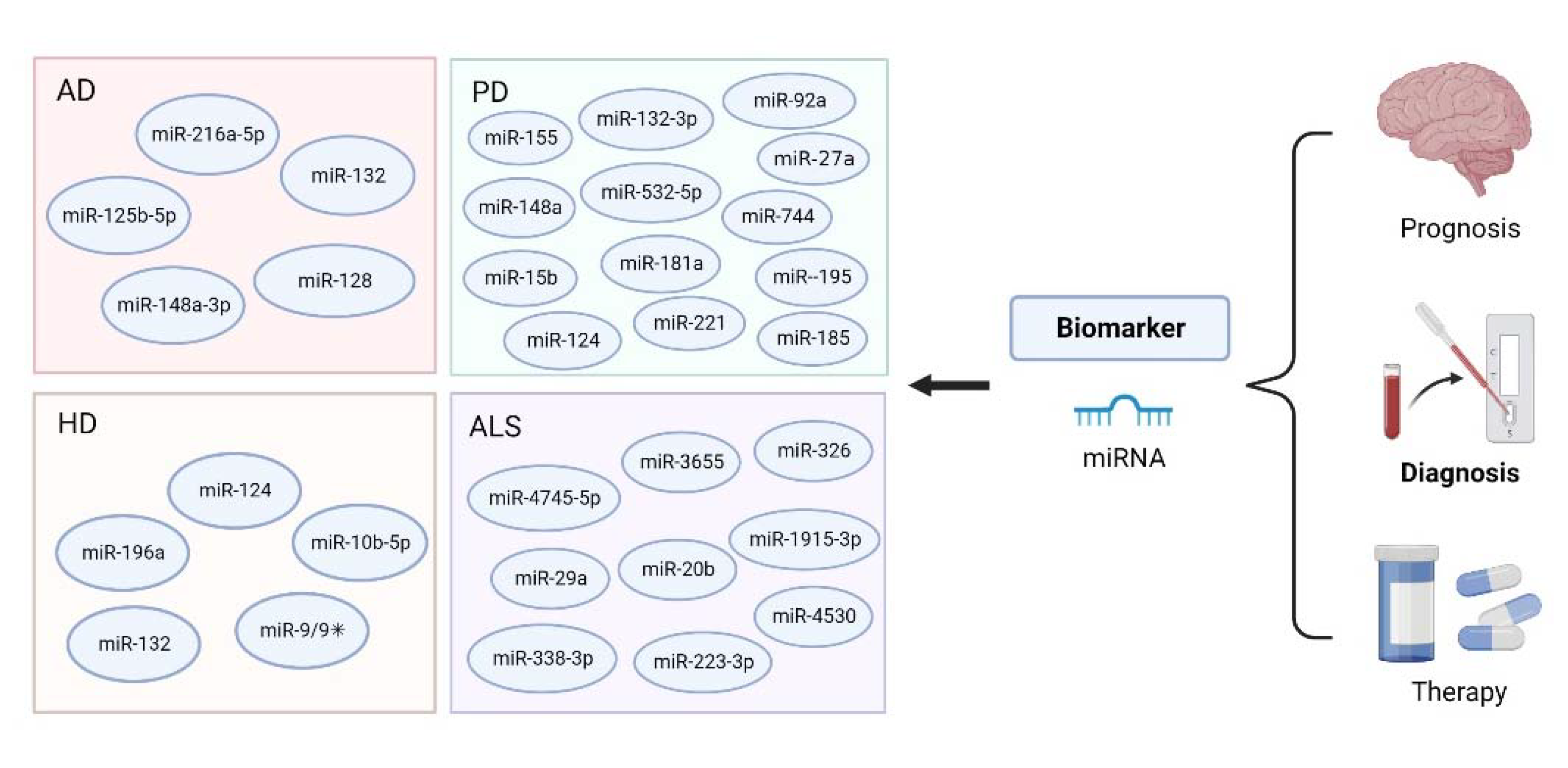
3. MiRNA-Based Bioanalysis in the Diagnosis of Neurodegenerative Diseases
3.1. miRNA-Based Bioanalysis in AD
3.2. miRNA-Based Bioanalysis in PD
3.3. miRNA-Based Bioanalysis in ALS
3.4. miRNA-Based Bioanalysis in HD
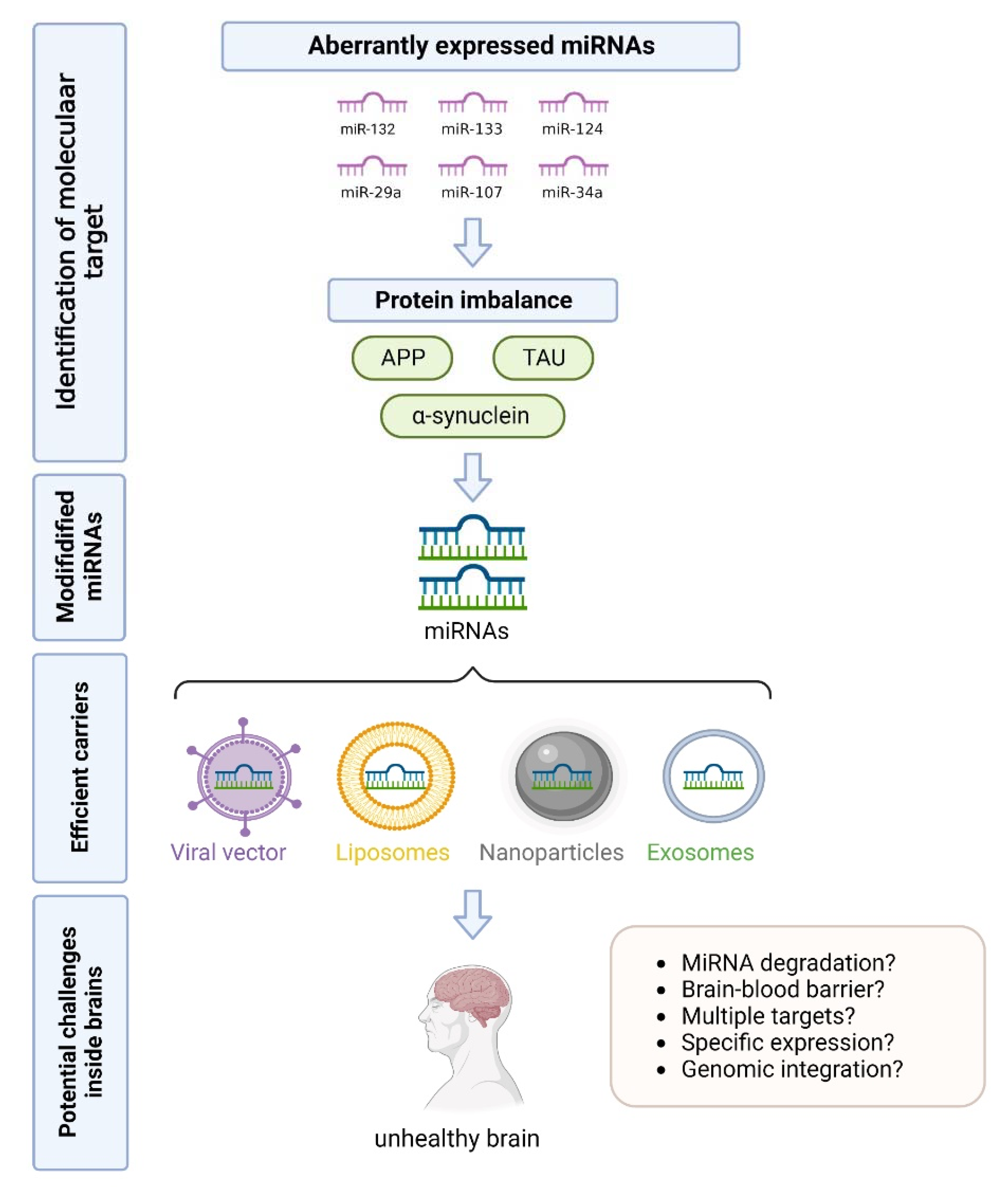
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, X.; Bian, Z. MicroRNA-21 is a versatile regulator and potential treatment target in central nervous system disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef] [PubMed]
- De Jager, P.L.; Bennett, D.A. An inflection point in gene discovery efforts for neurodegenerative diseases: From syndromic diagnoses toward endophenotypes and the epigenome. JAMA Neurol. 2013, 70, 719–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Yang, G.; Yan, Q.Q.; Zhao, J.; Li, S. Exosome-encapsulated microRNAs as promising biomarkers for Alzheimer’s disease. Rev. Neurosci. 2019, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kritsilis, M.; Rizou, S.V.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collaborators, G.D. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [Green Version]
- Loffreda, A.; Nizzardo, M.; Arosio, A.; Ruepp, M.D.; Calogero, R.A.; Volinia, S.; Galasso, M.; Bendotti, C.; Ferrarese, C.; Lunetta, C.; et al. MiR-129-5p: A key factor and therapeutic target in Amyotrophic lateral sclerosis. Prog. Neurobiol. 2020, 190, 101803. [Google Scholar] [CrossRef]
- Oh, Y.M.; Lee, S.W.; Kim, W.K.; Chen, S.; Church, V.A.; Cates, K.; Li, T.; Zhang, B.; Dolle, R.E.; Dahiya, S.; et al. Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy. Nat. Neurosci. 2022, 25, 1420–1433. [Google Scholar] [CrossRef]
- Yang, H.; Liu, M.; Jiang, H.; Zeng, Y.; Jin, L.; Luan, T.; Deng, Y.; He, N.; Zhang, G.; Zeng, X. Copy number variation analysis based on gold magnetic nanoparticles and fluorescence multiplex ligation-dependent probe amplification. J. Biomed. Nanotechnol. 2017, 13, 655–664. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Xie, S.; Tan, H.; Tan, W.; Li, N.; Xu, L.; Xu, J. Highly fluorescent N -doped carbon quantum dots derived from bamboo stems for selective detection of Fe3+ ions in biological systems. J. Biomed. Nanotechnol. 2021, 17, 312–321. [Google Scholar] [CrossRef]
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current signal amplification strategies in aptamer-based electrochemical biosensor: A review. Chin. Chem. Lett. 2021, 32, 1593–1602. [Google Scholar] [CrossRef]
- Tang, Y.; Ali, Z.; Dai, J.; Liu, X.; Wu, Y.; Chen, Z.; He, N.; Li, S.; Wang, L. Single-nucleotide polymorphism genotyping of exoS in pseudomonas aeruginosa using dual-color fluorescence hybridization and magnetic separation. J. Biomed. Nanotechnol. 2018, 14, 206–214. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, L.; Tan, M.; Pan, T.; He, H.; Wang, Y.; He, X.; Li, W.; Tang, L.; Nie, L. Two-photon fluorescent nanomaterials and their applications in biomedicine. J. Biomed. Nanotechnol. 2021, 17, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xi, L.; Tan, T.; Jin, L.; Wang, Z.; He, N. A novel aptamer-based histochemistry assay for specific diagnosis of clinical breast cancer tissues. Chin. Chem. Lett. 2021, 32, 1726–1730. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Y.; Qu, G.; Chen, Y.; Shang, J.; Feng, Z.; Zheng, J.; Yang, F.; He, N. Cell microarray chip system for accurate, rapid diagnosis and target treatment of breast cancer cells SK-BR-3. Chin. Chem. Lett. 2019, 30, 1043–1050. [Google Scholar] [CrossRef]
- Liu, H.; Dong, H.; Chen, Z.; Lin, L.; Chen, H.; Li, S.; Deng, Y. Magnetic nanoparticles enhanced microarray detection of multiple foodborne pathogens. J. Biomed. Nanotechnol. 2017, 13, 1333–1343. [Google Scholar] [CrossRef]
- Mou, X.; Chen, Z.; Li, T.; Liu, M.; Liu, Y.; Ali, Z.; Li, S.; Zhu, Y.; Li, Z.; Deng, Y. A highly sensitive strategy for low-abundance hepatitis B virus detection via one-step nested polymerase chain reaction, chemiluminescence technology and magnetic separation. J. Biomed. Nanotechnol. 2019, 15, 1832–1838. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Deng, Y.; Lin, L.; He, N. Development of a magnetic nanoparticles microarray for simultaneous and simple detection of foodborne pathogens. J. Biomed. Nanotechnol. 2013, 9, 1254–1260. [Google Scholar] [CrossRef]
- Yang, H.; Liang, W.; Si, J.; Li, Z.; He, N. Long spacer arm-functionalized magnetic nanoparticle platform for enhanced chemiluminescent detection of hepatitis B virus. J. Biomed. Nanotechnol. 2014, 10, 3610–3619. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, L.; Liu, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S. Immunosensors based on nanomaterials for detection of tumor markers. J. Biomed. Nanotechnol. 2018, 14, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Yang, H.; Chen, S.; Ma, G.; Zhang, X.; Zhu, M.; Yu, J.; Singh, R.; Zhang, Y.; et al. Ultrasensitive detection of gastric cancer plasma microRNAs via magnetic beads-based chemiluminescent assay. J. Biomed. Nanotechnol. 2017, 13, 1272–1280. [Google Scholar] [CrossRef]
- Wang, W.; Deng, Y.; Li, S.; Liu, H.; Lu, Z.; Zhang, L.; Lin, L.; Xu, L. A novel acetylcholine bioensor and its electrochemical behavior. J. Biomed. Nanotechnol. 2013, 9, 736–740. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhang, L.; Lu, Z.; Li, S.; Xu, L. Preparation and electrochemical behavior of L-glutamate electrochemical biosensor. J. Biomed. Nanotechnol. 2013, 9, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Huang, H.; Guo, W.; Zhang, C.; Chen, Z.; Li, S.; Ma, L.; Deng, Y. Recent progress in black phosphorus sensors. J. Biomed. Nanotechnol. 2020, 16, 1045–1064. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnology 2020, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Li, Z.; He, N.; Zhang, L.; Ma, C.; Li, X.; Li, C.; Wang, Z.; Deng, Y.; He, L. Preparation of functional magnetic nanoparticles mediated with PEG-4000 and application in Pseudomonas aeruginosa rapid detection. J. Biomed. Nanotechnol. 2013, 9, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Liu, H.; Wang, Y.; Su, X.; Jin, L.; Wu, Y.; Deng, Y.; Li, S.; Chen, Z.; Chen, H.; et al. Fast and accurate control strategy for portable nucleic acid detection (PNAD) system based on magnetic nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 407–415. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Chen, Z.; Hu, Z.; Fang, Y.; Liao, P.; Deng, Y.; He, N. Performance evaluation of a novel sample in-answer out (SIAO) system based on magnetic nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, C.; Wang, F.; Ma, N.; Li, X.; Li, Z.; Deng, Y.; Wang, Z.; Xi, Z.; Tang, Y.; et al. Magnetic nanoparticles-based extraction and verification of nucleic acids from different sources. J. Biomed. Nanotechnol. 2013, 9, 703–709. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tong, Z.; Tan, B.; He, X.; Zhang, T.; Guo, Y.; Jin, L.; He, N.; Li, S.; Chen, Z. Rapid detection of dNA methylation with a novel real-Time fluorescence recombinase-aided amplification assay. J. Biomed. Nanotechnol. 2021, 17, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, T.; Yang, H.; Li, T.; Nie, L.; Mou, X.; Deng, Y.; He, N.; Li, Z.; Wang, L.; et al. A portable multi-channel turbidity system for rapid detection of pathogens by loop-mediated isothermal amplification. J. Biomed. Nanotechnol. 2018, 14, 198–205. [Google Scholar] [CrossRef]
- Peng, L.H.; Zhou, L.Q.; Chen, X.; Piao, X. A computational study of potential miRNA-disease association inference based on ensemble learning and kernel ridge regression. Front. Bioeng. Biotechnol. 2020, 8, 40. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Li, Z.J.; Shi, Y.; Deng, J.; Bai, J.; Ma, L.; Zeng, X.X.; Feng, S.S.; Ren, J.L.; et al. Unravelling the role of lncRNA WT1-AS/miR-206/NAMPT axis as prognostic biomarkers in lung adenocarcinoma. Biomolecules 2021, 11, 203. [Google Scholar] [CrossRef]
- Li, W.; Jia, M.X.; Deng, J.; Wang, J.H.; Lin, Q.L.; Tang, J.X.; Zeng, X.X.; Cai, F.; Ma, L.; Su, W.; et al. Down-regulation of microRNA-200b is a potential prognostic marker of lung cancer in southern-central Chinese population. Saudi J. Biol. Sci. 2019, 26, 173–177. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ action through miRNA editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [Green Version]
- Michlewski, G.; Caceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2019, 25, 1–16. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godlewski, J.; Lenart, J.; Salinska, E. MicroRNA in brain pathology: Neurodegeneration the other side of the brain cancer. Noncoding RNA 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freilich, R.W.; Woodbury, M.E.; Ikezu, T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE 2013, 8, e79416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Hou, K.; Ji, T.; Wang, X.; Liu, Y.; Zheng, Y.; Xu, J.; Hou, Y.; Chi, G. The role of exosomal microRNAs in central nervous system diseases. Mol. Cell. Biochem. 2021, 476, 2111–2124. [Google Scholar] [CrossRef]
- Mancuso, R.; Agostini, S.; Hernis, A.; Zanzottera, M.; Bianchi, A.; Clerici, M. Circulatory miR-223-3p discriminates between Parkinson’s and Alzheimer’s patients. Sci. Rep. 2019, 9, 9393. [Google Scholar] [CrossRef] [Green Version]
- Hoss, A.G.; Lagomarsino, V.N.; Frank, S.; Hadzi, T.C.; Myers, R.H.; Latourelle, J.C. Study of plasma-derived miRNAs mimic differences in Huntington’s disease brain. Mov. Disord. 2015, 30, 1961–1964. [Google Scholar] [CrossRef] [Green Version]
- Liguori, M.; Nuzziello, N.; Introna, A.; Consiglio, A.; Licciulli, F.; D’Errico, E.; Scarafino, A.; Distaso, E.; Simone, I.L. Dysregulation of microRNAs and target genes networks in peripheral blood of patients with sporadic Amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2018, 11, 288. [Google Scholar] [CrossRef]
- Khezri, M.R.; Yousefi, K.; Zolbanin, N.M.; Ghasemnejad-Berenji, M. MicroRNAs in the pathophysiology of Alzheimer’s disease and Parkinson’s disease: An overview. Mol. Neurobiol. 2022, 59, 1589–1603. [Google Scholar] [CrossRef]
- Gascon, E.; Gao, F.B. Cause or Effect: Misregulation of microRNA pathways in neurodegeneration. Front. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Herranz, H.; Cohen, S.M. MicroRNAs and gene regulatory networks: Managing the impact of noise in biological systems. Genes Dev. 2010, 24, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhao, Q.; Yin, Y. MiR-133b is a potential diagnostic biomarker for Alzheimer’s disease and has a neuroprotective role. Exp. Ther. Med. 2019, 18, 2711–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Yang, W.; Wang, T.; Mao, C.; Guo, L.; Xiao, J.; He, N. Coaxially electrospun core/shell structured poly(L-lactide) acid/chitosan nanofibers for potential drug carrier in tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Huang, R.; Deng, Y.; He, N. Progress in selection and biomedical applications of aptamers. J. Biomed. Nanotechnol. 2014, 10, 3043–3062. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, H.; He, N.; Deng, Y. Effects of surface modifications on the physicochemical properties of iron oxide nanoparticles and their performance as anticancer drug carriers. Chin. Chem. Lett. 2018, 29, 1829–1833. [Google Scholar] [CrossRef]
- Walgrave, H.; Zhou, L.; De Strooper, B.; Salta, E. The promise of microRNA-based therapies in Alzheimer’s disease: Challenges and perspectives. Mol. Neurodegener. 2021, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Viswambharan, V.; Thanseem, I.; Vasu, M.M.; Poovathinal, S.A.; Anitha, A. miRNAs as biomarkers of neurodegenerative disorders. Biomark. Med. 2017, 11, 151–167. [Google Scholar] [CrossRef]
- Hoye, M.L.; Regan, M.R.; Jensen, L.A.; Lake, A.M.; Reddy, L.V.; Vidensky, S.; Richard, J.P.; Maragakis, N.J.; Rothstein, J.D.; Dougherty, J.D.; et al. Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain. 2018, 141, 2561–2575. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Zuccato, C.; Belyaev, N.D.; Guest, D.J.; Cattaneo, E.; Buckley, N.J. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol. Dis. 2008, 29, 438–445. [Google Scholar] [CrossRef]
- Waller, R.; Goodall, E.F.; Milo, M.; Cooper-Knock, J.; Da Costa, M.; Hobson, E.; Kazoka, M.; Wollff, H.; Heath, P.R.; Shaw, P.J.; et al. Serum miRNAs miR-206, 143–3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS). Neurobiol. Aging 2017, 55, 123–131. [Google Scholar] [CrossRef]
- Madadi, S.; Saidijam, M.; Yavari, B.; Soleimani, M. Downregulation of serum miR-106b: A potential biomarker for Alzheimer disease. Arch. Physiol. Biochem. 2022, 128, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Yang, Z.; Yang, F.; Wang, X.; Tan, J.; Liao, B. Long noncoding RNA NEAT1 aggravates Aβ-induced neuronal damage by targeting miR-107 in Alzheimer’s disease. Yonsei Med. J. 2019, 60, 640–650. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, Z.; Wang, J.; Feng, H. Expression relationship and significance of NEAT1 and miR-27a-3p in serum and cerebrospinal fluid of patients with Alzheimer’s disease. BMC Neurol. 2022, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Yu, M. MicroRNA-4722-5p and microRNA-615-3p serve as potential biomarkers for Alzheimer’s disease. Exp. Ther. Med. 2022, 23, 241. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, H.; Dong, W.; Quan, X.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Qin, C. miR-29c regulates BACE1 protein expression. Brain Res. 2011, 1395, 108–115. [Google Scholar] [CrossRef]
- Zhu, H.C.; Wang, L.M.; Wang, M.; Song, B.; Tan, S.; Teng, J.F.; Duan, D.X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-beta production by targeting BACE1. Brain Res. Bull. 2012, 88, 596–601. [Google Scholar] [CrossRef]
- Wang, D.; Fei, Z.; Luo, S.; Wang, H. MiR-335-5p inhibits beta-amyloid (abeta) accumulation to attenuate cognitive deficits through targeting c-jun-N-terminal kinase 3 in Alzheimer’s disease. Curr. Neurovasc. Res. 2020, 17, 93–101. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef]
- Zhong, Z.; Yuan, K.; Tong, X.; Hu, J.; Song, Z.; Zhang, G.; Fang, X.; Zhang, W. MiR-16 attenuates beta-amyloid-induced neurotoxicity through targeting beta-site amyloid precursor protein-cleaving enzyme 1 in an Alzheimer’s disease cell model. Neuroreport 2018, 29, 1365–1372. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Deng, W.; Liu, Y.; Qin, C. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012, 1455, 103–113. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Xie, F.; Wang, X.; Hou, Y.; Wang, X.; Liu, J. Overexpression of miR-26a-5p Suppresses tau phosphorylation and abeta accumulation in the Alzheimer’s disease mice by targeting DYRK1A. Curr. Neurovasc. Res. 2020, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Luo, Y.; Pi, D.; Xia, L.; Li, Z.; Tu, Q. MiR-340 reduces the accumulation of amyloid-beta through targeting BACE1 (beta-site amyloid precursor protein cleaving enzyme 1) in Alzheimer’s disease. Curr. Neurovasc. Res. 2020, 17, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Reddy, P.H. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 3808–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Liu, P.; Bai, H.; Li, X.; Xiao, J.; Yuan, Q.; Geng, S.; Yin, H.; Zhang, H.; et al. MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. Eur. J. Pharmacol. 2019, 843, 134–144. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, Q.; Jiang, H.; Zeng, L.; Li, Z.; Liu, R. MicroRNA-200a-3p mediates neuroprotection in Alzheimer-related deficits and attenuates amyloid-beta overproduction and tau hyperphosphorylation via coregulating BACE1 and PRKACB. Front. Pharmacol. 2019, 10, 806. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Wu, Y.; Li, L.; Liu, C. MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer’s disease by targeting heat shock protein B8. J. Neural Transm. 2020, 127, 339–346. [Google Scholar] [CrossRef]
- Mai, H.; Fan, W.; Wang, Y.; Cai, Y.; Li, X.; Chen, F.; Chen, X.; Yang, J.; Tang, P.; Chen, H.; et al. Intranasal administration of miR-146a agomir rescued the pathological process and cognitive impairment in an AD mouse model. Mol. Ther. Nucleic Acids 2019, 18, 681–695. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Li, Z.H.; Li, X.; Zheng, T.; Zhang, D.K. MicroRNA-592 blockade inhibits oxidative stress injury in Alzheimer’s disease astrocytes via the KIAA0319-mediated Keap1/Nrf2/ARE signaling pathway. Exp. Neurol. 2020, 324, 113128. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, L.; Zheng, J.; Wang, K.; Deng, H.; Liu, P.; Chen, L.; Mu, H. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci. Lett. 2017, 655, 21–27. [Google Scholar] [CrossRef]
- Duan, Q.; Si, E. MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer’s disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J. Cell. Biochem. 2019, 120, 15891–15905. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Y.; Wang, B.; Huang, J.; Li, Q. MiR-34a-5p and miR-125b-5p attenuate abeta-induced neurotoxicity through targeting BACE1. J. Neurol. Sci. 2020, 413, 116793. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tu, Q.; Liu, M. MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol. Med. Rep. 2018, 18, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, K.Y.; Park, Y.; McLean, C.; Park, J.; Lee, J.H.; Lee, K.H.; Kim, B.C.; Huh, Y.H.; Lee, K.H.; et al. Enhanced Expression of microRNA-1273g-3p contributes to Alzheimer’s disease pathogenesis by regulating the expression of mitochondrial Genes. Cells 2021, 10, 2697. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Su, L. MiR-539-5p decreases amyloid beta-protein production, hyperphosphorylation of tau and memory impairment by regulating PI3K/Akt/GSK-3beta pathways in APP/PS1 double transgenic mice. Neurotox. Res. 2020, 38, 524–535. [Google Scholar] [CrossRef]
- Han, C.; Guo, L.; Yang, Y.; Guan, Q.; Shen, H.; Sheng, Y.; Jiao, Q. Mechanism of microRNA-22 in regulating neuroinflammation in Alzheimer’s disease. Brain Behav. 2020, 10, e01627. [Google Scholar] [CrossRef] [Green Version]
- Doxakis, E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010, 285, 12726–12734. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Liu, G.; Jin, S.M.; Parisiadou, L.; Xie, C.; Yu, J.; Sun, L.; Ma, B.; Ding, J.; Vancraenenbroeck, R.; et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013, 22, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Xi, D.Z.; Wang, Y.H. MicroRNA-599 regulates the development of Parkinson’s disease through mediating LRRK2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 724–731. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef]
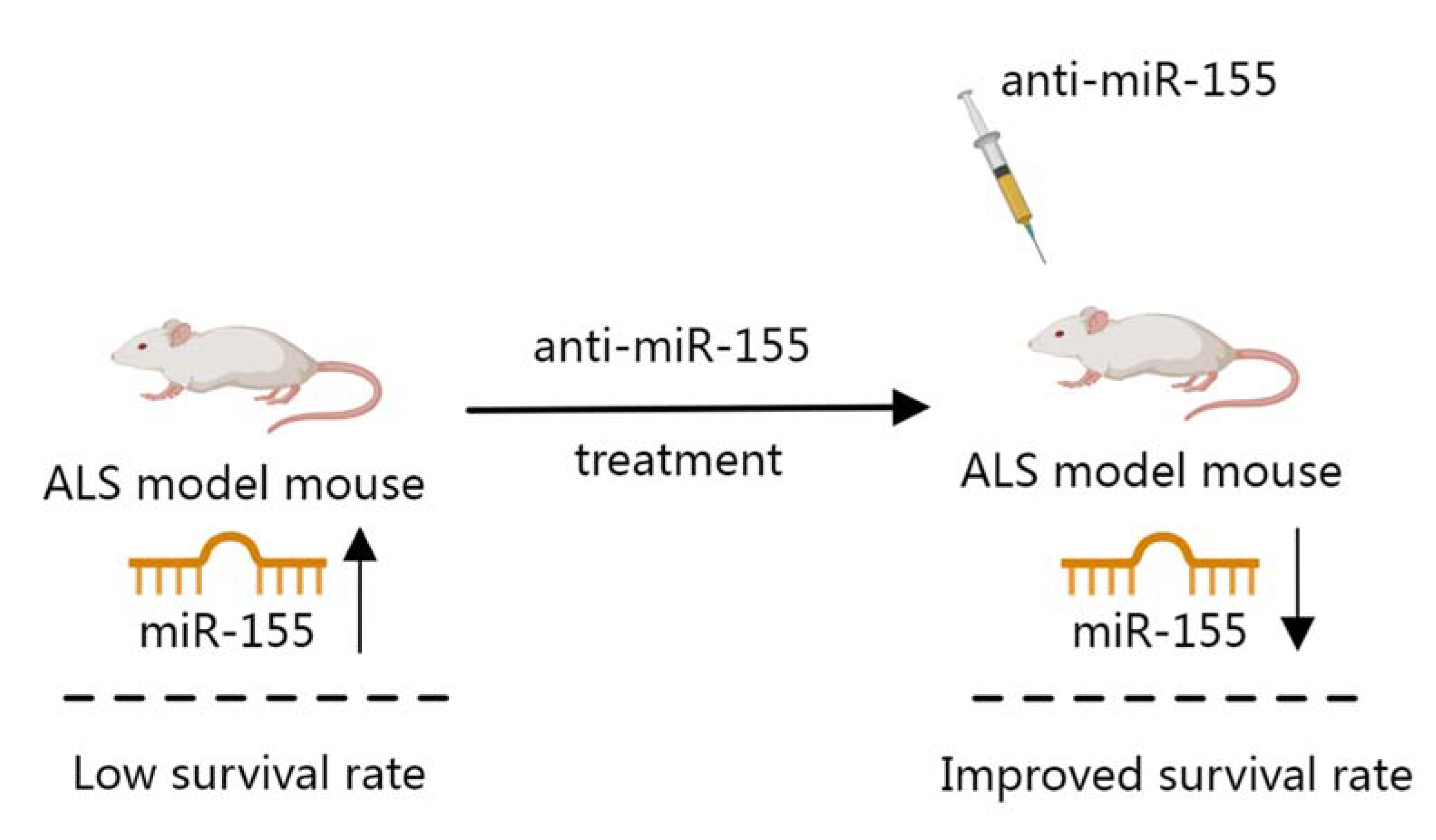
- Koval, E.D.; Shaner, C.; Zhang, P.; du Maine, X.; Fischer, K.; Tay, J.; Chau, B.N.; Wu, G.F.; Miller, T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013, 22, 4127–4135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, Z.C.E.; Campos-Melo, D.; Strong, M.J. MiR-105 and miR-9 regulate the mRNA stability of neuronal intermediate filaments. Implications for the pathogenesis of Amyotrophic lateral sclerosis (ALS). Brain Res. 2019, 1706, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.R.; Latourelle, J.C.; Bockholt, J.H.; Bregu, J.; Smock, J.; Paulsen, J.S.; Myers, R.H.; PREDICT-HD CSF Ancillary Study Investigators. MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology 2018, 90, e264–e272. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.H.; Li, C.L.; Lin, H.L.; Tsai, S.J.; Lai, Y.Y.; Chang, Y.F.; Cheng, P.H.; Chen, C.M.; Yang, S.H. The potential regulatory mechanisms of miR-196a in Huntington’s disease through bioinformatic analyses. PLoS ONE 2015, 10, e0137637. [Google Scholar] [CrossRef] [Green Version]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci. 2008, 28, 14341–14346. [Google Scholar] [CrossRef] [Green Version]
- Kocerha, J.; Xu, Y.; Prucha, M.S.; Zhao, D.; Chan, A.W. MicroRNA-128a dysregulation in transgenic Huntington’s disease monkeys. Mol. Brain. 2014, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, R.H.; Petersen, M.H.; Willert, C.W.; Heinrich, M.; Nymann, N.; Dall, M.; Treebak, J.T.; Bjorkqvist, M.; Silahtaroglu, A.; Hasholt, L.; et al. Perturbations in the p53/miR-34a/SIRT1 pathway in the R6/2 Huntington’s disease model. Mol. Cell. Neurosci. 2018, 88, 118–129. [Google Scholar] [CrossRef]
- Fukuoka, M.; Takahashi, M.; Fujita, H.; Chiyo, T.; Popiel, H.A.; Watanabe, S.; Furuya, H.; Murata, M.; Wada, K.; Okada, T.; et al. Supplemental treatment for Huntington’s disease with miR-132 that is deficient in Huntington’s disease Brain. Mol. Ther. Nucleic Acids 2018, 11, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Buckley, N.J. Gene dysregulation in Huntington’s disease: REST, microRNAs and beyond. Neuromol. Med. 2009, 11, 183–199. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Chen, Z.; Jin, L.; Wu, Z.; Yan, J.; Zhao, X.; Cai, L.; Deng, Y.; Guo, Y.; et al. The point-of-care-testing of nucleic acids by chip, cartridge and paper sensors. Chin. Chem. Lett. 2021, 32, 3675–3686. [Google Scholar] [CrossRef]
- Xiao, C.; Guo, Y.; Zhao, K.; Liu, S.; He, N.; He, Y.; Guo, S.; Chen, Z. Prognostic value of machine learning in patients with acute myocardial infarction. J. Cardiovasc. Dev. Dis. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, X.; Zhang, T.; Zhao, K.; Xiao, C.; Tong, Z.; Jin, L.; He, N.; Deng, Y.; Li, S.; et al. Highly sensitive smartphone-based detection of listeria monocytogenes using SYTO9. Chin. Chem. Lett. 2022, 33, 1933–1935. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, K.; He, Z.; Luo, X.; Qin, Z.; Tan, Y.; Zheng, X.; Wu, Z.; Deng, Y.; Chen, H.; et al. Development and evaluation of a thermostatic nucleic acid testing device based on magnesium pyrophosphate precipitation for detecting enterocytozoon hepatopenaei. Chin. Chem. Lett. 2022, 33, 4053–4056. [Google Scholar] [CrossRef]
- Prasad, K.N. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer’s disease. Mech. Ageing Dev. 2016, 153, 41–47. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, Z. Alzheimer’s disease and microRNA-132: A widespread pathological factor and potential therapeutic target. Front. Neurosci. 2021, 15, 687973. [Google Scholar] [CrossRef]
- Amakiri, N.; Kubosumi, A.; Tran, J.; Reddy, P.H. Amyloid beta and microRNAs in Alzheimer’s disease. Front. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef] [Green Version]
- Slota, J.A.; Booth, S.A. MicroRNAs in neuroinflammation: Implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA 2019, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, S.; Schäfer, M.; Behl, C. Estrogen-induced cell signalling in a cellular model of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2003, 84, 301–305. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zheng, C.Y.; Yan, H.; Wang, Z.F.; Tang, L.L.; Gao, X.; Tang, X.C. Potential therapeutic targets of huperzine a for Alzheimer’s disease and vascular dementia. Chem. Biol. Interact. 2008, 175, 396–402. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Ramírez, C.M.; Lee, S.-M.; Hoe, H.-S.; Fernández-Hernando, C.; Kim, J. miR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp. Neurol. 2012, 235, 476–483. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Wang, G.Q.; Wang, N.N.; Yu, Q.Y.; Liu, R.L.; Shi, W.Q. The long-non-coding RNA NEAT1 is a novel target for Alzheimer’s disease progression via miR-124/BACE1 axis. Neurol. Res. 2019, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Z.; Zhao, L.; Zhao, W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer’s disease via modulating the expression of miR-106b. Biomed. Pharmacother. 2017, 92, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, P.T.; Wang, W.X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J. Alzheimers Dis. 2010, 21, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, G.; Zhang, N.; Li, F.; Shi, L.; Li, H. Isovitexin modulates autophagy in Alzheimer’s disease via miR-107 signalling. Transl. Neurosci. 2020, 11, 391–401. [Google Scholar] [CrossRef]
- Liu, W.; Liu, C.; Zhu, J.; Shu, P.; Yin, B.; Gong, Y.; Qiang, B.; Yuan, J.; Peng, X. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol. Aging 2012, 33, 522–534. [Google Scholar] [CrossRef]
- Vilardo, E.; Barbato, C.; Ciotti, M.; Cogoni, C.; Ruberti, F. MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J. Biol. Chem. 2010, 285, 18344–18351. [Google Scholar] [CrossRef] [Green Version]
- Miya Shaik, M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Lebouvier, T.; Scales, T.M.; Williamson, R.; Noble, W.; Duyckaerts, C.; Hanger, D.P.; Reynolds, C.H.; Anderton, B.H.; Derkinderen, P. The microtubule-associated protein tau is also phosphorylated on tyrosine. J. Alzheimers Dis. 2009, 18, 1–9. [Google Scholar] [CrossRef]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta 2005, 1739, 216–223. [Google Scholar] [CrossRef]
- Wong, H.K.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. MiR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.-Y.; Hyman, B.T.; Bacskai, B.J. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between microRNAs and oxidative stress in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Xanthos, D.N.; Sandkuhler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef]
- Lu, C.E.; Liu, Y.; Sun, B.; Sun, Y.E.; Hou, B.; Zhang, Y.; Ma, Z.; Gu, X. Intrathecal injection of JWH-015 attenuates bone cancer pain via time-dependent modification of pro-inflammatory cytokines expression and astrocytes activity in spinal cord. Inflammation 2015, 38, 1880–1890. [Google Scholar] [CrossRef]
- Craft, J.M.; Watterson, D.M.; Van Eldik, L.J. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia 2006, 53, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Pizza, V.; Agresta, A.; D’Acunto, C.W.; Festa, M.; Capasso, A. Neuroinflamm-aging and neurodegenerative diseases: An overview. CNS Neurol. Disord. Drug Targets 2011, 10, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Varnum, M.M.; Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch. Immunol. Ther. Exp. 2012, 60, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-vivo measurement of activated microglia in dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Fillit, H.; Ding, W.H.; Buee, L.; Kalman, J.; Altstiel, L.; Lawlor, B.; Wolf-Klein, G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991, 129, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef]
- Tarassishin, L.; Loudig, O.; Bauman, A.; Shafit-Zagardo, B.; Suh, H.S.; Lee, S.C. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia 2011, 59, 1911–1922. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Lesage, S.; Brice, A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009, 18, R48–R59. [Google Scholar] [CrossRef]
- Leitão, A.D.G.; Rudolffi-Soto, P.; Chappard, A.; Bhumkar, A.; Lau, D.; Hunter, D.J.B.; Gambin, Y.; Sierecki, E. Selectivity of Lewy body protein interactions along the aggregation pathway of α-synuclein. Commun. Biol. 2021, 4, 1124. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, T.; Zhang, B. Exosomes in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.E.; Wang, Y.; Kong, B.; Woo, T.U.; Iyer, L.K.; Sonntag, K.C. Midbrain dopamine neurons in Parkinson’s disease exhibit a dysregulated miRNA and target-gene network. Brain Res. 2015, 1618, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burré, J. The synaptic function of α-synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Pu, J. Alpha-synuclein in Parkinson’s Disease: From pathogenetic dysfunction to potential clinical application. Park. Dis. 2016, 2016, 1720621. [Google Scholar] [CrossRef] [Green Version]
- Lashuel, H.A.; Petre, B.M.; Wall, J.; Simon, M.; Nowak, R.J.; Walz, T.; Lansbury, P.T., Jr. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 2002, 322, 1089–1102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Zhang, J.L.; Duan, Y.L.; Zhang, Q.S.; Li, G.F.; Zheng, D.L. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed. Pharmacother. 2015, 74, 252–256. [Google Scholar] [CrossRef]
- Sahay, S.; Ghosh, D.; Singh, P.K.; Maji, S.K. Alteration of structure and aggregation of α-synuclein by familial Parkinson’s disease associated mutations. Curr. Protein Pept. Sci. 2017, 18, 656–676. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yang, H.; Guo, J.; Li, N. Circulating microRNAs and long non-coding RNAs as potential diagnostic biomarkers for Parkinson’s disease. Front. Mol. Neurosci. 2021, 14, 631553. [Google Scholar] [CrossRef]
- Nies, Y.H.; Mohamad Najib, N.H.; Lim, W.L.; Kamaruzzaman, M.A.; Yahaya, M.F.; Teoh, S.L. MicroRNA dysregulation in Parkinson’s disease: A Narrative Review. Front. Neurosci. 2021, 15, 660379. [Google Scholar] [CrossRef]
- Evans, B.; Furlong, H.A.T.; de Lencastre, A. Parkinson’s disease and microRNAs—Lessons from model organisms and human studies. Exp. Gerontol. 2021, 155, 111585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Kuo, H.C. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, K.J.; Murray, T.K.; Bengoa-Vergniory, N.; Cordero-Llana, O.; Cooper, J.; Buckley, A.; Wade-Martins, R.; Uney, J.B.; O’Neill, M.J.; Wong, L.F.; et al. Loss of microRNA-7 regulation leads to α-synuclein accumulation and dopaminergic neuronal loss in vivo. Mol. Ther. 2017, 25, 2404–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consales, C.; Cirotti, C.; Filomeni, G.; Panatta, M.; Butera, A.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Benassi, B. Fifty-hertz magnetic field affects the epigenetic modulation of the miR-34b/c in neuronal cells. Mol. Neurobiol. 2018, 55, 5698–5714. [Google Scholar] [CrossRef]
- Tarale, P.; Daiwile, A.P.; Sivanesan, S.; Stöger, R.; Bafana, A.; Naoghare, P.K.; Parmar, D.; Chakrabarti, T.; Krishnamurthi, K. Manganese exposure: Linking down-regulation of miRNA-7 and miRNA-433 with α-synuclein overexpression and risk of idiopathic Parkinson’s disease. Toxicol. In Vitro 2018, 46, 94–101. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2015, 589, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Steer, E.K.; Chu, C.T. ERKed by LRRK2: A cell biological perspective on hereditary and sporadic Parkinson’s disease. Biochim. Biophys. Acta. 2014, 1842, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Esteves, A.R.; Swerdlow, R.H.; Cardoso, S.M. LRRK2, a puzzling protein: Insights into Parkinson’s disease pathogenesis. Exp. Neurol. 2014, 261, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Gehrke, S.; Imai, Y.; Sokol, N.; Lu, B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature 2010, 466, 637–641. [Google Scholar] [CrossRef] [Green Version]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as biomarkers in Amyotrophic lateral sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef]
- Henriques, A.; Pitzer, C.; Schneider, A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: Where do we stand? Front. Neurosci. 2010, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucchia, M.; Ramirez, A.; Parente, V.; Simone, C.; Nizzardo, M.; Magri, F.; Dametti, S.; Corti, S. Therapeutic development in Amyotrophic lateral sclerosis. Clin. Ther. 2015, 37, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, F.; Marrero, A.; O’Connell, C.; Morin, P., Jr. MicroRNAs as potential circulating biomarkers for Amyotrophic lateral sclerosis. J. Mol. Neurosci. 2015, 56, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Aloizou, A.M.; Siokas, V.; Patrinos, G.P.; Deretzi, G.; Mitsias, P.; Aschner, M.; Tsatsakis, A. The role of microRNAs in patients with Amyotrophic lateral sclerosis. J. Mol. Neurosci. 2018, 66, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, A.; Leonardis, L.; Zidar, J.; Koritnik, B.; Dolenc-Groselj, L.; Ristic Kovacic, S.; Curk, T.; Rogelj, B. Differential expression of microRNAs and other small RNAs in muscle tissue of patients with ALS and healthy age-matched controls. Sci. Rep. 2018, 8, 5609. [Google Scholar] [CrossRef]
- Sumitha, R.; Sidhu, R.J.; Sathyaprabha, T.N.; Nalini, A.; Raju, T.R.; Alladi, P.A. Differential expression of microRNA-206 in the gastrocnemius and biceps brachii in response to CSF from sporadic amyotrophic lateral sclerosis patients. J. Neurol. Sci. 2014, 345, 254–256. [Google Scholar] [CrossRef]
- Rastegar-Moghaddam, S.H.; Ebrahimzadeh-Bideskan, A.; Shahba, S.; Malvandi, A.M.; Mohammadipour, A. MicroRNA-22: A novel and potent biological therapeutics in neurological disorders. Mol. Neurobiol. 2022, 59, 2694–2701. [Google Scholar] [CrossRef]
- Chu, E.M.; O’Neill, M.; Purkayastha, D.D.; Knight, C. Huntington’s disease: A forensic risk factor in women. J. Clin. Mov. Disord. 2019, 6, 3. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.Y.; Dong, Y.; Zhang, Y.B.; Cheng, H.R.; Gan, S.R.; Liu, Z.J.; Ni, W.; Burgunder, J.M.; Yang, X.W.; et al. Clinical and genetic profiles in chinese patients with Huntington’s Disease: A ten-year multicenter study in china. Aging Dis. 2019, 10, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, G.; Wu, D.; Lu, H.; Hou, Z.; Ross, C.A.; Yang, Y.; Zhang, J.; Duan, W. Resting-state functional MRI reveals altered brain connectivity and its correlation with motor dysfunction in a mouse model of Huntington’s disease. Sci Rep. 2017, 7, 16742. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.O.; Surmeier, D.J. Enhanced GABAergic inhibition of cholinergic interneurons in the zQ175(+/-) mouse model of Huntington’s Disease. Front Syst. Neurosci. 2020, 14, 626412. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, M.H.; Lee, S.J.; Lee, K.H.; Kim, M.J.; Kim, J.S.; Cho, J.W. Decreased metabolism in the cerebral cortex in early-stage Huntington’s disease: A possible biomarker of disease progression? J. Clin. Neurol. 2013, 9, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, J.M.; Rego, A.C. Mechanisms of neurodegeneration in Huntington’s disease. Eur. J. Neurosci. 2008, 27, 2803–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, A.L.; de Souza, L.C.; Rocha, N.P.; Furr-Stimming, E.; Lauterbach, E.C. Revisiting the neuropsychiatry of Huntington’s disease. Dement Neuropsychol. 2016, 10, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Solberg, O.K.; Filkukova, P.; Frich, J.C.; Feragen, K.J.B. Age at death and causes of death in patients with Huntington disease in norway in 1986-2015. J. Huntingtons. Dis. 2018, 7, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Kachian, Z.R.; Cohen-Zimerman, S.; Bega, D.; Gordon, B.; Grafman, J. Suicidal ideation and behavior in Huntington’s disease: Systematic review and recommendations. J. Affect. Disord. 2019, 250, 319–329. [Google Scholar] [CrossRef]
- Shah, R.; Lee, S.C.; Strasser, R.B.; Grossman, C. An Australian neuro-palliative perspective on Huntington’s disease: A case report. BMC Palliat Care 2021, 20, 53. [Google Scholar] [CrossRef]
- Carbo, M.; Brandi, V.; Pascarella, G.; Staid, D.S.; Colotti, G.; Polticelli, F.; Ilari, A.; Morea, V. Bioinformatics analysis of ras homologue enriched in the striatum, a potential target for Huntington’s disease therapy. Int. J. Mol. Med. 2019, 44, 2223–2233. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, J.; Yu, D.; Aiba, Y.; Lee, S.; Pendergraff, H.; Boubaker, J.; Artates, J.W.; Lagier-Tourenne, C.; Lima, W.F.; et al. Exploring the effect of sequence length and composition on allele-selective inhibition of human huntingtin expression by single-stranded silencing RNAs. Nucleic Acid. Ther. 2014, 24, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Hoss, A.G.; Kartha, V.K.; Dong, X.; Latourelle, J.C.; Dumitriu, A.; Hadzi, T.C.; Macdonald, M.E.; Gusella, J.F.; Akbarian, S.; Chen, J.F.; et al. MicroRNAs located in the Hox gene clusters are implicated in Huntington’s disease pathogenesis. PLoS Genet. 2014, 10, e1004188. [Google Scholar] [CrossRef]
- Martí, E.; Pantano, L.; Bañez-Coronel, M.; Llorens, F.; Miñones-Moyano, E.; Porta, S.; Sumoy, L.; Ferrer, I.; Estivill, X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010, 38, 7219–7235. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wu, Y.R.; Chen, C.M. Down-regulation of miR-9* in the peripheral leukocytes of Huntington’s disease patients. Orphanet J. Rare Dis. 2017, 12, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as peripheral biomarkers in aging and age-related diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar] [CrossRef] [PubMed]
- Soldati, C.; Bithell, A.; Johnston, C.; Wong, K.Y.; Stanton, L.W.; Buckley, N.J. Dysregulation of REST-regulated coding and non-coding RNAs in a cellular model of Huntington’s disease. J. Neurochem. 2013, 124, 418–430. [Google Scholar] [CrossRef]
- Candelise, N.; Baiardi, S.; Franceschini, A.; Rossi, M.; Parchi, P. Towards an improved early diagnosis of neurodegenerative diseases: The emerging role of in vitro conversion assays for protein amyloids. Acta Neuropathol. Commun. 2020, 8, 117. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Trubiani, O.; Bramanti, P.; Mazzon, E. Salivary biomarkers: Future approaches for early diagnosis of neurodegenerative diseases. Brain Sci. 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Tong, Y.; Li, J.; Wang, Y.; Gao, F.; Li, H.; Wang, C.; Du, L.; Jiang, Y. Ultrasensitive photoelectrochemical biosensor based on black/red phosphorus heterojunction@Bi2Te3 hybrid and enzymatic signal amplification for the detection of colorectal cancer-related piRNA-823. Sens. Actuators B Chem. 2022, 368, 132244. [Google Scholar] [CrossRef]
- Grasso, M.; Piscopo, P.; Crestini, A.; Confaloni, A.; Denti, M.A. Circulating microRNAs in neurodegenerative diseases. Exp. Suppl. 2015, 106, 151–169. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, S.; Kenny, A.; Medina, M.; Engel, T.; Jimenez-Mateos, E.M. MicroRNAs in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2017, 334, 309–343. [Google Scholar] [CrossRef]
- Karnati, H.K.; Panigrahi, M.K.; Gutti, R.K.; Greig, N.H.; Tamargo, I.A. MiRNAs: Key players in neurodegenerative disorders and epilepsy. J. Alzheimer’s Dis. 2015, 48, 563–580. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Ge, H.; Li, K. Aberrant expression of miR-148a-3p in Alzheimer’s disease and its protective role against amyloid-beta induced neurotoxicity. Neurosci. Lett. 2021, 756, 135953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, W.; Xu, Y.; Li, D.; Xue, Q. Serum miR-128 serves as a potential diagnostic biomarker for Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2021, 17, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Shao, P. MiR-216a-5p ameliorates learning-memory deficits and neuroinflammatory response of Alzheimer’s disease mice via regulation of HMGB1/NF-κB signaling. Brain Res. 2021, 1766, 147511. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hu, S.; Wu, Z.; Liu, J.; Li, S. The role of miR-132 in regulating neural stem cell proliferation, differentiation and neuronal maturation. Cell. Physiol. Biochem. 2018, 47, 2319–2330. [Google Scholar] [CrossRef]
- Jia, M.; Wang, X.; Zhang, H.; Ye, C.; Ma, H.; Yang, M.; Li, Y.; Cui, C. MicroRNA-132 in the adult dentate gyrus is involved in opioid addiction via modifying the differentiation of neural stem cells. Neurosci. Bull. 2019, 35, 486–496. [Google Scholar] [CrossRef]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The relation of the brain-derived neurotrophic factor with microRNAs in neurodegenerative diseases and ischemic stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef]
- Ding, H.; Huang, Z.; Chen, M.; Wang, C.; Chen, X.; Chen, J.; Zhang, J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Park. Relat. Disord. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Gong, X.; Huang, M.; Chen, L. Mechanism of miR-132-3p promoting neuroinflammation and dopaminergic neurodegeneration in Parkinson’s disease. eNeuro 2022, 9, 1. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, J.; Tang, P.; Tu, N.; Wang, K.; Wu, G. Overexpression of miR-185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol. Med. Rep. 2018, 17, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. MicroRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J. Neurosci. 2016, 36, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. Molecular biomarkers and their implications for the early diagnosis of selected neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 4610. [Google Scholar] [CrossRef] [PubMed]
- Magen, I.; Yacovzada, N.S.; Yanowski, E.; Coenen-Stass, A.; Grosskreutz, J.; Lu, C.H.; Greensmith, L.; Malaspina, A.; Fratta, P.; Hornstein, E. Circulating miR-181 is a prognostic biomarker for amyotrophic lateral sclerosis. Nat. Neurosci. 2021, 24, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Manfellotto, F.; Fiorentino, G.; Annunziata, A.; Biffali, E.; Pannone, R.; Federico, A. Wide-ranging analysis of microRNA profiles in sporadic Amyotrophic lateral sclerosis using next-generation sequencing. Front. Genet. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, Q.; Chen, X.; Li, C.; Cao, B.; Ou, R.; Hadano, S.; Shang, H.F. Aberration of miRNAs expression in leukocytes from sporadic Amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, K.; Mitchem, M.R.; Jimenez-Mateos, E.M.; Henshall, D.C.; Concannon, C.G.; Prehn, J.H. Increased expression of microRNA-29a in ALS mice: Functional analysis of its inhibition. J. Mol. Neurosci. 2014, 53, 231–241. [Google Scholar] [CrossRef]
- Gagliardi, D.; Comi, G.P.; Bresolin, N.; Corti, S. MicroRNAs as regulators of cell death mechanisms in Amyotrophic lateral sclerosis. J. Cell. Mol. Med. 2019, 23, 1647–1656. [Google Scholar] [CrossRef]
- Klatt, C.L.; Theis, V.; Hahn, S.; Theiss, C.; Matschke, V. Deregulated miR-29b-3p correlates with tissue-specific activation of intrinsic apoptosis in an animal model of Amyotrophic lateral sclerosis. Cells 2019, 8, 1077. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhang, C.; Guan, Y.; Chen, Y.; Lu, Q.; Jie, L.; Gao, H.; Du, H.; Zhang, H.; Liu, Y.; et al. Screening the expression characteristics of several miRNAs in G93A-SOD1 transgenic mouse: Altered expression of miRNA-124 is associated with astrocyte differentiation by targeting Sox2 and Sox9. J. Neurochem. 2018, 145, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Martinez, B.; Peplow, P.V. Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies. Neural Regen. Res. 2021, 16, 2159–2169. [Google Scholar] [CrossRef]
- Cheng, P.H.; Li, C.L.; Chang, Y.F.; Tsai, S.J.; Lai, Y.Y.; Chan, A.W.; Chen, C.M.; Yang, S.H. MiR-196a ameliorates phenotypes of Huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am. J. Hum. Genet. 2013, 93, 306–312. [Google Scholar] [CrossRef]
- Her, L.S.; Mao, S.H.; Chang, C.Y.; Cheng, P.H.; Chang, Y.F.; Yang, H.I.; Chen, C.M.; Yang, S.H. MiR-196a enhances neuronal morphology through suppressing RANBP10 to provide neuroprotection in Huntington’s Disease. Theranostics 2017, 7, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Hoss, A.G.; Labadorf, A.; Beach, T.G.; Latourelle, J.C.; Myers, R.H. microRNA Profiles in Parkinson’s Disease Prefrontal Cortex. Front Aging Neurosci. 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoss, A.G.; Labadorf, A.; Latourelle, J.C.; Kartha, V.K.; Hadzi, T.C.; Gusella, J.F.; MacDonald, M.E.; Chen, J.-F.; Akbarian, S.; Weng, Z.; et al. miR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genom. 2015, 8, 10. [Google Scholar] [CrossRef] [PubMed]
| Neurodegenerative Diseases | MiRNAs | Models | Expression | Pathway Regulation | References |
|---|---|---|---|---|---|
| AD | miR-106b | Sera of AD patients | Downregulated | Aβ deposition | [61] |
| miR-107 | SH-SY5Y and SK-N-SH cells treated with Aβ1-42 | [62] | |||
| miR-27a-3p | Sera and CSF of AD patients | [63] | |||
| miR-4722-5p, miR-615-3p | Blood of AD patients and PC12 cells treated with Aβ25-35 | [64] | |||
| miR-29c | miR-29c transgenic mice | [65] | |||
| miR-195 | SAMP8 mice and N2a/WT cells | [66] | |||
| miR-335-5p | APP/PS1 transgenic mice, APP/PS1 transgenic cells | [67] | |||
| miR-124 | PC12 cells and hippocampal neurons treated with Aβ1-42 | [68] | |||
| miR-16 | Brains of AD patients and PC12 cells treated with Aβ42 | [69] | |||
| miR-153 | APPswe/PSΔE9 transgenic mice | [70] | |||
| miR-26a-5p | APPswe/PS1 transgenic mice | [71] | |||
| miR-340 | SH-SY5Y/APPswe cells | [72] | |||
| miR-455-3p | Sera of AD patients | Upregulated | [73] | ||
| miR-128 | APP/PSA/Tau transgenic mice and N2a cells | [74] | |||
| miR-200a-3p | APP/PS1 mice, SAMP8 mice, SAMR1 mice, blood of AD patients | Downregulated | Tau protein phosphorylation | [75] | |
| miR-132 | Brains of AD patients | [76] | |||
| miR-425-5p | Postmortem brains of AD patients, HEK293/tau cells, N2a/APP cells | [77] | |||
| miR-146a | APP/PS1 transgenic mice | Upregulated | [78] | ||
| miR-592 | AD rats established by D-galactose and Aβ25-35 injection | Downregulated | Oxidative stress | [79] | |
| miR-144 | SH-SY5Y cells treated with Aβ1-42 | [80] | |||
| miR-25 | AD mice established by Aβ1-42 | [81] | |||
| miR-34a-5p, miR-125b-5p | Sera of AD patients and N2a cells treated with Aβ25-35 | [82] | |||
| miR-125b | APPswe/Δ9 transgenic cells | Upregulated | [83] | ||
| miR-1273g-3p | Plasma and CSF of AD patients | [84] | |||
| miR-539-5p | CSF of AD patients and APP/PS1 transgenic mice | [85] | |||
| miR-22 | Blood of AD patients and APP/PS1 transgenic mice | Upregulated | Neuroinflammation | [86] | |
| PD | miR-7, miR-153 | HEK293 cells transfected with miR-7, miR-153 | Downregulated | α-synuclein | [87] |
| miR-205 | Frontal cortex of PD patients | Upregulated | LRRK2 expression | [88] | |
| miR-599 | SH-SY5Y cells treated with MPP+ | / | [89] | ||
| ALS | miR-206, miR-155 | G93A-SOD1 transgenic mice | Upregulated | / | [90,91] |
| miR-9, miR-105 | Spinal cord of ALS patients | Downregulated | INA, NEFL, PRPH | [92] | |
| HD | miR-520f-3p, miR-135b-3p, miR-4317, miR-3928-5p, miR-8082, miR-140-5p | CSF of HD patients | Upregulated | / | [93] |
| miR-196a | HD transgenic mice | / | [94] | ||
| miR-124a, miR-132 | Brains of HD patients | Downregulated | / | [59] | |
| miR-9 | Cortices of HD patients | [95] | |||
| miR-128a | Brains of HD monkeys | [96] | |||
| miR-34a, miR-132 | R6/2 transgenic mice | [97,98] | |||
| miR-124, miR-132 | Brains of HD patients and cortex and hippocampus of R6/2 transgenic mice | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, Z.; Chen, H.; Deng, Y.; Li, S.; Jin, L. Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases. Biosensors 2022, 12, 1074. https://doi.org/10.3390/bios12121074
Zhang J, Chen Z, Chen H, Deng Y, Li S, Jin L. Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases. Biosensors. 2022; 12(12):1074. https://doi.org/10.3390/bios12121074
Chicago/Turabian StyleZhang, Juan, Zhu Chen, Hui Chen, Yan Deng, Song Li, and Lian Jin. 2022. "Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases" Biosensors 12, no. 12: 1074. https://doi.org/10.3390/bios12121074
APA StyleZhang, J., Chen, Z., Chen, H., Deng, Y., Li, S., & Jin, L. (2022). Recent Advances in the Roles of MicroRNA and MicroRNA-Based Diagnosis in Neurodegenerative Diseases. Biosensors, 12(12), 1074. https://doi.org/10.3390/bios12121074





