Naked-Eye Detection of Food-Borne Pathogens Using Multiplex Hyperbranched Rolling Circle Amplification and Magnetic Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sample
2.2. Ligation
2.3. HRCA Reaction
2.4. Naked-Eye Detection
2.5. Limit of Detection
3. Results and Discussion
3.1. Amplification Reaction
3.2. Magnetic Particles Selection
3.3. Magnetic Particles Amount
3.4. Limits of Detection
3.5. Food-Borne Pathogen Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riley, L.W. Extraintestinal Foodborne Pathogens. Annu. Rev. Food Sci. Technol. 2020, 11, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishaq, A.R.; Manzoor, M.; Hussain, A.; Altaf, J.; Rehman, S.U.; Javed, Z.; Afzal, I.; Noor, A.; Noor, F. Prospect of microbial food borne diseases in Pakistan: A review. Braz. J. Biol. 2021, 81, 940–953. [Google Scholar] [CrossRef]
- Nelluri, K.D.D.; Thota, N.S. Challenges in Emerging Food-Borne Diseases. In Book Food Safety and Preservation, 2nd ed.; Alexandru, M.G., Alina, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–268. [Google Scholar]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a Paper-Based Analytical Device for Colorimetric Detection of Select Foodborne Pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Aboubakr, H.; Goyal, S. Involvement of Egyptian Foods in Foodborne Viral Illnesses: The Burden on Public Health and Related Environmental Risk Factors: An Overview. Food. Environ. Virol. 2019, 11, 315–339. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Ding, H.; Jiang, C.; Geng, Y.; Sun, X.; Jing, J.; Gao, H.; Wang, Z.; Dong, C. Recombinase polymerase amplification with polymer flocculation sedimentation for rapid detection of Staphylococcus aureus in food samples. Int. J. Food Microbiol. 2020, 331, 108691. [Google Scholar] [CrossRef]
- Raj, V.; Vijayan, A.N.; Joseph, K. Cysteine capped gold nanoparticles for naked eye detection of E. coli bacteria in UTI patients. Sens. Bio-Sens. Res. 2015, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Lee, S.; Ahn, M.M.; Kang, I.S.; Park, K.-H.; Jeon, S. Colorimetric detection of pathogenic bacteria using platinum-coated magnetic nanoparticle clusters and magnetophoretic chromatography. Anal. Chim. Acta 2015, 883, 61–66. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, H.; Jeong, J.; Kwon, J.N.; Jung, Y.; Chung, B.H. Label-free and naked eye detection of PNA/DNA hybridization using enhancement of gold nanoparticles. Chem. Commun. 2010, 46, 3315–3317. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Y.; Zhou, X.; Yao, B.; Fang, Q. Naked-eye detection of nucleic acids through rolling circle amplification and magnetic particle mediated aggregation. Biosens. Bioelectron. 2013, 47, 515–519. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Sokullu, E.; Ng, A.; Braescuac, L.; Zourob, M. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst 2014, 139, 482–487. [Google Scholar] [CrossRef]
- Ohk, S.-H.; Bhunia, A.K. Multiplex fiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol. 2013, 33, 166–171. [Google Scholar] [CrossRef]
- Shi, X.; Yu, L.; Lin, C.; Li, K.; Chen, J.; Qin, H. Biotin exposure-based immunomagnetic separation coupled with sodium dodecyl sulfate, propidium monoazide, and multiplex real-time PCR for rapid detection of viable Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes in milk. J. Dairy Sci. 2021, 104, 6588–6597. [Google Scholar] [CrossRef]
- Maier, C.; Hofmann, K.; Huptas, C.; Scherer, S.; Wenning, M.; Luecking, G. Simultaneous quantification of the most common and proteolytic Pseudomonas species in raw milk by multiplex qPCR. Appl. Microbiol. Biotechnol. 2021, 105, 1693–1708. [Google Scholar] [CrossRef]
- Li, F.; Ye, Q.; Chen, M.; Shang, Y.; Zhang, J.; Ding, Y.; Xue, L.; Wu, S.; Wang, J.; Pang, R.; et al. Real-time PCR identification of Listeria monocytogenes serotype 4c using primers for novel target genes obtained by comparative genomic analysis. LWT—Food Sci. Technol. 2021, 138, 110774. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Y.; Du, Y.; Wang, Z.; Liu, Z.; Wang, H.; Zhao, G.; Ma, Y.; Zheng, M. Rapid Detection of Diarrheagenic Escherichia coli by a New Multiplex Real-Time Quantitative PCR Assay. Appl. Biochem. Microbiol. 2020, 56, 748–757. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Zhao, T.; He, X.; Ke, Y.; Liu, W.; Zou, D. Multiplex real-time SYBR Green I PCR assays for simultaneous detection of 15 common enteric pathogens in stool samples. Mol. Cell Probes 2020, 53, 101619. [Google Scholar] [CrossRef]
- Suo, B.; He, Y.; Tu, S.-I.; Shi, X. A Multiplex Real-Time Polymerase Chain Reaction for Simultaneous Detection of Salmonella spp., Escherichia coli O157, and Listeria monocytogenes in Meat Products. Foodborne Pathog. Dis. 2010, 7, 619–628. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Li, G.; Shen, B.; He, N.; Ma, C.; Elingarami, S.; Li, Z. Synthesis and Characterization of Fe3O4@SiO2 Core–Shell Magnetic Microspheres for Extraction of Genomic DNA from Human Whole Blood. J. Nanosci. Nanotechnol. 2011, 11, 10295–10301. [Google Scholar] [CrossRef] [PubMed]

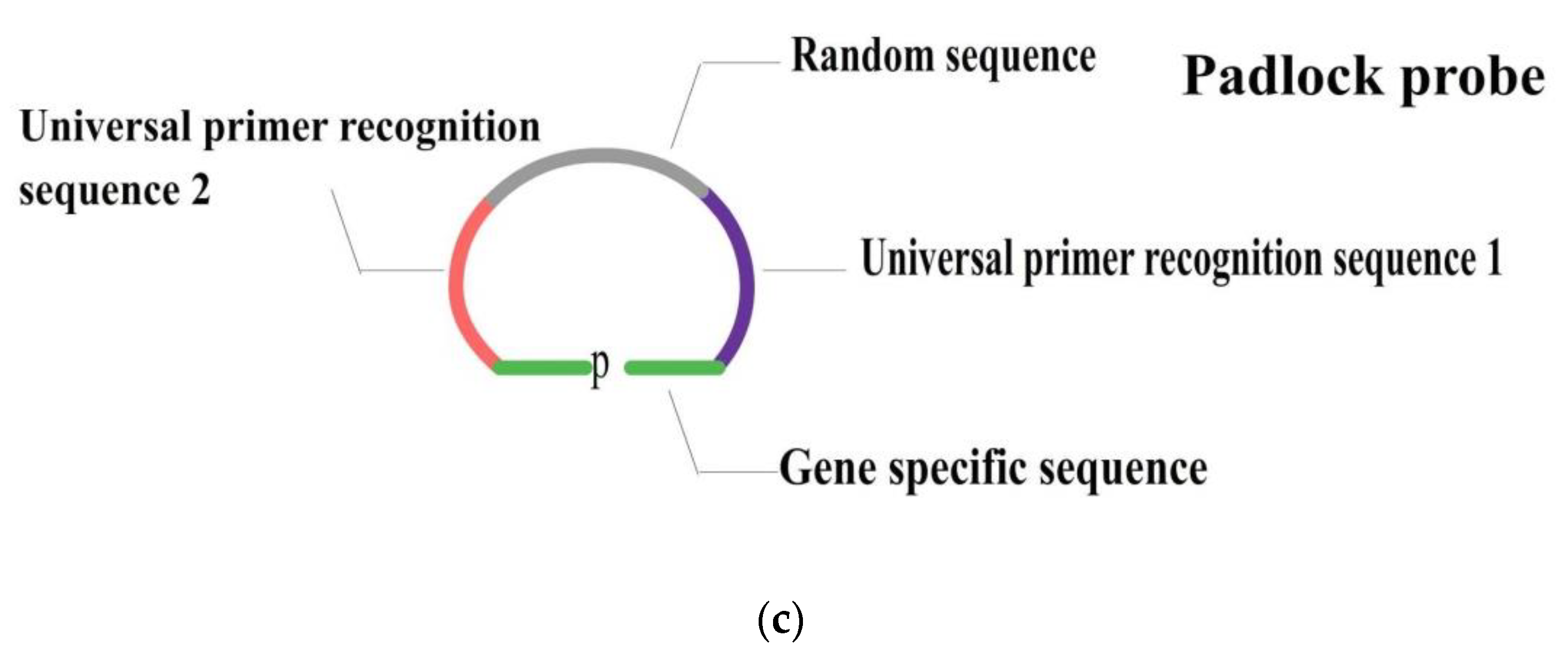
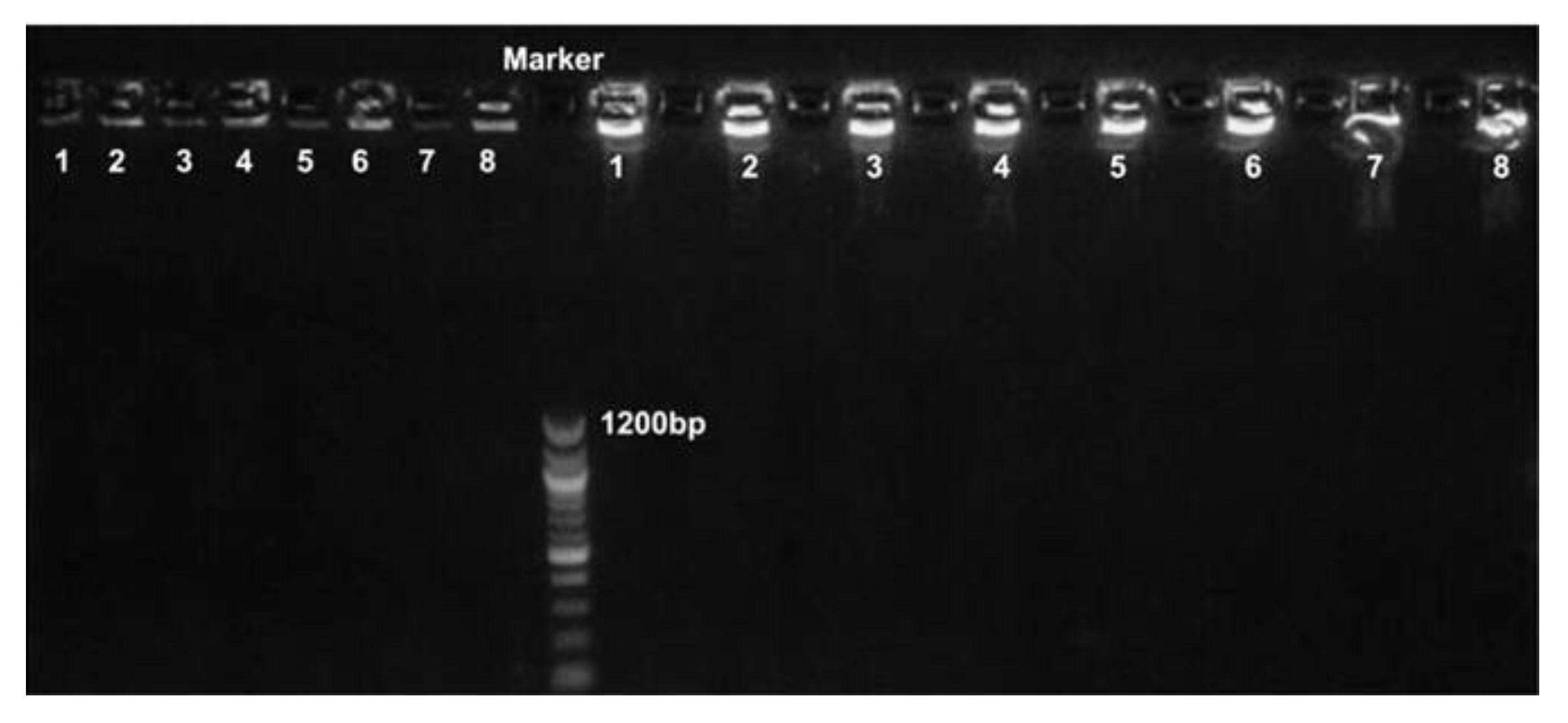




| Reference Strain | Strain | uidA | rfbe | stx1 | eae | ipaH | LT | ST |
|---|---|---|---|---|---|---|---|---|
| E. coli | CICC 10305 | + | - | - | - | - | - | - |
| EIEC | CICC 10661 | + | - | - | - | + | - | - |
| EPEC | CICC 10664 | + | - | - | + | - | - | - |
| ETEC | CICC 10667 | + | - | - | - | - | + | + |
| EHEC | CICC 21530 | + | + | + | + | - | - | - |
| Target Gene | Sequence Name | Sequence |
|---|---|---|
| uidA | Padlock probe | p-ATCACCATTCCCGGCGCGTCTCGTGGCTGAGAGCCTGGTAGTCGGAACTGGCAGGCAGGACGCACGCGTAAGCCGTTTTCATCGGTA |
| Primer 1 | CGTCTCGTGGCTGAGAG | |
| Primer 2 | TTACGCGTGCGTCCTGC | |
| Simulate target | CGCCGGGAATGGTGATTACCGATGAAAACGGC | |
| ST | Padlock probe | p-CATGCTTTCAGGACTACGCTCCATGATAGGCACGCCTGGTAGTCGGAACGTGGCAGGATTAGCTAGCCGATGTCCGCAGTAATTGCTACTATT |
| Primer 1 | GCTCCATGATAGGCACG | |
| Primer 2 | GGACATCGGCTAGCTAATC | |
| Simulate target | GTAGTCCTGAAAGCATGAATAGTAGCAATTACTGC | |
| LT | Padlock probe | p-GTTCCTCTCGCGTGATCTAACGGAGGCTAAGTTCCCTGGTAGTCGGAACGTGGCAGCGCAAGGCACCTCCTGCCAAAGCCGGTTTGT |
| Primer 1 | TAACGGAGGCTAAGTTC | |
| Primer 2 | AGGAGGTGCCTTGCG | |
| Simulate target | GATCACGCGAGAGGAACACAAACCGGCTTTGGC | |
| IpaH | Padlock probe | p-GGAAAGGCGGTCAAGGAACCACGTAAGGCCGTATCGACCTGGTAGTCGGAACGTGGCAGGTGTCAAGGCTTCACGCTGCTGGCAGAGACGGTATC |
| Primer 1 | CACGTAAGGCCGTATCGA | |
| Primer 2 | GCGTGAAGCCTTGACAC | |
| Simulate target | GTTCCTTGACCGCCTTTCCGATACCGTCTCTGCCAGCA | |
| eae | Padlock probe | p-CCGTTCCATAATGTTGTTGTGAAGCGTAGGCACCTGGTAGTCGGAACGTGGCAGAGTAAGGTCCTACCGGTCTGCAGATTAACCTCTG |
| Primer 1 | TTGTGAAGCGTAGGCA | |
| Primer 2 | CCGGTAGGACCTTACT | |
| Simulate target | CAACATTATGGAACGGCAGAGGTTAATCTGCAGA | |
| rfbE | Padlock probe | p-GTAATAGTTTTATTTCCAGAGCAGTCCTACCTTGCCCTGGTAGTCGGAACGTGGCAGAGGCAAGCGCGCATCTCCACCTTCACCTGTAG |
| Primer 1 | GAGCAGTCCTACCTTGC | |
| Primer 2 | AGATGCGCGCTTGCCT | |
| Simulate target | TGGAAATAAAACTATTACTACAGGTGAAGGTGG | |
| Stx1 | Padlock probe | p-CAGACAATGTAACCGCTGCCAAGCCTGATCGTCTGCCTGGTAGTCGGAACGTGGCAGGTGTTCGAGCACGCTCCGTGGTATAGCTACTGTCAC |
| Primer 1 | CCAAGCCTGATCGTCTG | |
| Primer 2 | GGAGCGTGCTCGAACAC | |
| Simulate target | CAGCGGTTACATTGTCTGGTGACAGTAGCTATACCAC | |
| Negative Control | Padlock probe | p-GCTGACCTCGGCATGGACGCATGCCTTCTAACCCTGGTAGTCGGAACGTGGCAGCGCTTAGTAGCTCCACAGGTAGCACTATGCC |
| Primer 1 | ACGCATGCCTTCTAAC | |
| Primer 2 | GTGGAGCTACTAAGCG | |
| Simulate target | CCATGCCGAGGTCAGCGGCATAGTGCTACCT |
| Strain | Gene | Detection Limit (CFU/mL) |
|---|---|---|
| EHEC | 1000 | |
| eae | 1000 | |
| uidA | 1000 | |
| rfbe | 10 | |
| stx1 | 100 | |
| ETEC | 10,000 | |
| LT | 10,000 | |
| ST | 10,000 | |
| uidA | 1000 | |
| EPEC | 1000 | |
| eae | 1000 | |
| uidA | 100 | |
| EIEC | 10,000 | |
| ipaH | 10,000 | |
| uidA | 100 | |
| E. coli | 100 | |
| uidA | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Liu, H.; Pan, W.; Wang, M.; Ren, J.; Chen, Z.; Chen, H.; Deng, Y.; Li, S. Naked-Eye Detection of Food-Borne Pathogens Using Multiplex Hyperbranched Rolling Circle Amplification and Magnetic Particles. Biosensors 2022, 12, 1075. https://doi.org/10.3390/bios12121075
Tang C, Liu H, Pan W, Wang M, Ren J, Chen Z, Chen H, Deng Y, Li S. Naked-Eye Detection of Food-Borne Pathogens Using Multiplex Hyperbranched Rolling Circle Amplification and Magnetic Particles. Biosensors. 2022; 12(12):1075. https://doi.org/10.3390/bios12121075
Chicago/Turabian StyleTang, Congli, Hongna Liu, Wenjing Pan, Meiling Wang, Jie Ren, Zhu Chen, Hui Chen, Yan Deng, and Song Li. 2022. "Naked-Eye Detection of Food-Borne Pathogens Using Multiplex Hyperbranched Rolling Circle Amplification and Magnetic Particles" Biosensors 12, no. 12: 1075. https://doi.org/10.3390/bios12121075
APA StyleTang, C., Liu, H., Pan, W., Wang, M., Ren, J., Chen, Z., Chen, H., Deng, Y., & Li, S. (2022). Naked-Eye Detection of Food-Borne Pathogens Using Multiplex Hyperbranched Rolling Circle Amplification and Magnetic Particles. Biosensors, 12(12), 1075. https://doi.org/10.3390/bios12121075





