Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EuPSM
2.3. Antibody Modification on EuPSM
2.4. Preparation of EuPSM-LFIA
2.5. Optimization of the Running Buffer and Antibody Concentration on T-Line and C-Line
2.6. Detection of CRP Samples
2.7. Detection of Clinical Samples
3. Results and Discussion
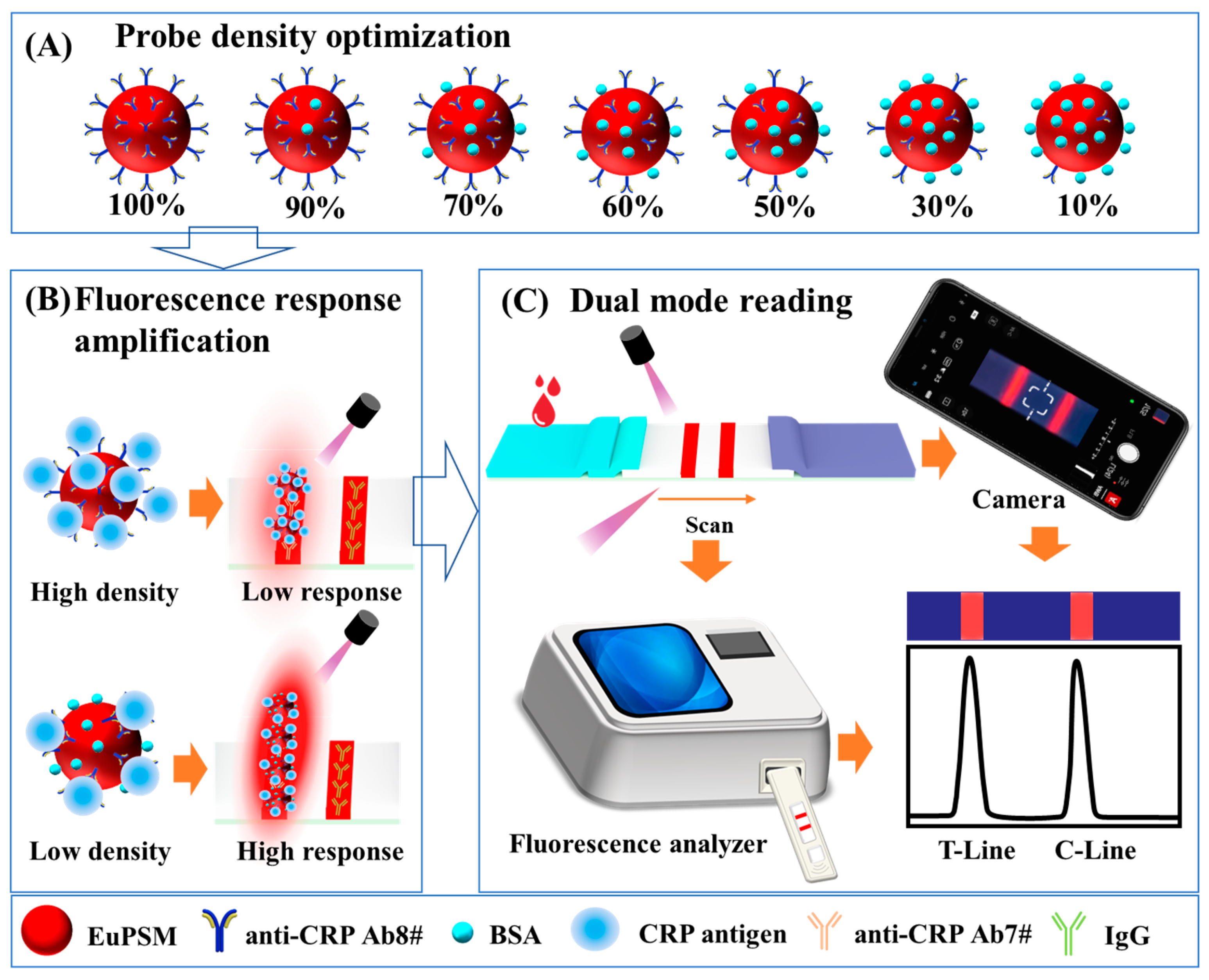
3.1. Optimization of Modified Antibodies on EuPSM for High-Sensitivity LFIA
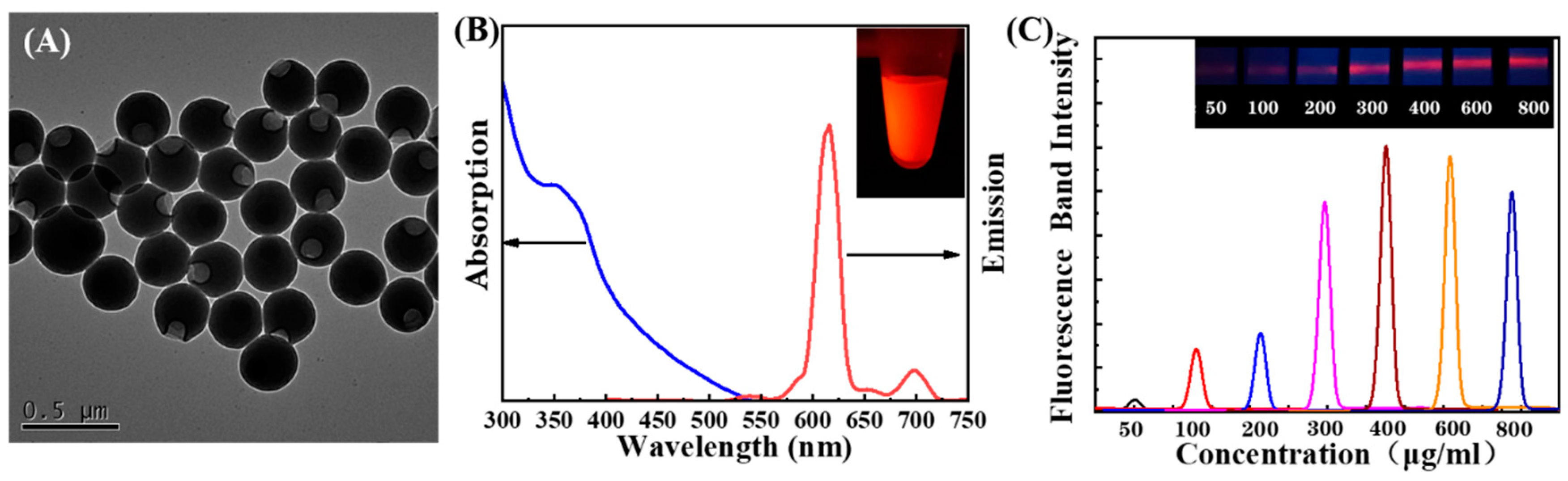
3.2. Characterization of EuPSM
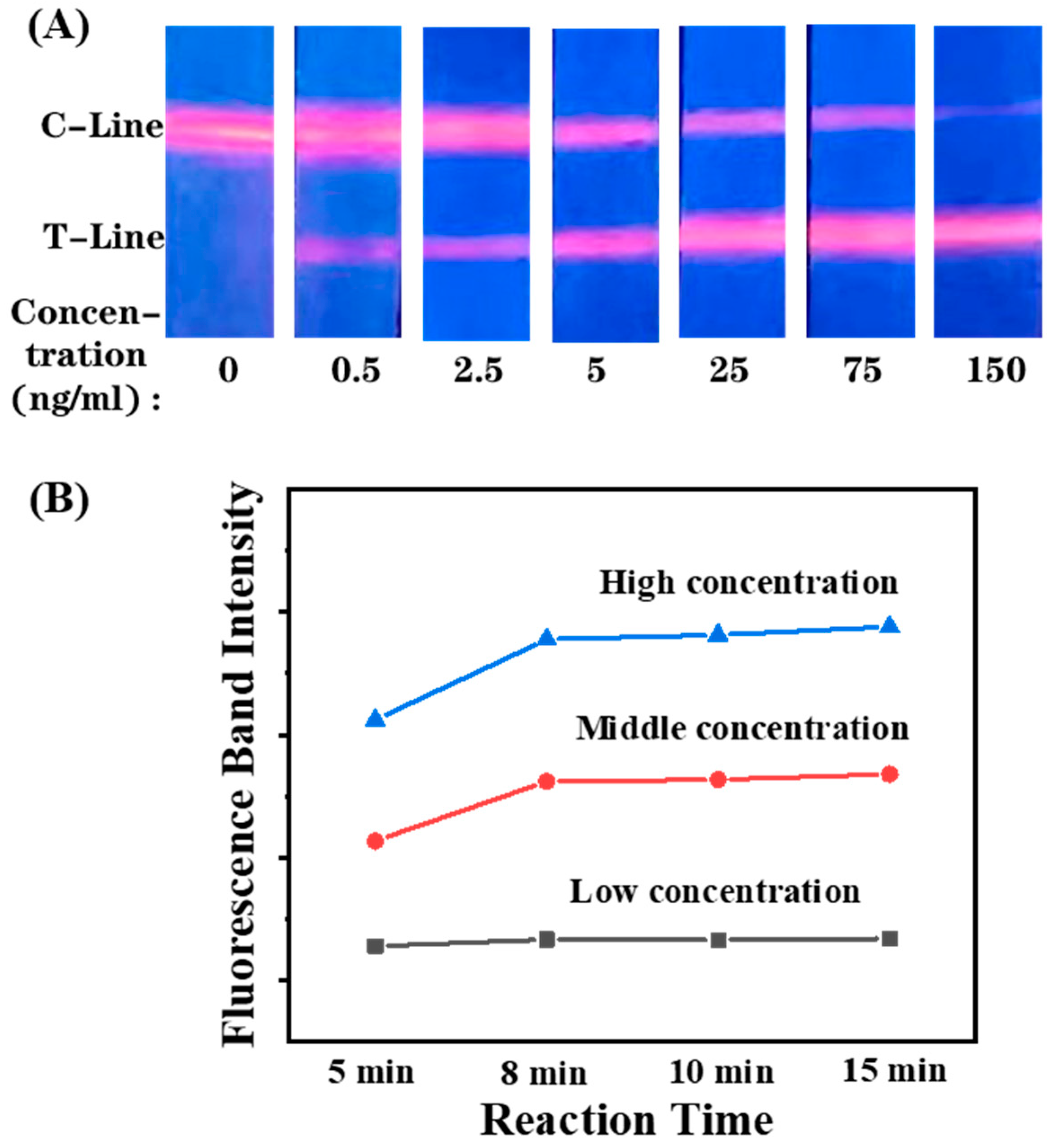
3.3. Feasibility Detection of the LFIA
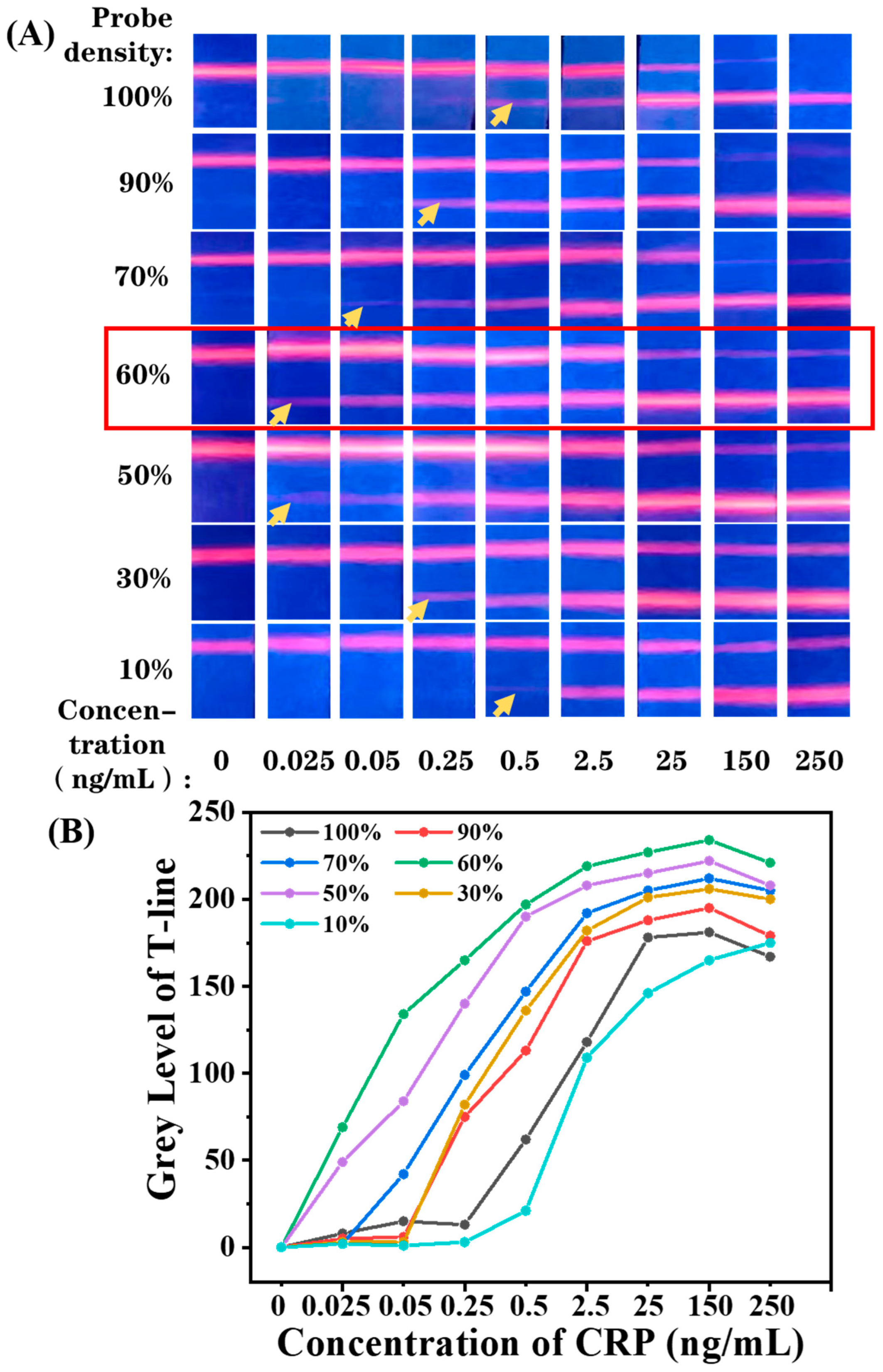
3.4. Optimization of Antibody Density on EuPSM
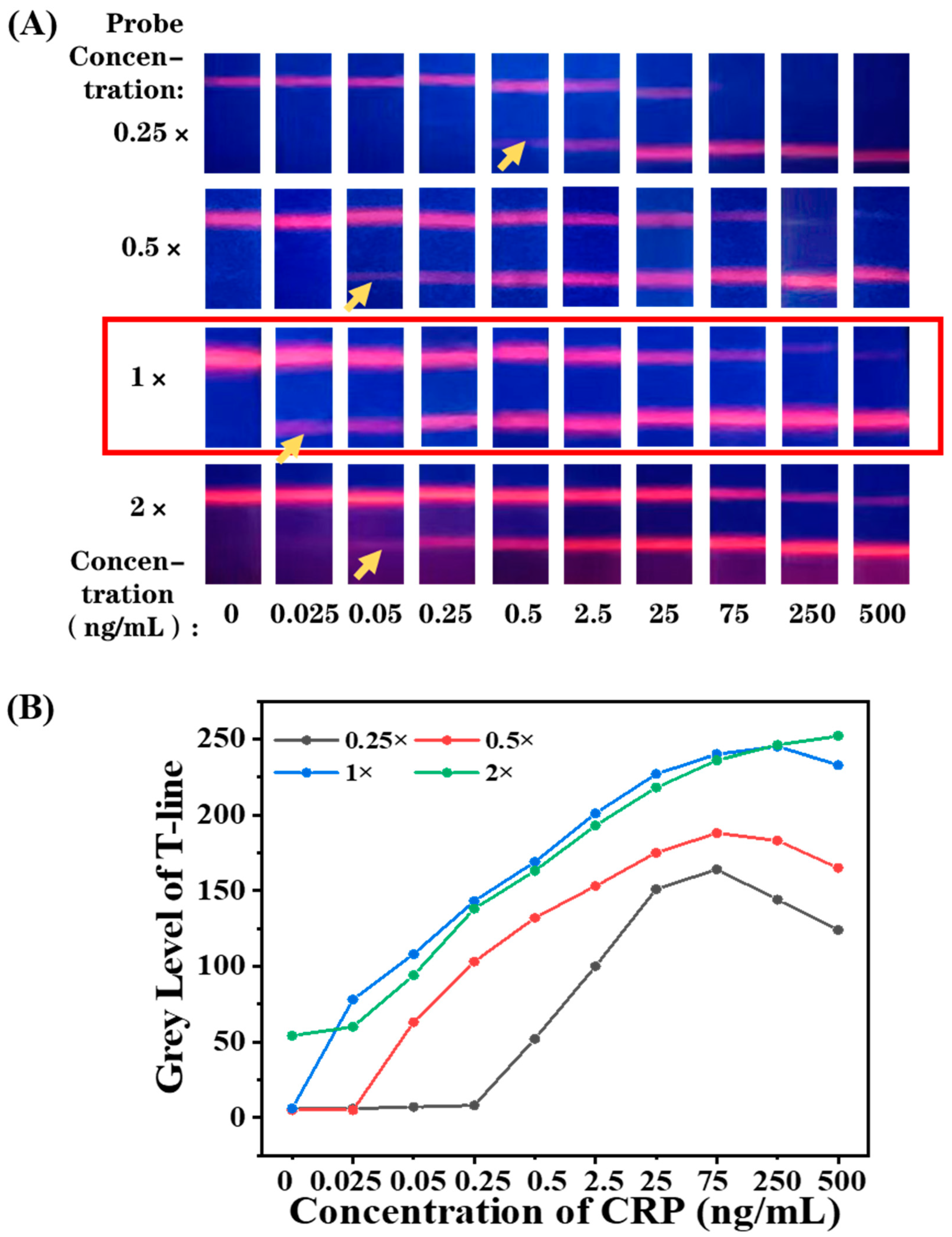
3.5. Optimization of EuPSM-Antibody Concentration
3.6. Development of a Benchtop Fluorescence Analyzer
3.7. Clinical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2022.
- Tang, Q.; Xu, J.; Wei, S.; Chen, H.; Chen, J.; Zhang, H.; Liu, L. Label-free and highly sensitive detection of CRP based on the combination of nicking endonuclease-assisted signal amplification and capillary electrophoresis-UV assay. Anal. Chim. Acta 2022, 1221, 340131. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Kim, W.; Park, B.; Oh, S.Y.; Kim, T.; Huh, Y.S.; Hwangbo, C.K.; Han, Y.K. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: A predictive strategy for heart failure. Biosens. Bioelectron. 2019, 142, 111549. [Google Scholar] [CrossRef]
- Jarczewska, M.; Rebis, J.; Gorski, L.; Malinowska, E. Development of DNA aptamer-based sensor for electrochemical detection of C-reactive protein. Talanta 2018, 189, 45–54. [Google Scholar] [CrossRef]
- Hindenberg, S.; Klenner-Gastreich, S.; Kneier, N.; Zielinsky, S.; Gommeren, K.; Bauer, N.; Moritz, A. Evaluation of a species-specific C-reactive protein assay for the dog on the ABX Pentra 400 clinical chemistry analyzer. BMC Vet. Res. 2017, 13, 146. [Google Scholar] [CrossRef]
- Morioka, K.; Sato, H.; Kuboyama, M.; Yanagida, A.; Shoji, A. Quantification of CRP in human serum using a handheld fluorescence detection system for capillary-based ELISA. Talanta 2021, 224, 121725. [Google Scholar] [CrossRef]
- Kim, C.H.; Ahn, J.H.; Kim, J.Y.; Choi, J.M.; Lim, K.C.; Park, T.J.; Heo, N.S.; Lee, H.G.; Kim, J.W.; Choi, Y.K. CRP detection from serum for chip-based point-of-care testing system. Biosens. Bioelectron. 2013, 41, 322–327. [Google Scholar] [CrossRef]
- Yen, C.W.; de Puig, H.; Tam, J.O.; Gomez-Marquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored silver nanoparticles for multiplexed disease diagnostics: Distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef]
- Zhan, L.; Guo, S.Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef]
- Grant, B.D.; Anderson, C.E.; Williford, J.R.; Alonzo, L.F.; Glukhova, V.A.; Boyle, D.S.; Weigl, B.H.; Nichols, K.P. SARS-CoV-2 Coronavirus Nucleocapsid Antigen-Detecting Half-Strip Lateral Flow Assay Toward the Development of Point of Care Tests Using Commercially Available Reagents. Anal. Chem. 2020, 92, 11305–11309. [Google Scholar] [CrossRef]
- Wu, K.H.; Huang, W.C.; Shyu, R.H.; Chang, S.C. Silver nanoparticle-base lateral flow immunoassay for rapid detection of Staphylococcal enterotoxin B in milk and honey. J. Inorg. Biochem. 2020, 210, 111163. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, J.; Yin, X.; Li, Y.; Shu, R.; Wang, J.; Zhang, D. Vanadium Disulfide Nanosheet Boosts Optical Signal Brightness as a Superior Enzyme Label to Improve the Sensitivity of Lateral Flow Immunoassay. Anal. Chem. 2022, 94, 8693–8703. [Google Scholar] [CrossRef]
- Bu, T.; Jia, P.; Sun, X.; Liu, Y.; Wang, Q.; Wang, L. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples. Sens. Actuators B 2020, 320, 128440. [Google Scholar] [CrossRef]
- Kaur, J.; Vergara, A.; Rossi, M.; Gravagnuolo, A.M.; Valadan, M.; Corrado, F.; Conte, M.; Gesuele, F.; Giardina, P.; Altucci, C. Electrostatically driven scalable synthesis of MoS2–graphene hybrid films assisted by hydrophobins. RSC Adv. 2017, 7, 50166–50175. [Google Scholar] [CrossRef]
- Jin, B.; Li, Z.; Zhao, G.; Ji, J.; Chen, J.; Yang, Y.; Xu, R. Upconversion fluorescence-based paper disc for multiplex point-of-care testing in water quality monitoring. Anal. Chim. Acta 2022, 1192, 339388. [Google Scholar] [CrossRef]
- Jin, B.; Yang, Y.; He, R.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, F.; Shi, C.; He, C.-X.; Li, Z. Lanthanide chelate-encapsulated polystyrene nanoparticles for rapid and quantitative immunochromatographic assay of procalcitonin. RSC Adv. 2016, 6, 103463–103470. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, S.; Chen, T.; Li, J.; Shen, H.; Chai, Y.; Li, L.S. Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal. Chim. Acta 2018, 1008, 1–7. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ma, X.; Guo, J. A sensitive and quantitative prognosis of C-reactive protein at picogram level using mesoporous silica encapsulated core-shell up-conversion nanoparticle based lateral flow strip assay. Talanta 2021, 230, 122335. [Google Scholar] [CrossRef]
- Li, X.; Pan, Z.; Li, M.; Jia, X.; Zhang, S.; Lin, H.; Liu, J.; Ma, L. Europium chelate-labeled lateral flow assay for rapid and multiple detection of beta-lactam antibiotics by the penicillin-binding protein. Anal. Methods 2020, 12, 3645–3653. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Li, Y.; Yuan, W.; Li, F.Y. One-step polymerized lanthanide-based polystyrene microsphere for sensitive lateral flow immunoassay. J. Rare Earths 2021, 39, 11–18. [Google Scholar] [CrossRef]
- Lahtinen, S.; Lyytikäinen, A.; Sirkka, N.; Päkkilä, H.; Soukka, T. Improving the sensitivity of immunoassays by reducing non-specific binding of poly(acrylic acid) coated upconverting nanoparticles by adding free poly(acrylic acid). Microchim. Acta 2018, 185, 220. [Google Scholar] [CrossRef] [PubMed]
- Hlaváček, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horák, D.; Skládal, P.; Foret, F.; Gorris, H.H. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Avitabile, A.; Della Ventura, B.; Funari, R.; Ambrosio, A.; Maddalena, P.; Valadan, M.; Velotta, R.; Altucci, C. Nano- and femtosecond UV laser pulses to immobilize biomolecules onto surfaces with preferential orientation. Appl. Phys. A 2014, 117, 185–190. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Liu, X.; Trofimchuk, E.; Feng, S.; Ma, T.; Gao, X.; Fang, S.; Lu, X. Advantage of Eu(3+)-Doped Polystyrene Microspheres Compared with Colloidal Gold Used in Immunochromatographic Assays for the Detection of Melamine in Milk. J. Food Sci. 2017, 82, 694–697. [Google Scholar] [CrossRef]
- Movilli, J.; Choudhury, S.S.; Schonhoff, M.; Huskens, J. Enhancement of Probe Density in DNA Sensing by Tuning the Exponential Growth Regime of Polyelectrolyte Multilayers. Chem. Mater. 2020, 32, 9155–9166. [Google Scholar] [CrossRef]
- Lavin, A.; Vicente, J.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaria, B.; Maigler, M.V.; Hernandez, A.L.; Ramirez, Y. On the Determination of Uncertainty and Limit of Detection in Label-Free Biosensors. Sensors 2018, 18, 2038. [Google Scholar] [CrossRef]
- Cai, Y.; Kang, K.; Liu, Y.; Wang, Y.; He, X. Development of a lateral flow immunoassay of C-reactive protein detection based on red fluorescent nanoparticles. Anal. Biochem. 2018, 556, 129–135. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.L.; Wen, C.Y.; Tang, M.; Wu, L.L.; Liu, C.; Zhu, L.; Pang, D.W. Sensitive and Quantitative Detection of C-Reaction Protein Based on Immunofluorescent Nanospheres Coupled with Lateral Flow Test Strip. Anal. Chem. 2016, 88, 6577–6584. [Google Scholar] [CrossRef]
- Cheng, X.; Pu, X.; Jun, P.; Zhu, X.; Zhu, D.; Chen, M. Rapid and quantitative detection of C-reactive protein using quantum dots and immunochromatographic test strips. Int. J. Nanomed. 2014, 9, 5619–5626. [Google Scholar]
- Rong, Z.; Xiao, R.; Xing, S.; Xiong, G.; Yu, Z.; Wang, L.; Jia, X.; Wang, K.; Cong, Y.; Wang, S. SERS-based lateral flow assay for quantitative detection of C-reactive protein as an early bio-indicator of a radiation-induced inflammatory response in nonhuman primates. Analyst 2018, 143, 2115–2121. [Google Scholar] [CrossRef]
- Czilwik, G.; Vashist, S.K.; Klein, V.; Buderer, A.; Roth, G.; von Stetten, F.; Zengerle, R.; Mark, D. Magnetic chemiluminescent immunoassay for human C-reactive protein on the centrifugal microfluidics platform. RSC Adv. 2015, 5, 61906–61912. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, R.; Feng, K.; Li, J.; Mao, Q.; Yuan, H.; Shen, H.; Chai, X.; Li, L.S. Highly sensitive and accurate detection of C-reactive protein by CdSe/ZnS quantum dot-based fluorescence-linked immunosorbent assay. J. Nanobiotechnol. 2017, 15, 35. [Google Scholar] [CrossRef] [PubMed]


| Methods | LOD (ng/mL) | Upper Limit (ng/mL) | Detection Time (min) | Reference |
|---|---|---|---|---|
| Fluorescent-based LFA | 91 | 1.6 × 105 | 3 | [28] |
| Fluorescent-based LFA | 3.89 | 1.6 × 103 | 30 | [29] |
| QDs-based LFA | 0.3 | 103 | 3 | [18] |
| QDs-based LFA | 0.25 | 300 | 15 | [30] |
| SERS based LFA | 0.01 | 103 | unknown | [31] |
| Core-shell modified UCNPs-LFA | 0.05 | 50 | 8 | [19] |
| Magnetic chemiluminescent immunoassay | 1.5 | 81 | 30 | [32] |
| QDs-based immunosorbent assay | 0.45 | 400 | 50 | [33] |
| Probe density regulated EuPSM-LFA | 0.00076 | 250 | 8 | Our work |
| Numbering | Actual Concentration (ng/mL) | Detection Concentration (ng/mL) | Deviation (SD/M) |
|---|---|---|---|
| 1 | 21.3 | 19.98 | 6.19% |
| 2 | 34.6 | 33.45 | 1.80% |
| 3 | 7.22 | 6.62 | 8.30% |
| 4 | 70.16 | 64.75 | 7.71% |
| 5 | 9.96 | 8.85 | 11.15% |
| 6 | 91.12 | 77.57 | 14.87% |
| 7 | 119.9 | 111.21 | 7.24% |
| 8 | 12.76 | 11.62 | 8.99% |
| 9 | 17.78 | 17.73 | 0.28% |
| 10 | 213.9 | 231.62 | 8.28% |
| 11 | 243.12 | 239.44 | 1.51% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.; Du, Z.; Zhang, C.; Yu, Z.; Wang, X.; Hu, J.; Li, Z. Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection. Biosensors 2022, 12, 977. https://doi.org/10.3390/bios12110977
Jin B, Du Z, Zhang C, Yu Z, Wang X, Hu J, Li Z. Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection. Biosensors. 2022; 12(11):977. https://doi.org/10.3390/bios12110977
Chicago/Turabian StyleJin, Birui, Zhiguo Du, Chuyao Zhang, Zhao Yu, Xuemin Wang, Jie Hu, and Zedong Li. 2022. "Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection" Biosensors 12, no. 11: 977. https://doi.org/10.3390/bios12110977
APA StyleJin, B., Du, Z., Zhang, C., Yu, Z., Wang, X., Hu, J., & Li, Z. (2022). Eu-Chelate Polystyrene Microsphere-Based Lateral Flow Immunoassay Platform for hs-CRP Detection. Biosensors, 12(11), 977. https://doi.org/10.3390/bios12110977





