A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management
Abstract
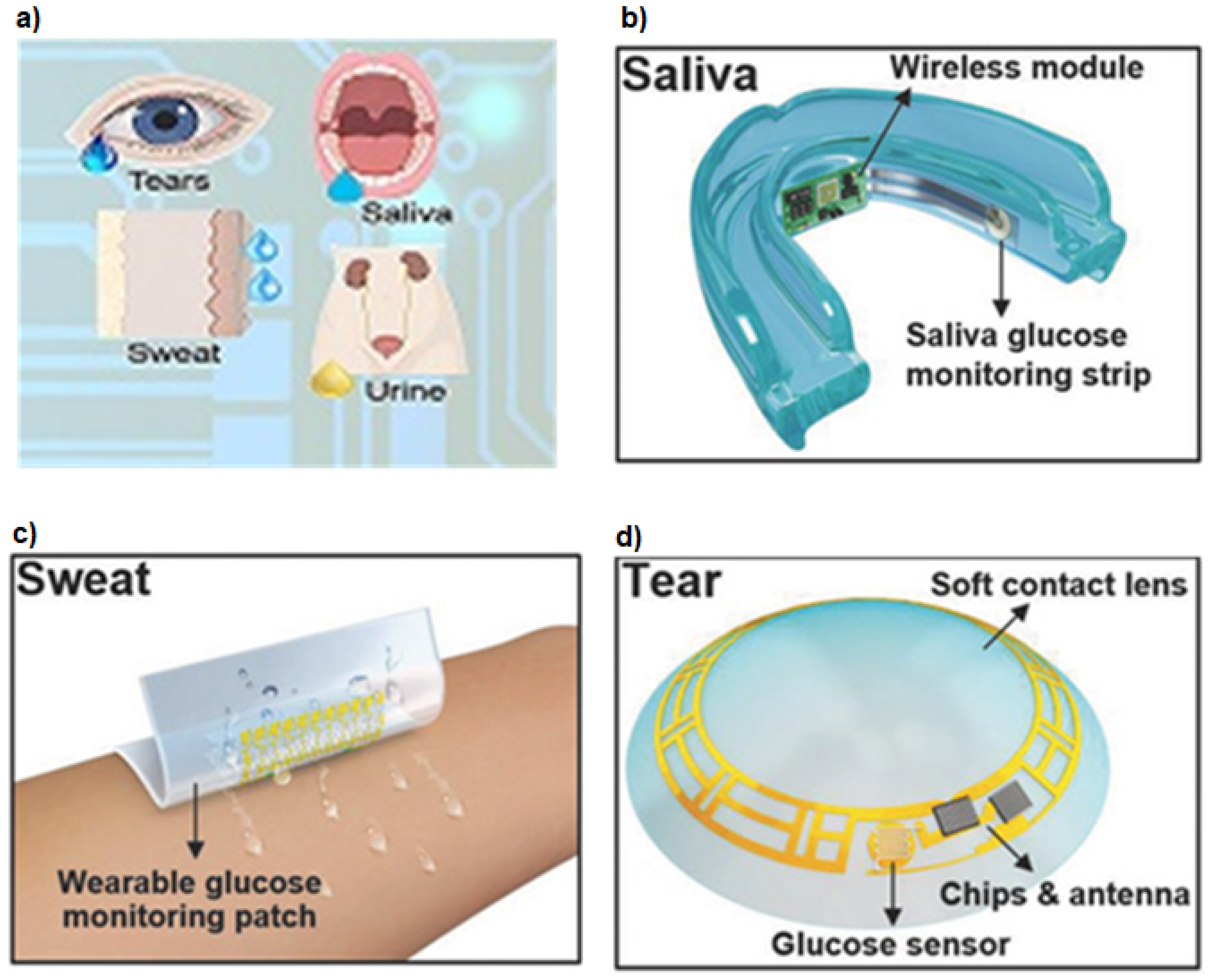
1. Introduction
2. Background
2.1. Diabetes Management via Conventional Blood Pricking Invasive Device
2.2. Diabetes Management via Minimally Invasive Continuous Glucose Monitoring
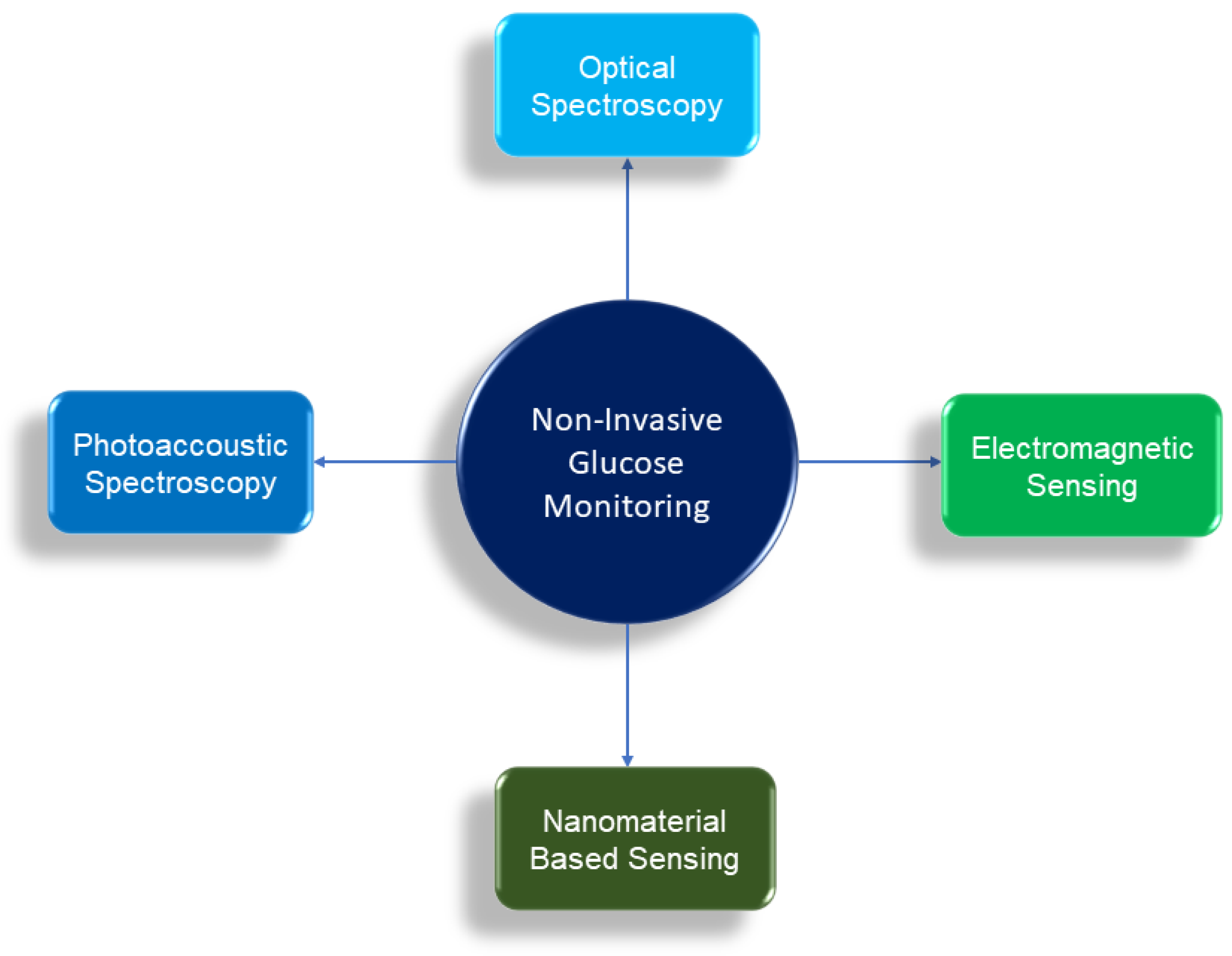
3. Principles of Non Invasive Glucose Monitoring
- Optical Spectroscopy (Optical Detection)
- Photoacoustic Spectroscopy (Acoustic Detection)
- Electromagnetic Sensing (Electromagnetic Detection)
- Nanomaterial Based Sensing (Electrochemical Detection)
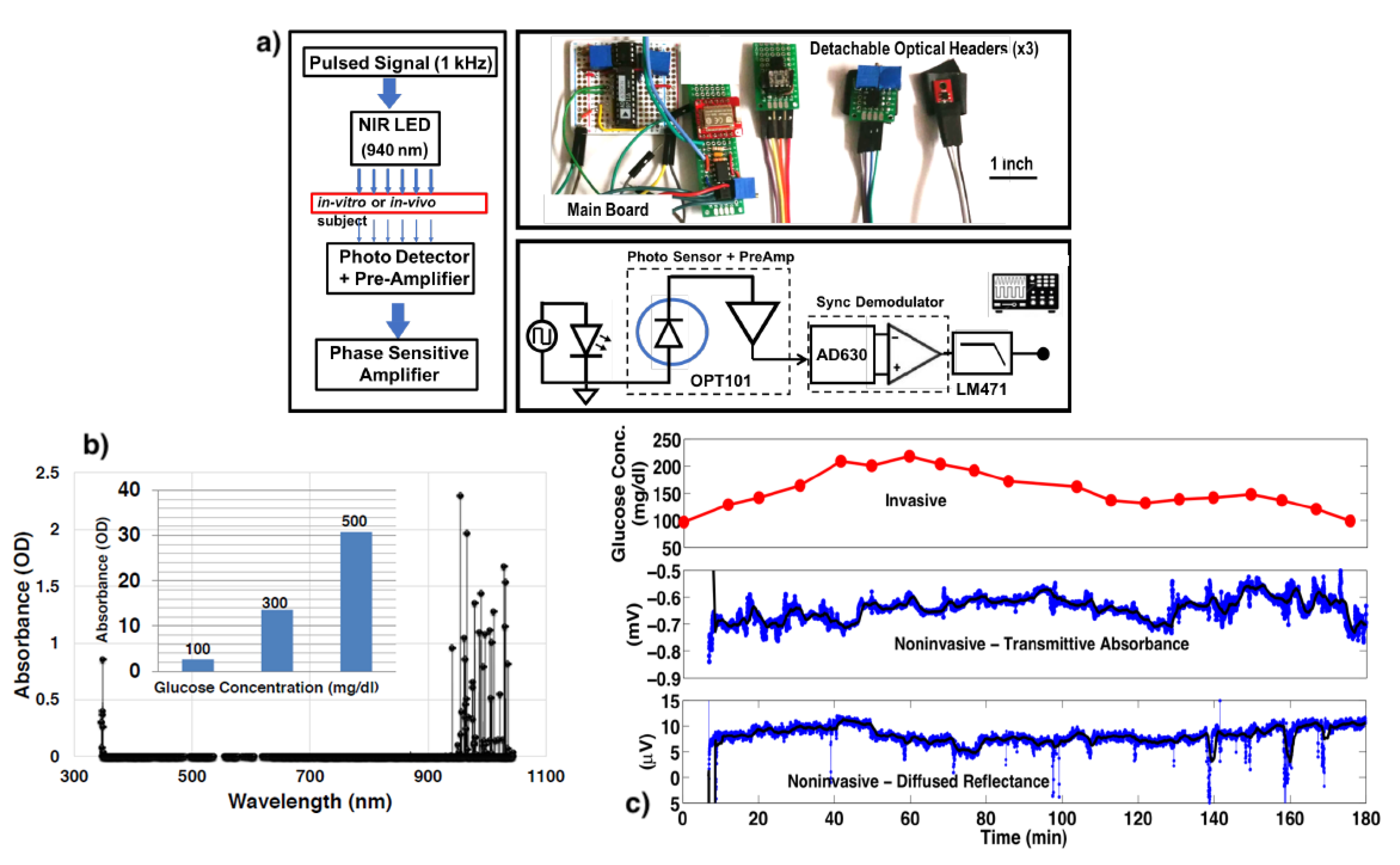
3.1. Optical Spectroscopy
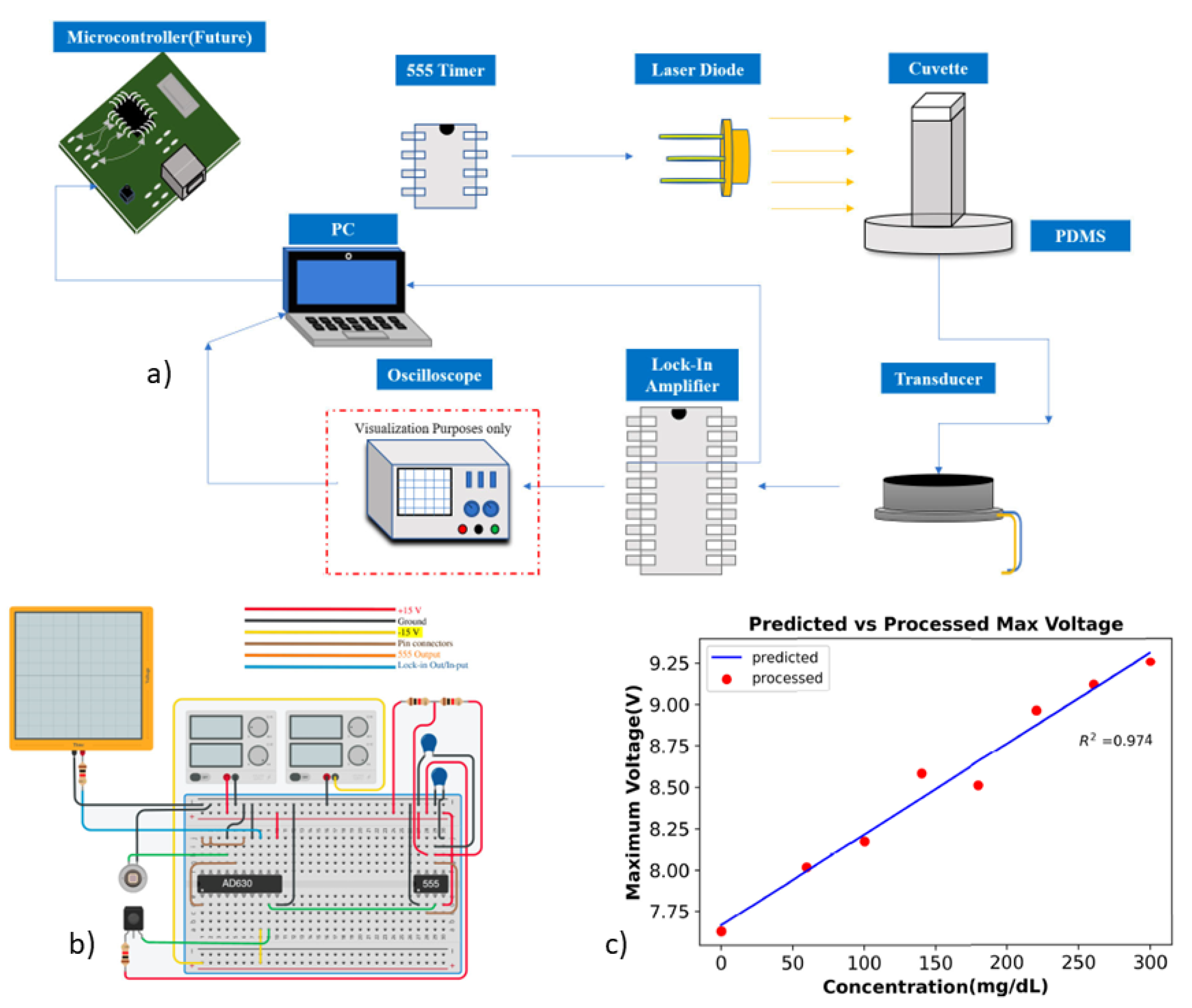
3.2. Photoacoustic Spectroscopy
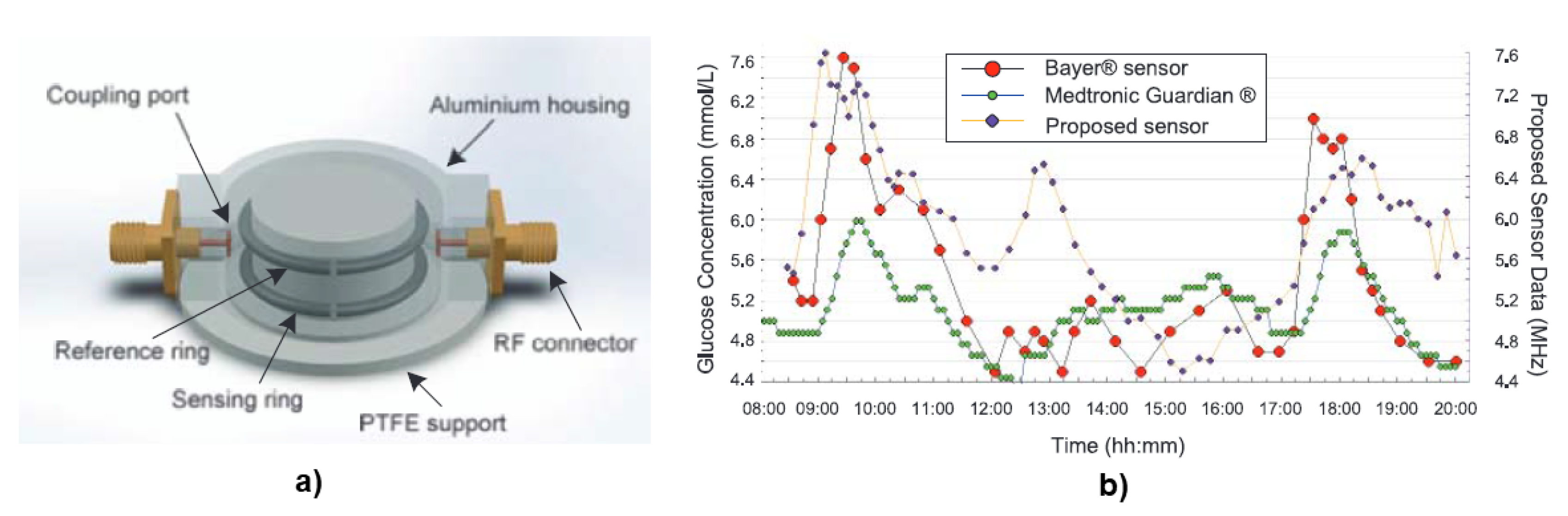
3.3. Electromagnetic Sensing
3.4. Nanomaterial Based Sensing
4. Optical Spectroscopy
5. Photoacoustic Spectroscopy
6. Electromagnetic Sensing
6.1. Planar Microwave Resonant Sensors
6.2. Antenna as Sensors
7. Nanomaterial Based Sensing
8. Hybrid, Integrated and Other Methods
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes Overview, World Health Organization. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 19 March 2022).
- National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 19 August 2022).
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine; Mc-Graw Hill Education: New York City, NY, USA, 2011. [Google Scholar]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A. Type 2 diabetes and risk of cognitive impairment and dementia. Curr. Neurol. Neurosci. 2007, 7, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Vittinghoff, E.; Sellmeyer, D.E.; Feingold, K.R.; de Rekeneire, N.; Strotmeyer, E.S.; Shorr, R.I.; Vinik, A.I.; Odden, M.C.; Park, S.W.; et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care 2008, 33, 391–396. [Google Scholar] [CrossRef]
- McCoy, R.G.; Houten, H.K.V.; Ziegenfuss, J.Y.; Shah, N.D.; Wermers, R.A.; Smith, S.A. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012, 35, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2010, 174, 678–686. [Google Scholar] [CrossRef]
- Mouri, M.; Badireddy, M. Hyperglycemia; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lizzo, J.M.; Goyal, A.; Gupta, V. Adult Diabetic Ketoacidosis; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic hyperosmolar State. Endotext. 2021. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279052/ (accessed on 15 October 2022).
- Bolla, A.; Priefer, R. Blood glucose monitoring- an overview of current and future non-invasive devices. Diabetes Metab. Syndr. 2017, 14, 739–751. [Google Scholar] [CrossRef]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef]
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E. Microwave Planar Resonant Solutions for Glucose Concentration Sensing: A Systematic Review. Appl. Sci. 2021, 11, 7018. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Gusev, M.; Poposka, L.; Spasevski, G.; Kostoska, M.; Koteska, B.; Simjanoska, M.; Ackovska, N.; Stojmenski, A.; Tasic, J.; Trontelj, J.; et al. Noninvasive Glucose Measurement Using Machine Learning and Neural Network Methods and Correlation with Heart Rate Variability. J. Sens. 2020, 2020, 9628281. [Google Scholar] [CrossRef]
- Bondar, J.L.; Mead, D.C. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin. Chem. 1974, 20, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Finger Prick. Available online: https://dtc.ucsf.edu/types-of-diabetes/type1/treatment-of-type-1-diabetes/monitoring-diabetes/monitoring-your-blood/ (accessed on 19 March 2022).
- Bond, M.M.; Richards-Kortum, R.R. Drop-to-Drop Variation in the Cellular Components of Fingerprick Blood: Implications for Point-of-Care Diagnostic Development. Am. J. Clin. Pathol. 2015, 144, 885–894. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, C.E.F.; Wolf, B. Current Development in Non-Invasive Glucose Monitoring. Med. Eng. Phys. 2008, 30, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, Y.; Ammar, A. A System for Blood Glucose Monitoring and Smart Insulin Prediction. IEEE Sens. J. 2021, 21, 13895–13909. [Google Scholar] [CrossRef]
- Laakso, M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 1999, 48, 937–942. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef]
- Dexom CGM. Available online: https://www.dexcom.com/g6/how-it-works (accessed on 19 March 2022).
- Libre CGM. Available online: https://www.freestylelibre.us/cgm-difference/benefits-of-cgm.html (accessed on 19 March 2022).
- Eversense CGM. Available online: https://www.eversensediabetes.com/why-eversense-cgm (accessed on 12 August 2022).
- Messer, L.H.; Berget, C.; Beatson, C.; Polsky, S.; Forlenza, G.P. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol. Ther. 2018, 20, S2-54–S2-64. [Google Scholar] [CrossRef]
- Christensen, M.O.; Berg, A.K.; Rytter, K.; Hommel, E.; Thyssen, J.P.; Svensson, J.; Nørgaard, K. Skin problems due to treatment with technology are associated with increased disease burden among adults with type 1 diabetes. Diabetes Technol. Ther. 2019, 21, 215–221. [Google Scholar] [CrossRef]
- Mastrototaro, J.; Shin, J.; Marcus, A.; Sulur, G. STAR 1 Clinical Trial Investigators The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol. Ther. 2008, 10, 385–390. [Google Scholar] [CrossRef]
- Engler, R.; Routh, T.L.; Lucisano, J.Y. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: Results from patient preference surveys. Clin. Diabetes 2018, 36, 50–58. [Google Scholar] [CrossRef]
- Messer, L.H.; Tanenbaum, M.L.; Cook, P.F.; Wong, J.J.; Hanes, S.J.; Driscoll, K.A.; Hood, K.K. Cost, hassle, and on-body experience: Barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol. Ther. 2020, 22, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S. Spectroscopic and Clinical Aspects of Noninvasive Glucose Measurements. Clin. Chem. 1999, 45, 165–177. [Google Scholar] [CrossRef]
- Omidniaee, A.; Karimi, S.; Farmani, A. Surface Plasmon Resonance-Based SiO2 Kretschmann Configuration Biosensor for the Detection of Blood Glucose. Silicon 2021, 14, 3081–3090. [Google Scholar] [CrossRef]
- MacKenzie, H.A.; Ashton, H.S.; Spiers, S.; Shen, Y.; Freeborn, S.S.; Hannigan, J.; Lindberg, J.; Rae, P. Advances in Photoacoustic Noninvasive Glucose Testing. Clin. Chem. 1999, 45, 1587–1595. [Google Scholar] [CrossRef]
- Debye, P.J.W. Polar Molecules; Dover Publications: Mineola, NY, USA, 1960. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Adhikari, K.K.; Dhakal, R.; Chuluunbaatar, Z.; Wang, C.; Kim, E.-S. Rapid, sensitive and reusable detection of glucose by a robust radiofrequency passive device biosensor chip. Nat. Sci. Rep. 2015, 5, 7807. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, K.; Kim, N.Y. Ultrahigh sensitive mediator free biosensor based on a microfabricated microwave sensor for the detection of micromolar glucose concentrations. IEEE Trans. Microw. Theory Tech. 2016, 64, 319–327. [Google Scholar] [CrossRef]
- Makaram, P.; Owens, D.; Aceros, J. Trends in Nanomaterial-Based Non-Invasive Diabetes Sensing Technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef]
- Haxha, S.; Jhoja, J. Optical Based Noninvasive Glucose Monitoring Sensor Prototype. IEEE Photonics J. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Asekar, M.S. Development of Portable Non-Invasive Blood Glucose Measuring Device Using NIR Spectroscopy. In Proceedings of the 2018 Second International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 14–15 June 2018; pp. 572–575. [Google Scholar]
- Zheng, T.; Li, W.; Liu, Y.; Ling, B.W.-K. A noninvasive blood glucose measurement system by Arduino and near-infrared. In Proceedings of the 2016 IEEE International Conference on Consumer Electronics-China (ICCE-China), Guangzhou, China, 19–21 December 2016; pp. 1–3. [Google Scholar]
- Maruo, K.; Tsurugi, M.; Chin, J.; Ota, T.; Arimoto, H.; Yamada, Y.; Tamura, M.; Ishii, M.; Ozaki, Y. Noninvasive Blood Glucose Assay Using a Newly Developed Near-Infrared System. IEEE J. Sel. Top. Quantum Electron. 2003, 9, 322–330. [Google Scholar] [CrossRef]
- Arefin, M.S.; Khan, A.H.; Islam, R. Non-invasive Blood Glucose Determination using Near Infrared LED in Diffused Reflectance Method. In Proceedings of the 2018 10th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 20–22 December 2018; pp. 93–96. [Google Scholar]
- Udara, S.S.W.I.; Alwis, A.K.D.; Silva, K.M.W.K.; Ananda, U.V.D.M.A.; Kahandawaarachchi, K.A.D.C.P. DiabiTech- Non-Invasive Blood Glucose Monitoring System. In Proceedings of the 2019 International Conference on Advancements in Computing (ICAC), Malabe, Sri Lanka, 5–7 December 2019; pp. 145–150. [Google Scholar]
- Lawand, K.; Parihar, M.; Patil, S.N. Design and development of infrared LED based non invasive blood glucometer. In Proceedings of the 2015 Annual IEEE India Conference (INDICON), New Delhi, India, 17–20 December 2015; pp. 1–6. [Google Scholar]
- Noor, Y.; Mohd, N.; Mohd, Z.Z.; Zuli, J.M.; Md, Y.Z.; Rehman, L.A.; Hafiz, L.M.; Hafizulfika, H.M. Noninvasive glucose level determination using diffuse reflectance near infrared spectroscopy and chemometrics analysis based on in vitro sample and human skin. In Proceedings of the 2014 IEEE Conference on Systems, Process and Control (ICSPC 2014), Kuala Lumpur, Malaysia, 12–14 December 2014; pp. 30–35. [Google Scholar]
- Laha, S.; Kaya, S.; Dhinagar, N.; Kelestemur, Y.; Puri, V. A Compact Continuous non-Invasive Glucose Monitoring System with Phase-Sensitive Front End. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Cardoso, S.D.S.; Machado, M.B.; Ruzicki, J.C.M. A Non-Invasive Infrared Glucose Monitor Double Wavelength Based. IEEE Lat. Am. Trans. 2020, 18, 1572–1580. [Google Scholar] [CrossRef]
- Abidin, M.T.B.Z.; Rosli, M.K.R.; Shamsuddin, S.A.B.; Madzhi, N.K. Initial quantitative comparison of 940nm and 950nm infrared sensor performance for measuring glucose non-invasively. In Proceedings of the 2013 IEEE International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), Kuala Lumpur, Malaysia, 25–27 November 2013; pp. 1–6. [Google Scholar]
- Kassem, A.; Hamad, M.; Harbieh, G.G.; Moucary, C.E. A Non-Invasive Blood Glucose Monitoring Device. In Proceedings of the 2020 IEEE 5th Middle East and Africa Conference on Biomedical Engineering (MECBME), Amman, Jordan, 27–29 October 2020; pp. 1–4. [Google Scholar]
- Shokrekhodaei, M.; Cistola, D.P.; Roberts, R.C.; Quinones, S. Non-Invasive Glucose Monitoring Using Optical Sensor and Machine Learning Techniques for Diabetes Applications. IEEE Access 2021, 9, 73029–73045. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.; Tsai, C.W. Skin Impedance Measurement in Wearable Non-invasive Optical Blood Glucose Monitors. In Proceedings of the 2020 IEEE 2nd International Workshop on System Biology and Biomedical Systems (SBBS), Taichung, Taiwan, 3–4 December 2020; pp. 1–4. [Google Scholar]
- Yu, Y.; Huang, J.; Zhu, J.; Liang, S. An Accurate Noninvasive Blood Glucose Measurement System Using Portable Near-Infrared Spectrometer and Transfer Learning Framework. IEEE Sens. J. 2021, 21, 3506–3519. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Zhang, Y. Non-invasive blood glucose measurement scheme based on near-infrared spectroscopy. In Proceedings of the 2017 Conference on Lasers and Electro-Optics Pacific Rim (CLEO-PR), Singapore, 31 July–4 August 2017; pp. 1–4. [Google Scholar]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Noninvasive in vivo glucose sensing on human subjects using mid-infrared light. Biomed. Opt. Express 2014, 5, 2397–2404. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Pan, Z.; Shimamoto, S. Non-invasive Blood Glucose Measurement Based on mid-Infrared Spectroscopy. In Proceedings of the 2020 IEEE 17th Annual Consumer Communications & Networking Conference (CCNC), Las Vegas, NV, USA, 10–13 January 2020; pp. 1–5. [Google Scholar]
- Koyama, S.; Miyauchi, Y.; Horiguchi, T.; Ishizawa, H. Non-invasive blood glucose measurement based on ATR infrared spectroscopy. In Proceedings of the 2008 SICE Annual Conference, Chofu, Japan, 20–22 August 2008; pp. 321–324. [Google Scholar]
- Koyama, S.; Miyauchi, Y.; Horiguchi, T.; Ishizawa, H. Non-invasive measurement of blood glucose of diabetic based on IR spectroscopy. In Proceedings of the SICE Annual Conference, Taipei, Taiwan, 18–21 August 2010; pp. 3425–3426. [Google Scholar]
- Morikawa, T.; Saiki, F.; Ishizawa, H.; Toba, E. Noninvasive Measurement of Blood Glucose Based on Optical Sensing and Internal Standard Method. In Proceedings of the IEEE Instrumentation and Measurement Technology Conference Proceedings, Ottawa, ON, Canada, 16–19 May 2005; pp. 1433–1437. [Google Scholar]
- Song, K.; Ha, U.; Park, S.; Yoo, H.-J. An impedance and multi-wavelength near-infrared spectroscopy IC for non-invasive blood glucose estimation. IEEE J.-Solid-State Circuits 2015, 50, 1025–1037. [Google Scholar] [CrossRef]
- Dantu, V.; Vempati, J.; Srivilliputhur, S. Non-invasive blood glucose monitor based on spectroscopy using a smartphone. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3695–3698. [Google Scholar]
- Hsieh, H.V.; Pfeiffer, Z.A.; Amiss, T.J.; Sherman, D.B.; Pitner, J.B. Direct detection of glucose by surface plasmon resonance with bacterial glucose/galactose-binding protein. Biosens. Bioelectron. 2004, 19, 653–660. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Arora, V.; Sapra, S.; Gupta, B.D. Localized Surface Plasmon Resonance-Based Fiber Optic U-Shaped Biosensor for the Detection of Blood Glucose. Plasmonics 2012, 7, 261–268. [Google Scholar] [CrossRef]
- Panda, A.; Pukhrambam, P.D.; Keiser, G. Performance analysis of graphene-based surface plasmon resonance biosensor for blood glucose and gas detection. Appl. Phys. 2020, 126, 153. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Li, Y.; Zhu, H.; Banerjee, P.; Zhou, S. Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 2012, 33, 7115–7125. [Google Scholar] [CrossRef]
- Pravdin, A.B.; Spivak, V.A.; Yakovlev, D.A. On the possibility of noninvasive polarimetric determination of glucose content in skin. Opt. Spectrosc. 2016, 120, 45–49. [Google Scholar] [CrossRef]
- Jin, Y.; Yin, Y.; Li, C.; Liu, H.; Shi, J. Non-Invasive Monitoring of Human Health by Photoacoustic Spectroscopy. Sensors 2022, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Wadamori, N.; Shinohara, R.; Ishihara, Y. Photoacoustic depth profiling of a skin model for non-invasive glucose measurement. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 5644–5647. [Google Scholar]
- Kulkarni, O.C.; Mandal, P.; Das, S.S.; Banerjee, S. A Feasibility Study on Noninvasive Blood Glucose Measurement Using Photoacoustic Method. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Camou, S.; Ueno, Y.; Tamechika, E. Towards non-invasive and continuous monitoring of blood glucose level based on CW photoacoustics: New concept for selective and sensitive measurements of aqueous glucose. In Proceedings of the Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011; pp. 193–197. [Google Scholar]
- Tanaka, Y.; Tajima, T.; Seyama, M.; Waki, K. Differential Continuous Wave Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring. IEEE Sens. J. 2020, 20, 4453–4458. [Google Scholar] [CrossRef]
- Shaikh, F.; Haworth, N.; Wells, R.; Bishop, J.; Chatterjee, S.K.; Banerjee, S.; Laha, S. Compact Instrumentation for Accurate Detection and Measurement of Glucose Concentration Using Photoacoustic Spectroscopy. IEEE Access 2022, 10, 31885–31895. [Google Scholar] [CrossRef]
- Pai, P.P.; Sanki, P.K.; Banerjee, S. A photoacoustics based continuous non-invasive blood glucose monitoring system. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Turin, Italy, 7–9 May 2015; pp. 106–111. [Google Scholar]
- Pai, P.P.; Sanki, P.K.; Sahoo, S.K.; De, A.; Bhattacharya, S.; Banerjee, S. Cloud Computing-Based Non-Invasive Glucose Monitoring for Diabetic Care. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 663–676. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, W.; He, Q.; Zhao, H. A new approach to non-invasive blood glucose measurement based on 2 dimension photoacoustic spectrum. In Proceedings of the International Conference on Electronics Instrumentation & Information Systems (EIIS), Harbin, China, 3–5 June 2017; pp. 1–5. [Google Scholar]
- Priya, B.L.; Jayalakshmy, S.; Bhuvaneshwar, R.; Kumar, J.K. Non—Invasive Blood Glucose Monitoring based on Visible LASER Light. In Proceedings of the 2018 3rd International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 15–16 October 2018; pp. 938–941. [Google Scholar]
- Ali, H.; Bensaali, F.; Jaber, F. Novel Approach to Non-Invasive Blood Glucose Monitoring based on Transmittance and Refraction of Visible Laser Light. IEEE Access 2017, 5, 9163–9177. [Google Scholar] [CrossRef]
- Naam, H.A.A.; Idrees, M.O.; Awad, A.; Abdalsalam, O.S.; Moham, F. Non invasive blood glucose measurement based on Photo-Acoustic Spectroscopy. In Proceedings of the 2015 International Conference on Computing, Control, Networking, Electronics and Embedded Systems Engineering (ICCNEEE), Khartoum, Sudan, 7–9 September 2015; pp. 1–4. [Google Scholar]
- Jahana, T.; Higa, H. Non-Invasive Blood Glucose Monitoring Device Using Photoacoustic Spectroscopy. In Proceedings of the 5th International Conference on Intelligent Informatics and Biomedical Sciences (ICIIBMS), Okinawa, Japan, 18–20 November 2020; pp. 85–88. [Google Scholar]
- Allen, T.J.; Beard, P.C. High power visible light emitting diodes as pulsed excitation sources for biomedical photoacoustics. Biomed. Opt. Express 2016, 7, 1260–1270. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shigeta, Y.; Wang, X. Light Emitting Diodes based Photoacoustic Imaging and Potential Clinical Applications. Nat. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Simulating the Effects of Skin Thickness and Fingerprints to Highlight Problems With Non-Invasive RF Blood Glucose Sensing From Fingertips. IEEE Sens. J. 2017, 17, 7553–7560. [Google Scholar] [CrossRef]
- Choi, H.; Nylon, J.; Luzio, S.; Beutler, J.; Porch, A. Design of continuous non-invasive blood glucose monitoring sensor based on a microwave split ring resonator. In Proceedings of the IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications, London, UK, 8–10 December 2014; pp. 1–3. [Google Scholar]
- Schwerthoeffer, U.; Weigel, R.; Kissinger, D. Highly sensitive microwave resonant near-field sensor for precise aqueous glucose detection in microfluidic medical applications. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference Proceedings, Montevideo, Uruguay, 12–15 May 2014; pp. 919–922. [Google Scholar]
- Cebedio, M.C.; Rabioglio, L.A.; Gelosi, I.E.; Ribas, R.A.; Uriz, A.J.; Moreira, J.C. Analysis and Design of a Microwave Coplanar Sensor for Non-Invasive Blood Glucose Measurements. IEEE Sens. J. 2020, 20, 10572–10581. [Google Scholar] [CrossRef]
- Oloyo, A.A.; Hu, Z. A highly sensitive microwave resonator for non-invasive blood glucose level detection. In Proceedings of the IEEE European Conference on Antennas and Propagation (EuCAP 2018), London, UK, 9–13 April 2018; pp. 1–5. [Google Scholar]
- Qin, K.; He, Y.; Pei, Y.; Cai, X.; Luo, Y. A Microwave Biosensor for Non-invasive Blood Glucose Detection with Accuracy Enhancement. In Proceedings of the International Applied Computational Electromagnetics Society Symposium, Nanjing, China, 8–11 August 2019; pp. 1–2. [Google Scholar]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquié, G.; Deshours, F.; Kokabi, H.; Shubair, R.M. Non-Invasive Real-Time Monitoring of Glucose Level Using Novel Microwave Biosensor Based on Triple-Pole CSRR. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Morshidi, W.H.W.; Zaharudin, Z.; Khan, S.; Nordin, A.N.; Shaikh, F.A.; Adam, I.; Kader, K.A. Inter-digital sensor for non-invasive blood glucose monitoring. In Proceedings of the IEEE International Conference on Innovative Research and Development (ICIRD), Bangkok, Thailand, 11–12 May 2018; pp. 1–6. [Google Scholar]
- Xiao, X.; Li, Q. A Noninvasive Measurement of Blood Glucose Concentration by UWB Microwave Spectrum. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1040–1043. [Google Scholar] [CrossRef]
- Zhou, Y.; Qing, X.; See, T.S.P.; Chin, F.; Karim, M.F. Transmission characterization of glucose solutions at Ku-band for non-invasive glucose monitoring. In Proceedings of the Progress in Electromagnetics Research Symposium, Singapore, 19–22 November 2017; pp. 2925–2928. [Google Scholar]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-Based Noninvasive Concentration Measurements for Biomedical Applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Paul, B.; Manuel, M.P.; Alex, Z.C. Design and development of non invasive blood glucose measurement system. In Proceedings of the Physics and Technology of Sensors International Symposium (ISPTS), Pune, India, 7–10 March 2012; pp. 43–46. [Google Scholar]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and In Vitro Interference Test of Microwave Noninvasive Blood Glucose Monitoring Sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef]
- Hofmann, M.; Fersch, T.; Weigel, R.; Fischer, G.; Kissinger, D. A novel approach to non-invasive blood glucose measurement based on RF transmission. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 30–31 May 2011; pp. 39–42. [Google Scholar]
- Satish, K.S.; Anand, S. Design of microstrip sensor for non invasive blood glucose monitoring. In Proceedings of the International Conference on Emerging Trends & Innovation in ICT (ICEI), Pune, India, 3–5 February 2017; pp. 5–8. [Google Scholar]
- Jiang, F.; Li, S.; Yu, Y.; Cheng, Q.S.; Koziel, S. Sensitivity optimization of antenna for non-invasive blood glucose monitoring. In Proceedings of the International Applied Computational Electromagnetics Society Symposium (ACES), Suzhou, China, 1–4 August 2017; pp. 1–2. [Google Scholar]
- Buford, R.J.; Green, E.C.; McClung, M.J. A microwave frequency sensor for non-invasive blood-glucose measurement. In Proceedings of the IEEE Sensors Applications Symposium, Atlanta, GA, USA, 12–14 February 2008; pp. 4–7. [Google Scholar]
- Baghbani, R.; Ashoorirad, M.; Pourziad, A. Microwave sensor for noninvasive glucose measurements design and implementation of a novel linear. IET Wirel. Sens. Syst. 2015, 5, 51–57. [Google Scholar] [CrossRef]
- Freer, B.; Venkataraman, J. Feasibility study for non-invasive blood glucose monitoring. In Proceedings of the IEEE Antennas and Propagation Society International Symposium, Toronto, ON, Canada, 11–17 July 2010; pp. 1–4. [Google Scholar]
- Zeising, S.; Kirchner, J.; Khalili, H.F.; Ahmed, D.; Lübke, M.; Thalmayer, A.; Fischer, G. Towards Realisation of a Non-Invasive Blood Glucose Sensor Using Microstripline. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; pp. 1–6. [Google Scholar]
- Raj, S.; Kishore, N.; Upadhyay, G.; Tripathi, S.; Tripathi, V.S. A Novel Design of CSRR Loaded Truncated Patch Antenna for Non-Invasive Blood Glucose Monitoring System. In Proceedings of the 2018 IEEE MTT-S International Microwave and RF Conference (IMaRC), Kolkata, India, 28–30 November 2018; pp. 1–4. [Google Scholar]
- Gouzouasis, I.; Cano-Garcia, H.; Sotiriou, I.; Saha, S.; Palikaras, G.; Kosmas, P.; Kallos, E. Detection of varying glucose concentrations in water solutions using a prototype biomedical device for millimeter-wave non-invasive glucose sensing. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–4. [Google Scholar]
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.; Kosmas, P.; et al. A Glucose Sensing System Based on Transmission Measurements at Millimetre Waves using Micro strip Patch Antennas. Nat. Sci. Rep. 2017, 7, 6855. [Google Scholar] [CrossRef] [PubMed]
- Sreenivas, C.; Laha, S. Compact Continuous Non-Invasive Blood Glucose Monitoring using Bluetooth. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Wang, T.-T.; Huang, X.-F.; Huang, H.; Luo, P.; Qing, L.-S. Nanomaterial based optical and electrochemical-biosensors for urine glucose detection: A comprehensive review. Adv. Sens. Energy Mater. 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef]
- Lin, W.J.; Lin, Y.S.; Chang, H.T.; Unnikrishnan, B.; Huang, C.C. Electrocatalytic CuBr@CuO nanoparticles based salivary glucose probes. Biosens. Bioelectron. 2021, 194, 113610. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Adhikari, M.D.; Chung, M.; Tran, T.D.; Kim, J.; Kim, M.I. Magnetic Nanoparticles-Embedded Enzyme-Inorganic Hybrid Nanoflowers with Enhanced Peroxidase-Like Activity and Substrate Channeling for Glucose Biosensing. Adv. Healthc. Mater. 2019, 8, 1801507. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 121, 2344–2348. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.-T.; Wang, X.-Y.; Guo, F.-N.; Wen, H.; Yang, T.; Wang, J.-H. Enhanced peroxidase-like activity of AuNPs loaded graphitic carbon nitride nanosheets for colorimetric biosensing. Anal. Chim. Acta 2019, 1091, 69–75. [Google Scholar] [CrossRef]
- Vivekananth, R.; Babu, R.S.; Prasanna, K.; Lee, C.W.; Kalaivani, R.A. Non-enzymatic glucose sensing platform using self assembled cobalt oxide/graphene nanocomposites immobilized graphite modified electrode. J. Mater. Sci. Mater. Electron. 2018, 29, 6763–6770. [Google Scholar] [CrossRef]
- Mitsumori, M.; Yamaguchi, M.; Kano, Y. A new approach to noninvasive measurement of blood glucose using saliva analyzing system. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 1 November 1998; pp. 1767–1770. [Google Scholar]
- Yamaguchi, M.; Mitsumori, M.; Kano, Y. Development of noninvasive procedure for monitoring blood glucose levels using saliva. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 1 November 1998; pp. 1763–1766. [Google Scholar]
- Chakraborty, P.; Dhar, S.; Deka, N.; Debnath, K.; Mondal, S.P. Non-enzymatic salivary glucose detection using porous CuO nanostructures. Sens. Actuators B Chem. 2020, 302, 1–7. [Google Scholar] [CrossRef]
- Chakraborty, P.; Deka, N.; Patra, D.C.; Debnath, K.; Mondal, S.P. Salivary glucose sensing using highly sensitive and selective non-enzymatic porous NiO nanostructured electrodes. Surf. Interfaces 2021, 26, 101324. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, X.; Heinig, N.F.; Thomas, J.P.; Zhang, L.; Leung, K.T. Nonenzymatic Saliva-Range Glucose Sensing Using Electrodeposited Cuprous Oxide Nanocubes on a Graphene Strip. ACS Appl. Nano Mater. 2021, 4, 4790–4799. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dhar, S.; Debnath, K.; Majumder, T.; Mondal, S.P. Non-enzymatic and non-invasive glucose detection using Au nanoparticle decorated CuO nanorods. Sens. Actuators B Chem. 2019, 283, 776–785. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Lu, Y.; Sheng, K.; Liu, W.; Chen, C.; Li, Y.; Dong, B.; Song, H. Engineered IrO2@NiO core–shell nanowires for sensitive non-enzymatic detection of trace glucose in saliva. Anal. Chem. 2016, 88, 12346–12353. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Zhu, G.; Wang, T.; Yu, D.; Yu, H. One-Step Synthesis of Copper-Platinum Nanoparticles Modified Electrode for Non-Enzymatic Salivary Glucose Detection. Available online: https://ssrn.com/abstract=4164980 (accessed on 20 August 2022).
- Alam, F.; Jalal, A.H.; Forouzanfar, S.; Karabiyik, M.; Rabiei Baboukani, A.; Pala, N. Flexible and Linker-Free Enzymatic Sensors Based on Zinc Oxide Nanoflakes for Noninvasive L-Lactate Sensing in Sweat. IEEE Sens. J. 2020, 20, 5102–5109. [Google Scholar] [CrossRef]
- Lin, K.-C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Molecularly Imprinted Sensor based on Ag-Au NPs/SPCE for Lactate Determination in Sweat for Healthcare and Sport Monitoring. Int. J. Electrochem. Sci. 2021, 16, 2. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Abrar, M.A.; Dong, Y.; Lee, P.K.; Kim, W.S. Bendable Electro-chemical Lactate Sensor Printed with Silver Nano-particles. Nat. Sci. Rep. 2016, 6, 30565. [Google Scholar] [CrossRef]
- Anderson, K.; Poulter, B.; Dudgeon, J.; Li, S.E.; Ma, X.A. A highly sensitive nonenzymatic glucose biosensor based on the regulatory effect of glucose on electrochemical behaviors of colloidal silver nanoparticles on MoS2. Sensors 2017, 17, 1807. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, H.J.; Park, S.; Lee, D.Y.; Chung, E. Tear glucose measurement by reflectance spectrum of a nanoparticle embedded contact lens. Nat. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, Z.A.; Rabbani, M.; Foroushani, M.S. Green Fabrication of Nonenzymatic Glucose Sensor Using Multi-Walled Carbon Nanotubes Decorated with Copper (II) Oxide Nanoparticles for Tear Fluid Analysis. Appl. Biochem. Biotechnol. 2022, 194, 3689–3705. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Li, Y.; Liu, M.; Qiao, J.; Wang, D.; Cao, H.; He, W.; Feng, Y.; Yang, Z. Detection of glucose in diabetic tears by using gold nanoparticles and MXene composite surface-enhanced Raman scattering substrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120432. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, S.; Park, S.; Jeong, I.K.; Kang, J.; Kim, Y.R.; Lee, D.Y.; Chung, E. Optical Assessment of Tear Glucose by Smart Biosensor Based on Nanoparticle Embedded Contact Lens. Nano Lett. 2021, 21, 8933–8940. [Google Scholar] [CrossRef]
- Lee, W.C.; Koh, E.H.; Kim, D.H.; Park, S.G.; Jung, H.S. Plasmonic contact lens materials for glucose sensing in human tears. Sens. Actuators B Chem. 2021, 344, 130297. [Google Scholar] [CrossRef]
- Noviosense. Available online: https://noviosense.com/noviosense/ (accessed on 19 August 2022).
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Laha, S.S.; Thorat, N.D.; Singh, G.; Sathish, C.I.; Yi, J.; Dixit, A.; Vinu, A. Rare-Earth Doped Iron Oxide Nanostructures for Cancer Theranostics: Magnetic Hyperthermia and Magnetic Resonance Imaging. Small 2022, 18, 2104855. [Google Scholar] [CrossRef]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.-K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar]
- Kalaiselvan, C.R.; Laha, S.S.; Somvanshi, S.B.; Tabish, T.A.; Thorat, N.D.; Sahu, N.K. Manganese ferrite (MnFe2O4) nanostructures for cancer theranostics. Coord. Chem. Rev. 2022, 473, 1262–1268. [Google Scholar] [CrossRef]
- Laha, S.S.; Naik, A.R.; Kuhn, E.R.; Alvarez, M.; Sujkowski, A.; Wessells, R.J.; Jena, B.P. Nanothermometry measure of muscle efficiency. Nano Lett. 2017, 17, 214809. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 19, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Han, Z.; Zhu, J.J. Helical carbon nanotubes: Intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chem. Eur. J. 2011, 17, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, J.; Zhang, C. Zinc ferrite based gas sensors: A review. Ceram. Int. 2019, 45, 11143–11157. [Google Scholar] [CrossRef]
- Sakhuja, N.; Jha, R.; Laha, S.S.; Rao, A.; Bhat, N. Fe3O4 Nanoparticle-Decorated WSe2 Nanosheets for Selective Chemiresistive Detection of Gaseous Ammonia at Room Temperature. ACS Appl. Nano Mater. 2020, 3, 11160–11171. [Google Scholar] [CrossRef]
- Luaibi, A.Y.; Al-Ghusain, A.J.; Rahman, A.; Al-Sayah, M.H.; Al-Nashash, H.A. Noninvasive blood glucose level measurement using nuclear magnetic resonance. In Proceedings of the Noninvasive Blood Glucose Level Measurement Using Nuclear Magnetic Resonance, Muscat, Oman, 1–4 February 2015; pp. 1–4. [Google Scholar]
- Albalat, A.L.; Alaman, M.B.S.; Diez, M.C.D.; Martinez-Millana, A.; Salcedo, V.T. Non-Invasive Blood Glucose Sensor: A Feasibility Study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 3–6 November 2019; pp. 1179–1182. [Google Scholar]
- Nanayakkara, N.D.; Munasingha, S.C.; Ruwanpathirana, G.P. Non-Invasive Blood Glucose Monitoring using a Hybrid Technique. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 30 May–1 June 2018; pp. 7–12. [Google Scholar]
- Periyasamy, R.; Anand, S. A study on non-invasive blood glucose estimation—An approach using capacitance measurement technique. In Proceedings of the International Conference on Signal Processing, Communication, Power and Embedded System (SCOPES), Paralakhemundi, India, 3–5 October 2016; pp. 847–850. [Google Scholar]
- Dutta, A.; Chandra Bera, S.; Das, K. A non-invasive microcontroller based estimation of blood glucose concentration by using a modified capacitive sensor at low frequency. AIP Adv. 2019, 9, 105027. [Google Scholar] [CrossRef]
- Suseela, S.; Wahid, P. Non Invasive Monitoring of Blood Glucose Using Saliva as a Diagnostic Fluid. In Proceedings of the Southeast Conference, St. Petersburg, FL, USA, 19–22 April 2018; pp. 1–3. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laha, S.; Rajput, A.; Laha, S.S.; Jadhav, R. A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management. Biosensors 2022, 12, 965. https://doi.org/10.3390/bios12110965
Laha S, Rajput A, Laha SS, Jadhav R. A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management. Biosensors. 2022; 12(11):965. https://doi.org/10.3390/bios12110965
Chicago/Turabian StyleLaha, Soumyasanta, Aditi Rajput, Suvra S. Laha, and Rohan Jadhav. 2022. "A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management" Biosensors 12, no. 11: 965. https://doi.org/10.3390/bios12110965
APA StyleLaha, S., Rajput, A., Laha, S. S., & Jadhav, R. (2022). A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management. Biosensors, 12(11), 965. https://doi.org/10.3390/bios12110965





