Novel Pumping Methods for Microfluidic Devices: A Comprehensive Review
Abstract
1. Introduction
2. Passive (Non-Mechanical Systems)
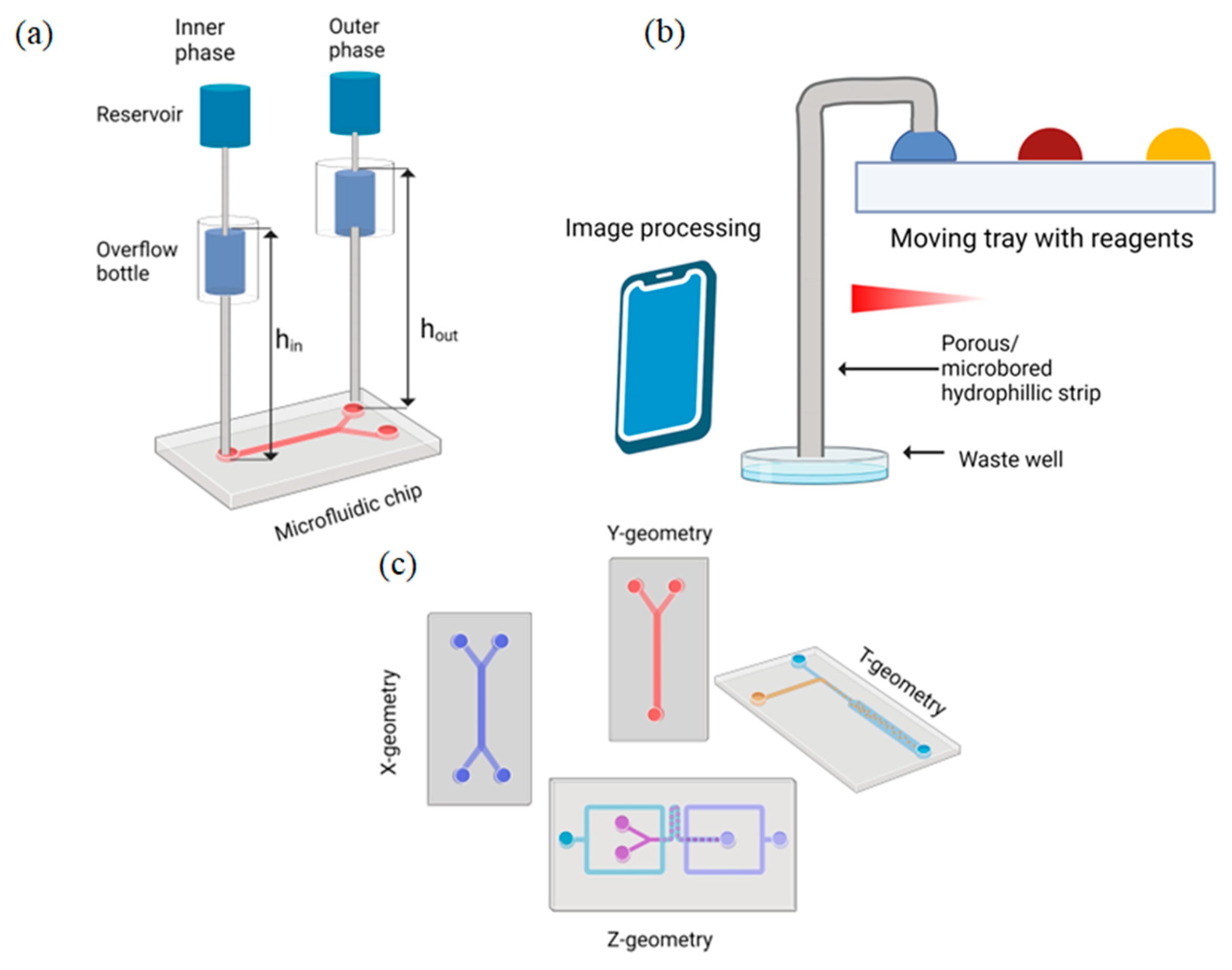
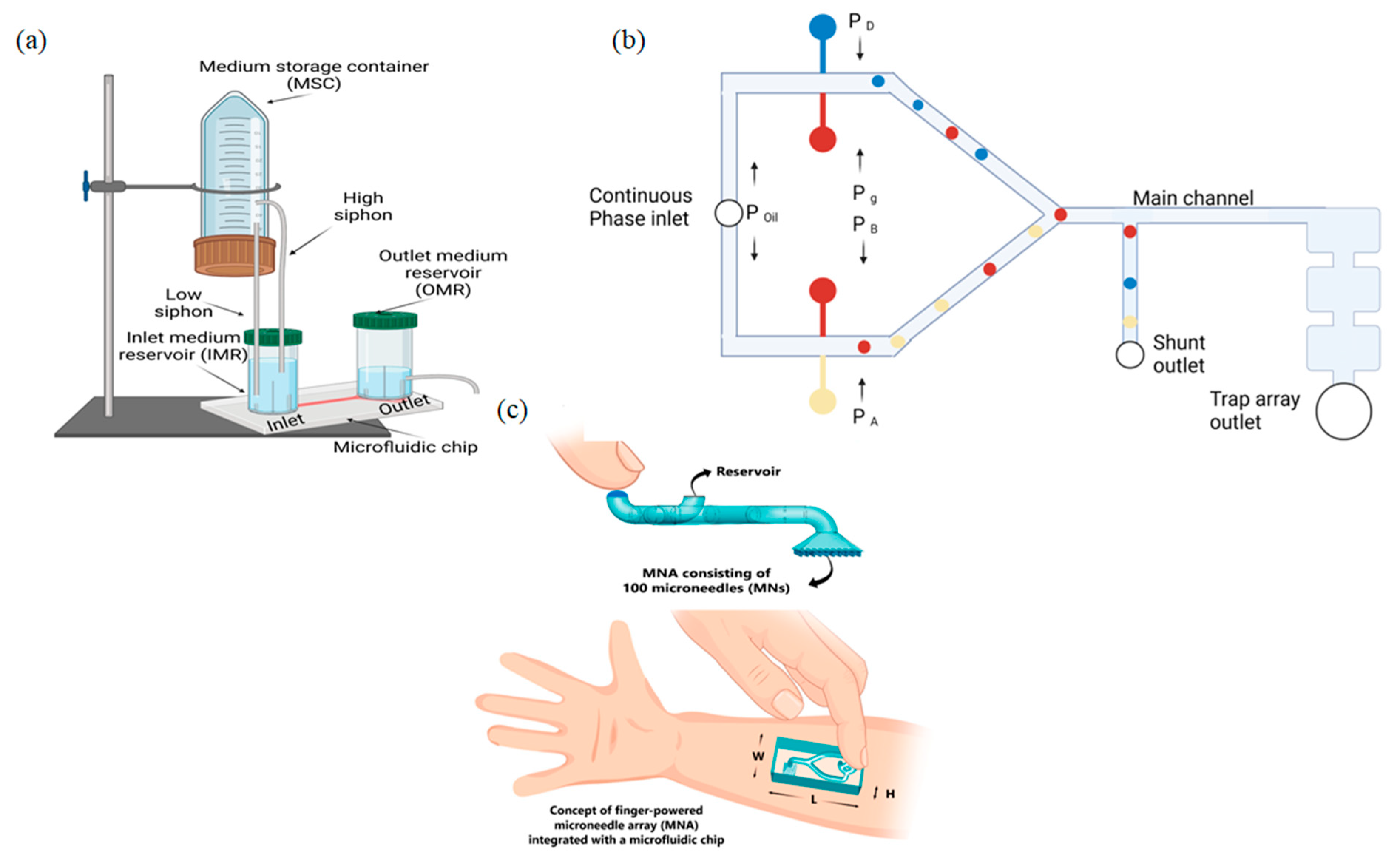
2.1. Gravity-Driven Flow
Limitations
2.2. Capillary Action Flow
Limitations
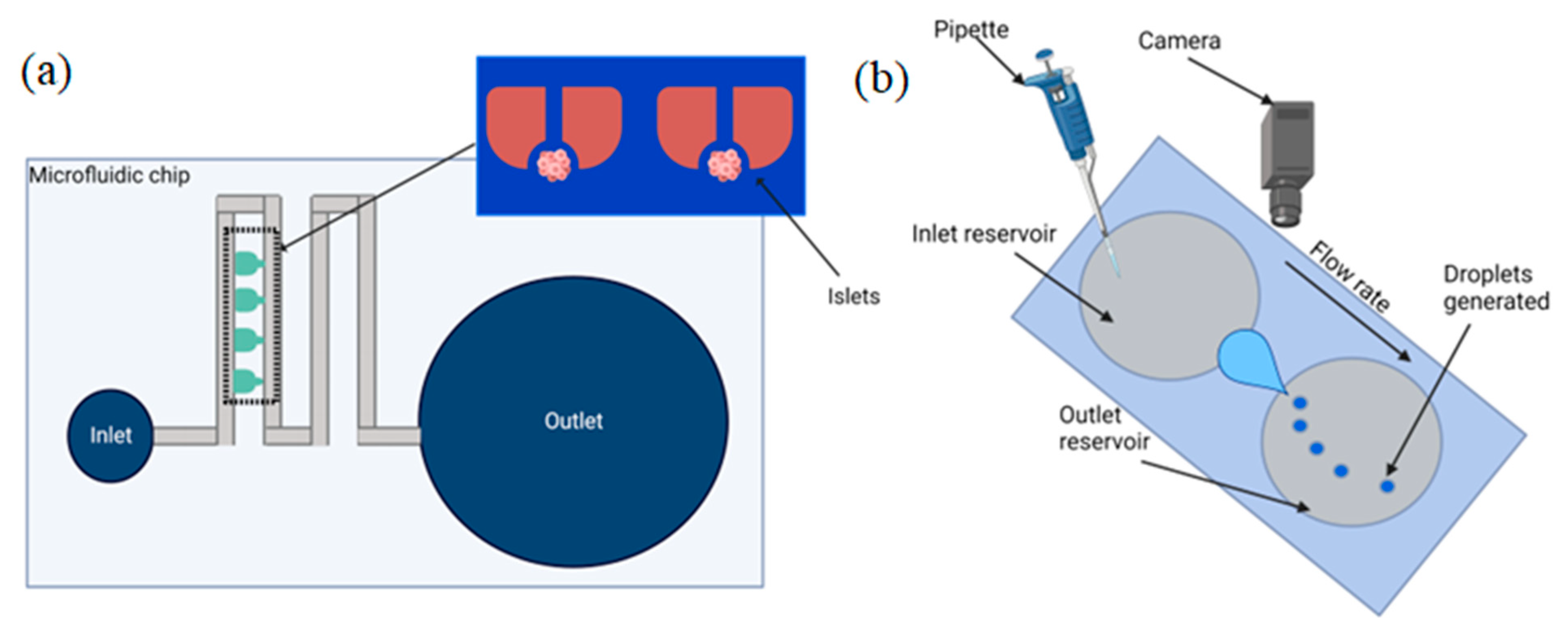
2.3. Surface Tension Flow
Limitations
2.4. Vacuum Driven
Limitations
2.5. Osmosis
Limitations
2.6. Pressure-Driven Systems
Limitations
3. Active (Mechanical Systems)
3.1. Pressure-Driven Systems
Limitations
3.2. Centrifugal Microfluidics
Limitations
3.3. Electrokinetic Platforms
Limitations
3.4. Acoustic Streaming
Limitations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dong, L.; Zhao, J.; Hu, X.; Shen, C.; Qiao, Y.; Zhang, X.; Wang, Y.; Ismagilov, R.F.; Liu, S.; et al. High-Throughput Single-Cell Cultivation on Microfluidic Streak Plates. Appl. Environ. Microbiol. 2016, 82, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Tehranirokh, M.; Kouzani, A.Z.; Francis, P.S.; Kanwar, J.R. Microfluidic devices for cell cultivation and proliferation. Biomicrofluidics 2013, 7, 051502. [Google Scholar] [CrossRef]
- Zhu, Z.; Frey, O.; Ottoz, D.S.; Rudolf, F.; Hierlemann, A. Microfluidic single-cell cultivation chip with controllable immobilization and selective release of yeast cells. Lab Chip 2012, 12, 906–915. [Google Scholar] [CrossRef]
- Dong, L.; Chen, D.-W.; Liu, S.-J.; Du, W. Automated Chemotactic Sorting and Single-cell Cultivation of Microbes using Droplet Microfluidics. Sci. Rep. 2016, 6, 24192. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef]
- Pawell, R.S.; Inglis, D.W.; Barber, T.J.; Taylor, R.A. Manufacturing and wetting low-cost microfluidic cell separation devices. Biomicrofluidics 2013, 7, 056501. [Google Scholar] [CrossRef]
- Herrmann, N.; Neubauer, P.; Birkholz, M. Spiral microfluidic devices for cell separation and sorting in bioprocesses. Biomicrofluidics 2019, 13, 061501. [Google Scholar] [CrossRef]
- Farahinia, A.; Zhang, W.J.; Badea, I. Novel microfluidic approaches to circulating tumor cell separation and sorting of blood cells: A review. J. Sci. Adv. Mater. Devices 2021, 6, 303–320. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Lim, Y.B.; Lee, S.C.; Han, J.; Lim, C.T. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat. Protoc. 2018, 13, 34–58. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.M.; Witek, M.A.; Kamande, J.W.; Soper, S.A. Materials and microfluidics: Enabling the efficient isolation and analysis of circulating tumour cells. Chem. Soc. Rev. 2017, 46, 4245–4280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, Y.; Zhao, Y.; Zhang, L.; Zhang, L.; Mao, H.; Huang, C. Nanotechnology-Assisted Isolation and Analysis of Circulating Tumor Cells on Microfluidic Devices. Micromachines 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, Z.; Wang, J.; Ren, Y.; Wu, A. Microfluidic applications on circulating tumor cell isolation and biomimicking of cancer metastasis. Electrophoresis 2020, 41, 933–951. [Google Scholar] [CrossRef]
- Descamps, L.; le Roy, D.; Deman, A.-L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 1981. [Google Scholar] [CrossRef]
- Bhat, M.P.; Thendral, V.; Uthappa, U.T.; Lee, K.; Kigga, M.; Altalhi, T.; Kurkuri, M.D.; Kant, K. Recent Advances in Microfluidic Platform for Physical and Immunological Detection and Capture of Circulating Tumor Cells. Biosensors 2022, 12, 220. [Google Scholar] [CrossRef]
- Rajawat, A.; Tripathi, S. Disease diagnostics using hydrodynamic flow focusing in microfluidic devices: Beyond flow cytometry. Biomed. Eng. Lett. 2020, 10, 241–257. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Fritz, Z.; Novik, E.M.; Yarmush, G.M.; Schloss, R.S.; Zahn, J.D.; Yarmush, M.L. Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology 2018, 6, 1–23. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, N.; Yang, X.; Peng, B.; Jiang, H. New advances in microfluidic flow cytometry. Electrophoresis 2019, 40, 1212–1229. [Google Scholar] [CrossRef]
- Stavrakis, S.; Holzner, G.; Choo, J.; deMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zheng, W.; Sun, J.; Zhang, W.; Jiang, X. Microfluidics for Manipulating Cells. Small 2013, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005, 26, R73–R98. [Google Scholar] [CrossRef]
- Mittal, R.; Woo, F.W.; Castro, C.S.; Cohen, M.A.; Karanxha, J.; Mittal, J.; Chhibber, T.; Jhaveri, V.M. Organ-on-chip models: Implications in drug discovery and clinical applications. J. Cell. Physiol. 2019, 234, 8352–8380. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, M.; Millet, S.; the ORCHID partners; van den Eijnden-van Raaij, J. Organ-on-Chip In Development:Towards a roadmap for Organs-on-Chip. Preprints 2019, 2019030031. [Google Scholar] [CrossRef]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Essaouiba, A.; Okitsu, T.; Kinoshita, R.; Jellali, R.; Shinohara, M.; Danoy, M.; Legallais, C.; Sakai, Y.; Leclerc, E. Development of a pancreas-liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem. Eng. J. 2020, 164, 107783. [Google Scholar] [CrossRef]
- Thacker, V.V.; Dhar, N.; Sharma, K.; Barrile, R.; Karalis, K.; McKinney, J.D. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife 2020, 9, e59961. [Google Scholar] [CrossRef]
- Abadpour, S.; Aizenshtadt, A.; Olsen, P.A.; Shoji, K.; Wilson, S.R.; Krauss, S.; Scholz, H. Pancreas-on-a-Chip Technology for Transplantation Applications. Curr. Diabetes Rep. 2020, 20, 72. [Google Scholar] [CrossRef]
- Wyatt Shields, C.; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Han, J.-I.; Park, J.-K. Inertial Microfluidics-Based Cell Sorting. BioChip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Sesen, M.; Whyte, G. Image-Based Single Cell Sorting Automation in Droplet Microfluidics. Sci. Rep. 2020, 10, 8736. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Singh, K.; O’Neill, A.; Jackson, C.; Morrison, A.; O’Brien, P. Development of a microfluidic device for fluorescence activated cell sorting. J. Micromech. Microeng. 2002, 12, 486. [Google Scholar] [CrossRef]
- Sia, S.K.; Kricka, L.J. Microfluidics and point-of-care testing. Lab Chip 2008, 8, 1982–1983. [Google Scholar] [CrossRef]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics Based Point-of-Care Diagnostics. Biotechnol. J. 2018, 13, 1700047. [Google Scholar] [CrossRef]
- Li, H.; Steckl, A.J. Paper Microfluidics for Point-of-Care Blood-Based Analysis and Diagnostics. Anal. Chem. 2019, 91, 352–371. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Evans, D.; Papadimitriou, K.; Vasilakis, N.; Pantelidis, P.; Kelleher, P.; Morgan, H.; Prodromakis, T. A Novel Microfluidic Point-of-Care Biosensor System on Printed Circuit Board for Cytokine Detection. Sensors 2018, 18, 4011. [Google Scholar] [CrossRef]
- Eluru, G.; Adhikari, J.V.; Chanda, P.; Gorthi, S.S. Hand-Powered Elastomeric Pump for Microfluidic Point-of-Care Diagnostics. Micromachines 2020, 11, 67. [Google Scholar] [CrossRef]
- Tay, A.; Pavesi, A.; Yazdi, S.R.; Lim, C.T.; Warkiani, M.E. Advances in microfluidics in combating infectious diseases. Biotechnol. Adv. 2016, 34, 404–421. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 2020, 16, 1903916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Liu, Y. Microfluidic-Based Approaches for Foodborne Pathogen Detection. Microorganisms 2019, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhang, C.; Jiang, H. Advances in microfluidic nanobiosensors for the detection of foodborne pathogens. LWT 2021, 151, 112172. [Google Scholar] [CrossRef]
- Gao, D.; Ma, Z.; Jiang, Y. Recent advances in microfluidic devices for foodborne pathogens detection. TrAC Trends Anal. Chem. 2022, 157, 116788. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Domachuk, P.; Tsioris, K.; Omenetto, F.G.; Kaplan, D.L. Bio-microfluidics: Biomaterials and Biomimetic Designs. Adv. Mater. 2010, 22, 249–260. [Google Scholar] [CrossRef]
- Kiran Raj, M.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef]
- Roy, E.; Galas, J.-C.; Veres, T. Thermoplastic elastomers for microfluidics: Towards a high-throughput fabrication method of multilayered microfluidic devices. Lab Chip 2011, 11, 3193–3196. [Google Scholar] [CrossRef]
- Giri, K.; Tsao, C.-W. Recent Advances in Thermoplastic Microfluidic Bonding. Micromachines 2022, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, P.; Xiao, G. The fabrication of polymer microfluidic devices using a solid-to-solid interfacial polyaddition. Polymer 2009, 50, 5358–5361. [Google Scholar] [CrossRef]
- Ma, J.; Yan, S.; Miao, C.; Li, L.; Shi, W.; Liu, X.; Luo, Y.; Liu, T.; Lin, B.; Wu, W.; et al. Paper Microfluidics for Cell Analysis. Adv. Healthc. Mater. 2019, 8, 1801084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhushan, P.; Agarwal, A.K.; Bhattacharya, S. A Historical Perspective on Paper Microfluidic Based Point-of-Care Diagnostics. In Paper Microfluidics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–5. [Google Scholar]
- Cosson, S.; Lutolf, M.P. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci. Rep. 2014, 4, 4462. [Google Scholar] [CrossRef] [PubMed]
- Goy, C.B.; Chaile, R.E.; Madrid, R.E. Microfluidics and hydrogel: A powerful combination. React. Funct. Polym. 2019, 145, 104314. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Han, W.; Chen, X. A review on microdroplet generation in microfluidics. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 247. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Tan, S.H.; Gañán-Calvo, A.M.; Tor, S.B.; Loh, N.H.; Nguyen, N.-T. Active droplet generation in microfluidics. Lab Chip 2016, 16, 35–58. [Google Scholar] [CrossRef]
- Lung-Ming, F.; Wei-Ching, F.; Ting-Fu, H.; Chia-Yen, L. A Magnetic Micropump Based on Ferrofluidic Actuation. Int. J. Autom. Smart Technol. 2014, 4, 77–82. [Google Scholar] [CrossRef]
- Lok, K.S.; Kwok, Y.C.; Lee, P.P.F.; Nguyen, N.-T. Ferrofluid plug as valve and actuator for whole-cell PCR on chip. Sens. Actuators B Chem. 2012, 166–167, 893–897. [Google Scholar] [CrossRef][Green Version]
- Lynn, N.S.; Dandy, D.S. Passive microfluidic pumping using coupled capillary/evaporation effects. Lab Chip 2009, 9, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Mavrogiannis, N.; Ibo, M.; Fu, X.; Crivellari, F.; Gagnon, Z. Microfluidics made easy: A robust low-cost constant pressure flow controller for engineers and cell biologists. Biomicrofluidics 2016, 10, 034107. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, D.R.; Li, X.; Shen, W. Flow control concepts for thread-based microfluidic devices. Biomicrofluidics 2011, 5, 014105. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A. Introduction to Fluid Dynamics for Microfluidic Flows BT—CMOS Biotechnology; Lee, H., Westervelt, R.M., Ham, D., Eds.; Springer: Boston, MA, USA, 2007; pp. 5–30. [Google Scholar]
- Madou, M.J. Fundamentals of Microfabrication and Nanotechnology, Three-Volume Set, 3rd ed.; CRC Press: Boca Rato, FL, USA, 2018. [Google Scholar]
- Goral, V.N.; Zhou, C.; Lai, F.; Yuen, P.K. A continuous perfusion microplate for cell culture. Lab Chip 2013, 13, 1039. [Google Scholar] [CrossRef]
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef]
- Marimuthu, M.; Kim, S. Pumpless steady-flow microfluidic chip for cell culture. Anal. Biochem. 2013, 437, 161–163. [Google Scholar] [CrossRef]
- Gao, W.; Liu, M.; Chen, S.; Zhang, C.; Zhao, Y. Droplet microfluidics with gravity-driven overflow system. Chem. Eng. J. 2019, 362, 169–175. [Google Scholar] [CrossRef]
- Reis, N.M.; Needs, S.H.; Jegouic, S.M.; Gill, K.K.; Sirivisoot, S.; Howard, S.; Kempe, J.; Bola, S.; Al-Hakeem, K.; Jones, I.M.; et al. Gravity-Driven Microfluidic Siphons: Fluidic Characterization and Application to Quantitative Immunoassays. ACS Sens. 2021, 6, 4338–4348. [Google Scholar] [CrossRef]
- Shin, J.-H.; Lee, G.-J.; Kim, W.; Choi, S. A stand-alone pressure-driven 3D microfluidic chemical sensing analytic device. Sens. Actuators B Chem. 2016, 230, 380–387. [Google Scholar] [CrossRef]
- Kao, Y.; Kaminski, T.S.; Postek, W.; Guzowski, J.; Makuch, K.; Ruszczak, A.; Stetten, F.; Zengerle, R.; Garstecki, P. Gravity-driven microfluidic assay for digital enumeration of bacteria and for antibiotic susceptibility testing. Lab Chip 2020, 20, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, Q.; Ma, S.; He, T.; Ai, X.; Hu, P.; Wang, Y.; Luo, G. A gravity-actuated technique for flexible and portable microfluidic droplet manipulation. Microfluid. Nanofluidics 2010, 9, 995–1001. [Google Scholar] [CrossRef]
- van Steijn, V.; Korczyk, P.M.; Derzsi, L.; Abate, A.R.; Weitz, D.A.; Garstecki, P. Block-and-break generation of microdroplets with fixed volume. Biomicrofluidics 2013, 7, 024108. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.K.; Abi-Samra, K.; Cho, Y.-K.; Takayama, S. Pumps for microfluidic cell culture. Electrophoresis 2014, 35, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef]
- Zimmermann, M.; Schmid, H.; Hunziker, P.; Delamarche, E. Capillary pumps for autonomous capillary systems. Lab Chip 2007, 7, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Novo, P.; Chu, V.; Conde, J.P. Integrated optical detection of autonomous capillary microfluidic immunoassays:a hand-held point-of-care prototype. Biosens. Bioelectron. 2014, 57, 284–291. [Google Scholar] [CrossRef]
- Epifania, R.; Soares, R.R.G.; Pinto, I.F.; Chu, V.; Conde, J.P. Capillary-driven microfluidic device with integrated nanoporous microbeads for ultrarapid biosensing assays. Sens. Actuators B Chem. 2018, 265, 452–458. [Google Scholar] [CrossRef]
- Hassan, S.-U.; Zhang, X. Design and Fabrication of Capillary-Driven Flow Device for Point-Of-Care Diagnostics. Biosensors 2020, 10, 39. [Google Scholar] [CrossRef]
- Ahi, E.E.; Torul, H.; Zengin, A.; Sucularlı, F.; Yıldırım, E.; Selbes, Y.; Suludere, Z.; Tamer, U. A capillary driven microfluidic chip for SERS based hCG detection. Biosens. Bioelectron. 2022, 195, 113660. [Google Scholar] [CrossRef]
- Gabrielse, G.; Fei, X.; Orozco, L.; Tjoelker, R.; Haas, J.; Kalinowsky, H.; Trainor, T.; Kells, W. Thousandfold improvement in the measured antiproton mass. Phys. Rev. Lett. 1990, 65, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Nourmohammadzadeh, M.; Elias, J.E.M.; Chan, M.; Chen, Z.; McGarrigle, J.J.; Oberholzer, J.; Wang, Y. A pumpless microfluidic device driven by surface tension for pancreatic islet analysis. Biomed. Microdevices 2016, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- de Groot, T.E.; Veserat, K.S.; Berthier, E.; Beebe, D.J.; Theberge, A.B. Surface-tension driven open microfluidic platform for hanging droplet culture. Lab Chip 2016, 16, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Calver, S.N.; Gaffney, E.A.; Walsh, E.J.; Durham, W.M.; Oliver, J.M. On the thin-film asymptotics of surface tension driven microfluidics. J. Fluid Mech. 2020, 901, A6. [Google Scholar] [CrossRef]
- Khor, J.W.; Lee, U.N.; Berthier, J.; Berthier, E.; Theberge, A.B. Interfacial tension driven open droplet microfluidics. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sierra, T.; Jang, I.; Noviana, E.; Crevillen, A.G.; Escarpa, A.; Henry, C.S. Pump-Free Microfluidic Device for the Electrochemical Detection of α 1 -Acid Glycoprotein. ACS Sens. 2021, 6, 2998–3005. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Zhang, P.; Hsieh, K.; Li, H.; Yang, S.; Wang, T. A vacuum-assisted, highly parallelized microfluidic array for performing multi-step digital assays. Lab Chip 2021, 21, 4716–4724. [Google Scholar] [CrossRef]
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, P.; Li, S.; Sha, Q.; Li, P.; Zeng, X.; Feng, X.; Du, W.; Liu, B. Hand-powered vacuum-driven microfluidic gradient generator for high-throughput antimicrobial susceptibility testing. Biosens. Bioelectron. 2022, 205, 114100. [Google Scholar] [CrossRef]
- Wang, A.; Boroujeni, S.M.; Schneider, P.J.; Christie, L.B.; Mancuso, K.A.; Andreadis, S.T.; Oh, K.W. An Integrated Centrifugal Degassed PDMS-Based Microfluidic Device for Serial Dilution. Micromachines 2021, 12, 482. [Google Scholar] [CrossRef]
- Liang, H.Q.; Hung, W.S.; Yu, H.H.; Hu, C.C.; Lee, K.R.; Lai, J.Y.; Xu, Z.K. Forward osmosis membranes with unprecedented water flux. J. Membr. Sci. 2017, 529, 47–54. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.; Go, M.; Park, J.Y. Stand-alone external power-free microfluidic fuel cell system harnessing osmotic pump for long-term operation. J. Micromech. Microeng. 2018, 28, 125005. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Chiang, Y.-Y. Bio-O-Pump: A novel portable microfluidic device driven by osmotic pressure. Sens. Actuators B Chem. 2019, 284, 736–743. [Google Scholar] [CrossRef]
- Iwai, K.; Sochol, R.D.; Lin, L. Finger-powered, pressure-driven microfluidic pump. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 1131–1134. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Phan, D.T.T.; Liu, J.; Chen, X.; Yang, B.; Hughes, C.C.W.; Zhang, W.; Lee, A.P. A hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function. Lab Chip 2018, 18, 2167–2177. [Google Scholar] [CrossRef]
- Schimel, T.M.; Nguyen, M.-A.; Sarles, S.A.; Lenaghan, S.C. Pressure-driven generation of complex microfluidic droplet networks. Microfluid. Nanofluidics 2021, 25, 78. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z. Microfluidic Passive Flow Regulatory Device with an Integrated Check Valve for Enhanced Flow Control. Micromachines 2019, 10, 653. [Google Scholar] [CrossRef]
- Park, J.; Park, J.-K. Finger-actuated microfluidic device for the blood cross-matching test. Lab Chip 2018, 18, 1215–1222. [Google Scholar] [CrossRef]
- Sarabi, M.R.; Ahmadpour, A.; Yetisen, A.K.; Tasoglu, S. Finger-Actuated Microneedle Array for Sampling Body Fluids. Appl. Sci. 2021, 11, 5329. [Google Scholar] [CrossRef]
- Li, C.G.; Dangol, M.; Lee, C.Y.; Jang, M.; Jung, H. A self-powered one-touch blood extraction system: A novel polymer-capped hollow microneedle integrated with a pre-vacuum actuator. Lab Chip 2015, 15, 382–390. [Google Scholar] [CrossRef]
- Li, C.G.; Lee, C.Y.; Lee, K.; Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices 2013, 15, 17–25. [Google Scholar] [CrossRef]
- Chen, P.; Chen, C.; Su, H.; Zhou, M.; Li, S.; Du, W.; Feng, X.; Liu, B. Integrated and finger-actuated microfluidic chip for point-of-care testing of multiple pathogens. Talanta 2021, 224, 121844. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, A.; Li, X.; Oh, K.W. Passive micropumping in microfluidics for point-of-care testing. Biomicrofluidics 2020, 14, 031503. [Google Scholar] [CrossRef] [PubMed]
- Juncker, D.; Schmid, H.; Drechsler, U.; Wolf, H.; Wolf, M.; Michel, B.; de Rooij, N.; Delamarche, E. Autonomous Microfluidic Capillary System. Anal. Chem. 2002, 74, 6139–6144. [Google Scholar] [CrossRef]
- Available online: https://ckp-rf.ru/catalog/usu/2512530/ (accessed on 21 September 2022).
- Lake, J.R.; Heyde, K.C.; Ruder, W.C. Low-cost feedback-controlled syringe pressure pumps for microfluidics applications. PLoS ONE 2017, 12, e0175089. [Google Scholar] [CrossRef]
- Zhang, Y.; Tseng, T.-M.; Schlichtmann, U. Portable all-in-one automated microfluidic system (PAMICON) with 3D-printed chip using novel fluid control mechanism. Sci. Rep. 2021, 11, 19189. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.; Melroy, G.; Mudugamuwa, A.; Sampath, P.; Premachandra, C.; Amarasinghe, R.; Dau, V. Design and development of a microfluidic droplet generator with vision sensing for lab-on-a-chip devices. Sens. Actuators A Phys. 2021, 332, 113047. [Google Scholar] [CrossRef]
- Joemaa, R.; Grosberg, M.; Rang, T.; Pardy, T. Low-cost, portable dual-channel pressure pump for droplet microfluidics. In Proceedings of the 2022 45th Jubilee International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 23–27 May 2022; pp. 205–211. [Google Scholar] [CrossRef]
- de Graaf, M.N.S.; Vivas, A.; van der Meer, A.D.; Mummery, C.L.; Orlova, V.V. Pressure-Driven Perfusion System to Control, Multiplex and Recirculate Cell Culture Medium for Organs-on-Chips. Micromachines 2022, 13, 1359. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Akhtar, A.S.; Pinto, I.F.; Lapins, N.; Barrett, D.; Sandh, G.; Yin, X.; Pelechano, V.; Russom, A. Sample-to-answer COVID-19 nucleic acid testing using a low-cost centrifugal microfluidic platform with bead-based signal enhancement and smartphone read-out. Lab Chip 2021, 21, 2932–2944. [Google Scholar] [CrossRef]
- Li, L.; Miao, B.; Li, Z.; Sun, Z.; Peng, N. Sample-to-Answer Hepatitis B Virus DNA Detection from Whole Blood on a Centrifugal Microfluidic Platform with Double Rotation Axes. ACS Sens. 2019, 4, 2738–2745. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Meng, X.; Wang, J.; Lu, Y.; Xu, Y.; Cheng, J. Comprehensive Study of the Flow Control Strategy in a Wirelessly Charged Centrifugal Microfluidic Platform with Two Rotation Axes. Anal. Chem. 2017, 89, 9315–9321. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Shen, M.; Lu, Y.; Cheng, J.; Xu, Y. Rapid and Automated Detection of Six Contaminants in Milk Using a Centrifugal Microfluidic Platform with Two Rotation Axes. Anal. Chem. 2019, 91, 7958–7964. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Naghdloo, A.; Besanjideh, M. Cancer cell enrichment on a centrifugal microfluidic platform using hydrodynamic and magnetophoretic techniques. Sci. Rep. 2021, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Gowda, H.N.; Kido, H.; Wu, X.; Shoval, O.; Lee, A.; Lorenzana, A.; Madou, M.; Hoffmann, M.; Jiang, S.C. Development of a proof-of-concept microfluidic portable pathogen analysis system for water quality monitoring. Sci. Total Environ. 2022, 813, 152556. [Google Scholar] [CrossRef] [PubMed]
- Hwu, A.T.; Madadelahi, M.; Nakajima, R.; Shamloo, E.; Perebikovsky, A.; Kido, H.; Jain, A.; Jasinskas, A.; Prange, S.; Felgner, P.; et al. Centrifugal disc liquid reciprocation flow considerations for antibody binding to COVID antigen array during microfluidic integration. Lab Chip 2022, 22, 2695–2706. [Google Scholar] [CrossRef]
- Brassard, D.; Geissler, M.; Descarreaux, M.; Tremblay, D.; Daoud, J.; Clime, L.; Mounier, M.; Charlebois, D.; Veres, T. Extraction of nucleic acids from blood: Unveiling the potential of active pneumatic pumping in centrifugal microfluidics for integration and automation of sample preparation processes. Lab Chip 2019, 19, 1941–1952. [Google Scholar] [CrossRef]
- Bengtsson, K.; Christoffersson, J.; Mandenius, C.-F.; Robinson, N.D. A clip-on electroosmotic pump for oscillating flow in microfluidic cell culture devices. Microfluid. Nanofluidics 2018, 22, 27. [Google Scholar] [CrossRef]
- Yen, P.; Lin, S.; Huang, Y.; Huang, Y.; Tung, Y.; Lu, S.; Lin, C. A Low-Power CMOS Microfluidic Pump Based on Travelling-Wave Electroosmosis for Diluted Serum Pumping. Sci. Rep. 2019, 9, 14794. [Google Scholar] [CrossRef]
- Scott, A.; Mills, D.; Birch, C.; Panesar, S.; Li, J.; Nelson, D.; Starteva, M.; Khim, A.; Root, B.; Landers, J.P. Automated microchannel alignment using innate opto-signature for microchip electrophoresis. Lab Chip 2019, 19, 3834–3843. [Google Scholar] [CrossRef]
- Han, C.-H.; Jang, J. Integrated microfluidic platform with electrohydrodynamic focusing and a carbon-nanotube-based field-effect transistor immunosensor for continuous, selective, and label-free quantification of bacteria. Lab Chip 2021, 21, 184–195. [Google Scholar] [CrossRef]
- Han, C.-H.; Ha, H.W.; Jang, J. Two-dimensional computational method for generating planar electrode patterns with enhanced volumetric electric fields and its application to continuous dielectrophoretic bacterial capture. Lab Chip 2019, 19, 1772–1782. [Google Scholar] [CrossRef]
- Zhu, C.; Maldonado, J.; Sengupta, K. CMOS-Based Electrokinetic Microfluidics With Multi-Modal Cellular and Bio-Molecular Sensing for End-to-End Point-of-Care System. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Aslan, Z. A practical microfluidic pump enabled by acoustofluidics and 3D printing. Microfluid. Nanofluidics 2021, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ning, Y.; Pan, S.; Liu, B.; Chang, Y.; Pang, W.; Duan, X. Mixing during Trapping Enabled a Continuous-Flow Microfluidic Smartphone Immunoassay Using Acoustic Streaming. ACS Sens. 2021, 6, 2386–2394. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, X.; Zhu, J.; Luo, Z.; Liang, L.; Yang, D.; Chen, Y.; Chen, L.; Zheng, Y.; Hu, Q.; et al. On-chip rapid drug screening of leukemia cells by acoustic streaming. Lab Chip 2021, 21, 4005–4015. [Google Scholar] [CrossRef]
- Tachibana, H.; Saito, M.; Tsuji, K.; Yamanaka, K.; Hoa, L.Q.; Tamiya, E. Self-propelled continuous-flow PCR in capillary-driven microfluidic device: Microfluidic behavior and DNA amplification. Sens. Actuators B Chem. 2015, 206, 303–310. [Google Scholar] [CrossRef]
- Li, W.; Sheng, W.; Wegener, E.; Du, Y.; Li, B.; Zhang, T.; Jordan, R. Capillary Microfluidic-Assisted Surface Structuring. ACS Macro Lett. 2020, 9, 328–333. [Google Scholar] [CrossRef]
- Gervais, L.; Hitzbleck, M.; Delamarche, E. Capillary-driven multiparametric microfluidic chips for one-step immunoassays. Biosens. Bioelectron. 2011, 27, 64–70. [Google Scholar] [CrossRef]
- Xu, L.; Lee, H.; Pinheiro, M.V.B.; Schneider, P.; Jetta, D.; Oh, K.W. Phaseguide-assisted blood separation microfluidic device for point-of-care applications. Biomicrofluidics 2015, 9, 014106. [Google Scholar] [CrossRef]

| Method | Power Supply | “Off-Chip” Elements | Type of MCU/PC | Method of Pressure Control | Feature | Price | Reference |
|---|---|---|---|---|---|---|---|
| Pressure-driven (Syringe pump) | 10 V | Yes | Arduino-board, Raspberry Pi 2 | PID-controller, Bang-bang method | Stability of the device to within ±1% | USD 110 | [109] |
| Pressure-driven (Air/gas interception) | 5 V, 7.7 W | No | Raspberry Pi 3 B+ | Self-made program | All-in-one 3D-printed, based on gas interception system | EUR 340 | [110] |
| Pressure-driven (Flow-focusing geometry) | 6 V, 2 W | Yes | Atmega328P | - | Flow-focusing method for droplet generation | - | [111] |
| Pressure-driven (Dual-channel pressure pump) | 3.7 V, 1330 mW | No | ESP-32 board | PID-controller | Wireless communication, sensitive PID controller, compactness | EUR 250 | [112] |
| Pressure-driven (Pneumatic pressure controllers) | No information available | Yes | MCU | PID-controller | Cheap and highly accurate system | ~USD 150 | [113] |
| Centrifugal microfluidics (Portable bead-based platform) | No information available | Yes | Arduino-board | PID-controller | Combined centrifugal and heating modules serve as complete package for sample-to-answer analysis | ~USD 250 | [114] |
| Electroosmotic pump | 13 mW | No | - | - | Low power consumption, low dimensions, high accurate control both of micro- and macroparticles | No information available | [122] |
| CMOS electroosmotic pump | 1.74 mW | No | - | - | Very low power consumption, compact CMOS compatible process | [123] |
| Technique | Flow Rate | Advantages | Limitations | Typical Applications | Reference |
|---|---|---|---|---|---|
| Passive Systems | |||||
| Gravity-driven | µL/min | Stable pressure input and continuous fluid injection, rapid straightforward fluid driving | Too difficult to control pressure gradient between the inlet and outlet of the chip | Droplet generations systems, generating complex emulsions, bacteria enumeration, cell isolation | [71,74,75] |
| Capillary action | nL/min~µL/min | Stable flow rate, “auto-stop” mechanism, low consumption of the reagent, ultra-cheap fabrication | Complex structure, depends on the concentration of the liquid, channel surface needs to be modified by surfactant with multiple procedures, it is not easy to control the flow rate | Capillary pumps, autonomous capillary systems, POC-diagnostics, indicators for chemical reactions | [81,82,133,134,135] |
| Surface tension | µL/min | Low shear stress, high working period | Requires timely replenishment of the solution, complicated fabrication process | Long-term cultivating systems, droplet generation systems | [85,86,87,88] |
| Vacuum driven | nL/min | Compactness, combines driving of the fluid and reactions inside the chip | Requires specific vacuum storage, difficult to manufacture, disposable systems | Sample loading systems, POC diagnostics, air-bubble removal | [90,91,92,93,136] |
| Osmotic | µL/min | Continuous flow, which can last for more than a week | Unstable and inaccurate flow rate | Drug delivery systems, POC systems | [94,95,96] |
| Pressure-driven | µL/min~mL/min | User-friendly easy-to-use device, low-cost, accurate flow control | Human error—device, contains large components, not accurate flow rate, low repeatability | Cell separation, POC diagnostics, cell removal, droplet generation | [98,103,104,105,106] |
| Active Systems | |||||
| Pressure-driven | µL/min~mL/min | Easy-to-fabricate, easily accessible materials, simple working principle, high level of integration with computer | Dimensions of the system, unidirectional flow (syringe pumps), requires high accurate flow control systems | Cell separation, cell cultivation, diagnostics, droplet generation, autonomous systems | [109,110,111,112,113] |
| Centrifugal driven | µL/min~mL/min | Simultaneous multiple testings, simple working principle, biocompatible | Too complicated fabrication process | Cell enrichment, cell sorting, sample-to-answer assays, chemical lysis | [114,115,118,119,120] |
| Electrokinetic | µL/min | Effective highly accurate manipulation of the fluid, compactness, low power consumption | Too complicated fabrication and creation processes | Oscillating flow systems, POC systems | [122,123,127] |
| Acoustic | µL/min | Biocompatible, contactless nature of the device | Requires complex structure and expensive acoustic converters | Biomedical and chemical applications | [128,130,131,132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iakovlev, A.P.; Erofeev, A.S.; Gorelkin, P.V. Novel Pumping Methods for Microfluidic Devices: A Comprehensive Review. Biosensors 2022, 12, 956. https://doi.org/10.3390/bios12110956
Iakovlev AP, Erofeev AS, Gorelkin PV. Novel Pumping Methods for Microfluidic Devices: A Comprehensive Review. Biosensors. 2022; 12(11):956. https://doi.org/10.3390/bios12110956
Chicago/Turabian StyleIakovlev, Aleksei P., Alexander S. Erofeev, and Petr V. Gorelkin. 2022. "Novel Pumping Methods for Microfluidic Devices: A Comprehensive Review" Biosensors 12, no. 11: 956. https://doi.org/10.3390/bios12110956
APA StyleIakovlev, A. P., Erofeev, A. S., & Gorelkin, P. V. (2022). Novel Pumping Methods for Microfluidic Devices: A Comprehensive Review. Biosensors, 12(11), 956. https://doi.org/10.3390/bios12110956





