Chitosan Micro-Membranes with Integrated Gold Nanoparticles as an LSPR-Based Sensing Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
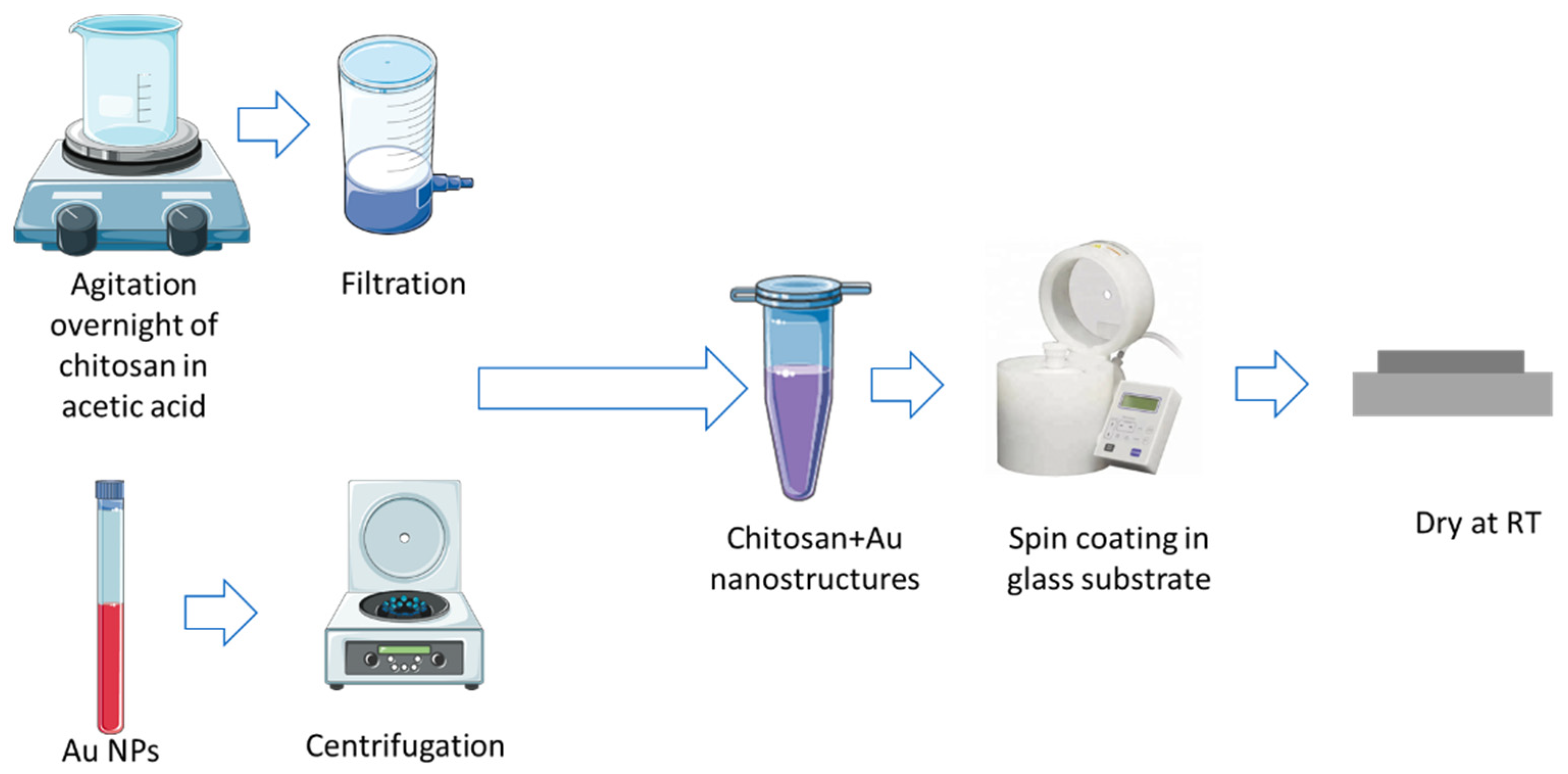
2.2.1. Chitosan Purification
2.2.2. Chitosan/Au-NPs Membrane Production
2.2.3. Surface Characterization Using Atomic Force Microscopy (AFM)
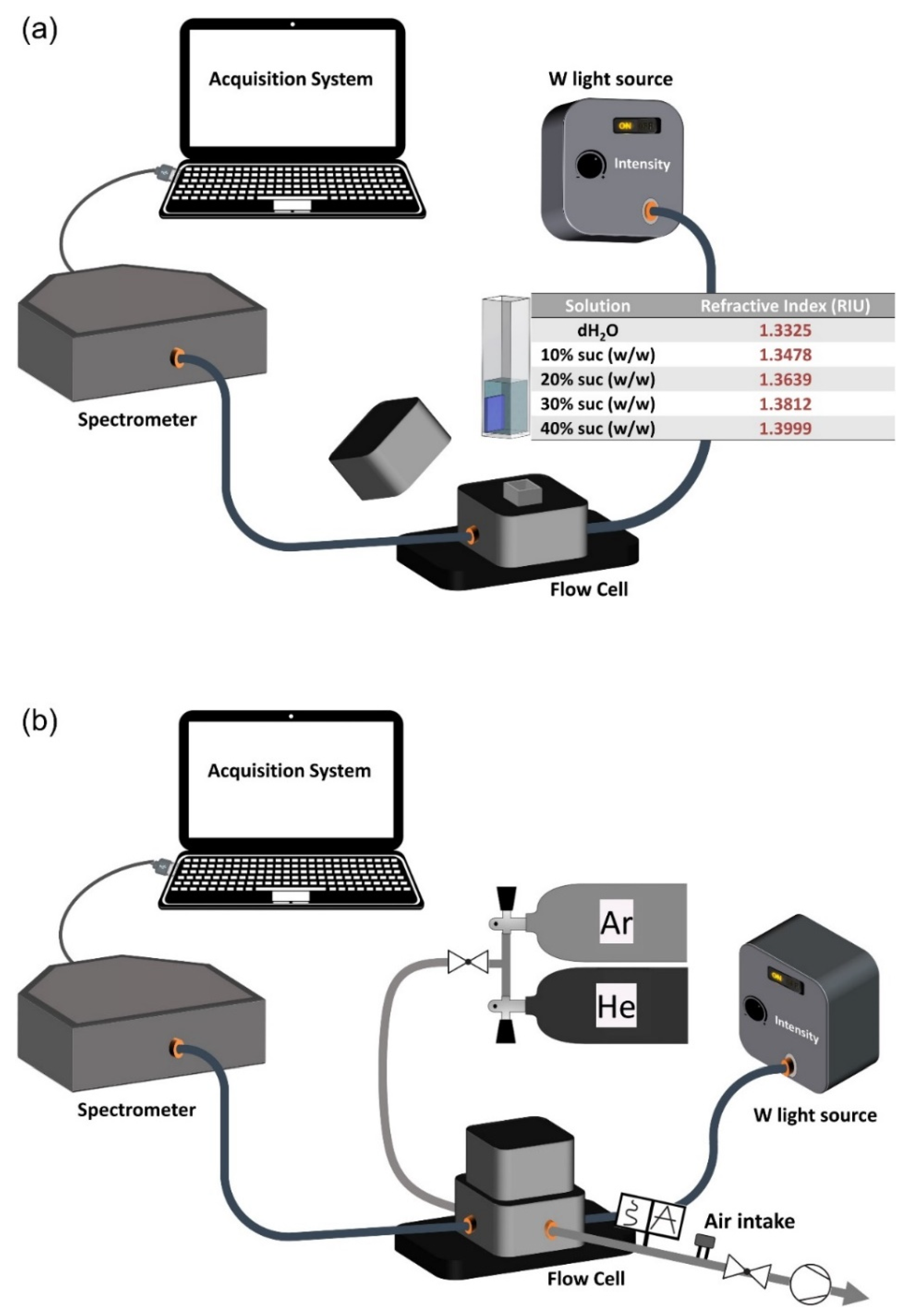
2.2.4. Sensitivity Tests Using HR-LSPR Spectroscopy
- Liquid Sensitivity Tests
- Gas Sensitivity Tests
3. Results and Discussion
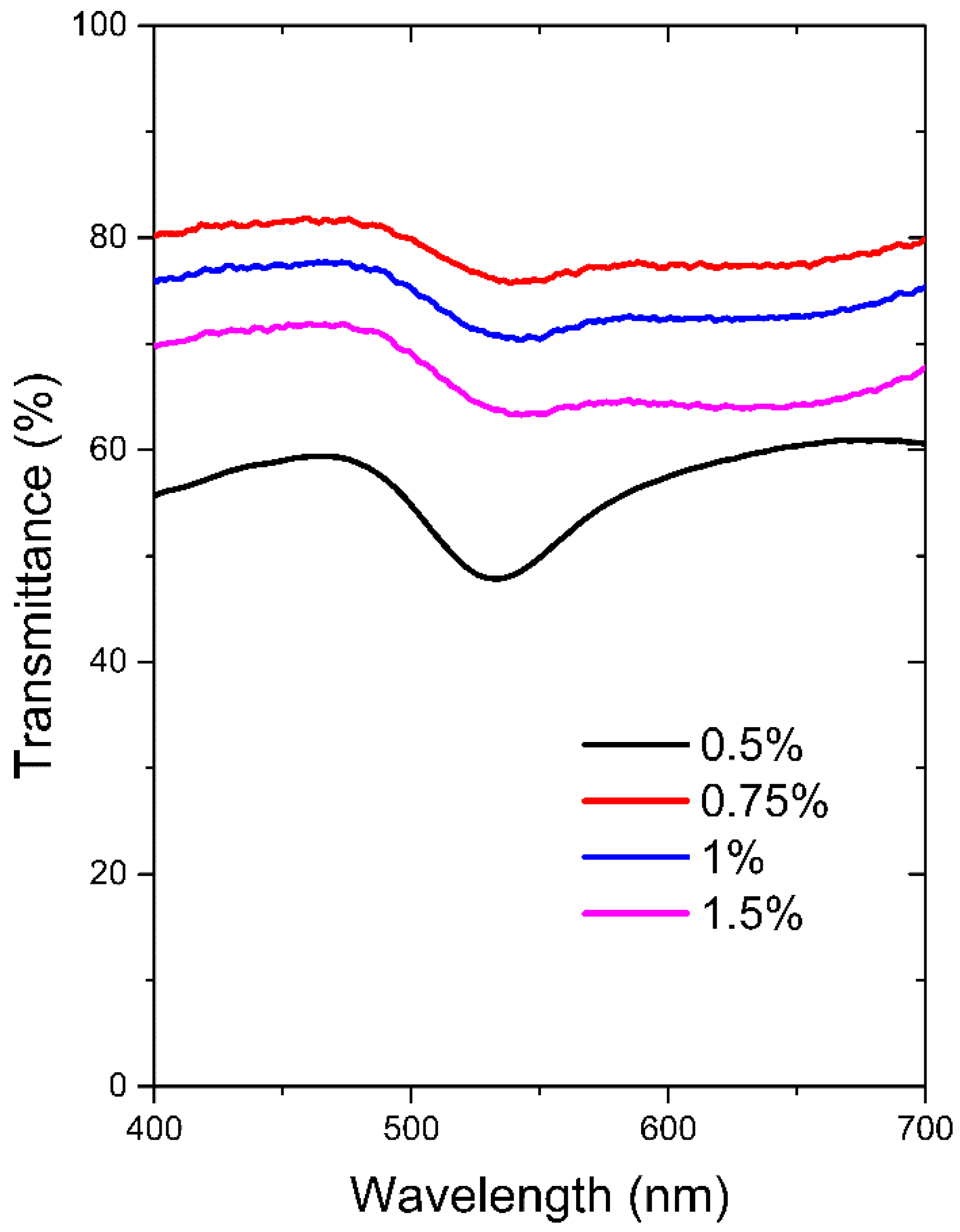
3.1. Optical (T-LSPR) Response of Chitosan/Au-NPs Membranes
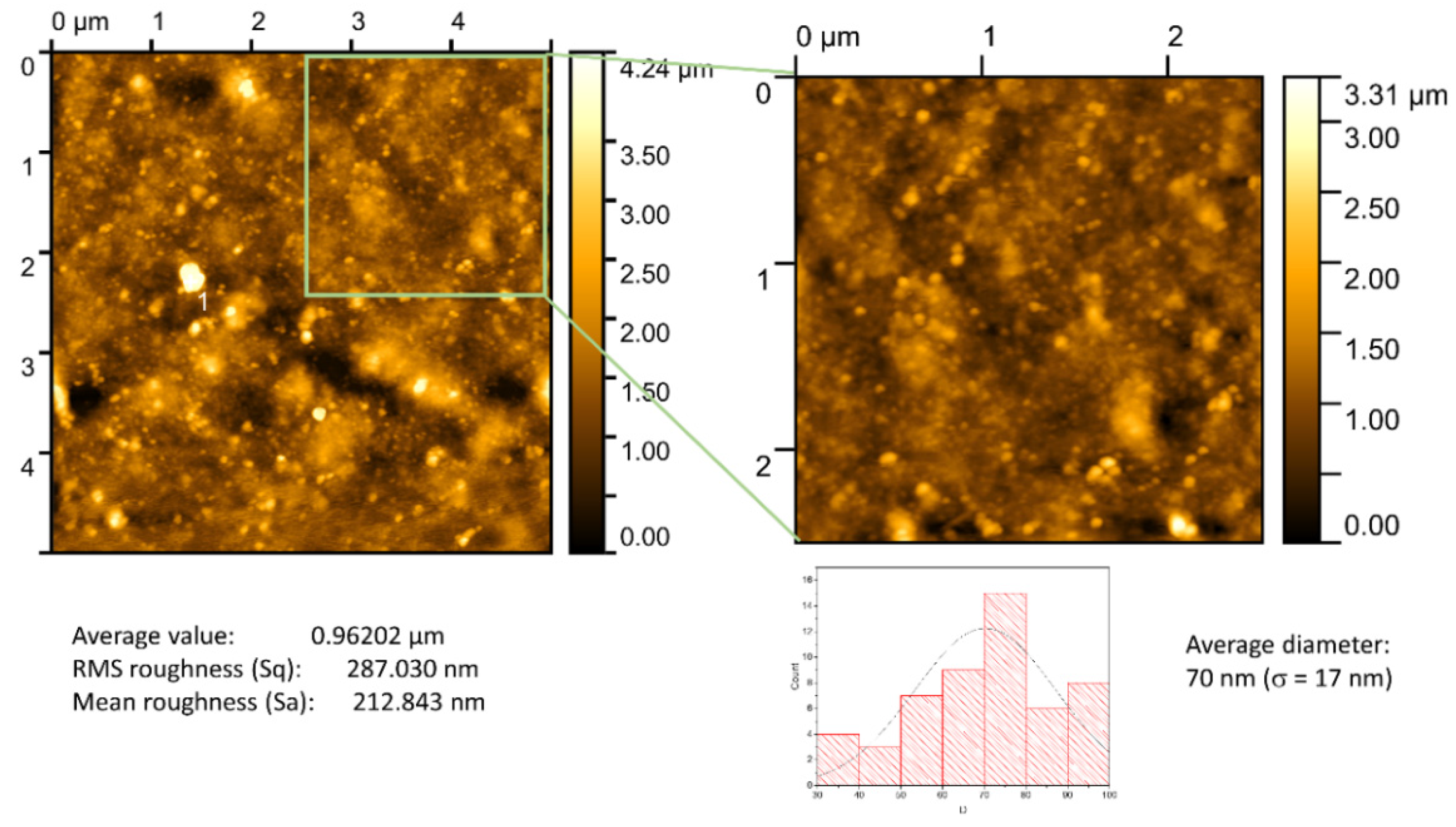
3.2. Surface Characterization of Chitosan (0.5%)/Au-NPs Membrane
3.3. T-LSPR Liquid Sensing of Chitosan (0.5%)/Au-NPs Membrane
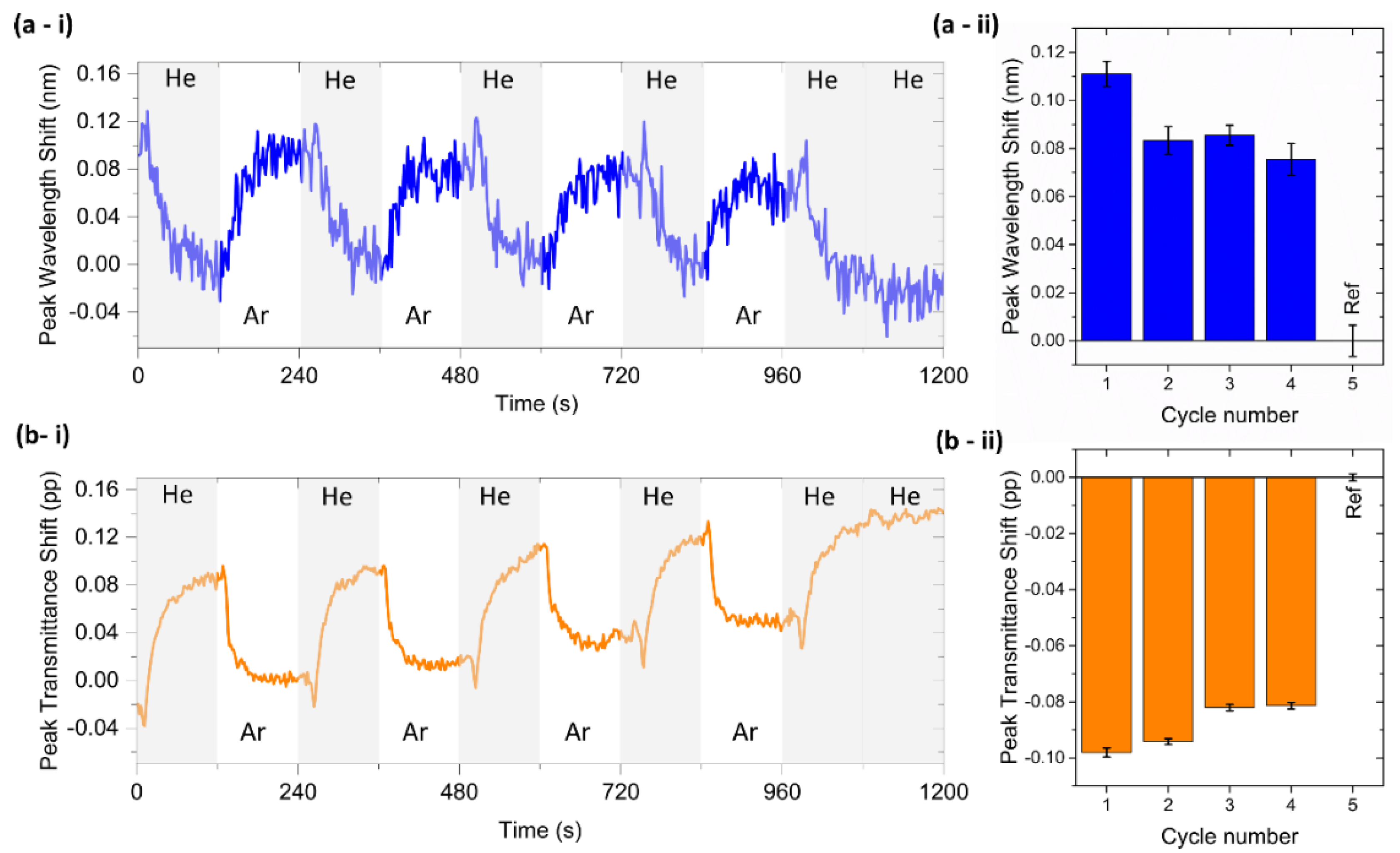
3.4. T-LSPR Gas Sensing of Chitosan (0.5%)/Au-NPs Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.S.; Borges, J.; Lopes, C.; Pereira, R.M.S.; Vasilevskiy, M.I.; Vaz, F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Appl. Sci. 2021, 11, 5388. [Google Scholar] [CrossRef]
- Ha, M.; Kim, J.H.; You, M.; Li, Q.; Fan, C.; Nam, J.M. Multicomponent Plasmonic Nanoparticles: From Heterostructured Nanoparticles to Colloidal Composite Nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent Advances of Plasmonic Nanoparticles and Their Applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Proença, M.; Rodrigues, M.S.; Borges, J.; Vaz, F. Optimization of Au:CuO Nanocomposite Thin Films for Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem. 2020, 92, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Meira, D.I.; Domingues, R.P.; Rodrigues, M.S.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin Films of Au-Al2O3 for Plasmonic Sensing. Appl. Surf. Sci. 2020, 500, 144035. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Borges, J.; Meira, D.I.; Costa, D.; Rodrigues, M.S.; Rebelo, R.; Correlo, V.M.; Vaz, F.; Reis, R.L. Development of Label-Free Plasmonic Au-TiO2 Thin Film Immunosensor Devices. Mater. Sci. Eng. C 2019, 100, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, P.; Meira, D.I.; Costa-Barbosa, A.; Costa, D.; Rodrigues, M.S.; Borges, J.; Machado, A.V.; Cavaleiro, A.; Sampaio, P.; Vaz, F. Immobilization of Streptavidin on a Plasmonic Au-TiO2 Thin Film towards an LSPR Biosensing Platform. Nanomater 2022, 12, 1526. [Google Scholar] [CrossRef]
- Sun, L.L.; Leo, Y.S.; Zhou, X.; Ng, W.; Wong, T.I.; Deng, J. Localized Surface Plasmon Resonance Based Point-of-Care System for Sepsis Diagnosis. Mater. Sci. Energy Technol. 2020, 3, 274–281. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.-P.; Wang, W.; Shen, Y.; Guo, J.-S. Surface Plasmon Resonance for Water Pollutant Detection and Water Process Analysis. TrAC Trends Anal. Chem. 2016, 85, 153–165. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Guo, Q.; Sun, X.; Zhang, H.; Wang, S.; Birech, Z.; Hu, J. An Efficient LSPR Method to Quantitatively Detect Dimethoate: Development, Characterization and Evaluation. PLoS ONE 2020, 15, e0239632. [Google Scholar] [CrossRef]
- Wang, W.; You, Y.; Gunasekaran, S. LSPR-Based Colorimetric Biosensing for Food Quality and Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5829–5855. [Google Scholar] [CrossRef] [PubMed]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Kasani, S.; Curtin, K.; Wu, N. A Review of 2D and 3D Plasmonic Nanostructure Array Patterns: Fabrication, Light Management and Sensing Applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Meira, D.I.; Sampaio, P.; Dias, J.P.; Pedrosa, P.; Martin, N.; Bundaleski, N.; Teodoro, O.M.N.D.; et al. Nanocomposite Thin Films Based on Au-Ag Nanoparticles Embedded in a CuO Matrix for Localized Surface Plasmon Resonance Sensing. Appl. Surf. Sci. 2019, 484, 152–168. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Vaz, F. Plasmonic Strain Sensors Based on Au-TiO2 Thin Films on Flexible Substrates. Sensors 2022, 22, 1375. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Vaz, F. Enhancing the Sensitivity of Nanoplasmonic Thin Films for Ethanol Vapor Detection. Materials 2020, 13, 870. [Google Scholar] [CrossRef]
- Xiang, G.; Zhang, N.; Zhou, X. Localized Surface Plasmon Resonance Biosensing with Large Area of Gold Nanoholes Fabricated by Nanosphere Lithography. Nanoscale Res. Lett. 2010, 5, 818–822. [Google Scholar] [CrossRef]
- Miranda, B.; Rea, I.; Dardano, P.; De Stefano, L.; Forestiere, C. Recent Advances in the Fabrication and Functionalization of Flexible Optical Biosensors: Toward Smart Life-Sciences Applications. Biosensors 2021, 11, 107. [Google Scholar] [CrossRef]
- Karakouz, T.; Vaskevich, A.; Rubinstein, I. Polymer-Coated Gold Island Films as Localized Plasmon Transducers for Gas Sensing. J. Phys. Chem. 2008, 112, 14530–14538. [Google Scholar] [CrossRef]
- Hanif, M.; Juluri, R.R.; Fojan, P.; Popok, V. Polymer Films with Size-Selected Silver Nanoparticles as Plasmon Resonance-Based Transducers for Protein Sensing. Biointerface Res. Appl. Chem. 2016, 6, 1564–1568. [Google Scholar]
- Bhattarai, J.K.; Uddin Maruf, M.H.; Stine, K.J. Plasmonic-Active Nanostructured Thin Films. Processes 2020, 8, 115. [Google Scholar] [CrossRef]
- Rivero, P.J.; Hernaez, M.; Goicoechea, J.; Matias, I.R.; Arregui, F.J. Optical Fiber Refractometers Based on Localized Surface Plasmon Resonance (LSPR) and Lossy Mode Resonance (LMR). In Proceedings of the 23rd International Conference on Optical Fibre Sensors, Santander, Spain, 2–6 June 2014; Volume 9157, pp. 681–684. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Optical Fiber Sensors Based on Polymeric Sensitive Coatings. Polymers 2018, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Pereira, R.M.S.; Vasilevskiy, M.I.; Borges, J.; Vaz, F. NANOPTICS: In-Depth Analysis of NANomaterials for OPTICal Localized Surface Plasmon Resonance Sensing. SoftwareX 2020, 12, 100522. [Google Scholar] [CrossRef]
- Kedem, O.; Vaskevich, A.; Rubinstein, I. Critical Issues in Localized Plasmon Sensing. J. Phys. Chem. C 2014, 118, 8227–8244. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications-A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Lopatina, L.I.; Tsarkova, L.A. PH-Triggered Aggregation Behavior of Hybrid Chitosan Assemblies with Controlled Density Distribution of Gold Nanoparticles. Colloid Polym. Sci. 2019, 297, 339–350. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Y.; Huo, B.; Zhao, X.; Huang, H.; Li, S.; Bai, J.; Liang, J.; Gao, Z. A Copper Monosulfide-Nanoparticle-Based Fluorescent Probe for the Sensitive and Specific Detection of Ochratoxin A. Talanta 2021, 222, 121678. [Google Scholar] [CrossRef]
- Majdi, H.; Salehi, R.; Pourhassan-Moghaddam, M.; Mahmoodi, S.; Poursalehi, Z.; Vasilescu, S. Antibody Conjugated Green Synthesized Chitosan-Gold Nanoparticles for Optical Biosensing. Colloids Interface Sci. Commun. 2019, 33, 100207. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alsuhybani, M.; Algamdi, M.S.; Alquppani, D.; Mashhour, I.; Alshammari, M.S.; Alsohaimi, I.H.; Alraddadi, T.S. Evaluating the Performance of Chitosan and Chitosan-Palm Membrane for Water Treatment: Preparation, Characterization and Purification Study. J. Taibah Univ. Sci. 2021, 15, 77–86. [Google Scholar] [CrossRef]
- Proença, M.; Rodrigues, M.S.; Meira, D.I.; Castro, M.C.R.; Rodrigues, P.V.; Machado, A.V.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Optimization of Au:CuO Thin Films by Plasma Surface Modification for High-Resolution LSPR Gas Sensing at Room Temperature. Sensors 2022, 22, 7043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Mark, A.G.; Alarcón-Correa, M.; Kim, I.; Oswald, P.; Lee, T.-C.; Fischer, P. Dispersion and Shape Engineered Plasmonic Nanosensors. Nat. Commun. 2016, 7, 11331. [Google Scholar] [CrossRef] [PubMed]
- Djurić, Z.; Jokić, I.; Milovanović, G. Signal-to-Noise Ratio in Adsorption-Based Microfluidic Bio/Chemical Sensors. In Proceedings of the 30th Anniversary Eurosensors Conference–Eurosensors 2016, Budapest, Hungary, 4–7 September 2016; Volume 168, pp. 642–645. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meira, D.I.; Proença, M.; Rebelo, R.; Barbosa, A.I.; Rodrigues, M.S.; Borges, J.; Vaz, F.; Reis, R.L.; Correlo, V.M. Chitosan Micro-Membranes with Integrated Gold Nanoparticles as an LSPR-Based Sensing Platform. Biosensors 2022, 12, 951. https://doi.org/10.3390/bios12110951
Meira DI, Proença M, Rebelo R, Barbosa AI, Rodrigues MS, Borges J, Vaz F, Reis RL, Correlo VM. Chitosan Micro-Membranes with Integrated Gold Nanoparticles as an LSPR-Based Sensing Platform. Biosensors. 2022; 12(11):951. https://doi.org/10.3390/bios12110951
Chicago/Turabian StyleMeira, Diana I., Manuela Proença, Rita Rebelo, Ana I. Barbosa, Marco S. Rodrigues, Joel Borges, Filipe Vaz, Rui L. Reis, and Vitor M. Correlo. 2022. "Chitosan Micro-Membranes with Integrated Gold Nanoparticles as an LSPR-Based Sensing Platform" Biosensors 12, no. 11: 951. https://doi.org/10.3390/bios12110951
APA StyleMeira, D. I., Proença, M., Rebelo, R., Barbosa, A. I., Rodrigues, M. S., Borges, J., Vaz, F., Reis, R. L., & Correlo, V. M. (2022). Chitosan Micro-Membranes with Integrated Gold Nanoparticles as an LSPR-Based Sensing Platform. Biosensors, 12(11), 951. https://doi.org/10.3390/bios12110951









