Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein
Abstract
1. Introduction
2. Materials and Methods
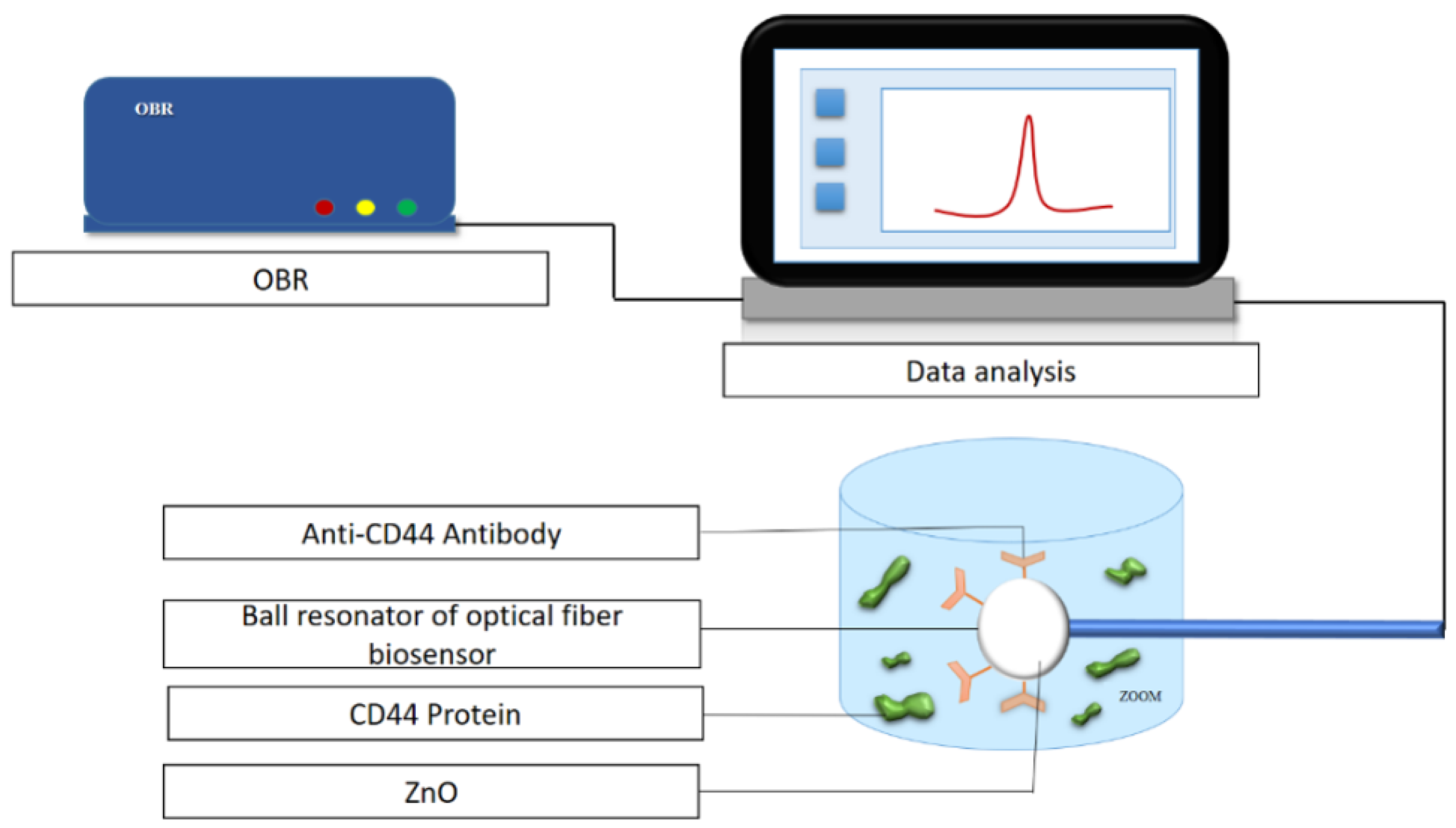
2.1. Equipment
2.2. Fabrication and Characterization of Ball Resonator Optical Fiber Sensor
2.3. Ball Resonator Optical Fiber Surface Coating with the ZnO Thin Layer
2.4. Functionalization of the ZnO-Based Ball Resonator Optical Biosensor with CD44 Antibody
2.5. CD44 Detection
2.6. Specificity Analysis
2.7. Regeneration Studies
3. Results and Discussion
3.1. Surface Characterization of Ball Resonator Optical Fiber Biosensor
3.2. Characterization of Synthesized ZnO Thin Layer
3.3. Detection of CD44 Biomarker
3.4. Specificity Analysis
3.5. Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Fiber Optic Sensors Market 2020–2025. BCC Research 2021. Available online: https://www.researchandmarkets.com/reports/5321748/global-fiber-optic-sensors-market-2020-2025?utm_source=BW&utm_medium=PressRelease&utm_code=g6rqq5&utm_campaign=1577126+-+Global+Fiber+Optic+Sensors+Market+Report+2021%3a+10.9%25+CAGR+Forecast+Between+2020+and+2025%2c+with+Market+Forecast+to+Reach++%244.9+Billion+by+2025+&utm_exec=cari18prd (accessed on 5 September 2022).
- Socorro-Leránoz, A.B.; Santano, D.; Del Villar, I.; Matias, I.R. Trends in the design of wavelength-based optical fibre biosensors (2008–2018). Biosens. Bioelectron. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.Y.; Caucheteur, C. Tilted fiber Bragg grating sensors. Laser Photonics Rev. 2013, 7, 83–108. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Annamdas, V.G.M. Review on Developments in Fiber Optical Sensors and Applications. Int. J. Mater. Eng. 2011, 1, 1–16. [Google Scholar] [CrossRef]
- Correia, R.; James, S.; Lee, S.-W.; Morgan, S.P.; Korposh, S. Biomedical application of optical fibre sensors. J. Opt. 2018, 20, 073003. [Google Scholar] [CrossRef]
- Liang, H.; Wang, J.; Zhang, L.; Liu, J.; Wang, S. Review of Optical Fiber Sensors for Temperature, Salinity, and Pressure Sensing and Measurement in Seawater. Sensors 2022, 22, 5363. [Google Scholar] [CrossRef]
- Tang, F.; Zhou, G.; Li, H.-N.; Verstrynge, E. A review on fiber optic sensors for rebar corrosion monitoring in RC structures. Constr. Build. Mater. 2021, 313, 125578. [Google Scholar] [CrossRef]
- Ruffin, P.B. A review of fiber optics technology for military applications. Proc. SPIE 2000, 10299, 24. [Google Scholar] [CrossRef]
- He, X.; Pan, Y.; You, H.; Lu, Z.; Gu, L.; Liu, F.; Yi, D.; Zhang, M. Fibre optic seismic sensor for down-well monitoring in the oil industry. Measurement 2018, 123, 145–149. [Google Scholar] [CrossRef]
- Sun, D.; Ran, Y.; Wang, G. Label-Free Detection of Cancer Biomarkers Using an in-line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating. Sensors 2017, 17, 2559. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Ashikbayeva, Z.; Myrkhiyeva, Z.; Nugmanova, A.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Label-free fiber-optic spherical tip biosensor to enable picomolar-level detection of CD44 protein. Sci. Rep. 2021, 11, 19583. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, M.; Bis, C.; Scharer, J.M.; Moo-Young, M.; Luong, J.H. Fiber-Optic Biosensors-Trends and Advances. Anal. Sci. 2000, 16, 677–692. [Google Scholar] [CrossRef]
- Battisha, I.K.; Wahab, H.A.; Salama, A.A.; El Saeid, A.A.; Willander, M.; Nur, O. Semiconductor ZnO nano-rods thin film grown on silver wire for hemoglobin biosensor fabrication. New J. Glass Ceram. 2015, 5, 9–15. [Google Scholar] [CrossRef]
- Nowak, E.; Szybowicz, M.; Stachowiak, A.; Koczorowski, W.; Schulz, D.; Paprocki, K.; Fabisiak, K.; Los, S. A comprehensive study of structural and optical properties of ZnO bulk crystals and polycrystalline films grown by sol-gel method. Appl. Phys. A 2020, 126, 552. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, J.-H.; Hsu, D.-X.; Wang, S.-H.; Lin, S.-Y.; Hsueh, T.-J. Enhanced non-enzymatic glucose biosensor of ZnO nanowires via decorated Pt nanoparticles and illuminated with UV/green light emitting diodes. Sens. Actuators B Chem. 2017, 238, 150–159. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef]
- Mahmoud, A.; Echabaane, M.; Omri, K.; Boudon, J.; Saviot, L.; Millot, N.; Chaabane, R.B. Cu-Doped ZnO Nanoparticles for Non-Enzymatic Glucose Sensing. Molecules 2021, 26, 929. [Google Scholar] [CrossRef]
- Khan, M.; Nagal, V.; Masrat, S.; Tuba, T.; Tripathy, N.; Parvez, M.K.; Al-Dosari, M.S.; Khosla, A.; Furukawa, H.; Hafiz, A.K.; et al. Wide-Linear Range Cholesterol Detection Using Fe2O3 Nanoparticles Decorated ZnO Nanorods Based Electrolyte-Gated Transistor. J. Electrochem. Soc. 2022, 169, 7. [Google Scholar] [CrossRef]
- Nagal, V.; Kumar, V.; Khan, M.; AlOmar, S.Y.; Tripathy, N.; Singh, K.; Khosla, A.; Ahmad, N.; Hafiz, A.K.; Ahmad, R. A highly sensitive uric acid biosensor based on vertically arranged ZnO nanorods on a ZnO nanoparticle-seeded electrode. New J. Chem. 2021, 45, 18863–18870. [Google Scholar] [CrossRef]
- Khotmungkhun, K.; Chaengsawang, W.; Srikhirin, T.; Poo-arporn, R.P.; Doungchawee, G.; Subannajui, K. Bulk ZnO Produced from Ultrafast Synthesis of ZnO Nanorods by Microwave Plasma for Antimicrobial Elimination of Leptospirosis. ACS Appl. Nano Mater. 2022, 5, 4462–4472. [Google Scholar] [CrossRef]
- Liang, Y.-H.; Chang, C.-C.; Chen, C.-C.; Chu-Su, Y.; Lin, C.-W. Development of an Au/ZnO thin film surface plasmon resonance-based biosensor immunoassay for the detection of carbohydrate antigen 15-3 in human saliva. Clin. Biochem. 2012, 45, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Peng, J.; Lai, X.; Wu, Q.; Cao, Y.; Ding, L.; Tu, J. The Au/ZnSe/ZnO Heterojunction Improves the Electron Transfer Behavior to Enhance the Detection Performance of Ascorbic Acid. J. Alloy. Compd. 2021, 873, 159721. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yang, J.; Zheng, Z.; Peng, M.; Ng, K.-M.; Chen, Y.; Huang, L.; Gao, W. Sensitive photoelectrochemical detection of colitoxin DNA based on NCDs@CuO/ZnO heterostructured nanocomposites with efficient separation capacity of photo-induced carriers. Mikrochim. Acta 2022, 189, 166. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Wang, X.; Zhang, Y.; Chen, Z. Voltammetric aptamer based detection of HepG2 tumor cells by using an indium tin oxide electrode array and multifunctional nanoprobes. Microchim. Acta 2017, 184, 3487–3496. [Google Scholar] [CrossRef]
- Kanga, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Ning, Z.; Long, Z.; Yang, G.; Xing, L.; Xue, X. Self-Powered Wearable Biosensor in a Baby Diaper for Monitoring Neonatal Jaundice through a Hydrovoltaic-Biosensing Coupling Effect of ZnO Nanoarray. Biosensors 2022, 12, 164. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zheng, L.; Guo, W. Expression of CD44 and p-AKT in pancreatic head cancer. World J. Surg. Oncol. 2015, 13, 334. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Lin, H.; Zhang, L.; Zhang, H.; Wang, Q.; Hu, F.; Li, J.; Li, B.; Zhang, T. Expression of CD44 in Tumor Tissue and Serum of Small Cell Lung Cancer and Its Clinical Prognostic Significance. Chin. J. Lung Cancer 2021, 24, 583–590. [Google Scholar] [CrossRef]
- Kong, Y.; Lyu, N.; Wu, J.; Tang, H.; Xie, X.; Yang, L.; Li, X.; Wei, W.; Xie, X. Breast cancer stem cell markers CD44 and ALDH1A1 in serum: Distribution and prognostic value in patients with primary breast cancer. J. Cancer 2018, 9, 3728–3735. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, D.; Kuku, G.; Selvaraju, R.; Nestor, M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumor Biol. 2013, 35, 2053–2062. [Google Scholar] [CrossRef]
- Nimse, S.B.; Sonawane, M.D.; Song, K.S.; Kim, T. Biomarker detection technologies and future directions. Analyst 2016, 141, 740–755. [Google Scholar] [CrossRef]
- Pushpesh, R.; Mohd, A.S.; Shalu, Y.; Raju, K. An Electrochemical Immunosensor Based on Gold-Graphene Oxide Nanocomposites with Ionic Liquid for Detecting the Breast Cancer CD44 Biomarker. ACS Appl. Mater. Interfaces 2022, 18, 20802–20812. [Google Scholar] [CrossRef]
- Bobo, F.; Qi, F.; Min, C.; Tingting, W.; Jinshen, W.; Hongmin, M.; Qin, W. Photoelectrochemical Biosensor for Sensitive Detection of Soluble CD44 Based on the Facile Construction of a Poly (ethylene glycol)/Hyaluronic Acid Hybrid Antifouling Interface. ACS Appl. Mater. Interfaces 2019, 11, 24764–24770. [Google Scholar] [CrossRef]
- Shaimerdenova, M.; Ayupova, T.; Sypabekova, M.; Tosi, D. Fiber optic refractive index sensors based on a ball resonator and optical backscatter interrogation. Sensors 2020, 20, 6199. [Google Scholar] [CrossRef]
- Tosi, D.; Ashikbayeva, Z.; Bekmurzayeva, A.; Myrkhiyeva, Z.; Rakhimbekova, A.; Ayupova, T.; Shaimerdenova, M. Optical Fiber Ball Resonator Sensor Spectral Interrogation through Undersampled KLT: Application to Refractive Index Sensing and Cancer Biomarker Biosensing. Sensors 2021, 21, 6721. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzayeva, A.; Ashikbayeva, Z.; Assylbekova, N.; Myrkhiyeva, Z.; Dauletova, A.; Ayupova, T.; Shaimerdenova, M.; Tosi, D. Ultra-wide, attomolar-level limit detection of CD44 biomarker with a silanized optical fiber biosensor. Biosens. Bioelectron. 2022, 208, 114217. [Google Scholar] [CrossRef]
- Kedruk, Y.Y.; Bobkov, A.A.; Gritsenko, L.V.; Moshnikov, V.A. Investigation of the Properties of Zinc Oxide by the Method of impedance spectroscopy. Glass Phys. Chem. 2022, 48, 123–129. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K.; Ramesh Babu, R.; Sethuraman, K.; Moorthy Babu, S. Hydrothermally Grown ZnO Nanoparticles for Effective Photocatalytic Activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Hall, B.; Zanchet, D.; Ugarte, D. Estimating Nanoparticle Size from Diffraction Measurements. J. Appl. Crystallogr. 2000, 33, 1335–1341. [Google Scholar] [CrossRef]
- He, B.B. Introduction to two-dimensional X-ray diffraction. Powder Diffr. 2003, 18, 71–85. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Albert, J.; Lepinay, S.; Caucheteur, C.; DeRosa, M.C. High resolution grating-assisted surface plasmon resonance fiber optic aptasensor. Methods 2013, 63, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a uniform metrological assessment of grating-based optical fiber sensors: From refractometers to biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paltusheva, Z.U.; Ashikbayeva, Z.; Tosi, D.; Gritsenko, L.V. Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein. Biosensors 2022, 12, 1015. https://doi.org/10.3390/bios12111015
Paltusheva ZU, Ashikbayeva Z, Tosi D, Gritsenko LV. Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein. Biosensors. 2022; 12(11):1015. https://doi.org/10.3390/bios12111015
Chicago/Turabian StylePaltusheva, Zhaniya U., Zhannat Ashikbayeva, Daniele Tosi, and Lesya V. Gritsenko. 2022. "Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein" Biosensors 12, no. 11: 1015. https://doi.org/10.3390/bios12111015
APA StylePaltusheva, Z. U., Ashikbayeva, Z., Tosi, D., & Gritsenko, L. V. (2022). Highly Sensitive Zinc Oxide Fiber-Optic Biosensor for the Detection of CD44 Protein. Biosensors, 12(11), 1015. https://doi.org/10.3390/bios12111015





