Abstract
Diabetes mellitus has become a worldwide epidemic, and it is expected to become the seventh leading cause of death by 2030. In response to the increasing number of diabetes patients worldwide, glucose biosensors with high sensitivity and selectivity have been developed for rapid detection. The selectivity, high sensitivity, simplicity, and quick response of electrochemical biosensors have made them a popular choice in recent years. This review summarizes the recent developments in electrodes for non-enzymatic glucose detection using carbon nanofiber (CNF)-based nanocomposites. The electrochemical performance and limitations of enzymatic and non-enzymatic glucose biosensors are reviewed. Then, the recent developments in non-enzymatic glucose biosensors using CNF composites are discussed. The final section of the review provides a summary of the challenges and perspectives, for progress in non-enzymatic glucose biosensors.
1. Introduction
In recent decades, diabetes mellitus has become a worldwide epidemic [1]. Globally, diabetes is expected to become the seventh leading cause of death by 2030, according to reports [2,3,4,5]. Generally, a blood glucose range of less than 100 mg/dL is considered normal. If the blood glucose level of a patient is higher than 126 mg/dL on two consecutive fasting blood glucose tests, the patient is considered diabetic [6,7]. In the human body, glucose levels can be categorized into two types. First, low glucose levels, known as hypoglycemia, can lead to fainting, comas, and even death, when blood sugar levels drop below normal levels. Hyperglycemia is the second phenomenon that undesirably influences the kidneys, eyes, nerves, and blood vessels, causing blood sugar levels to rise abnormally [8,9,10]. As a result of insufficient insulin production in the body (Type-1 diabetes) or not being able to use the insulin produced in the body (Type-2 diabetes), diabetes is defined by hyperglycemia, or a high blood glucose level [11,12,13]. The hormone insulin aids the cells of the body in absorbing glucose in the blood. Glucose concentrations in the blood can rise to a level where the kidneys, eyes, nerves, and heart can be damaged, in both Type-1 and Type-2 diabetes, if the body is not provided with insulin [14,15].
In response to the increasing number of diabetes patients worldwide, glucose biosensors with high sensitivity and selectivity have been developed for rapid detection [16,17]. Biosensors are devices that transform biological events into electrical signals [18,19,20]. A variety of techniques have been proposed for glucose monitoring, but electrochemical biosensors are preferred, owing to their ease of use, low cost, and ability to be combined with portable devices to provide numerical analysis [21,22,23,24]. Two kinds of electrochemical glucose biosensors are available: enzymatic and non-enzymatic. In an enzymatic biosensor, glucose oxidase enzyme (GOx) is used for the detection of glucose; whereas in non-enzymatic biosensors, the glucose detection mechanism is based on electrocatalytic activities [25,26,27,28].
Carbon nanofiber (CNF) is similar to CNTs in structure and properties, but it is easier to produce, has a lower cost, and provides improved functionality [29,30,31]. CNFs are carbon nanomaterials with diameter in the nanometer range and length in the micron range [32]. Much attention has been given to nanocomposites, owing to their exceptional properties [33,34,35,36]. Modification of bare electrode surfaces using nanocomposite materials comprising nanomaterials and matrices takes advantage of the properties of both nanomaterials and matrices [37,38,39]. Conductive nanocomposites are used in numerous biomedical applications, including biosensors and actuators [40,41,42]. CNFs have good thermal conductivity, outstanding electrical conductivity, high porosity, and high surface area, making them excellent substrates and supporting matrices for embedding nanoparticles for the synthesis of CNF-based nanocomposites [43,44,45]. CNFs are considered a strong substrate for non-enzymatic biosensors, because of their easy manufacturing process, high electrical conductivity, and high specific surface area. Additionally, CNF-based electrodes can be prepared as free-standing, without the addition of a binder or glassy carbon electrode (GCE) being applied to the actual glucometer as a strip [46,47].
Electrospinning and catalytic vapor deposition growth (CVD) are the two main methods for fabricating CNFs [48]. Electrospinning produces nanofibers by uniaxial stretching of a viscoelastic solution. Electrospinning uses electrostatic forces to stretch the solution as it solidifies, unlike dry-spinning and melt-spinning. Fibers are formed by drawing the solution, as long as the electrospinning jet is fed with a sufficient solution. Therefore, fiber formation is continuous, without interruption of the electrospinning jet [49]. Electrospinning has been used to produce nanofibers of various polymers, with diameters ranging from tens of nanometers to a few micrometers, and in various forms, such as nonwoven mats (webs) and yarns. A continuous nanofiber can be produced from polymer solutions or melts using this relatively simple and low-cost method [50]. Through the electrospinning technique, CNFs can be easily produced via the carbonization of polymer nanofibers, which are called electrospun CNFs (ECNFs) [32,51]. Polyacrylonitrile (PAN) is the most widely used polymer precursor for the electrospinning of carbon nanofibers, owing to its easy processing, excellent mechanical properties, and high carbon yield [52,53]. CNFs can also be made by direct carbonization of other polymers, such as cellulose, chitin, lignin, and chitosan NFs [54,55]. CVD can also produce carbon nanofibers with different structures and properties from those made by electrospinning, called vapor-grown carbon fibers (VGCF) [50]. In CVD, CNFs are synthesized using a catalyst to grow carbon in a 1D pattern on a substrate [56]. As a result of their high electrical conductivity, mechanical properties, and specific surface area, CNFs have numerous potential applications in a broad range of industries, such as energy storage devices (supercapacitors and batteries), the reinforcement of nanocomposites, biomedicine, textiles, filtration, drug delivery, and especially biosensing [57,58,59,60]. Figure 1 illustrates some applications of CNFs in different fields.
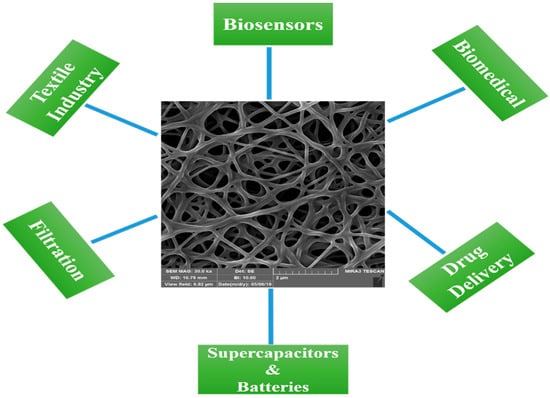
Figure 1.
Different applications of CNFs.
Figure 2 illustrates the characteristics of CNFs that make them ideal diagnostic electrodes for biosensors. Owing to their high conductivity, CNFs facilitate and accelerate electron transfer in the electrode, which results in higher biosensor sensitivity [60]. Additionally, CNF-based electrodes possess high mechanical properties, resulting in the high strength of the biosensor electrodes. Furthermore, carbon nanofibers are easy to synthesize and produce, which reduces the cost of manufacturing biosensor electrodes [61]. Moreover, owing to the large specific surface area of carbon nanofibers, a large number of nanoparticles can be embedded on their surface, with a uniform dispersion, thus enhancing the electrocatalytic properties of the carbon nanofiber electrodes and increasing the sensitivity of the biosensor [62].
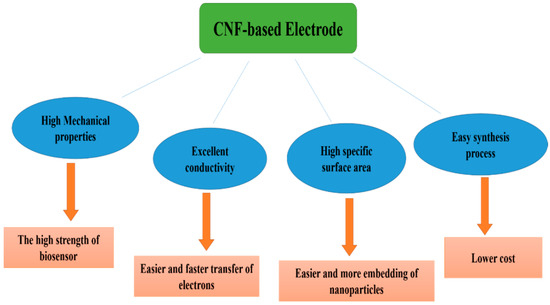
Figure 2.
The characteristic properties of CNFs and the effect of the properties on the performance of the fabricated biosensor.
After a brief discussion on enzymatic and non-enzymatic biosensors, non-enzymatic biosensors of glucose based on nanocomposites of CNF are discussed. Subsequently, the challenges associated with non-enzymatic glucose biosensors and their advantages are summarized.
2. Enzymatic Electrochemical Biosensors for Glucose Sensing
Glucose oxidase (GOx) is a common enzyme used in enzymatic biosensors [63,64,65]. In the 1960s, Clark and Lyons described the first enzyme-based biosensor [66]. Enzyme-based biosensors are widely used and extensively studied, owing to their excellent selectivity and sensitivity [67]. There are three types of enzymatic glucose biosensors [68,69]. In the first type, an amperometric technique is used, which involves immobilizing a catalytic enzyme, to reduce oxygen to hydrogen peroxide. Oxygen dependence and a high operating potential are the main disadvantages of this kind of biosensor [70,71]. The next type of glucose biosensor is designed to overcome the oxygen deficiency under a low oxygen pressure and high applied potential. GOx is immobilized on redox mediators in these biosensors [72,73]. Typical electron redox mediators include ferrocene derivatives, ferrocyanide, conducting organic salts, and quinones [74,75]. In the third type of biosensor, electrons are transferred directly between the immobilized enzyme active sites and the electrode surfaces [76]. Compared with the first and second types of glucose biosensors, the third type exhibits deteriorated detection properties, especially a smaller linear range [26,67]. The properties of GOx influence the performance of enzymatic biosensors. Studies have shown that GOx is unstable at pH values lower than 2 and higher than 8. Furthermore, humidity and temperatures above 40 °C significantly reduce the activity of enzymes. Moreover, enzyme immobilization methods are complex and costly [77,78]. Recent research has increasingly focused on non-enzymatic glucose biosensors, because of the disadvantages of GOx [79]. Non-enzymatic biosensors work by directly oxidizing glucose on an electrode that possesses electrocatalytic activity and contains a transition metal center [80,81].
3. Non-Enzymatic Electrochemical Biosensors for Glucose Sensing
Owing to the inherent disadvantages of traditional glucose enzymatic biosensors, such as their high fabrication costs, poor enzyme stability, pH value dependence, and other dedicated limitations, non-enzymatic glucose sensors are gaining increased attention. Considering the drawbacks of enzyme-based biosensors, researchers are developing new electrochemical biosensors without using any biological molecules such as enzymes; that is, non-enzymatic biosensors [82,83,84]. Unlike enzymatic sensors, non-enzymatic glucose biosensors do not suffer from enzyme immobilization problems. Furthermore, the stability of non-enzymatic biosensors is higher, owing to the absence of enzymes. An enhanced sensitivity and specificity of detection can be achieved through functionalized nanomaterials acting as catalysts or immobilization platforms. The use of enzyme-less materials based on straight glucose electro-oxidation led to the fourth-generation glucose biosensor [85,86]. Compared with their enzyme counterparts, these biosensors exhibit considerably enhanced electrocatalytic performance for glucose detection, owing to the incorporation of nanostructured metal or metal oxide on the electrode surfaces [87,88,89]. The key step in designing non-enzymatic biosensors is selecting the appropriate catalyst for glucose detection [90,91]. The sensitivity, selectivity, and stability of certain non-enzymatic electrochemical glucose biosensors have been well-established, through extensive research. In addition, non-enzymatic glucose biosensors are cost-effective, stable, reproducible, and simple to develop, without any complicated enzyme immobilization methods [92,93,94]. There are several types of non-enzymatic electro-catalysts, including metallic nanoparticles, metal oxide nanoparticles, bimetallic/alloys nanostructure, carbon nanomaterials, and metal/carbon nanomaterial-based nanocomposites [61,62,95,96].
4. Non-Enzymatic Glucose Biosensors Based on Nanocomposites of CNF
Recently, CNFs have been considered excellent substrates for embedding metal and metal oxide nanoparticles as sensitive nanocomposites for biosensors, owing to their high specific surface area and porosity, high mechanical properties, and high electrical conductivity [31,97,98,99]. Owing to advantages such as a facile and environmentally friendly fabrication process, high conductivity, high surface area, and high porosity, CNFs have been widely used as electrodes for biosensors. The large specific surface area of the CNF allows a large number of nanoparticles and catalysts to be incorporated onto its surface, enhancing the electrocatalytic performance when reacting with a target analyte [100,101]. Moreover, adding metal nanoparticles or metal oxide nanoparticles to the CNF matrix increases the electrocatalytic activity of the biosensor and enhances the biosensor performance, in terms of the limit of detection (LOD), linear range (LR), and sensitivity.
With the growing number of diabetic patients, it is necessary to develop a blood glucose measuring device. Commercial glucometer strips are usually coated with enzymes. The instability of enzymes in environmental conditions, the difficulty of immobilizing enzymes on electrode surfaces, and the high cost of enzymes have motivated researchers to develop non-enzymatic biosensors. A substrate is used to embed the catalyst in non-enzymatic biosensors. Carbon nanofibers (CNFs) are a good substrate for non-enzymatic biosensors, because of their easy manufacturing process, high electrical conductivity, and high specific surface area. Additionally, CNF-based electrodes can be prepared free-standing, without the addition of a binder or GCE, to use with the actual glucometer as a strip. Various nanostructures have been attached to the CNF surface as electro-catalysts, including noble metals, nickel-based nanoparticles, cobalt-based nanoparticles, and copper-based nanoparticles [102,103,104,105]. Table 1 summarizes all the non-enzymatic glucose biosensors that use CNF composite electrodes.

Table 1.
CNF-based non-enzymatic glucose biosensors and their detection performances.
4.1. Mono Metallic (Metal Oxide)/CNF Nanocomposites as Non-Enzymatic Glucose Biosensors
Non-enzymatic biosensors with CNF nanocomposites are typically made using nickel, cobalt, copper, platinum, nickel oxide, cobalt oxide, and copper oxide nanoparticles. Liu et al. [122] proposed a glucose biosensor with Ni nanoparticle-loaded CNF electrodes by combining electrospinning with thermal treatment. Owing to the large surface of CNF and the high electrocatalytic activity of Ni nanoparticles, the Ni/CNF electrode showed an excellent electrocatalytic performance for oxidizing glucose.
As shown in Figure 3, the Ni/CNF electrode exhibits a pair of redox peaks in the blank NaOH solution, corresponding to the Ni(II)/Ni(III) redox couple. An increase in the glucose concentration also led to a greater voltammetric response. As a result, Ni/CNF electrodes showed an adequate electrocatalytic activity. Additionally, the CNF electrode showed no response current to glucose, whereas the Ni/CNF electrode showed oxidation/reduction peaks, indicating that Ni nanoparticles play a key role in electrocatalytic sensing [133]. The figure also shows that the peak current in the Ni/CNF nanocomposite electrode changed with the glucose concentration, indicating that the biosensor is highly sensitive. Moreover, the results indicate that Ni/CNF electrodes do not exhibit significant changes in their current response when glucose is oxidized, which indicates that the electrode is highly resistant to surface fouling and has excellent selectivity, making it a good candidate for developing a low-cost non-enzymatic glucose biosensor. The presented sensor has a sensitivity of 420.4 μA·mM−1·cm−2, an LOD of 10−3 mM, and an LR of 2 × 10−3–2.5 mM.
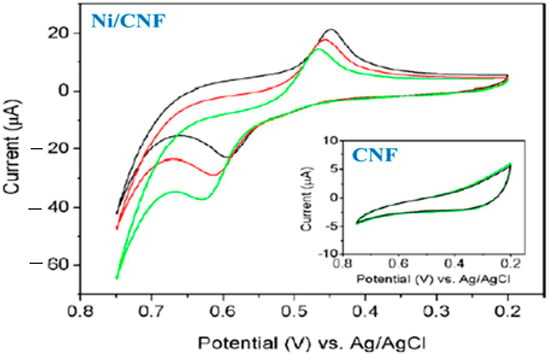
Figure 3.
CVs of the Ni/CNF nanocomposite without (dark line) and with 2 mM (red line) and 4 mM (green line) glucose. Inset shows the CVs of pure CNF without (dark line) and with glucose. Reprinted from Ref. [122], with permission from Elsevier.
Biosensors have been constructed using a variety of nanostructures, including nanoplatelets, nanoflakes, nanorods, nanoribbons, nanowires, and nanotubes because of their interesting properties, such as a large surface-to-volume ratio, high porosity, excellent electrocatalytic properties, and high surface area [24,134,135]. Chen et al. [106] constructed a biosensor using a nanocomposite of Ni(OH)2 nanoplatelets and ECNF. Figure 4 shows the steps involved in manufacturing Ni(OH)2/ECNF nanocomposites. Initially, CNFs are synthesized using an electrospinning technique, and then Ni(OH)2 nanoplatelets are deposited on the surface of CNFs. A conductive substrate such as CNF facilitates the electron transfer and improves the sensitivity of biosensors. CNFs are also capable of embedding more nanoparticles with good dispersion, owing to their large specific surface area. In addition, Ni(OH)2 with the morphology of nanoplatelets increases the effective specific surface area of the electrode and results in an easier absorption of glucose molecules on the electrode surface, thereby improving the performance of the biosensor. EIS analysis of this group showed that Ni(OH)2/ECNF-modified nanocomposites had smaller Rct values than pure Ni(OH)2, suggesting that the ECNF substrate considerably enhanced the conductivity of the nanocomposite.
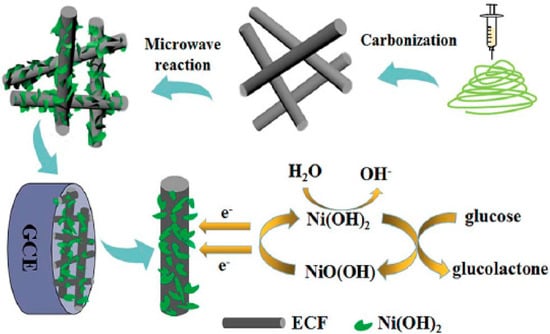
Figure 4.
Ni(OH)2/ECNF nanocomposite fabrication steps. Reprinted from reference [106], with permission from the Royal Society of Chemistry.
Ni(OH)2/ECNF electrodes displayed smaller anodic peak potentials and higher peak currents than pure Ni(OH)2 electrodes, suggesting that the high electrical conductivity of the ECNF matrix facilitated the redox processes of Ni(II)/Ni(III). Furthermore, one-dimensional ECNFs can prevent aggregation of Ni(OH)2 nanoparticles and may simplify the diffusion of the electrolyte into Ni(OH)2 nanoplatelet electrodes. Thus, when the excellent electrochemical performance of ECNFs, such as their high conductivity and high surface area, is combined with the electrocatalytic properties of Ni(OH)2 nanoplatelets, a synergistic effect is achieved.
Nanofillers with small diameters (d) and long lengths (l) have a high aspect ratio (l/d) and large specific surface area; hence, they have excellent electron-transfer properties and electrocatalytic activity [136]. An enzyme-less glucose biosensor was developed by Ye et al. [115] using ECNFs with various diameters as Pt-catalyst supports. In ECNFs with a smaller diameter, more Pt atoms are observed on the surface of the ECNF, owing to the high surface area. In addition, a ECNF with a smaller diameter has a greater electrochemical activity and superior electrocatalytic performance for the detection of glucose. Thus, a Pt/ECNF nanocomposite electrode with a smaller ECNF diameter shows a better response current and higher sensitivity (Figure 5). For nanofibers with smaller diameters, the slope of the calibration curve is higher, indicating that the sensitivity increases with the decreasing diameter of the CNFs. In other words, reducing the fiber diameter increases the aspect ratio, which improves the properties, especially the electrical conductivity. Carbon nanofibers with higher electrical conductivity result in a faster electron transfer on the electrode surface, resulting in improved biosensor sensitivity and response times. In addition, according to the chronoamperometric diagram, each time glucose is added and a step is formed, the current remains constant, until the next glucose injection, showing the high stability of the biosensors.
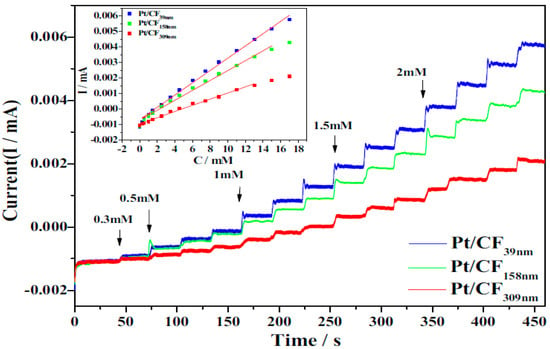
Figure 5.
Amperometric analysis and corresponding calibration plots (inset) of Pt/ECNF nanocomposites with different diameters of ECNF. Reprinted from reference [115], with permission from Elsevier.
Zhang et al. [117] presented a nanocomposite of nano-cupric oxide (CuONPs) on a CNF surface for fabricating a non-enzymatic glucose biosensor. Figure 6 illustrates the glucose detection mechanism of a CuONPs-CNF nanocomposite. Glucose was rapidly oxidized to gluconolactone by the copper (III) formed on the surface of the nanoparticles. Meanwhile, the oxidation peak current increased, whereas the reduction peak current decreased, owing to the feeding of Cu (III) agents and the formation of Cu (II) agents. Cu (III) may act as a mediator for electron transfer. CuONPs electrocatalysts and glucose molecules undergo an electrochemical reaction, releasing electrons and producing electrical signals proportional to the glucose concentration. The conductive substrate of CNFs promotes a faster electron transfer, enables CuONPs to disperse on their surface, and, as a supporting matrix, prevents the separation of nanoparticles from the electrode surfaces. The results of this study indicate that CuONPs can act as a strong electrocatalyst for glucose oxidation, thus enhancing the performance of the biosensor. The designed biosensor revealed a wide LR to glucose from 5 × 10−4 mM to 11.1 mM, with a high sensitivity of 2739 μA·mM−1·cm−2 and a low LOD of 2 × 10−4 mM.
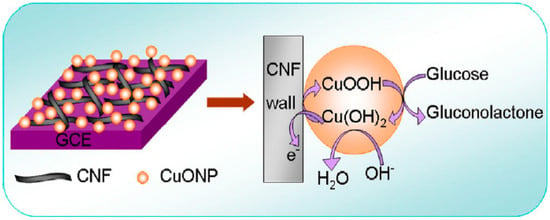
Figure 6.
Schematic illustration of glucose recognition mechanism based on a CuONPs-CNF nanocomposite. Reprinted from reference [117], with permission from Elsevier.
4.2. Hybrid Nanoparticle/CNF Nanocomposites as a Non-Enzymatic Glucose Biosensor
Researchers have focused on creating hybrid nanostructures composed of two or more different materials, to overcome the limitations of single-component nanoparticles, improve their properties, achieve novel properties that are not possible with single-component nanoparticles, and achieve multiple functionalities. Nanostructures with hybrid compositions have superior electrochemical properties than nanoparticles with single components. There is a synergistic effect between the components, which produces these results. Consequently, it is expected that combining two or more nanoparticles with ECNF matrices could be a unique approach for fabricating advanced electrode materials for non-enzymatic glucose detection [60,137,138].
Ding et al. [107] developed a nanocomposite of CoFe2O4 nanoparticles and ECNFs via electrospinning and subsequent heat treatment process. ECNFs with outstanding conductivity functioned as a substrate guiding the development of CoFe2O4 particles, thus improving the CoFe2O4 dispersion and forming a superior electron transportation path. Additionally, electrospun nanofibers can be used in the electrocatalytic reaction processes of CoFe2O4 as electron transport channels, owing to their large surface, electrical conductivity, and broad electrochemical window. The proposed nanocomposite electrode exhibits excellent detection properties, owing to the effects of CoFe2O4 nanoparticles and an ECNF matrix. It has an adequate sensitivity of 318 μA·mM−1·cm−2, a wide LR from 10−2 to 3.52 mM, and a low LOD of 3.25 × 10−4 mM.
Li et al. [112] prepared MCo (M = Cu, Fe, Ni, and Mn) nanoparticles embedded in CNFs as non-enzymatic glucose biosensors. Figure 7 represents the stages of the synthesis of nanocomposites and the fabrication of the biosensors. As shown in the figure, during the process of heat treatment, the PVP nanofibers are converted into CNFs, and at high temperatures, metal nanoparticles diffuse from the bulk of the CNFs to their surface, owing to the presence of a metal concentration gradient between the bulk and CNF surface [60,61]. The presence of nanoparticles on the surface of CNFs improves the electrocatalytic properties of the electrode surface in the reaction with glucose analyte and enhances the performance of the biosensor. Based on these results, CuCo/CNFs exhibit the highest catalytic ability, followed by Co/CNFs, NiCo/CNFs, FeCo/CNFs, and MnCo/CNFs. Additionally, according to the chronoamperometric plot shown in Figure 7, CuCo/CNFs display the highest sensing efficiency, even for the sensing of glucose in a real sample, owing to the structural advantages of the CNF matrix and the simultaneous effects of Co (III)/Co (IV) and Cu (II)/Cu (III) redox couples. The proposed biosensor has an LOD of 10−3 mM, high sensitivity of 507 μA·mM−1·cm−2, and an LR of 0.02–11 mM. Moreover, the proposed biosensor is neutral with other interfering species in blood and exhibits a high selectivity.
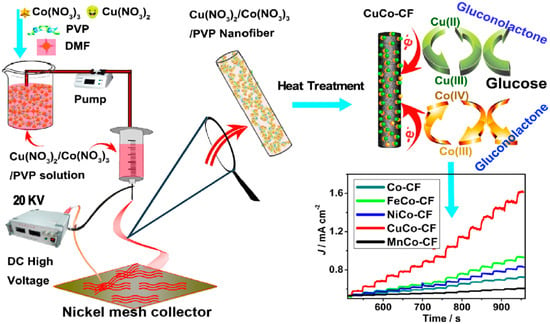
Figure 7.
Synthesis steps of Co/CNFs and MCo/CNFs (M = Fe, Ni, Cu, and Mn) nanocomposites, electrochemical mechanism of electrodes, and the results of amperometric analysis of biosensors. Reprinted from reference [112], with permission from Elsevier.
Yin et al. [116] developed an enzyme-less biosensor by decorating the surface of an ECNF matrix with binary nanoparticles of Co3O4 and MnO2 for glucose sensing. Compared with monometallic Co3O4 or MnO2 electrodes with ECNFs, binary MnO2-Co3O4/ECNF electrodes showed an outstanding consistency, with high porosity, large electrochemical surface area, increased conductivity, and high glucose electro-oxidation effectiveness. As shown in the EIS spectra in Figure 8, MnO2-Co3O4/ECNF electrodes exhibited the smallest Nyquist semicircle and the lowest Rct compared with ECNF electrodes electrodeposited with MnO2 or Co3O4. This indicates that the MnO2/Co3O4@ECNF electrodes exhibited the highest electron and mass transport efficiency. The permeable structure with a bigger surface for electrolyte ions could explain this decrease in resistance. Owing to the morphological and physical aspects of binary MnO2-Co3O4/ECNFs, the ion penetration to the electrode surface and conductivity improved. The binary metal oxides, as well as the ECNF matrix with high porosity, considerably enhanced the detection performance, with an outstanding sensitivity = 1159 μA·mM−1·cm−2 and a low LOD = 3 × 10−4 mM with LR = 0.005–10.9 mM.
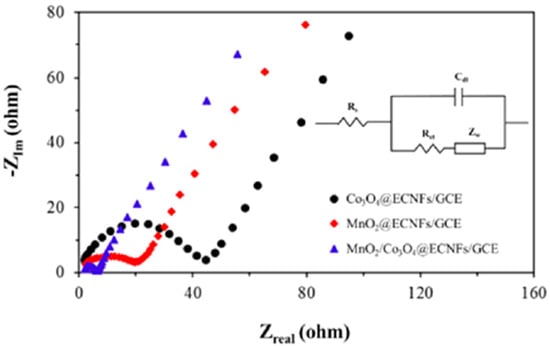
Figure 8.
Nyquist plots of electrodes in a 0.1 M KCl solution containing 5 mM [Fe(CN)6]3−/4−. Reprinted from reference [116], with permission from ACS.
Recently, our group [61] presented a glucose biosensor using metal and metal oxide nanoparticles of nickel, cobalt, and hybrid nanoparticles decorated on the surface of ECNFs. As shown in the SEM images, the nanoparticles were uniformly dispersed on the ECNF surface. The SEM image also shows that carbon nanofibers with nanometer diameters and micron lengths were synthesized, without beads. The results showed that the nickel nanoparticles exhibited better electrocatalytic properties in reaction with glucose than cobalt nanoparticles. Nanocomposites containing nickel had a higher electrical conductivity than those containing cobalt. The increased electrical conductivity of nickel metal, compared with cobalt metal, increased the electron transfer in glucose oxidation-reduction reactions, leading to stronger electrocatalytic properties and better sensitivity in biosensors. The nickel electrode sensitivity was 610.6 μA·mM−1·cm−2, which was much higher than the cobalt electrode sensitivity (236.85 μA·mM−1·cm−2). The electrocatalytic properties of the final biosensor with the hybrid nanoparticles were improved by increasing the percentage of nickel in the electrospinning solution. Moreover, the ECNF matrix acted as an excellent supporting substrate for embedding nanoparticles because of its high conductivity and large surface area. As a result, the electrode displayed excellent selectivity for detecting glucose, even in the presence of interfering agents (Figure 9). This high selectivity may be attributed to the lower applied potentials, which could be achieved because of the higher electrical conductivity and easier electron transfer of CNF-based nanocomposites.
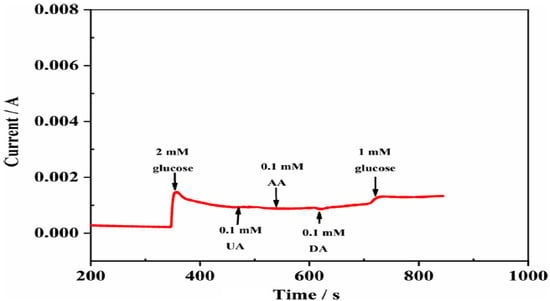
Figure 9.
The amperometric response of an electrode to successive injections of 1.0 and 2.0 mM glucose and 0.1 mM interferents of UA, AA, and DA in 0.1 M NaOH solution. Reprinted from reference [61], with permission from Springer.
Huan et al. [127] developed a nanocomposite of CuSn/CNFs as a biosensor for glucose recognition. They found that an appropriate amount of Sn considerably increases the electrocatalytic activity of Cu/CNFs for oxidation of glucose. Thus, by adding doped Sn to CuSn/CNF, large defects can be introduced to increase the electron transference rate and decrease the activation energy needed for the intermediate process. Therefore, CuSn/CNFs exhibited a higher oxidation current than Cu/CNFs for the detection of glucose [139]. The interaction between Cu and Sn nanoparticles on the CNF matrix enhanced the electrocatalytic activity of CuSn/CNFs for sensing. The biosensor exhibited an exceptional sensing performance for glucose, with a broad LR in the range from 10−4 to 9 mM, low LOD of 8 × 10−5 mM, and adequate sensitivity of 291.4 μA·mM−1·cm−2.
5. Conclusions and Future Challenges
This review has presented an overview of the recent advances in non-enzymatic electrochemical biosensing of glucose using CNF-based nanocomposites. A wide range of electrochemical biosensors with excellent analytical performance have been constructed using the attractive properties of CNFs, such as their high porosity, excellent conductivity, great mechanical properties, and high superficial area. The high electrical conductivity of CNFs improves the performance of biosensors. Adding metal nanoparticles or metal oxide nanoparticles to the CNF matrix increases the electrocatalytic activity of the biosensor, as well as its performance. In the initial research studies, metals such as Ni, Cu, Co, Pt, and Pd, and compounds such as nickel oxides, cupric oxide, and cobalt oxides were embedded into a CNF matrix for manufacturing nanocomposite electrodes. More recently, combinations of different metal compounds, such as alloys and bimetallic nanoparticles embedded into CNFs, have been explored for their synergistic effects and excellent electrocatalytic performance. However, a major obstacle to using non-enzymatic biosensors for blood samples is that most non-enzymatic glucose biosensors cannot catalyze glucose oxidation under physiological conditions. Thus, most non-enzymatic glucose biosensors reported in the literature use alkaline media to detect glucose, while the physiological pH of blood is 7.4. This is particularly relevant for the electrodes made of Cu, Ni, metal oxides, and carbon nanomaterials, despite their excellent sensitivity. Thus, these electrodes cannot be used to directly diagnose diabetes without a preliminary blood preparation. Another major challenge in non-enzymatic glucose biosensors is the selectivity of these devices. The GOx enzyme is specific for glucose detection in enzymatic glucose biosensors; however, in non-enzymatic glucose biosensors, interfering species in the blood, such as dopamine, uric acid, and ascorbic acid, may react with the electrode surface and cause errors in blood glucose detection. Consequently, it is important to choose a catalyst that has excellent electrocatalytic properties. There is ongoing research on non-enzymatic biosensors, but further research on electrodes with different electro-catalysts is needed, for their commercialization as the fourth generation of electrochemical glucose biosensors. Fabrication cost is the major concern in the commercialization of non-enzymatic electrodes. As a result, relatively less research has been done on this type of CNF-based biosensor. It would be beneficial to conduct further studies using new nanostructured materials as catalysts for reactions. However, in the last decade, this type of biosensor has attracted the attention of many researchers. Non-enzymatic glucose biosensors are still in the R&D stage and have not yet reached the commercialization stage. The most common type of glucometer available on the market is the enzymatic biosensor. In this regard, it is hoped that researchers in this field will develop fourth-generation commercial glucometers based on non-enzymatic biosensors.
Author Contributions
Conceptualization, A.M.-H., S.M.-H. and Y.Z.; methodology, A.M.-H., S.M.-H. and Y.Z.; software, A.M.-H. and Y.Z.; validation, A.M.-H., Y.Z., K.Y.R. and S.-J.P.; formal analysis, A.M.-H.; investigation, A.M.-H., S.M.-H. and Y.Z.; data curation, A.M.-H., S.M.-H. and Y.Z.; writing—original draft preparation, A.M.-H. and Y.Z.; writing—review and editing, A.M.-H., Y.Z., K.Y.R. and S.-J.P.; supervision, Y.Z. and K.Y.R.; project administration, K.Y.R.; funding acquisition, K.Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022M3J7A1062940).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bamgboje, D.; Christoulakis, I.; Smanis, I.; Chavan, G.; Shah, R.; Malekzadeh, M.; Violaris, I.; Giannakeas, N.; Tsipouras, M.; Kalafatakis, K.; et al. Continuous Non-Invasive Glucose Monitoring via Contact Lenses: Current Approaches and Future Perspectives. Biosensors 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Cano Perez, J.L.; Gutiérrez-Gutiérrez, J.; Perezcampos Mayoral, C.; Pérez-Campos, E.L.; Pina Canseco, M.d.S.; Tepech Carrillo, L.; Mayoral, L.P.-C.; Vargas Treviño, M.; Apreza, E.L.; Rojas Laguna, R. Fiber Optic Sensors: A Review for Glucose Measurement. Biosensors 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A Review of Recent Advances in Nonenzymatic Glucose Sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Novel SWCNTs-Mesoporous Silicon Nanocomposite as Efficient Non-Enzymatic Glucose Biosensor. Appl. Surf. Sci. 2021, 552, 149477. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Shaye, K.; Amir, T.; Shlomo, S.; Yechezkel, S. Fasting Glucose Levels within the High Normal Range Predict Cardiovascular Outcome. Am. Heart J. 2012, 164, 111–116. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Sweet Potato (Ipomoea Batatas L.) Leaf Polyphenols Ameliorate Hyperglycemia in Type 2 Diabetes Mellitus Mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Perlmuter, L.C.; Flanagan, B.P.; Shah, P.H.; Singh, S.P. Glycemic Control and Hypoglycemia: Is the Loser the Winner? Diabetes Care 2008, 31, 2072–2076. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.-K.; Yu, J.-Q.; Tan, R.; Yang, P.-L. Hypoglycemia and Mortality in Sepsis Patients: A Systematic Review and Meta-Analysis. Heart Lung 2021, 50, 933–940. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Flores-DelaToba, R.; González-González, M.; Rito-Palomares, M. Current Challenges and Future Trends of Enzymatic Paper-Based Point-of-Care Testing for Diabetes Mellitus Type 2. Biosensors 2021, 11, 482. [Google Scholar] [CrossRef]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 Diabetes Mellitus in Older Adults: Clinical Considerations and Management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]
- Von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and Future Therapies for Type 1 Diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Teju, V.; Sowmya, K.V.; Yuvanika, C.; Saikumar, K.; Krishna, T.B.D.S. Detection of Diabetes Melittus, Kidney Disease with ML. In Proceedings of the 2021 3rd International Conference on Advances in Computing, Communication Control and Networking (ICAC3N), Greater Noida, India, 17–18 December 2021; pp. 217–222. [Google Scholar]
- Jwad, S.M.; AL-Fatlawi, H.Y. Types of Diabetes and Their Effect on the Immune System. J. Adv. Pharm. Pract. 2022, 4, 21–30. [Google Scholar]
- Lin, M.-H.; Gupta, S.; Chang, C.; Lee, C.-Y.; Tai, N.-H. Carbon Nanotubes/Polyethylenimine/Glucose Oxidase as a Non-Invasive Electrochemical Biosensor Performs High Sensitivity for Detecting Glucose in Saliva. Microchem. J. 2022, 180, 107547. [Google Scholar] [CrossRef]
- Settu, K.; Chiu, P.-T.; Huang, Y.-M. Laser-Induced Graphene-Based Enzymatic Biosensor for Glucose Detection. Polymers 2021, 13, 2795. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Effects of Interphase Regions and Filler Networks on the Viscosity of PLA/PEO/Carbon Nanotubes Biosensor. Polym. Compos. 2019, 40, 4135–4141. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Park, S.-J. Simple Model for Hydrolytic Degradation of Poly (Lactic Acid)/Poly (Ethylene Oxide)/Carbon Nanotubes Nanobiosensor in Neutral Phosphate-Buffered Saline Solution. J. Biomed. Mater. Res. Part A 2019, 107, 2706–2717. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Effect of Contact Resistance on the Electrical Conductivity of Polymer Graphene Nanocomposites to Optimize the Biosensors Detecting Breast Cancer Cells. Sci. Rep. 2022, 12, 5406. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Electrochemical Biosensors Based on Polymer Nanocomposites for Detecting Breast Cancer: Recent Progress and Future Prospects. Adv. Colloid Interface Sci. 2022, 309, 102795. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Mohammad Haniff, M.A.S.; Mat Zaid, M.H.; Choon, O.P.; Mohd Razip Wee, M.F. Label Free Glucose Electrochemical Biosensor Based on Poly(3,4-Ethylenedioxy Thiophene): Polystyrene Sulfonate/Titanium Carbide/Graphene Quantum Dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef]
- Madden, J.; Barrett, C.; Laffir, F.R.; Thompson, M.; Galvin, P.; O’Riordan, A. On-Chip Glucose Detection Based on Glucose Oxidase Immobilized on a Platinum-Modified, Gold Microband Electrode. Biosensors 2021, 11, 249. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Development of a Theoretical Model for Estimating the Electrical Conductivity of a Polymeric System Reinforced with Silver Nanowires Applicable for the Biosensing of Breast Cancer Cells. J. Mater. Res. Technol. 2022, 18, 4894–4902. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Electrochemical Nonenzymatic Sensing of Glucose Using Advanced Nanomaterials. Microchim. Acta 2018, 185, 49. [Google Scholar] [CrossRef]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for Electrochemical Non-Enzymatic Glucose Biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Heli, H.; Pishahang, J. Cobalt Oxide Nanoparticles Anchored to Multiwalled Carbon Nanotubes: Synthesis and Application for Enhanced Electrocatalytic Reaction and Highly Sensitive Nonenzymatic Detection of Hydrogen Peroxide. Electrochim. Acta 2014, 123, 518–526. [Google Scholar] [CrossRef]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsiè, L. Advanced Promising Routes of Carbon/Metal Oxides Hybrids in Sensors: A Review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon Nanofiber-Based Three-Dimensional Nanomaterials for Energy and Environmental Applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Zhang, X.; Xie, G.; Su, Z.; Wei, G. Nanoporous Carbon Nanofibers Decorated with Platinum Nanoparticles for Non-Enzymatic Electrochemical Sensing of H2O2. Nanomaterials 2015, 5, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent Advances in Carbon Nanofibers and Their Applications—A Review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Zare, Y. Study on Interfacial Properties in Polymer Blend Ternary Nanocomposites: Role of Nanofiller Content. Comput. Mater. Sci. 2016, 111, 334–338. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Modeling of Stress Relaxation Modulus for a Nanocomposite Biosensor by Relaxation Time, Yield Stress, and Zero Complex Viscosity. JOM 2021, 73, 3693–3701. [Google Scholar] [CrossRef]
- Zare, Y. A Model for Tensile Strength of Polymer/Clay Nanocomposites Assuming Complete and Incomplete Interfacial Adhesion between the Polymer Matrix and Nanoparticles by the Average Normal Stress in Clay Platelets. RSC Adv. 2016, 6, 57969–57976. [Google Scholar] [CrossRef]
- Zare, Y.; Garmabi, H. Nonisothermal Crystallization and Melting Behavior of PP/Nanoclay/CaCO3 Ternary Nanocomposite. J. Appl. Polym. Sci. 2012, 124, 1225–1233. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Dependence of Z Parameter for Tensile Strength of Multi-Layered Interphase in Polymer Nanocomposites to Material and Interphase Properties. Nanoscale Res. Lett. 2017, 12, 42. [Google Scholar] [CrossRef]
- Zare, Y. “A” Interfacial Parameter in Nicolais-Narkis Model for Yield Strength of Polymer Particulate Nanocomposites as a Function of Material and Interphase Properties. J. Colloid Interface Sci. 2016, 470, 245–249. [Google Scholar] [CrossRef]
- Peng, W.; Rhim, S.; Zare, Y.; Rhee, K.Y. Effect of “Z” Factor for Strength of Interphase Layers on the Tensile Strength of Polymer Nanocomposites. Polym. Compos. 2019, 40, 1117–1122. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Experimental Data and Modeling of Storage and Loss Moduli for a Biosensor Based on Polymer Nanocomposites. Results Phys. 2020, 19, 103537. [Google Scholar] [CrossRef]
- Zare, Y.; Rhim, S.S.; Rhee, K.Y. The Interphase Degradation in a Nanobiosensor Including Biopolymers and Carbon Nanotubes. Sens. Actuators A Phys. 2021, 331, 112967. [Google Scholar] [CrossRef]
- Razavi, R.; Zare, Y.; Rhee, K.Y. A Two-Step Model for the Tunneling Conductivity of Polymer Carbon Nanotube Nanocomposites Assuming the Conduction of Interphase Regions. RSC Adv. 2017, 7, 50225–50233. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; You, T. Carbon Nanofiber Based Electrochemical Biosensors: A Review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Ruiz-Cornejo, J.C.; Sebastián, D.; Lázaro, M.J. Synthesis and Applications of Carbon Nanofibers: A Review. Rev. Chem. Eng. 2020, 36, 493–511. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon Nanofibers and Their Composites: A Review of Synthesizing, Properties and Applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, M.; Wei, X.; Sun, J.; Xu, D. Recent Advances in Morphology, Aperture Control, Functional Control and Electrochemical Sensors Applications of Carbon Nanofibers. Anal. Biochem. 2022, 656, 114882. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Liu, D.; Li, L. Carbon Nanofibers for Electroanalysis; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 27–53. [Google Scholar]
- Hu, G.; Zhang, X.; Liu, X.; Yu, J.; Ding, B. Strategies in Precursors and Post Treatments to Strengthen Carbon Nanofibers. Adv. Fiber Mater. 2020, 2, 46–63. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon Nanofibers Prepared via Electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef]
- Alegre, C.; Modica, E.; Di Blasi, A.; Di Blasi, O.; Busacca, C.; Ferraro, M.; Aricò, A.S.; Antonucci, V.; Baglio, V. NiCo-Loaded Carbon Nanofibers Obtained by Electrospinning: Bifunctional Behavior as Air Electrodes. Renew. Energy 2018, 125, 250–259. [Google Scholar] [CrossRef]
- Kausar, A. Polyacrylonitrile-Based Nanocomposite Fibers: A Review of Current Developments. J. Plast. Film. Sheeting 2019, 35, 295–316. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-Based Nanofibers—A State-of-the-Art Review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Manufacture and Application of Lignin-Based Carbon Fibers (LCFs) and Lignin-Based Carbon Nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Gaminian, H.; Montazer, M.; Bahi, A.; Karaaslan, M.; Ko, F. Capacitance Performance Boost of Cellulose-Derived Carbon Nanofibers via Carbon and Silver Nanoparticles. Cellulose 2019, 26, 2499–2512. [Google Scholar] [CrossRef]
- Lu, W.; He, T.; Xu, B.; He, X.; Adidharma, H.; Radosz, M.; Gasem, K.; Fan, M. Progress in Catalytic Synthesis of Advanced Carbon Nanofibers. J. Mater. Chem. A 2017, 5, 13863–13881. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, F.; Guan, J.; Wei, W.; Zou, L. Label-Free Amperometric Immunosensor Based on Versatile Carbon Nanofibers Network Coupled with Au Nanoparticles for Aflatoxin B1 Detection. Biosensors 2020, 11, 5. [Google Scholar] [CrossRef]
- Abdo, G.G.; Zagho, M.M.; Al Moustafa, A.-E.; Khalil, A.; Elzatahry, A.A. A Comprehensive Review Summarizing the Recent Biomedical Applications of Functionalized Carbon Nanofibers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1893–1908. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Tabar, F.A.; Mohammadpour-Haratbar, A.; Bazargan, A.M.; Mazinani, S.; Keihan, A.H.; Sharif, F. Preparation and Evaluation of Electrospun Carbon Nanofibers Infused by Metal Nanoparticles for Supercapacitor Electrodes Application. Synth. Met. 2021, 274, 116706. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Kiaeerad, P.; Mazinani, S.; Bazargan, A.M.; Sharif, F. Bimetallic Nickel-Cobalt Oxide Nanoparticle/Electrospun Carbon Nanofiber Composites: Preparation and Application for Supercapacitor Electrode. Ceram. Int. 2021, 48, 10015–10023. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mazinani, S.; Sharif, F.; Bazargan, A.M. Improving Nonenzymatic Biosensing Performance of Electrospun Carbon Nanofibers Decorated with Ni/Co Particles via Oxidation. Appl. Biochem. Biotechnol. 2022, 194, 2542–2564. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mosallanejad, B.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Co3O4 Nanoparticles Embedded in Electrospun Carbon Nanofibers as Free-Standing Nanocomposite Electrodes as Highly Sensitive Enzyme-Free Glucose Biosensors. Rev. Adv. Mater. Sci. 2022, 61, 744–755. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Q.; Liu, P.; Gopinath, S.C.B.; Zhang, L. Nanostructure-Mediated Glucose Oxidase Biofunctionalization for Monitoring Gestational Diabetes. Process Biochem. 2021, 110, 19–25. [Google Scholar] [CrossRef]
- Mahdizadeh, B.; Nouri, A.; Baharinikoo, L.; Lotfalipour, B. Enzymatic Glucose Biosensors: A Review on Recent Progress in Materials and Fabrication Techniques. Anal. Bioanal. Chem. Res. 2022, 9, 1–19. [Google Scholar]
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Hovancová, J.; Šišoláková, I.; Orivnaková, R.; Orivnak, A. Nanomaterial-Based Electrochemical Sensors for Detection of Glucose and Insulin. J. Solid State Electrochem. 2017, 21, 2147–2166. [Google Scholar] [CrossRef]
- Ou, L.; Liu, G.; Xia, N. Research Progress and Application Prospects of Electrochemical Glucose Sensors. Int. J. Electrochem. Sci. 2021, 16, 210633. [Google Scholar] [CrossRef]
- Hassan, I.U.; Salim, H.; Naikoo, G.A.; Awan, T.; Dar, R.A.; Arshad, F.; Tabidi, M.A.; Das, R.; Ahmed, W.; Asiri, A.M.; et al. A Review on Recent Advances in Hierarchically Porous Metal and Metal Oxide Nanostructures as Electrode Materials for Supercapacitors and Non-Enzymatic Glucose Sensors. J. Saudi Chem. Soc. 2021, 25, 101228. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose Biosensors in Clinical Practice: Principles, Limits and Perspectives of Currently Used Devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Awan, T.; Salim, H.; Arshad, F.; Hassan, I.U.; Pedram, M.Z.; Ahmed, W.; Faruck, H.L.; Aljabali, A.A.A.; Mishra, V.; et al. Fourth-Generation Glucose Sensors Composed of Copper Nanostructures for Diabetes Management: A Critical Review. Bioeng. Transl. Med. 2022, 7, e10248. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent Advances of Electrochemical and Optical Enzyme-Free Glucose Sensors Operating at Physiological Conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.; Yokoyama, K. Electrochemical Characterization of an Enzyme Electrode Based on a Ferrocene-Containing Redox Polymer. J. Electroanal. Chem. 1999, 468, 193–201. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-Enzymatic Electrochemical Sensing of Glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Chu, Z.; Liu, Y.; Xu, Y.; Shi, L.; Peng, J.; Jin, W. In-Situ Fabrication of Well-Distributed Gold Nanocubes on Thiol Graphene as a Third-Generation Biosensor for Ultrasensitive Glucose Detection. Electrochim. Acta 2015, 176, 162–171. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shin, J.H. Recent Developments in Nanostructure Based Electrochemical Glucose Sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose Detection Devices and Methods Based on Metal-Organic Frameworks and Related Materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Jeong, H.; Yoo, J.; Park, S.; Lu, J.; Park, S.; Lee, J. Non-Enzymatic Glucose Biosensor Based on Highly Pure TiO2 Nanoparticles. Biosensors 2021, 11, 149. [Google Scholar] [CrossRef]
- Duan, X.; Liu, K.; Xu, Y.; Yuan, M.; Gao, T.; Wang, J. Nonenzymatic Electrochemical Glucose Biosensor Constructed by NiCo2O4@ Ppy Nanowires on Nickel Foam Substrate. Sens. Actuators B Chem. 2019, 292, 121–128. [Google Scholar] [CrossRef]
- Savk, A.; Aydin, H.; Cellat, K.; Sen, F. A Novel High Performance Non-Enzymatic Electrochemical Glucose Biosensor Based on Activated Carbon-Supported Pt-Ni Nanocomposite. J. Mol. Liq. 2020, 300, 112355. [Google Scholar] [CrossRef]
- Azharudeen, A.M.; Karthiga, R.; Rajarajan, M.; Suganthi, A. Fabrication, Characterization of Polyaniline Intercalated NiO Nanocomposites and Application in the Development of Non-Enzymatic Glucose Biosensor. Arab. J. Chem. 2020, 13, 4053–4064. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, M.; Mishra, P.; Jahan, N.; Ahsan, M.A.; Ahmad, I.; Khan, M.R.; Watanabe, Y.; Syed, M.A.; Furukawa, H.; et al. Engineered Hierarchical CuO Nanoleaves Based Electrochemical Nonenzymatic Biosensor for Glucose Detection. J. Electrochem. Soc. 2021, 168, 17501. [Google Scholar] [CrossRef]
- Dong, M.; Hu, H.; Ding, S.; Wang, C.; Li, L. Flexible Non-Enzymatic Glucose Biosensor Based on CoNi2S4 Nanosheets Grown on Nitrogen-Doped Carbon Foam Substrate. J. Alloys Compd. 2021, 883, 160830. [Google Scholar] [CrossRef]
- Gamessa, T.W.; Suman, D.; Tadesse, Z.K. Blood Glucose Monitoring Techniques: Recent Advances, Challenges and Future Perspectives. Int. J. Adv. Technol. Eng. Explor. 2018, 5, 335–344. [Google Scholar] [CrossRef]
- Joung, J.; Kim, K. Monitoring Emerging Technologies for Technology Planning Using Technical Keyword Based Analysis from Patent Data. Technol. Forecast. Soc. Chang. 2017, 114, 281–292. [Google Scholar] [CrossRef]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on Recent Progress in Metal-Organic Framework-Based Materials for Fabricating Electrochemical Glucose Sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef] [PubMed]
- Osuna, V.; Vega-Rios, A.; Zaragoza-Contreras, E.A.; Estrada-Moreno, I.A.; Dominguez, R.B. Progress of Polyaniline Glucose Sensors for Diabetes Mellitus Management Utilizing Enzymatic and Non-Enzymatic Detection. Biosensors 2022, 12, 137. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Xie, F.; Liu, T.; Xie, L.; Sun, X.; Luo, Y. Metallic Nickel Nitride Nanosheet: An Efficient Catalyst Electrode for Sensitive and Selective Non-Enzymatic Glucose Sensing. Sens. Actuators B Chem. 2018, 255, 2794–2799. [Google Scholar] [CrossRef]
- Waqas, M.; Lan, J.; Zhang, X.; Fan, Y.; Zhang, P.; Liu, C.; Jiang, Z.; Wang, X.; Zeng, J.; Chen, W. Fabrication of Non-Enzymatic Electrochemical Glucose Sensor Based on Pd-Mn Alloy Nanoparticles Supported on Reduced Graphene Oxide. Electroanalysis 2020, 32, 1226–1236. [Google Scholar] [CrossRef]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Baskoutas, S.; Kim, S.H.; Abaker, M.; Al-Hajry, A.; Al-Sayari, S.A. Fabrication of Highly Sensitive Non-Enzymatic Glucose Biosensor Based on ZnO Nanorods. Sci. Adv. Mater. 2011, 3, 901–906. [Google Scholar] [CrossRef]
- Rafique, N.; Asif, A.H.; Hirani, R.A.K.; Wu, H.; Shi, L.; Zhang, S.; Sun, H. Binder Free 3D Core-Shell NiFe Layered Double Hydroxide (LDH) Nanosheets (NSs) Supported on Cu Foam as a Highly Efficient Non-Enzymatic Glucose Sensor. J. Colloid Interface Sci. 2022, 615, 865–875. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.; Choi, M.M.F. A Sensitive AgNPs/CuO Nanofibers Non-Enzymatic Glucose Sensor Based on Electrospinning Technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Khan, M.; Nagal, V.; Nakate, U.T.; Khan, M.R.; Khosla, A.; Ahmad, R. Engineered CuO Nanofibers with Boosted Non-Enzymatic Glucose Sensing Performance. J. Electrochem. Soc. 2021, 168, 67507. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Tixier-Mita, A.; Toshiyoshi, H. Electrocatalytic Activity Enhancement of Au NPs-TiO2 Electrode via a Facile Redistribution Process towards the Non-Enzymatic Glucose Sensors. Sens. Actuators B Chem. 2020, 319, 128279. [Google Scholar] [CrossRef]
- Vellayappan, M.V.; Venugopal, J.R.; Ramakrishna, S.; Ray, S.; Ismail, A.F.; Mandal, M.; Manikandan, A.; Seal, S.; Jaganathan, S.K. Electrospinning Applications from Diagnosis to Treatment of Diabetes. RSC Adv. 2016, 6, 83638–83655. [Google Scholar] [CrossRef]
- Al Mamun, K.A.; Tulip, F.S.; MacArthur, K.; McFarlane, N.; Islam, S.K.; Hensley, D. Vertically Aligned Carbon Nanofiber Based Biosensor Platform for Glucose Sensor. Int. J. High Speed Electron. Syst. 2014, 23, 1450006. [Google Scholar] [CrossRef]
- Pinyou, P.; Conzuelo, F.; Sliozberg, K.; Vivekananthan, J.; Contin, A.; Pöller, S.; Plumeré, N.; Schuhmann, W. Coupling of an Enzymatic Biofuel Cell to an Electrochemical Cell for Self-Powered Glucose Sensing with Optical Readout. Bioelectrochemistry 2015, 106, 22–27. [Google Scholar] [CrossRef]
- Abouali, S.; Akbari Garakani, M.; Zhang, B.; Xu, Z.-L.; Kamali Heidari, E.; Huang, J.; Huang, J.; Kim, J.-K. Electrospun Carbon Nanofibers with in Situ Encapsulated Co3O4 Nanoparticles as Electrodes for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 13503–13511. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Z.-L.; He, Y.-B.; Abouali, S.; Garakani, M.A.; Heidari, E.K.; Kang, F.; Kim, J.-K. Exceptional Rate Performance of Functionalized Carbon Nanofiber Anodes Containing Nanopores Created by (Fe) Sacrificial Catalyst. Nano Energy 2014, 4, 88–96. [Google Scholar] [CrossRef]
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and Utilization of Co3O4 Doped Carbon Nanofiber for Fabrication of Hemoglobin-Based Electrochemical Sensor. Mater. Sci. Eng. C 2020, 107, 110209. [Google Scholar] [CrossRef]
- Loaiza, O.A.; Lamas-Ardisana, P.J.; Añorga, L.; Jubete, E.; Ruiz, V.; Borghei, M.; Cabañero, G.; Grande, H.J. Graphitized Carbon Nanofiber-Pt Nanoparticle Hybrids as Sensitive Tool for Preparation of Screen Printing Biosensors. Detection of Lactate in Wines and Ciders. Bioelectrochemistry 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Q.; Zhang, X.; Hou, H.; You, T. PdCo Alloy Nanoparticle-Embedded Carbon Nanofiber for Ultrasensitive Nonenzymatic Detection of Hydrogen Peroxide and Nitrite. J. Colloid Interface Sci. 2015, 450, 168–173. [Google Scholar] [CrossRef]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel Phenolic Biosensor Based on a Magnetic Polydopamine-Laccase-Nickel Nanoparticle Loaded Carbon Nanofiber Composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Guo, Q.; Wang, Z.; Liu, G.; Chen, S.; Hou, H. Preparation of Ni(OH)2 Nanoplatelet/Electrospun Carbon Nanofiber Hybrids for Highly Sensitive Nonenzymatic Glucose Sensors. RSC Adv. 2017, 7, 19345–19352. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, C.; Tian, X.; Zhang, M.; Zhang, J.; Sun, K.; Wu, Y.; Sun, H.; Pang, L.; Sha, F. Facile Synthesis of Aminophenylboronic Decorated Electrospun CoFe2O4 Spinel Nanofibers with Enhanced Electrocatalytic Performance for Glucose Electrochemical Sensor Application. Ceram. Int. 2021, 47, 19052–19062. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Hwang, S.-K.; Ranjith, K.S.; Cho, Y.; Huh, Y.S.; Han, Y.-K. A Facile Method for the Fabrication of Hierarchically Structured Ni2CoS4 Nanopetals on Carbon Nanofibers to Enhance Non-Enzymatic Glucose Oxidation. Microchim. Acta 2021, 188, 106. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Lu, Y.; Ding, Y.; Li, L.; Ren, Z.; Si, X.; Wu, Q. A Novel Non-Enzymatic Glucose Electrochemical Sensor Based on CNF@ Ni-Co Layered Double Hydroxide Modified Glassy Carbon Electrode. Microchem. J. 2019, 150, 104106. [Google Scholar] [CrossRef]
- Kim, S.G.; Jun, J.; Kim, Y.K.; Kim, J.; Lee, J.S.; Jang, J. Facile Synthesis of Co3O4-Incorporated Multichannel Carbon Nanofibers for Electrochemical Applications. ACS Appl. Mater. Interfaces 2020, 12, 20613–20622. [Google Scholar] [CrossRef]
- Li, L.; Zhou, T.; Sun, G.; Li, Z.; Yang, W.; Jia, J.; Yang, G. Ultrasensitive Electrospun Nickel-Doped Carbon Nanofibers Electrode for Sensing Paracetamol and Glucose. Electrochim. Acta 2015, 152, 31–37. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Xiong, Y.; Liu, X.; Nsabimana, A.; Bo, X.; Guo, L. Bimetallic MCo (M = Cu, Fe, Ni, and Mn) Nanoparticles Doped-Carbon Nanofibers Synthetized by Electrospinning for Nonenzymatic Glucose Detection. Sens. Actuators B Chem. 2015, 207, 614–622. [Google Scholar] [CrossRef]
- Rani, S.D.; Ramachandran, R.; Sheet, S.; Aziz, M.A.; Lee, Y.S.; Al-Sehemi, A.G.; Pannipara, M.; Xia, Y.; Tsai, S.-Y.; Ng, F.-L.; et al. NiMoO4 Nanoparticles Decorated Carbon Nanofiber Membranes for the Flexible and High Performance Glucose Sensors. Sens. Actuators B Chem. 2020, 312, 127886. [Google Scholar] [CrossRef]
- Roushani, M.; Sarabaegi, M.; Hosseini, H. Flexible NiP2@ Hollow N-Doped Nanocapsules/Carbon Nanofiber as a Freestanding Electrode for Glucose Sensing. Compos. Commun. 2021, 25, 100686. [Google Scholar] [CrossRef]
- Ye, J.-S.; Liu, Z.-T.; Lai, C.-C.; Lo, C.-T.; Lee, C.-L. Diameter Effect of Electrospun Carbon Fiber Support for the Catalysis of Pt Nanoparticles in Glucose Oxidation. Chem. Eng. J. 2016, 283, 304–312. [Google Scholar] [CrossRef]
- Yin, Z.; Allado, K.; Sheardy, A.T.; Ji, Z.; Arvapalli, D.; Liu, M.; He, P.; Zeng, X.; Wei, J. Mingled MnO2 and Co3O4 Binary Nanostructures on Well-Aligned Electrospun Carbon Nanofibers for Nonenzymatic Glucose Oxidation and Sensing. Cryst. Growth Des. 2021, 21, 1527–1539. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Dong, H.; Zhang, X.; Wang, W.; Chen, Z. In Situ Growth Cupric Oxide Nanoparticles on Carbon Nanofibers for Sensitive Nonenzymatic Sensing of Glucose. Electrochim. Acta 2013, 105, 433–438. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, S.; Lu, X. Amperometric Nonenzymatic Glucose Sensor Based on a Glassy Carbon Electrode Modified with a Nanocomposite Made from Nickel (II) Hydroxide Nanoplates and Carbon Nanofibers. Microchim. Acta 2014, 181, 365–372. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, T.; Liu, L.; He, Y.; Liu, D.; You, T. Hierarchically Porous NiCo2S4 Nanowires Anchored on Flexible Electrospun Graphitic Nanofiber for High-Performance Glucose Biosensing. J. Alloys Compd. 2020, 819, 153376. [Google Scholar] [CrossRef]
- Mei, Q.; Fu, R.; Ding, Y.; Wang, A.; Duan, D.; Ye, D. Electrospinning of Highly Dispersed Ni/CoO Carbon Nanofiber and Its Application in Glucose Electrochemical Sensor. J. Electroanal. Chem. 2019, 847, 113075. [Google Scholar] [CrossRef]
- Adabi, M.; Adabi, M. Electrodeposition of Nickel on Electrospun Carbon Nanofiber Mat Electrode for Electrochemical Sensing of Glucose. J. Dispers. Sci. Technol. 2021, 42, 262–269. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, H.; Hou, H.; You, T. Nonenzymatic Glucose Sensor Based on Renewable Electrospun Ni Nanoparticle-Loaded Carbon Nanofiber Paste Electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, D.; Zhang, X.; Li, L.; Hou, H.; Niwa, O.; You, T. Pd-Ni Alloy Nanoparticle/Carbon Nanofiber Composites: Preparation, Structure, and Superior Electrocatalytic Properties for Sugar Analysis. Anal. Chem. 2014, 86, 5898–5905. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Yang, J.; Liu, G.; Li, J.; Guo, L.; Chen, S.; Guo, Q. NiCo2O4 Nanoneedle-Decorated Electrospun Carbon Nanofiber Nanohybrids for Sensitive Non-Enzymatic Glucose Sensors. Sens. Actuators B Chem. 2018, 258, 920–928. [Google Scholar] [CrossRef]
- Lu, N.; Shao, C.; Li, X.; Miao, F.; Wang, K.; Liu, Y. CuO Nanoparticles/Nitrogen-Doped Carbon Nanofibers Modified Glassy Carbon Electrodes for Non-Enzymatic Glucose Sensors with Improved Sensitivity. Ceram. Int. 2016, 42, 11285–11293. [Google Scholar] [CrossRef]
- Saravanan, J.; Pannipara, M.; Al-Sehemi, A.G.; Talebi, S.; Periasamy, V.; Shah, S.S.; Aziz, M. Flower-like CuO/NiO Nanostructures Decorated Activated Carbon Nanofiber Membranes for Flexible, Sensitive, and Selective Enzyme-Free Glucose Detection. J. Mater. Sci. Mater. Electron. 2021, 32, 24775–24789. [Google Scholar] [CrossRef]
- Huan, K.; Li, Y.; Deng, D.; Wang, H.; Wang, D.; Li, M.; Luo, L. Composite-Controlled Electrospinning of CuSn Bimetallic Nanoparticles/Carbon Nanofibers for Electrochemical Glucose Sensor. Appl. Surf. Sci. 2022, 573, 151528. [Google Scholar] [CrossRef]
- Chai, A.-W.; Wang, C.-C.; Chen, C.-Y. Magnetic-Field-Induced Acicular Nickel Immobilized on Carbon Nanofibers as Electrodes for Electrochemical Glucose Sensing. J. Taiwan Inst. Chem. Eng. 2021, 129, 237–245. [Google Scholar] [CrossRef]
- Dey, B.; Ahmad, M.W.; Sarkhel, G.; Yang, D.-J.; Choudhury, A. Fabrication of Porous Nickel (II)-Based MOF@ Carbon Nanofiber Hybrid Mat for High-Performance Non-Enzymatic Glucose Sensing. Mater. Sci. Semicond. Process. 2022, 142, 106500. [Google Scholar] [CrossRef]
- Rathod, D.; Dickinson, C.; Egan, D.; Dempsey, E. Platinum Nanoparticle Decoration of Carbon Materials with Applications in Non-Enzymatic Glucose Sensing. Sens. Actuators B Chem. 2010, 143, 547–554. [Google Scholar] [CrossRef]
- Ye, D.; Liang, G.; Li, H.; Luo, J.; Zhang, S.; Chen, H.; Kong, J. A Novel Nonenzymatic Sensor Based on CuO Nanoneedle/Graphene/Carbon Nanofiber Modified Electrode for Probing Glucose in Saliva. Talanta 2013, 116, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, H.; Ren, C.; Zhang, M.; Sun, K. A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers. Materials 2020, 13, 2874. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, J.; Shi, W.; Wei, M. Facile Fabrication of Highly-Dispersed Nickel Nanoparticles with Largely Enhanced Electrocatalytic Activity. Electroanalysis 2013, 25, 1594–1598. [Google Scholar] [CrossRef]
- Lang, J.-W.; Kong, L.-B.; Wu, W.-J.; Liu, M.; Luo, Y.-C.; Kang, L. A Facile Approach to the Preparation of Loose-Packed Ni(OH)2 Nanoflake Materials for Electrochemical Capacitors. J. Solid State Electrochem. 2009, 13, 333–340. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef]
- Yao, Y.; Shiu, K.-K. Electron-Transfer Properties of Different Carbon Nanotube Materials, and Their Use in Glucose Biosensors. Anal. Bioanal. Chem. 2007, 387, 303–309. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, H.; Govorov, A.O.; Ouyang, M. Hierarchical Synthesis of Non-Centrosymmetric Hybrid Nanostructures and Enabled Plasmon-Driven Photocatalysis. Nat. Commun. 2014, 5, 4792. [Google Scholar] [CrossRef]
- Kiaeerad, P.; Naji, L. Synergistic Effect of Two Complexing Agents on the Hydrothermal Synthesis of Self-Supported ZnNiCo Oxide as Electrode Material in Supercapacitors. J. Electroanal. Chem. 2021, 901, 115779. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Santhan, A.; Tata, S.K.S. Fabrication of Sn-Doped ZnO Hexagonal Micro Discs Anchored on RGO for Electrochemical Detection of Anti-Androgen Drug Flutamide in Water and Biological Samples. Microchem. J. 2021, 160, 105689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).