A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of AuNPs@MIP@PANI@CNT/CNC@Textile Cortisol Sensor Patch
2.2. LbL Characterization of AuNPs@MIP@PANI@CNT/CNC@Textile Cortisol Sensor Patch
2.3. LbL Determination of Cortisol Sensor Patch Water Retention Abilities
2.4. Voltammetric Testing of Cortisol Sensor Patches
2.5. Sweat Analysis Using AuNPs@MIP@PANI@CNT/CNC@Textile Cortisol Sensor Patch
3. Results and Discussion
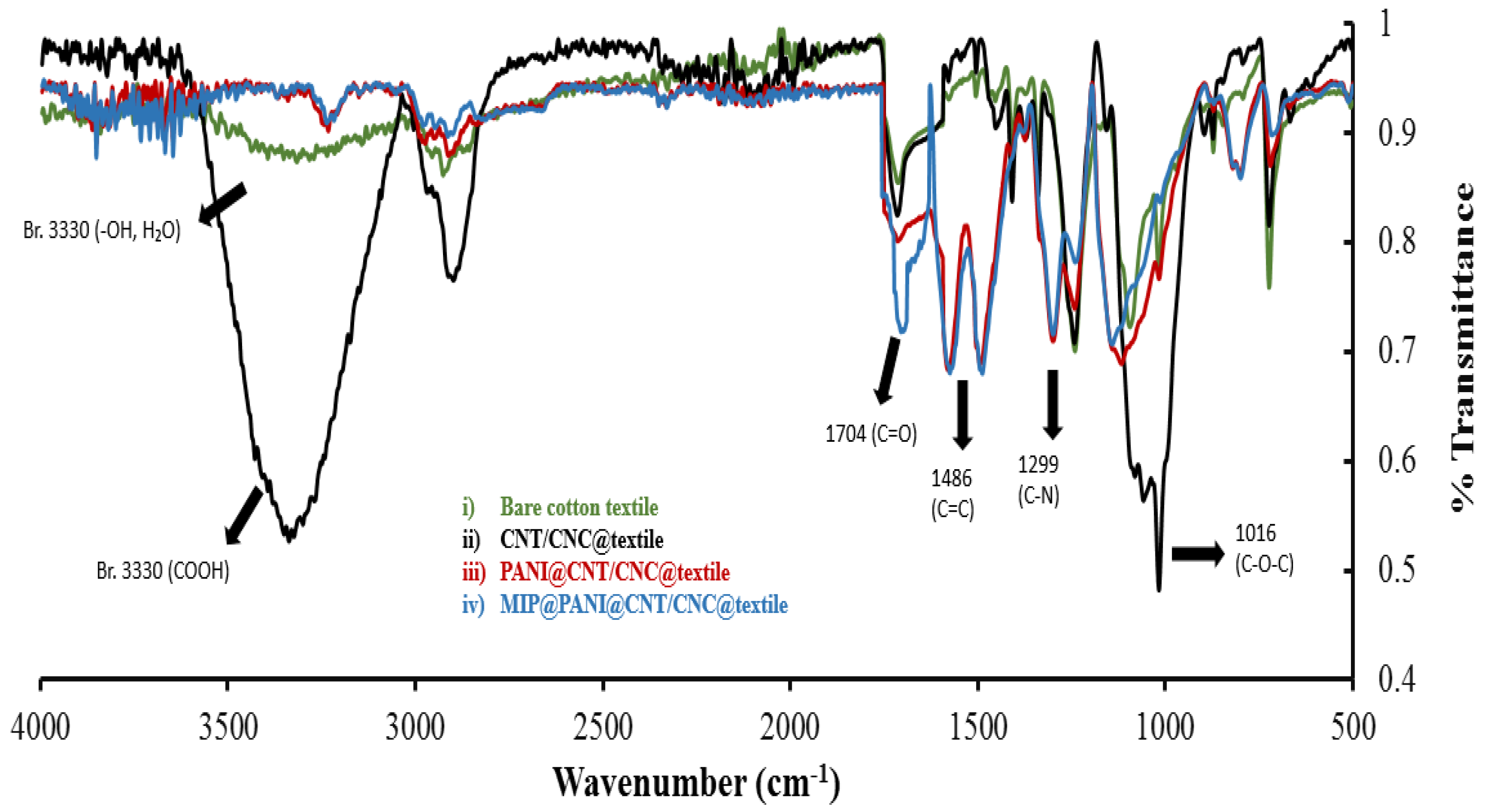
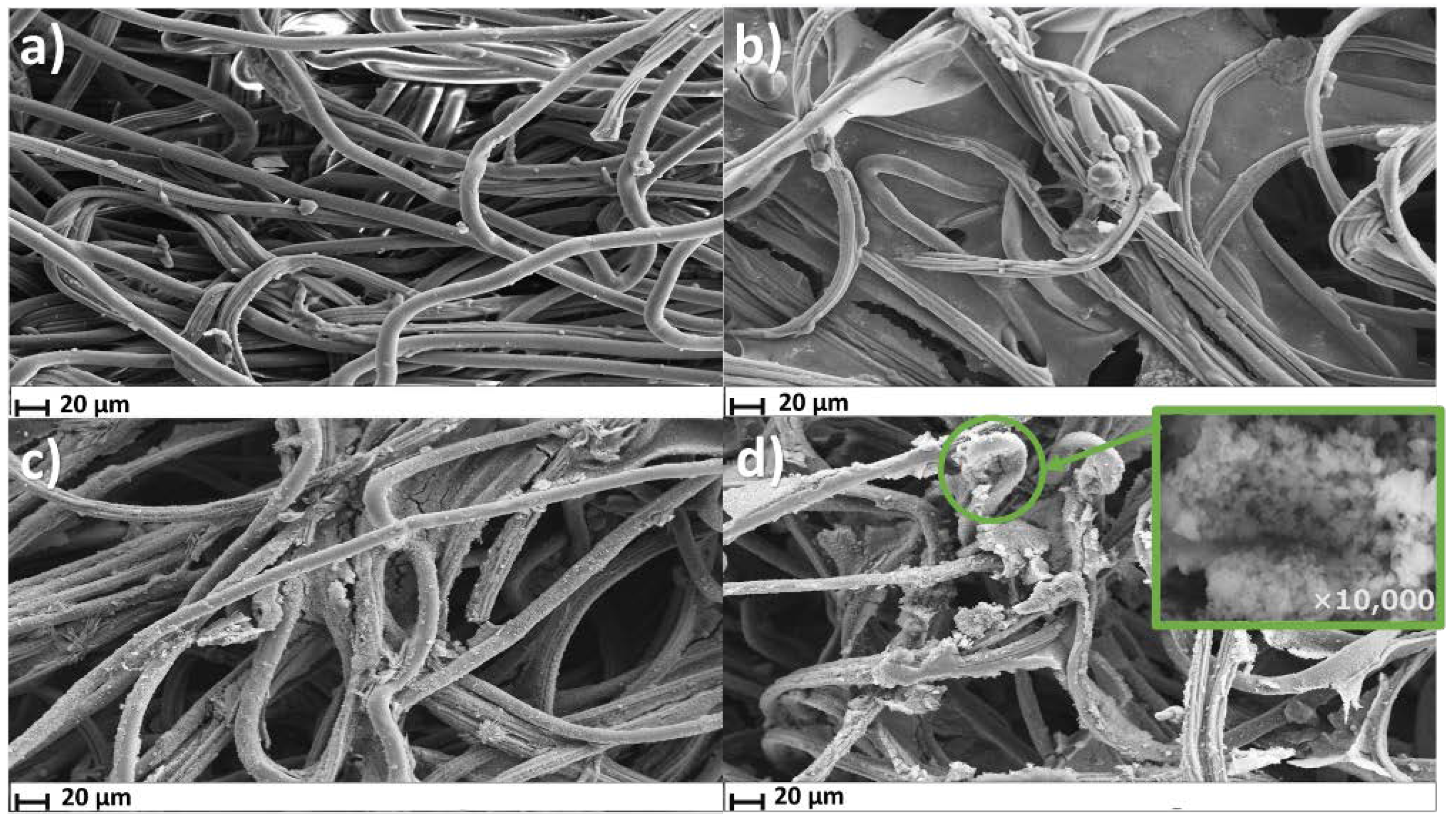
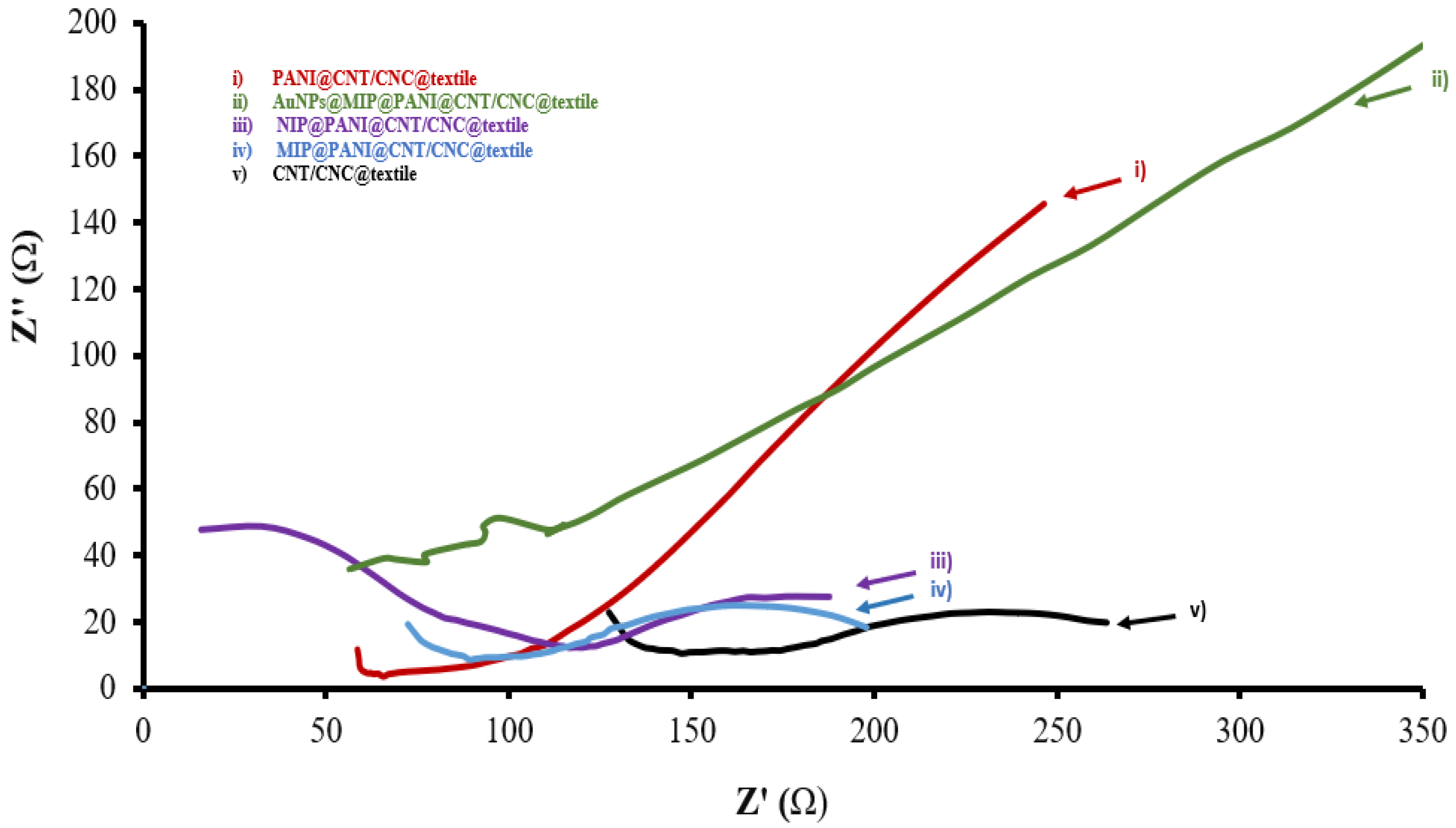
3.1. Stage-wise Characterization of MIP@PANI@CNT/CNC@Textile Cortisol Sensor Patch
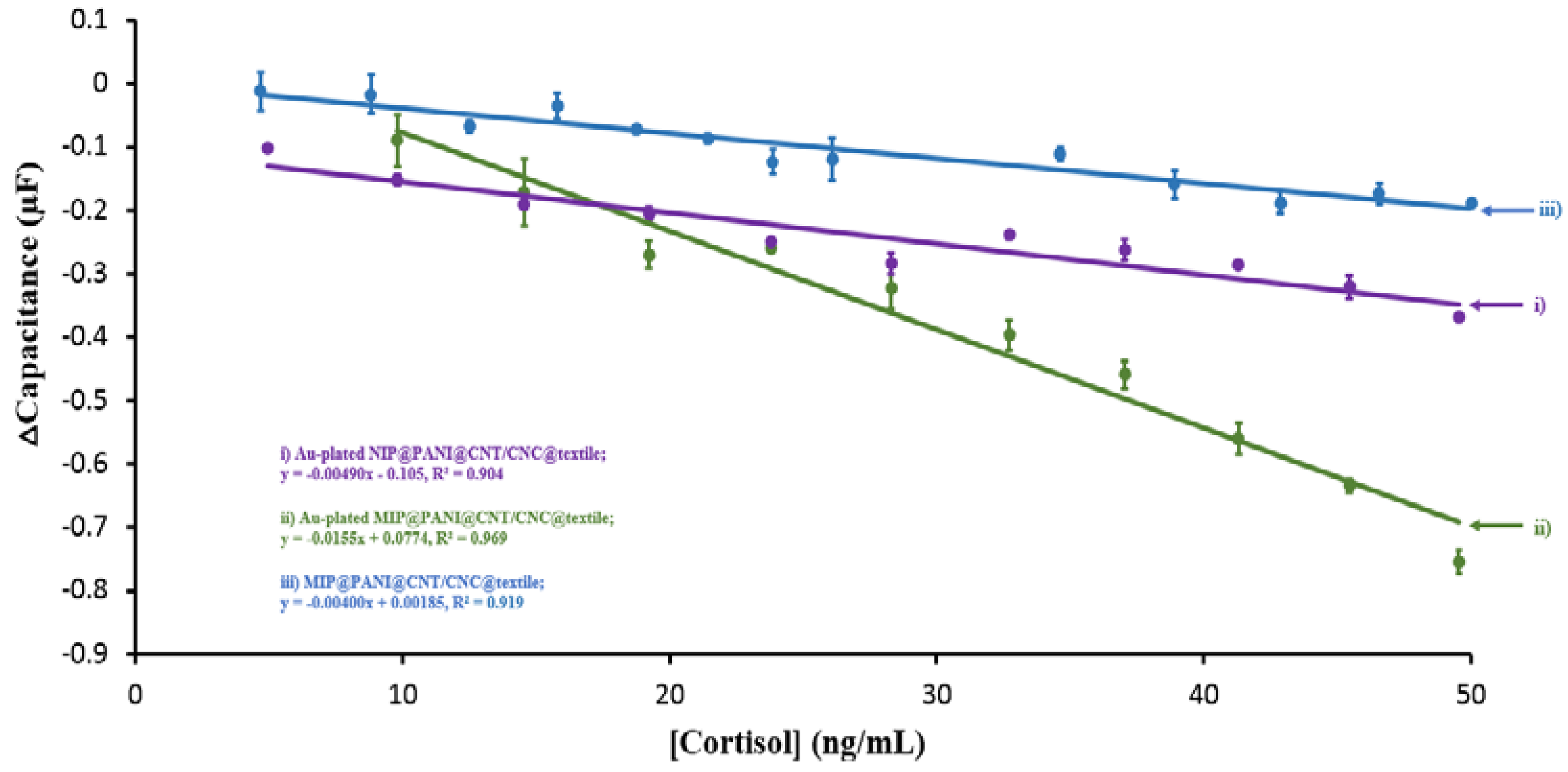
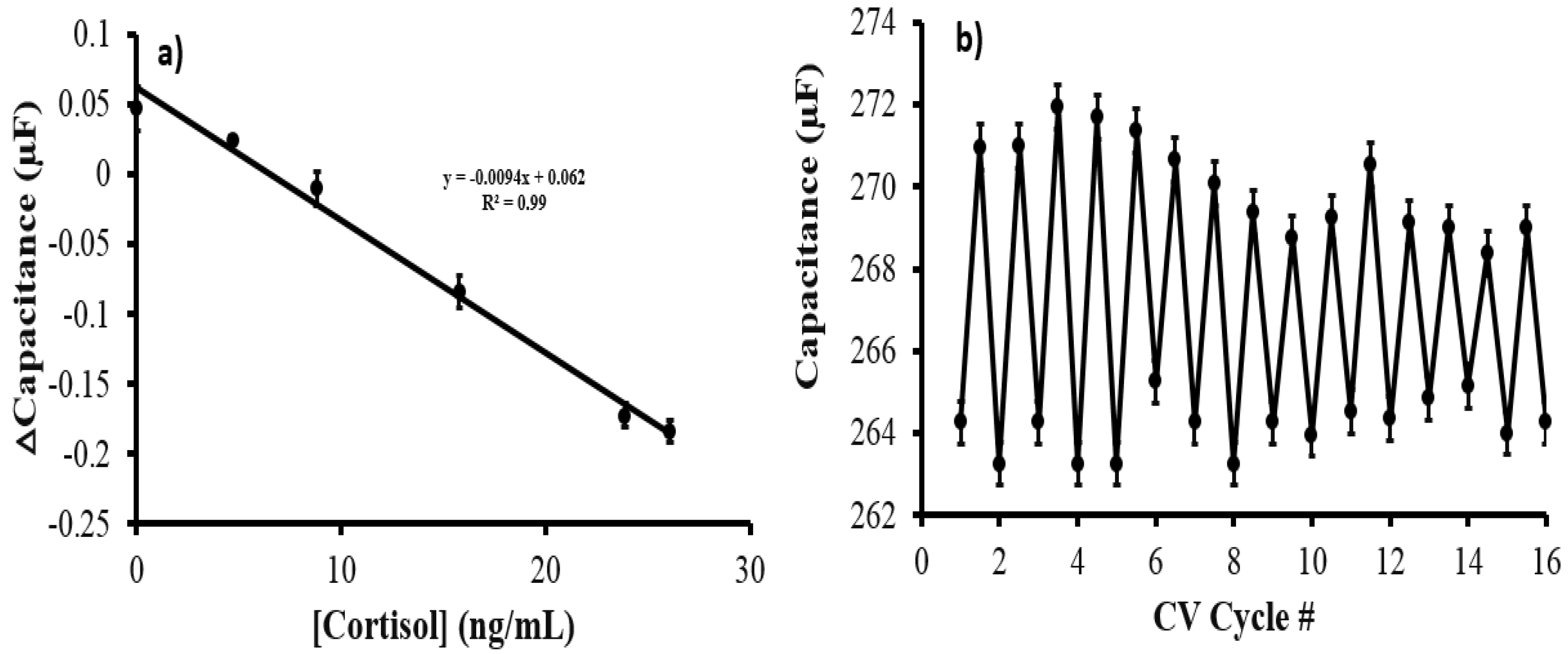
3.2. Performance Evaluation of the MIP Cortisol Sensor and NIP Patches
3.3. Evaluation of MIP@PANI@CNT/CNC@Textile Cortisol Sensor Selectivity
3.4. Evaluation of AuNPs@MIP@PANI@CNT/CNC@Textile Sensor for Sweat Analysis and Reusability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin Electronics from Scalable Fabrication of an Intrinsically Stretchable Transistor Array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, F.; Cai, P.; Wang, M.; He, K.; Luo, Y.; Li, Z.; Chen, G.; Ji, S.; Liu, Z.; et al. Mechanically Interlocked Hydrogel–Elastomer Hybrids for On-Skin Electronics. Adv. Funct. Mater. 2020, 30, 1909540. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.S.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and PH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef]
- Padmanathan, N.; Shao, H.; Razeeb, K.M. Multifunctional Nickel Phosphate Nano/Microflakes 3D Electrode for Electrochemical Energy Storage, Nonenzymatic Glucose, and Sweat PH Sensors. ACS Appl. Mater. Interfaces 2018, 10, 8599–8610. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Suzuki, M.; Tamiya, E.; Karube, I. Analysis of Metabolites in Sweat as a Measure of Physical Condition. Anal. Chim. Acta 1994, 289, 27–34. [Google Scholar] [CrossRef]
- Rayan, R.A.; Tasgkaris, C. IoT Technologies for Smart Healthcare. In Assistive Technology Intervention in Healthcare; CRC Press: Boca Raton, FL, USA, 2022; pp. 29–46. [Google Scholar]
- Ruth, J.; Willwacher, S.; Korn, O. Acceptance of Digital Sports: A Study Showing the Rising Acceptance of Digital Health Activities Due to the SARS-CoV-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 596. [Google Scholar] [CrossRef]
- Wells, C.R.; Townsend, J.P.; Pandey, A.; Fitzpatrick, M.C.; Crystal, W.S.; Moghadas, S.M.; Galvani, A.P. Quarantine and Testing Strategies for Safe Pandemic Travel. medRxiv 2021. [Google Scholar]
- Taskinsoy, J. The Great Pandemic of the 21st Century: The Stolen Lives. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Awotunde, J.B.; Jimoh, R.G.; AbdulRaheem, M.; Oladipo, I.D.; Folorunso, S.O.; Ajamu, G.J. IoT-Based Wearable Body Sensor Network for COVID-19 Pandemic. In Advances in Data Science and Intelligent Data Communcation Technologies for COVID-19: Innovative Solutions Against COVID-19; Hassanien, A.-E., Elghamrawy, S.M., Zelinka, I., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 378, pp. 253–275. [Google Scholar]
- Varma, P.; Junge, M.; Meaklim, H.; Jackson, M.L. Younger People Are More Vulnerable to Stress, Anxiety and Depression during COVID-19 Pandemic: A Global Cross-Sectional Survey. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110236. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive Label-Free Detection of Cortisol and Lactate Using Graphene Embedded Screen-Printed Electrode. Nanomicro Lett. 2018, 10, 41. [Google Scholar] [CrossRef]
- Jia, M.; Chew, W.M.; Feinstein, Y.; Skeath, P.; Sternberg, E.M. Quantification of Cortisol in Human Eccrine Sweat by Liquid Chromatography-Tandem Mass Spectrometry. Analyst 2016, 141, 2053–2060. [Google Scholar] [CrossRef]
- Tafet, G.E.; Idoyaga-Vargas, V.P.; Abulafia, D.P.; Calandria, J.M.; Roffman, S.S.; Chiovetta, A.; Shinitzky, M. Correlation between Cortisol Level and Serotonin Uptake in Patients with Chronic Stress and Depression. Cogn. Affect. Behav. Neurosci. 2001, 1, 388–393. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Gatti, R.; Antonelli, G.; Prearo, M.; Spinella, P.; Cappellin, E.; de Palo, E.F. Cortisol Assays and Diagnostic Laboratory Procedures in Human Biological Fluids. Clin. Biochem. 2009, 42, 1205–1217. [Google Scholar] [CrossRef]
- Putnam, S.K.; Lopata, C.; Thomeer, M.L.; Volker, M.A.; Rodgers, J.D. Salivary Cortisol Levels and Diurnal Patterns in Children with Autism Spectrum Disorder. J. Dev. Phys. Disabil. 2015, 27, 453–465. [Google Scholar] [CrossRef]
- Mugo, S.M.; Alberkant, J. Flexible Molecularly Imprinted Electrochemical Sensor for Cortisol Monitoring in Sweat. Anal. Bioanal. Chem. 2020, 412, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable Stringency Aptamer Selection and Gold Nanoparticle Assay for Detection of Cortisol. Anal. Bioanal. Chem. 2014, 406, 4637–4647. [Google Scholar] [CrossRef]
- Ramström, O.; Ye, L.; Mosbach, K. Artificial Antibodies to Corticosteroids Prepared by Molecular Imprinting. Chem. Biol. 1996, 3, 471–477. [Google Scholar] [CrossRef]
- Wood, L.; Ducroq, D.H.; Fraser, H.L.; Gillingwater, S.; Evans, C.; Pickett, A.J.; Rees, D.W.; John, R.; Turkes, A. Measurement of Urinary Free Cortisol by Tandem Mass Spectrometry and Comparison with Results Obtained by Gas Chromatography-Mass Spectrometry and Two Commercial Immunoassays. Ann. Clin. Biochem. 2008, 45, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Mugo, S.M.; Alberkant, J.; Bernstein, N.; Zenkina, O.V. Flexible Electrochemical Aptasensor for Cortisol Detection in Human Sweat. Anal. Methods 2021, 13, 4169–4173. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Povedano, E.; Krishnan, S.; Teymourian, H.; Tehrani, F.; Campuzano, S.; Dassau, E.; Wang, J. Simultaneous Cortisol/Insulin Microchip Detection Using Dual Enzyme Tagging. Biosens. Bioelectron. 2020, 167, 112512. [Google Scholar] [CrossRef] [PubMed]
- Dalirirad, S.; Han, D.; Steckl, A.J. Aptamer-Based Lateral Flow Biosensor for Rapid Detection of Salivary Cortisol. ACS Omega 2020, 5, 32890–32898. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole Cell Based Microcontact Imprinted Capacitive Biosensor for the Detection of Escherichia coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Mary, G.; Chand, N. The Use of Cotton Fibers as Reinforcements in Composites. In Biofiber Reinforcements in Composite Materials; Faruk, O., Sain, M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 320–341. [Google Scholar]
- Ibrahim, K.A. Synthesis and Characterization of Polyaniline and Poly(Aniline-Co-o-Nitroaniline) Using Vibrational Spectroscopy. Arab. J. Chem. 2017, 10, S2668–S2674. [Google Scholar] [CrossRef]
- Dhanjai; Mugo, S.M.; Lu, W. Modified Stainless Steel Microneedle Electrode for Polyphenolics Detection. Anal. Bioanal. Chem. 2020, 412, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Mugo, S.M.; Lu, W.; Wood, M.; Lemieux, S. Wearable Microneedle Dual Electrochemical Sensor for Simultaneous PH and Cortisol Detection in Sweat. Electrochem. Sci. Adv. 2021, 2, e2100039. [Google Scholar] [CrossRef]
- Portella, E.H.; Romanzini, D.; Angrizani, C.C.; Amico, S.C.; Zattera, A.J. Influence of Stacking Sequence on the Mechanical and Dynamic Mechanical Properties of Cotton/Glass Fiber Reinforced Polyester Composites. Mater. Res. 2016, 19, 542–547. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose Prepared by Acid Hydrolysis of Isolated Cellulose from Sugarcane Bagasse. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Solo, Indonesia, 8–9 September 2015; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012045. [Google Scholar]
- Azri, F.A.; Sukor, R.; Hajian, R.; Yusof, N.A.; Bakar, F.A.; Selamat, J. Modification Strategy of Screen-Printed Carbon Electrode with Functionalized Multi-Walled Carbon Nanotube and Chitosan Matrix for Biosensor Development. Asian J. Chem. 2017, 29, 31–36. [Google Scholar] [CrossRef]
- Stefanović, I.S.; Ekmeščić, B.M.; Maksin, D.D.; Nastasović, A.B.; Miladinović, Z.P.; Vuković, Z.M.; Micić, D.M.; Pergal, M.v. Structure, Thermal, and Morphological Properties of Novel Macroporous Amino-Functionalized Glycidyl Methacrylate Based Copolymers. Ind. Eng. Chem. Res. 2015, 54, 6902–6911. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on the Synthesized Mesoporous Carbon and Silver Nanoparticles Nanohybrid. Sens. Actuators B Chem. 2014, 203, 919–925. [Google Scholar] [CrossRef]
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.v.; Karyakina, E.E.; Karyakin, A.A. Nonenzymatic Sensor for Lactate Detection in Human Sweat. Anal. Chem. 2017, 89, 11198–11202. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; van Uum, S.H.M. The Detection of Cortisol in Human Sweat: Implications for Measurement of Cortisol in Hair. Ther. Drug Monit. 2014, 36, 30–34. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless MHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- Sonawane, A.; Mujawar, M.A.; Manickam, P.; Bhansali, S. Plasma-Induced Enhancement in Electronic Properties of Gold Nanoparticles: Application in Electrochemical Biosensing of Cortisol. ACS Appl. Electron. Mater. 2021, 3, 230–237. [Google Scholar] [CrossRef]
- Daniels, E.; Mustafa, Y.L.; Herdes, C.; Leese, H.S. Optimization of Cortisol-Selective Molecularly Imprinted Polymers Enabled by Molecular Dynamics Simulations. ACS Appl. Bio Mater. 2021, 4, 7243–7253. [Google Scholar] [CrossRef]



| Sensor Type | Electroactive Surface Area (cm2) | Rct (×103 Ω) |
|---|---|---|
| PANI@CNT/CNC@textile | 0.0120 | 0.703 |
| AuNPs@MIP@PANI@CNT/CNC@textile | 0.0264 | 1.59 |
| AuNPs@NIP@PANI@CNT/CNC@textile | 0.00497 | - |
| NIP@PANI@CNT/CNC@textile | 0.00469 | 3.16 |
| MIP@PANI@CNT/CNC@textile | 0.00529 | 2.23 |
| CNT/CNC@textile | 0.00114 | 29.2 |
| Sensor Type | Recognition Surface | Concentration Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|---|
| ZnO nanorod-integrated flexible carbon fibers | ZnONRs/CCY immunosensing platform | 1.0 × 10−6–1.0 × 103 | 9.8 × 10−8 | Madhu et al., 2020 [41] |
| Cortisol/insulin dual electrochemical immunosensor microchip | Alkaline phosphatase (ALP)-labeled competitive immunoassay | 0–250 | 13.4 | Vargas et al., 2020 [26] |
| Aptamer-based lateral flow biosensor | Cortisol aptamer | 0.5–15 | 0.37 | Dalirirad et al., 2020 [27] |
| Cortisol-specific DNA aptamer@CNT/CNC@PDMS sensor | Cortisol-specific DNA aptamer | 2.5–35 | 1.8 | Mugo et al., 2021 [25] |
| Cortisol MIP@CNT/CNC@ PDMS sensor | Cortisol-imprinted poly(GMA-co-EGDMA) | 10–66 | 2.0 ± 0.4 | Mugo et al., 2020 [21] |
| Graphene-based capacitive sensor | Carboxylate-rich pyrrole-derivative grafting | <10 | - | Torrente-Rodríguez et al., 2020 [40] |
| AuNP-basedcortisol sensor | Room-temperature plasma sintering technique | 5.0 × 10−4–30 | 0.12 | Sonawane et al., 2021 [42] |
| Cortisol-selective MIPs | MIP technique | 36.2–362 | - | Daniels et al., 2021 [43] |
| Au-plated MIP@PANI@CNT/CNC@textile cortisol sensor patch | Cortisol imprinted poly(GMA-co-EGDMA) with Au enhancement | 9.80–49.5 | 8.00 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugo, S.M.; Lu, W.; Robertson, S. A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat. Biosensors 2022, 12, 854. https://doi.org/10.3390/bios12100854
Mugo SM, Lu W, Robertson S. A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat. Biosensors. 2022; 12(10):854. https://doi.org/10.3390/bios12100854
Chicago/Turabian StyleMugo, Samuel M., Weihao Lu, and Scott Robertson. 2022. "A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat" Biosensors 12, no. 10: 854. https://doi.org/10.3390/bios12100854
APA StyleMugo, S. M., Lu, W., & Robertson, S. (2022). A Wearable, Textile-Based Polyacrylate Imprinted Electrochemical Sensor for Cortisol Detection in Sweat. Biosensors, 12(10), 854. https://doi.org/10.3390/bios12100854






