Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
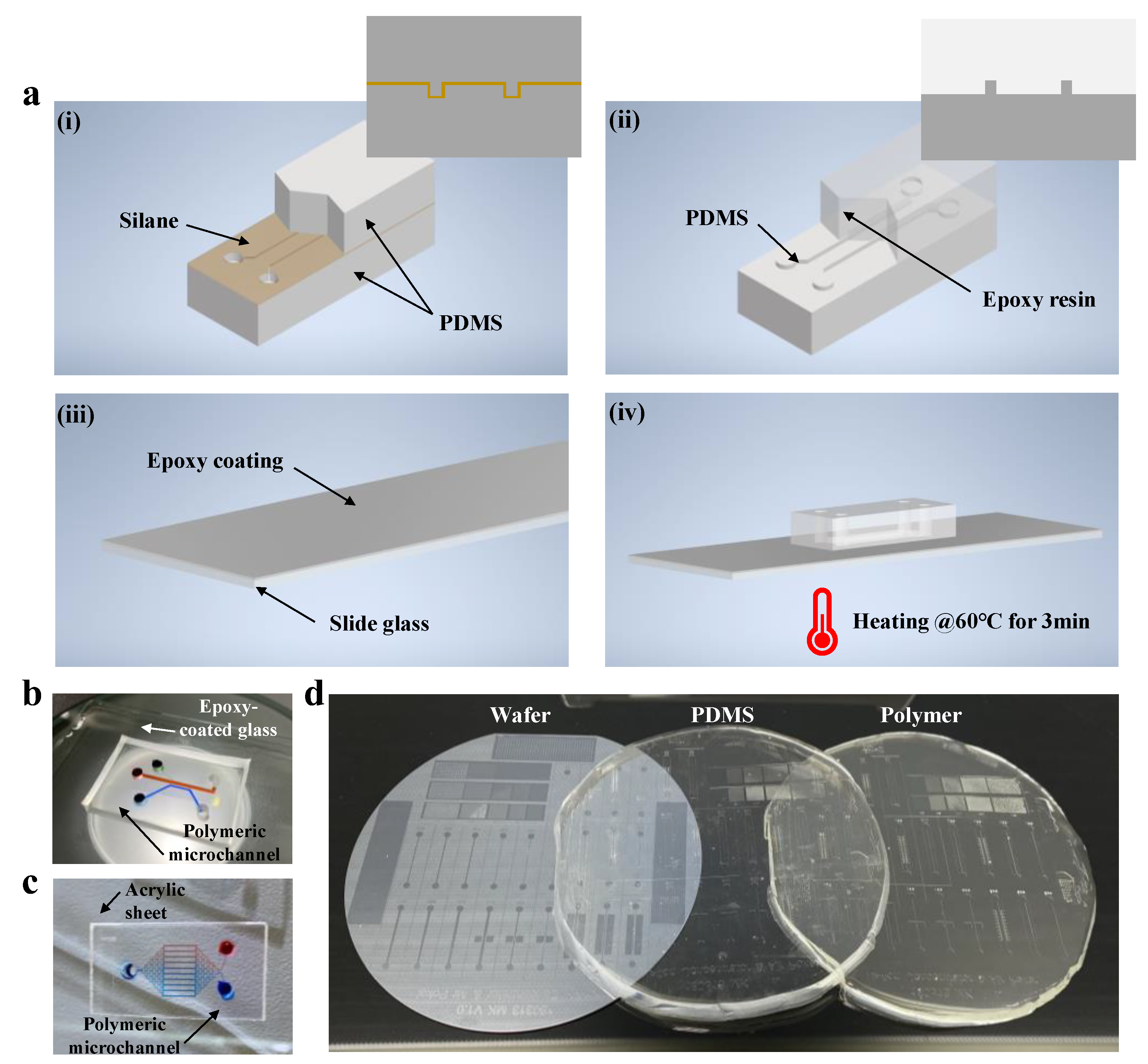
2.2. Device Design and Fabrication
2.3. Fabrication and Preparation of Polymeric Microfluidic Chips
2.4. Experimental Setup and Data Analysis
3. Results and Discussions
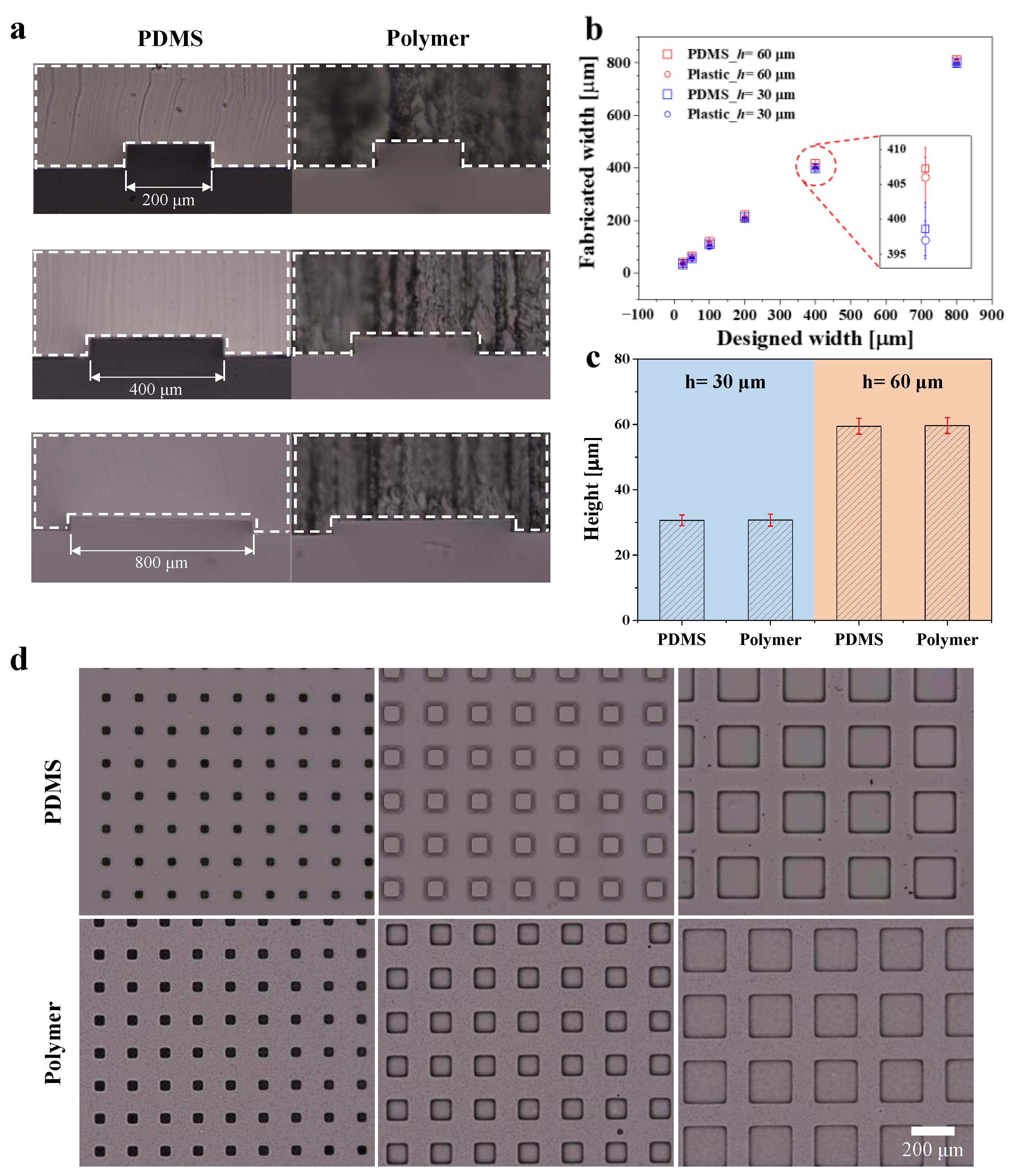
3.1. Dimension Comparison of Polymer and PDMS Devices
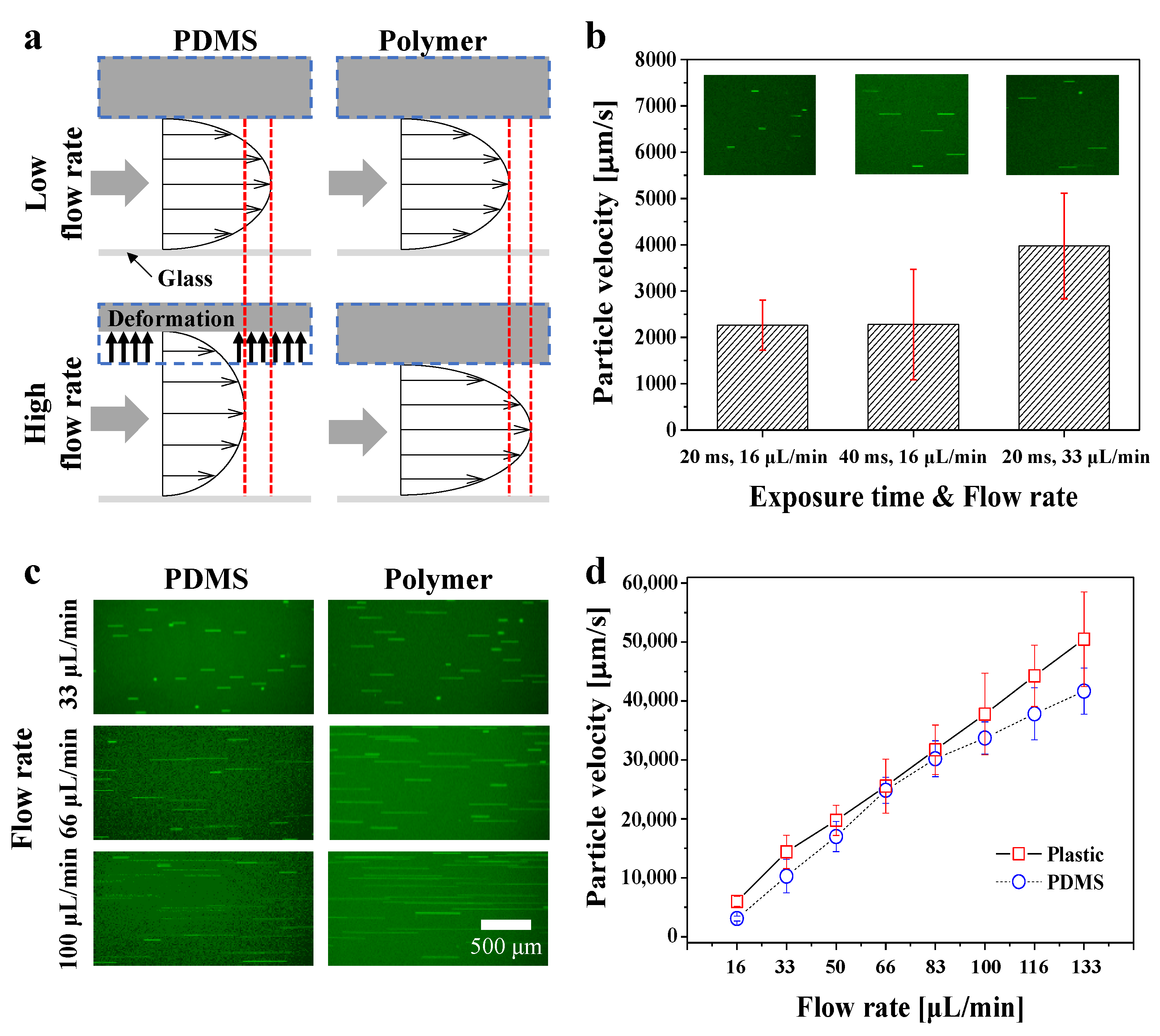
3.2. Channel Deformation at Various Flow Rates
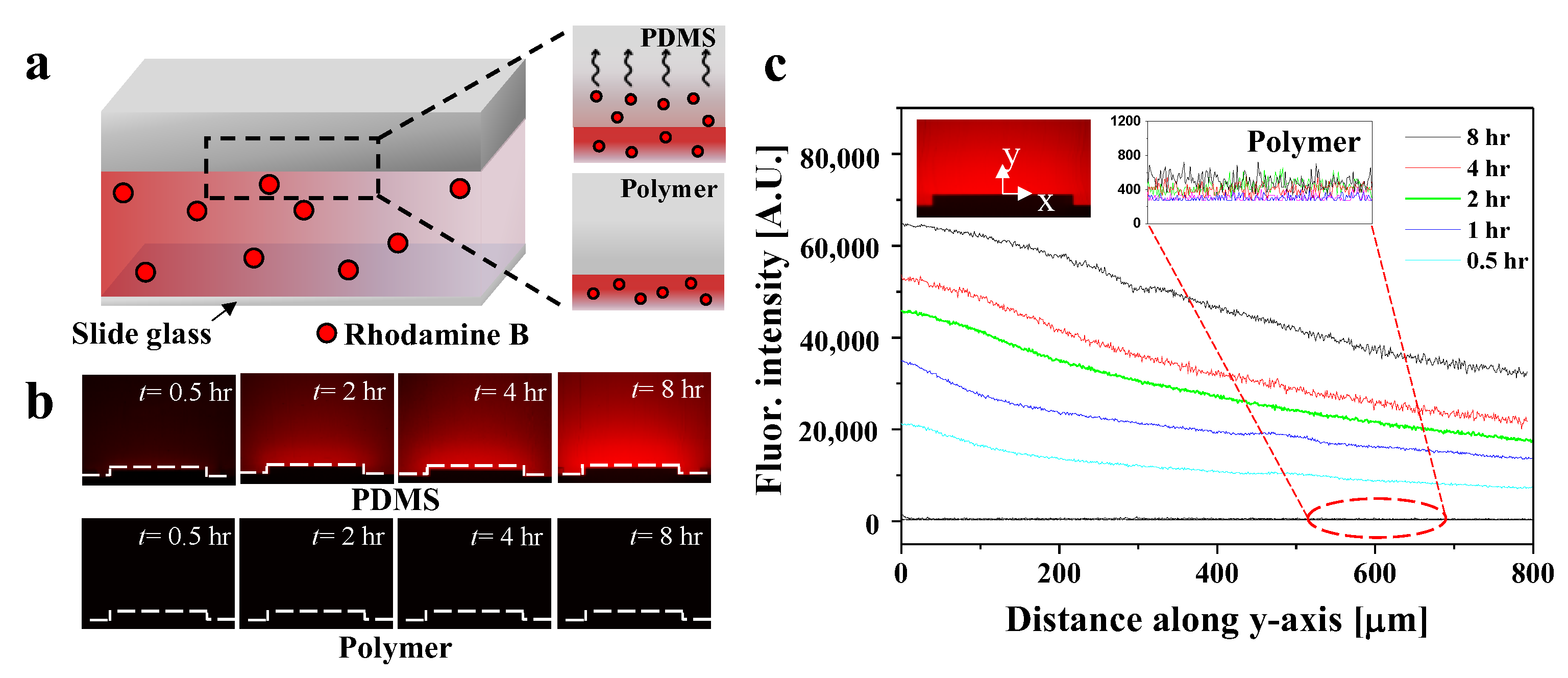
3.3. Visualization and Quantification of Molecular Adsorption
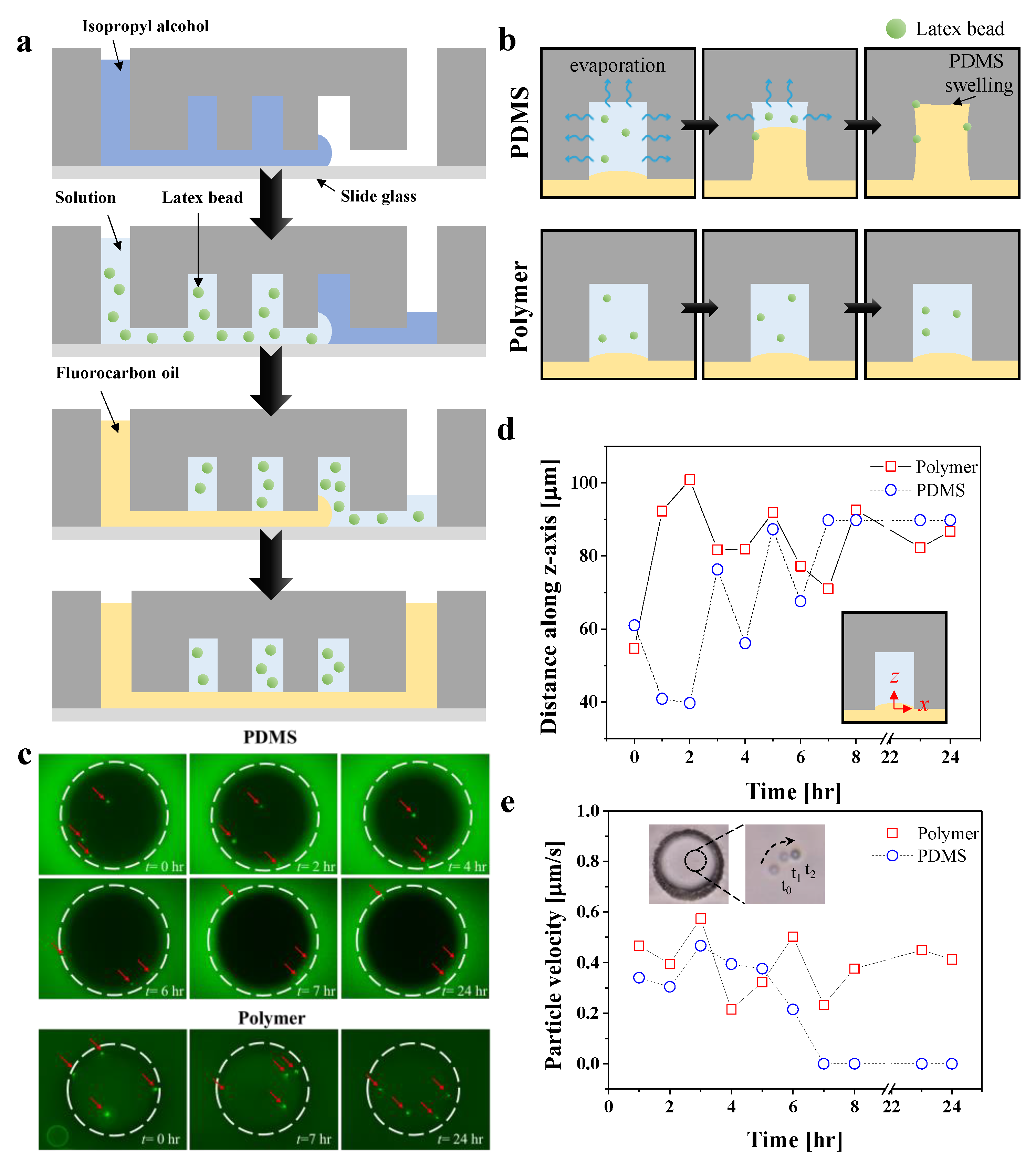
3.4. Particle Compartmentalization for Day-Long Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weigl, B.H.; Yager, P. Microfluidics—Microfluidic diffusion-based separation and detection. Science 1999, 283, 346–347. [Google Scholar] [CrossRef]
- Verpoorte, E.M.; Van Der Schoot, B.H.; Jeanneret, S.; Manz, A.; Widmer, H.M.; De Rooij, N.F. 3-Dimensional Micro-Flow Manifolds for Miniaturized Chemical-Analysis Systems. J. Micromechanics Microengineering 1994, 4, 246–256. [Google Scholar] [CrossRef]
- Sundberg, S.A. High-throughput and ultra-high-throughput screening: Solution- and cell-based approaches. Curr. Opin. Biotech. 2000, 11, 47–53. [Google Scholar] [CrossRef]
- McDonald, J.C.; Metallo, S.J.; Whitesides, G.M. Fabrication of a configurable, single-use microfluidic device. Anal. Chem. 2001, 73, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.Y.; Friend, J.R. Microfluidic Devices for Bioapplications. Small 2011, 7, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; Van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic Devices for Forensic DNA Analysis: A Review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Marle, L.; Greenway, G.M. Microfluidic devices for environmental monitoring. Trac-Trend Anal. Chem. 2005, 24, 795–802. [Google Scholar] [CrossRef]
- Yew, M.; Ren, Y.; Koh, K.S.; Sun, C.G.; Snape, C. A Review of State-of-the-Art Microfluidic Technologies for Environmental Applications: Detection and Remediation. Glob. Chall. 2019, 3, 1800060. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.H.; Nijhuis, C.A.; Gong, J.L.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zuo, P.; Ye, B.C. A low-cost and simple paper-based microfluidic device for simultaneous multiplex determination of different types of chemical contaminants in food. Biosens. Bioelectron. 2015, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Castillo-León, J. Microfluidics and Lab-on-a-Chip Devices: History and Challenges. In Lab-on-a-Chip Devices and Micro-Total Analysis Systems: A Practical Guide; Castillo-León, J., Svendsen, W.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Ren, K.N.; Zhou, J.H.; Wu, H.K. Materials for Microfluidic Chip Fabrication. Accounts Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Gomez-de Pedro, S.; Puyol, M.; Alonso-Chamarro, J. Continuous flow synthesis of nanoparticles using ceramic microfluidic devices. Nanotechnology 2010, 21, 415603. [Google Scholar] [CrossRef]
- Birol, H.; Maeder, T.; Jacq, C.; Straessler, S.; Ryser, P. Fabrication of low-temperature co-fired ceramics micro-fluidic devices using sacrificial carbon layers. Int. J. Appl. Ceram. Technol. 2005, 2, 364–373. [Google Scholar] [CrossRef]
- Guevara-Pantoja, P.E.; Chavez-Pineda, O.G.; Solis-Serrano, A.M.; Garcia-Cordero, J.L.; Caballero-Robledo, G.A. An affordable 3D-printed positioner fixture improves the resolution of conventional milling for easy prototyping of acrylic microfluidic devices. Lab Chip 2020, 20, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Heilman, S.; Wasserman, M.; Archer, S.; Shuler, M.L.; Wu, M.M. A hydrogel-based microfluidic device for the studies of directed cell migration. Lab Chip 2007, 7, 763–769. [Google Scholar] [CrossRef]
- Peng, R.; Li, D.Q. Fabrication of polydimethylsiloxane (PDMS) nanofluidic chips with controllable channel size and spacing. Lab Chip 2016, 16, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.K.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Wang, J.D.; Douville, N.J.; Takayama, S.; ElSayed, M. Quantitative Analysis of Molecular Absorption into PDMS Microfluidic Channels. Ann. Biomed. Eng. 2012, 40, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.J.C.; Weitz, D.A. Highly monodisperse conjugated polymer particles synthesized with drop-based microfluidics. Chem. Commun. 2011, 47, 12379–12381. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.J.; Lee, H.H. Surface modification of poly(dimethylsiloxane) for retarding swelling in organic solvents. Langmuir 2006, 22, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.N.; Dai, W.; Zhou, J.H.; Su, J.; Wu, H.K. Whole-Teflon microfluidic chips. Proc. Natl. Acad. Sci. USA 2011, 108, 8162–8166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Yang, C. Analysis of induced-charge electro-osmotic flow in a microchannel embedded with polarizable dielectric blocks. Phys. Rev. E 2009, 80, 046312. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.T.; Mathies, R.A. Ultra-High-Speed DNA Fragment Separations Using Microfabricated Capillary Array Electrophoresis Chips. Proc. Natl. Acad. Sci. USA 1994, 91, 11348–11352. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Erickson, D.; Ren, L.Q.; Li, D.Q. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J. Colloid. Interf. Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.M.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 016505. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lee, G.B.; Lin, Y.H.; Chang, G.L. A fast prototyping process for fabrication of microfluidic systems on soda-lime glass. J. Micromechanics Microengineering 2001, 11, 726–732. [Google Scholar] [CrossRef]
- Qu, B.Y.; Wu, Z.Y.; Fang, F.; Bai, Z.M.; Yang, D.Z.; Xu, S.K. A glass microfluidic chip for continuous blood cell sorting by a magnetic gradient without labeling. Anal. Bioanal. Chem. 2008, 392, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.B.; Xu, L.N.; Xu, Y.; Zhong, J.J.; Abedini, A.; Cheng, X.; Sinton, D. Disposable silicon-glass microfluidic devices: Precise, robust and cheap. Lab Chip 2018, 18, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef]
- Keller, N.; Nargang, T.M.; Runck, M.; Kotz, F.; Striegel, A.; Sachsenheimer, K.; Klemm, D.; Lange, K.; Worgull, M.; Richter, C.; et al. Tacky cyclic olefin copolymer: A biocompatible bonding technique for the fabrication of microfluidic channels in COC. Lab Chip 2016, 16, 1561–1564. [Google Scholar] [CrossRef]
- Bundgaard, F.; Nielsen, T.; Nilsson, D.; Shi, P.X.; Perozziello, G.; Kristensen, A.; Geschke, O. Cyclic olefin copolymer (COC/Topas((R)))—An exceptional material for exceptional lab-on-a-chip systems. Micro Total Anal. Syst. 2004, 2, 372–374. [Google Scholar]
- Jena, R.K.; Yue, C.Y.; Lam, Y.C. Micro fabrication of cyclic olefin copolymer (COC) based microfluidic devices. Microsyst. Technol. 2012, 18, 159–166. [Google Scholar] [CrossRef]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic Olefin Copolymer Microfluidic Devices for Forensic Applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Chung, S.; Kamm, R.D.; Charest, J.L. Hot embossing for fabrication of a microfluidic 3D cell culture platform. Biomed. Microdevices 2011, 13, 325–333. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Liang, F.P.; Qiao, Y.; Duan, M.Q.; Ju, A.; Lu, N.; Li, J.J.; Tu, J.; Lu, Z.H. Fabrication of a microfluidic chip based on the pure polypropylene material. RSC Adv. 2018, 8, 8732–8738. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.W.; Wang, Y.S.; Yao, X.; Mu, X.; Lu, X.D.; Zhou, J.H.; Cai, Z.W.; Ren, K.N. Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid, low-cost antimicrobial susceptibility test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Shirani, E.; Razmjou, A.; Tavassoli, H.; Landarani-Isfahan, A.; Rezaei, S.; Kajani, A.A.; Asadnia, M.; Hou, J.W.; Warkiani, M.E. Strategically Designing a Pumpless Microfluidic Device on an “Inert” Polypropylene Substrate with Potential Application in Biosensing and Diagnostics. Langmuir 2017, 33, 5565–5576. [Google Scholar] [CrossRef] [PubMed]
- Raffy, S.; Palleau, E.; Calvignac, B.; Brotons, G.; Lefebvre, G.; Rolley, N.; Teychene, S.; Viguier, B.; Cerezo, S.C.; Truan, G.; et al. “All in One” Epoxy-Based Microfluidic Chips at Your Fingertips. ACS Appl. Polym. Mater. 2021, 3, 801–810. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, Y.; Li, S.L.; Wang, Y.X.; Chen, H.W.; Cheng, J.; Liu, P. Enclosed casting of epoxy resin for rapid fabrication of rigid microfluidic chips. Sens. Actuat. B-Chem. 2017, 252, 785–793. [Google Scholar] [CrossRef]
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.T.; Ertl, P. Multi-layered, membrane-integrated microfluidics based on replica molding of a thiol-ene epoxy thermoset for organ-on-a-chip applications. Lab Chip 2015, 15, 4542–4554. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Haraldsson, T.; Oberg, K.; Malkoch, M.; van der Wijngaart, W. Beyond PDMS: Off-stoichiometry thiol-ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 2011, 11, 3136–3147. [Google Scholar] [CrossRef]
- Saunier, J.; Chodur, C.A.; Leclerc, B.; Larbes, L.; Yagoubi, N. Using COC as pharmaceutical or cosmetic storage material. II. Effect of electron beam radio-sterilization. J. Appl. Polym. Sci. 2008, 109, 1829–1839. [Google Scholar] [CrossRef]
- Yang, B.X.; Shi, J.H.; Pramoda, K.P.; Goh, S.H. Enhancement of the mechanical properties of polypropylene using polypropylene-grafted multiwalled carbon nanotubes. Compos. Sci. Technol. 2008, 68, 2490–2497. [Google Scholar] [CrossRef]
- Smooth-On. Available online: https://www.smooth-on.com/products/epoxacast-690/ (accessed on 16 August 2022).
- Koerner, T.; Brown, L.; Xie, R.X.; Oleschuk, R.D. Epoxy resins as stamps for hot embossing of microstructures and microfluidic channels. Sens. Actuat. B-Chem. 2005, 107, 632–639. [Google Scholar] [CrossRef]
- Chen, P.C.; Pan, C.W.; Lee, W.C.; Li, K.M. An experimental study of micromilling parameters to manufacture microchannels on a PMMA substrate. Int. J. Adv. Manuf. Technol. 2014, 71, 1623–1630. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Vazquez, E.; Rodriguez, C.A.; Elias-Zuniga, A.; Ciurana, J. An experimental analysis of process parameters to manufacture metallic micro-channels by micro-milling. Int. J. Adv. Manuf. Technol. 2010, 51, 945–955. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Ku, X.Y.; Zhang, Z.W.; Liu, X.L.; Chen, L.; Li, G. Low-cost rapid prototyping of glass microfluidic devices using a micromilling technique. Microfluid Nanofluid 2018, 22. [Google Scholar] [CrossRef]
- Owens, C.E.; Hart, A.J. High-precision modular microfluidics by micromilling of interlocking injection-molded blocks. Lab Chip 2018, 18, 890–901. [Google Scholar] [CrossRef]
- Thompson, C.S.; Abate, A.R. Adhesive-based bonding technique for PDMS microfluidic devices. Lab Chip 2013, 13, 632–635. [Google Scholar] [CrossRef]
- Ren, Y.D.; Ray, S.; Liu, Y.X. Reconfigurable Acrylic-tape Hybrid Microfluidics. Sci. Rep. 2019, 9, 4824. [Google Scholar] [CrossRef]
- Serra, M.; Pereiro, I.; Yamada, A.; Viovy, J.L.; Descroix, S.; Ferraro, D. A simple and low-cost chip bonding solution for high pressure, high temperature and biological applications. Lab Chip 2017, 17, 629–634. [Google Scholar] [CrossRef]
- Goh, C.S.; Tan, S.C.; May, K.T.; Chan, C.Z.; Ng, S.H. Adhesive Bonding of Polymeric Microfluidic Devices. In Proceedings of the 2009 11th Electronics Packaging Technology Conference (Eptc 2009), Singapore, 9–11 December 2009; pp. 737–740. [Google Scholar] [CrossRef]
- Ma, H.L.; Urbaczek, A.C.; de Souza, F.Z.R.; Leao, P.A.G.G.C.; Perussi, J.R.; Carrilho, E. Rapid Fabrication of Microfluidic Devices for Biological Mimicking: A Survey of Materials and Biocompatibility. Micromachines 2021, 12, 346. [Google Scholar] [CrossRef]
- Huang, X.W.; Zhu, Y.J.; Zhang, X.M.; Bao, Z.Y.; Lei, D.Y.; Yu, W.X.; Dai, J.Y.; Wang, Y. Clam-inspired nanoparticle immobilization method using adhesive tape as microchip substrate. Sens. Actuat. B-Chem. 2016, 222, 106–111. [Google Scholar] [CrossRef]
- Kim, M.; Wu, L.D.; Kim, B.; Hung, D.T.; Han, J. Continuous and High-Throughput Electromechanical Lysis of Bacterial Pathogens Using Ion Concentration Polarization. Anal. Chem. 2018, 90, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, J.W.; Lee, S.K.; Kim, T. Nanoscale Hydrodynamic Film for Diffusive Mass Transport Control in Compartmentalized Microfluidic Chambers. Anal. Chem. 2017, 89, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromechanics Microengineering 2014, 24, 035017. [Google Scholar] [CrossRef]
- Finck, H. Epoxy resins in electron microscopy. J. Biophys. Biochem. Cytol. 1960, 7, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Penn, L.S.; Chiao, T.T. Epoxy Resins. In Handbook of Composites; Lubin, G., Ed.; Springer: Boston, MA, USA, 1982; pp. 57–88. [Google Scholar] [CrossRef]
- Holden, M.A.; Kumar, S.; Beskok, A.; Cremer, P.S. Microfluidic diffusion diluter: Bulging of PDMS microchannels under pressure-driven flow. J. Micromechanics Microengineering 2003, 13, 412–418. [Google Scholar] [CrossRef]
- Zheng, X.; Silber-Li, Z.H. Measurement of velocity profiles in a rectangular microchannel with aspect ratio alpha=0.35. Exp. Fluids 2008, 44, 951–959. [Google Scholar] [CrossRef][Green Version]
- Hardy, B.S.; Uechi, K.; Zhen, J.; Kavehpour, H.P. The deformation of flexible PDMS microchannels under a pressure driven flow. Lab Chip 2009, 9, 935–938. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, C.H. Analysis of the wetting state of super-repellent fabrics with liquids of varying surface tension. RSC Adv. 2016, 6, 45884–45893. [Google Scholar] [CrossRef][Green Version]
- Dangla, R.; Gallaire, F.; Baroud, C.N. Microchannel deformations due to solvent-induced PDMS swelling. Lab Chip 2010, 10, 2972–2978. [Google Scholar] [CrossRef]
- Azizi, M.; Zaferani, M.; Dogan, B.; Zhang, S.Y.; Simpson, K.W.; Abbaspourrad, A. Nanoliter-Sized Microchamber/Microarray Microfluidic Platform for Antibiotic Susceptibility Testing. Anal. Chem. 2018, 90, 14137–14144. [Google Scholar] [CrossRef]
| PDMS | COC | PP | Epoxy | |
|---|---|---|---|---|
| Young’s modulus (MPa) | 2.05 ± 0.12 | 2.60 × 103 | 0.77 × 103 | 3.94 × 103 |
| Cure shrinkage | 0.5–2.5% | 0.1–0.5 % | 1.7–2.2% | 0.2% |
| Transparency | Good | Good | Medium | Good |
| Solvent resistance | Low (organic solvent) | Medium (non-polar organic solvent) | High | High |
| Reference | [18,20] | [35,37,38,48] | [40,41,42,49] | [45,50,51] |
| Elongation Limits (mm) | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Compressive Modulus (MPa) | Compressive Strength (MPa) | Gas Permeability | |
|---|---|---|---|---|---|---|
| PDMS [66] | 76.4 | 2.05 ± 0.12 | 6.25 ± 0.84 | 148.9 ± 5.47 | 40.1 ± 4.30 | Good |
| Epoxy resin [50] | 1.8 | 3.94 × 103 | 45.7 | 6.30 × 102 | 66.3 | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, M. Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments. Biosensors 2022, 12, 838. https://doi.org/10.3390/bios12100838
Lee J, Kim M. Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments. Biosensors. 2022; 12(10):838. https://doi.org/10.3390/bios12100838
Chicago/Turabian StyleLee, Jaeseok, and Minseok Kim. 2022. "Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments" Biosensors 12, no. 10: 838. https://doi.org/10.3390/bios12100838
APA StyleLee, J., & Kim, M. (2022). Polymeric Microfluidic Devices Fabricated Using Epoxy Resin for Chemically Demanding and Day-Long Experiments. Biosensors, 12(10), 838. https://doi.org/10.3390/bios12100838







