Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. TMS Conditions and EEG Recording System
2.4. EEG Preprocessing
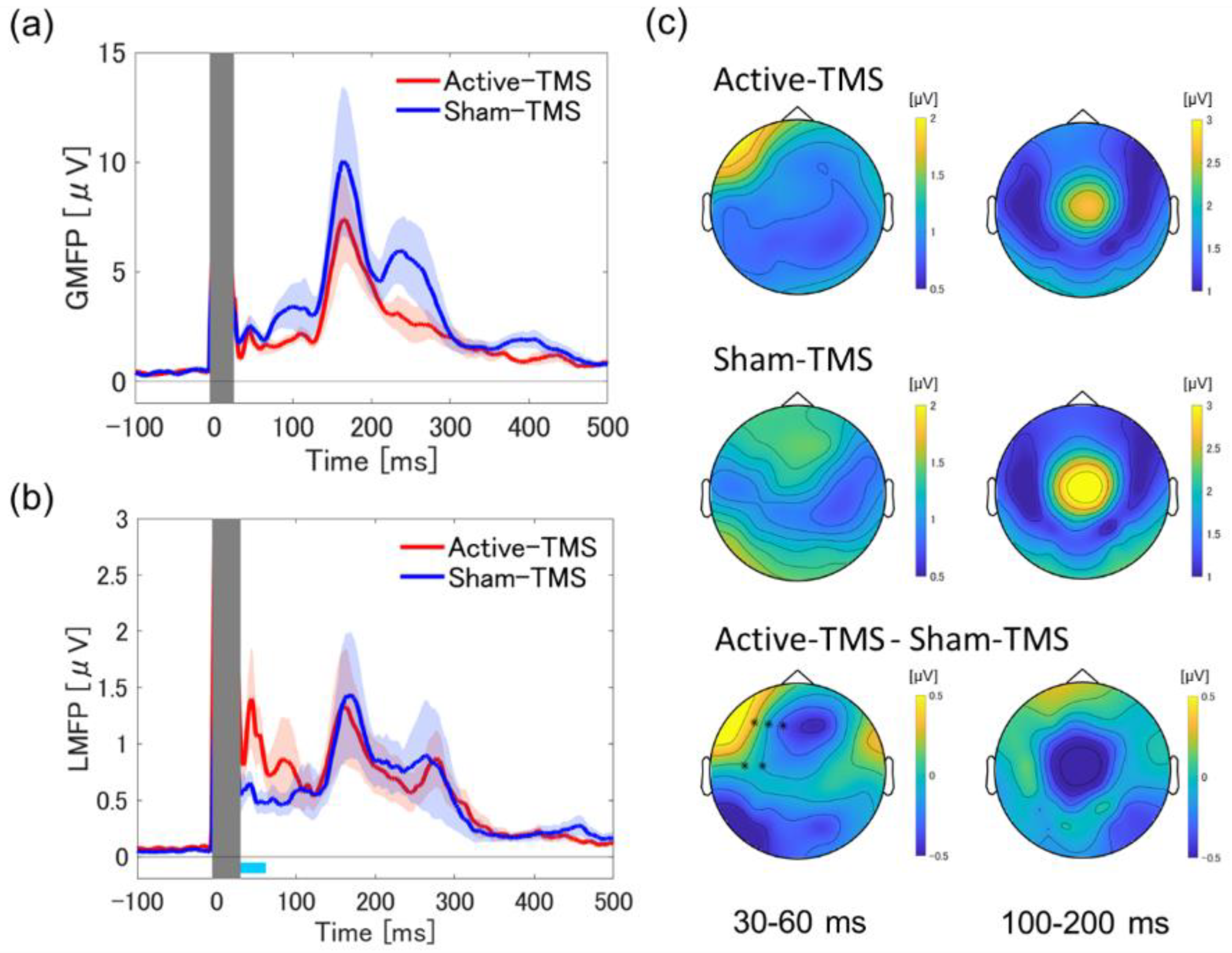
2.5. Global Mean Field Power and Local Mean Field Power Analysis
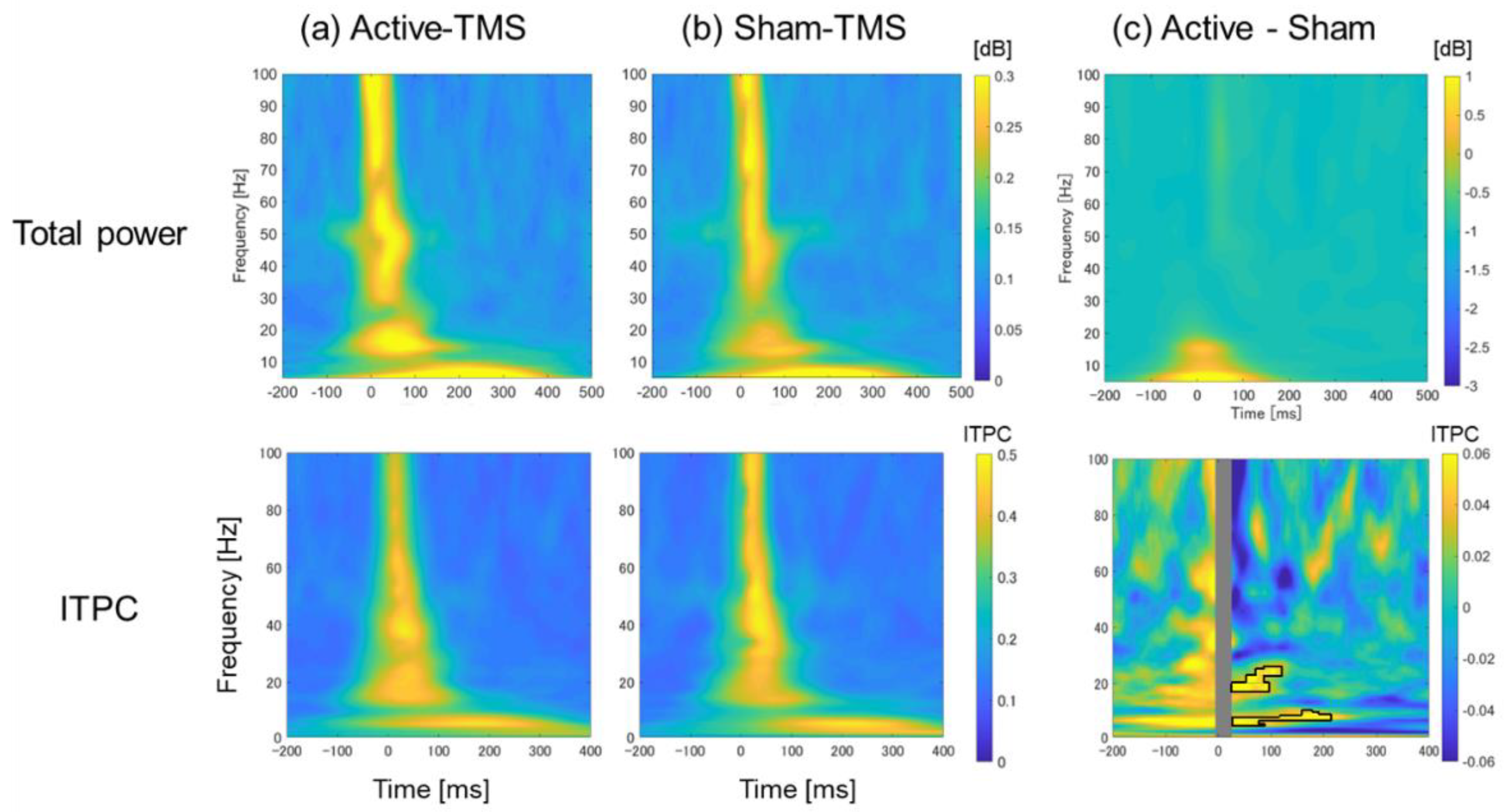
2.6. Time–Frequency Analyses
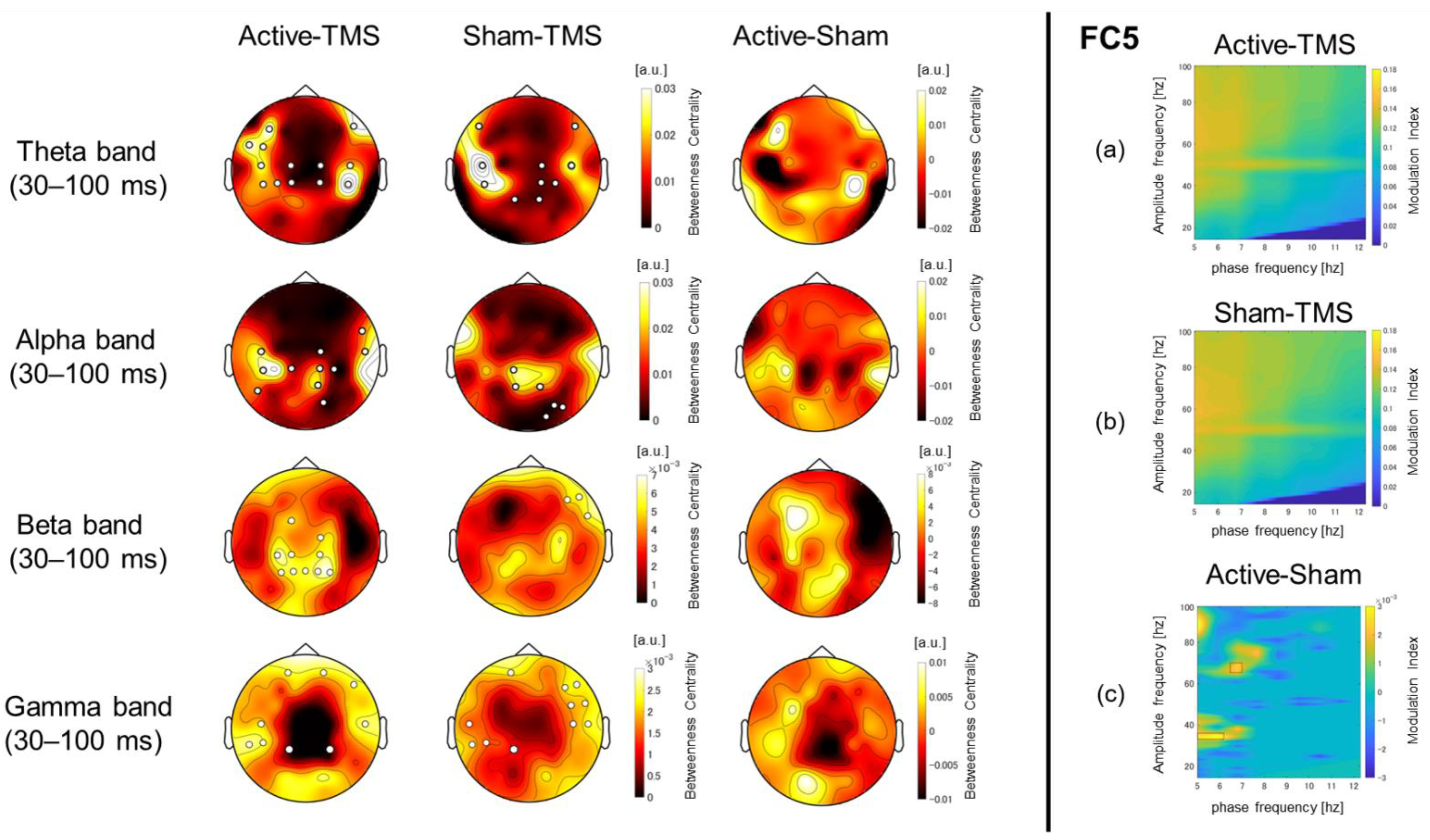
2.7. Connectivity and Network Analysis
2.8. Phase–Amplitude Coupling Analysis
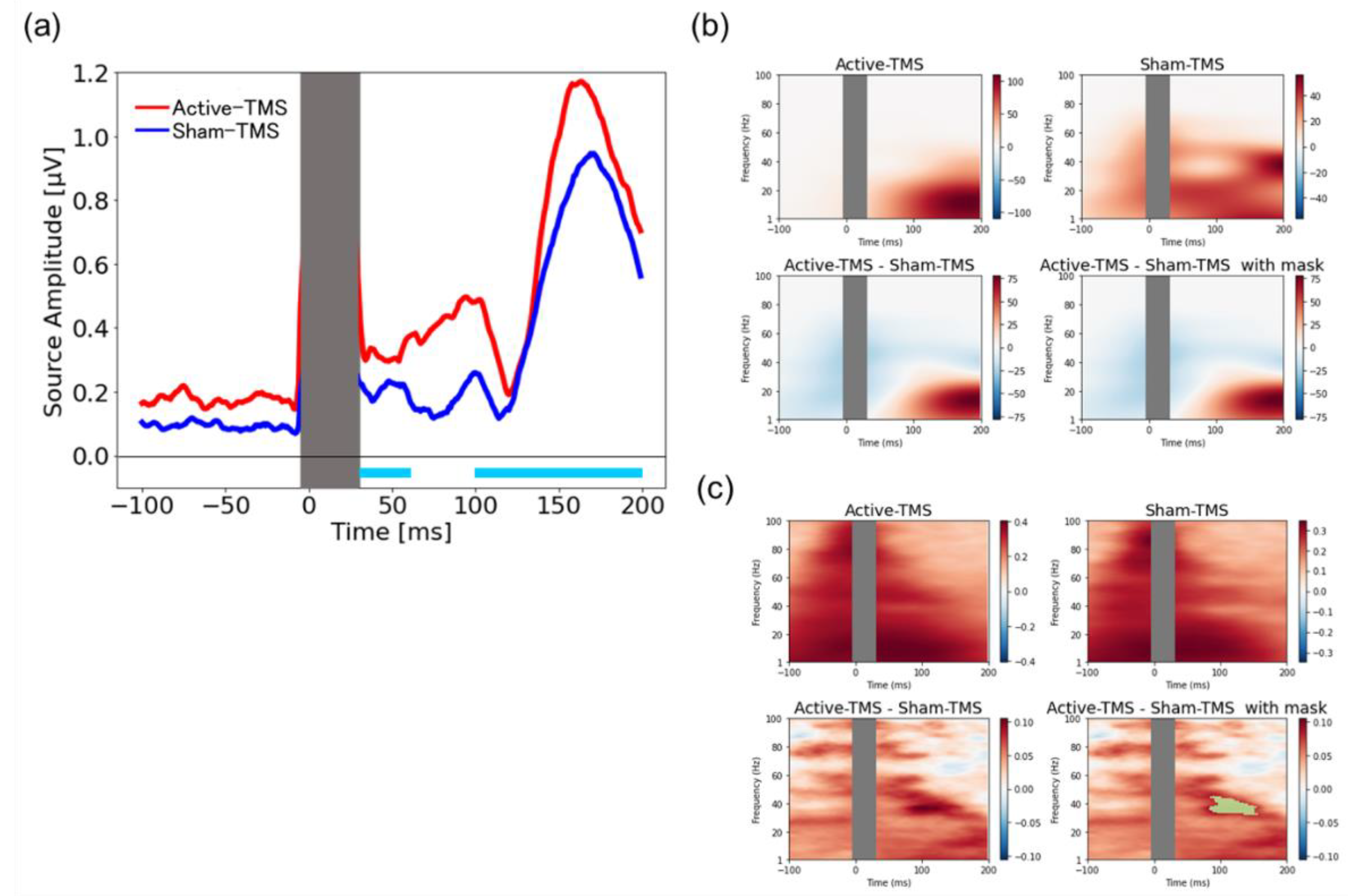
2.9. Source-Based Analysis
2.10. Statistical Analysis
3. Results
3.1. Global and Local Mean Field Power Analyses
3.2. Graph Theory-Based Network Analyses Using wPLI Values
3.3. Time–Frequency Analyses with Respect to Total Power and ITPC
3.4. Phase–Amplitude Cross-Frequency Coupling
3.5. Source-Based TMS-Elicited Response and Their Time–Frequency Analyses
3.6. Subjective Differences in Stimulus Sensation between Active and Sham Coils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. TMS-EEG: A Window into the Neurophysiological Effects of Transcranial Electrical Stimulation in Non-Motor Brain Regions. Neurosci. Biobehav. Rev. 2016, 64, 175–184. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal Responses to Magnetic Stimulation Reveal Cortical Reactivity and Connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef]
- Masuda, F.; Nakajima, S.; Miyazaki, T.; Yoshida, K.; Tsugawa, S.; Wada, M.; Ogyu, K.; Croarkin, P.E.; Blumberger, D.M.; Daskalakis, Z.J.; et al. Motor Cortex Excitability and Inhibitory Imbalance in Autism Spectrum Disorder Assessed with Transcranial Magnetic Stimulation: A Systematic Review. Transl. Psychiatry 2019, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, M.; Wada, M.; Nakajima, S.; Tsugawa, S.; Nakahara, T.; Blumberger, D.M.; Mimura, M.; Noda, Y. Transcranial Magnetic Stimulation Neurophysiology of Patients with Major Depressive Disorder: A Systematic Review and Meta-Analysis. Psychol. Med. 2021, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Nishida, H.; Nakajima, S.; Tsugawa, S.; Morita, S.; Yoshida, K.; Tarumi, R.; Ogyu, K.; Wada, M.; Kurose, S.; et al. Neurophysiological Biomarkers Using Transcranial Magnetic Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 121, 47–59. [Google Scholar] [CrossRef]
- Li, X.; Honda, S.; Nakajima, S.; Wada, M.; Yoshida, K.; Daskalakis, Z.J.; Mimura, M.; Noda, Y. TMS-EEG Research to Elucidate the Pathophysiological Neural Bases in Patients with Schizophrenia: A Systematic Review. J. Pers. Med. 2021, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Nakanishi, T.; Nakajima, S.; Li, X.; Wada, M.; Daskalakis, Z.J.; Goodman, M.S.; Blumberger, D.M.; Mimura, M.; Noda, Y. Insights of Neurophysiology on Unconscious State Using Combined Transcranial Magnetic Stimulation and Electroencephalography: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 131, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y. Toward the Establishment of Neurophysiological Indicators for Neuropsychiatric Disorders Using Transcranial Magnetic Stimulation-Evoked Potentials: A Systematic Review. Psychiatry Clin. Neurosci. 2020, 74, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical Utility and Prospective of TMS-EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, F.; Phillips, M.L. Examining and Modulating Neural Circuits in Psychiatric Disorders with Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments. Am. J. Psychiatry 2021, 178, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.-X.; Ma, M.-L.; Wang, C.-Z.; Iqbal, J.; Si, J.-J.; Xue, Y.-X.; Yang, J.-L. TMS-EEG: An Emerging Tool to Study the Neurophysiologic Biomarkers of Psychiatric Disorders. Neuropharmacology 2021, 197, 108574. [Google Scholar] [CrossRef] [PubMed]
- Conde, V.; Tomasevic, L.; Akopian, I.; Stanek, K.; Saturnino, G.B.; Thielscher, A.; Bergmann, T.O.; Siebner, H.R. The Non-Transcranial TMS-Evoked Potential Is an Inherent Source of Ambiguity in TMS-EEG Studies. Neuroimage 2019, 185, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pavon, J.C.; Metsomaa, J.; Mutanen, T.; Stenroos, M.; Mäki, H.; Ilmoniemi, R.J.; Sarvas, J. Uncovering Neural Independent Components from Highly Artifactual TMS-Evoked EEG Data. J. Neurosci. Methods 2012, 209, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Nikouline, V.; Ruohonen, J.; Ilmoniemi, R.J. The Role of the Coil Click in TMS Assessed with Simultaneous EEG. Clin. Neurophysiol. 1999, 110, 1325–1328. [Google Scholar] [CrossRef]
- Biabani, M.; Fornito, A.; Mutanen, T.P.; Morrow, J.; Rogasch, N.C. Characterizing and Minimizing the Contribution of Sensory Inputs to TMS-Evoked Potentials. Brain Stimul. 2019, 12, 1537–1552. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, M.; Reeves, J.A.; Hussain, S.J.; Zaghloul, K.A.; Wassermann, E.M. Identifying Site- and Stimulation-Specific TMS-Evoked EEG Potentials Using a Quantitative Cosine Similarity Metric. PLoS ONE 2020, 15, e0216185. [Google Scholar] [CrossRef]
- Korhonen, R.J.; Hernandez-Pavon, J.C.; Metsomaa, J.; Mäki, H.; Ilmoniemi, R.J.; Sarvas, J. Removal of Large Muscle Artifacts from Transcranial Magnetic Stimulation-Evoked EEG by Independent Component Analysis. Med. Biol. Eng. Comput. 2011, 49, 397–407. [Google Scholar] [CrossRef]
- Mutanen, T.; Mäki, H.; Ilmoniemi, R.J. The Effect of Stimulus Parameters on TMS–EEG Muscle Artifacts. Brain Stimul. 2013, 6, 371–376. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Thomson, R.H.; Farzan, F.; Fitzgibbon, B.M.; Bailey, N.W.; Hernandez-Pavon, J.C.; Daskalakis, Z.J.; Fitzgerald, P.B. Removing Artefacts from TMS-EEG Recordings Using Independent Component Analysis: Importance for Assessing Prefrontal and Motor Cortex Network Properties. Neuroimage 2014, 101, 425–439. [Google Scholar] [CrossRef]
- Ross, J.M.; Ozdemir, R.A.; Lian, S.J.; Fried, P.J.; Schmitt, E.M.; Inouye, S.K.; Pascual-Leone, A.; Shafi, M.M. A Structured ICA-Based Process for Removing Auditory Evoked Potentials. Sci. Rep. 2022, 12, 1391. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Di Santo, A.; Brown, K.; Ibáñez, J.; Casula, E.; Rawji, V.; Di Lazzaro, V.; Koch, G.; Rothwell, J. Disentangling EEG Responses to TMS due to Cortical and Peripheral Activations. Brain Stimul. 2021, 14, 4–18. [Google Scholar] [CrossRef]
- Belardinelli, P.; Biabani, M.; Blumberger, D.M.; Bortoletto, M.; Casarotto, S.; David, O.; Desideri, D.; Etkin, A.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Reproducibility in TMS-EEG Studies: A Call for Data Sharing, Standard Procedures and Effective Experimental Control. Brain Stimul. 2019, 12, 787–790. [Google Scholar] [CrossRef]
- Poorganji, M.; Zomorrodi, R.; Hawco, C.; Hill, A.T.; Hadas, I.; Rajji, T.K.; Chen, R.; Voineskos, D.; Daskalakis, A.A.; Blumberger, D.M.; et al. Differentiating Transcranial Magnetic Stimulation Cortical and Auditory Responses via Single Pulse and Paired Pulse Protocols: A TMS-EEG Study. Clin. Neurophysiol. 2021, 13, 1850–1858. [Google Scholar] [CrossRef]
- Siebner, H.R.; Conde, V.; Tomasevic, L.; Thielscher, A.; Bergmann, T.O. Distilling the Essence of TMS-Evoked EEG Potentials (TEPs): A Call for Securing Mechanistic Specificity and Experimental Rigor. Brain Stimul. 2019, 12, 1051–1054. [Google Scholar] [CrossRef]
- Fuggetta, G.; Fiaschi, A.; Manganotti, P. Modulation of Cortical Oscillatory Activities Induced by Varying Single-Pulse Transcranial Magnetic Stimulation Intensity over the Left Primary Motor Area: A Combined EEG and TMS Study. Neuroimage 2005, 27, 896–908. [Google Scholar] [CrossRef]
- Du, X.; Choa, F.-S.; Summerfelt, A.; Rowland, L.M.; Chiappelli, J.; Kochunov, P.; Hong, L.E. N100 as a Generic Cortical Electrophysiological Marker Based on Decomposition of TMS-Evoked Potentials across Five Anatomic Locations. Exp. Brain Res. 2017, 235, 69–81. [Google Scholar] [CrossRef]
- Herring, J.D.; Thut, G.; Jensen, O.; Bergmann, T.O. Attention Modulates TMS-Locked Alpha Oscillations in the Visual Cortex. J. Neurosci. 2015, 35, 14435–14447. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.C.; Desideri, D.; Belardinelli, P.; Zrenner, C.; Ziemann, U. Comparison of Cortical EEG Responses to Realistic Sham versus Real TMS of Human Motor Cortex. Brain Stimul. 2018, 11, 1322–1330. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial Magnetic Stimulation and Cortical Evoked Potentials: A TMS/EEG Co-Registration Study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Havlicek, J.; Phillips, D.; Nakajima, S.; Mimura, M.; Noda, Y. Development of an Advanced Sham Coil for Transcranial Magnetic Stimulation and Examination of Its Specifications. J. Pers. Med. 2021, 11, 1058. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Houser, C.M.; Reese, K.; Shotland, L.I.; Grafman, J.; Sato, S.; Valls-Solé, J.; Brasil-Neto, J.P.; Wassermann, E.M.; Cohen, L.G. Safety of Rapid-Rate Transcranial Magnetic Stimulation in Normal Volunteers. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 120–130. [Google Scholar] [CrossRef]
- ter Braack, E.M.; de Vos, C.C.; van Putten, M.J.A.M. Masking the Auditory Evoked Potential in TMS-EEG: A Comparison of Various Methods. Brain Topogr. 2015, 28, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, L.J.; Keller, C.J.; Wu, W.; Narayan, M.; Etkin, A. Test-Retest Reliability of Transcranial Magnetic Stimulation EEG Evoked Potentials. Brain Stimul. 2018, 11, 536–544. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open-Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- TESA Users Manual. Available online: https://nigelrogasch.gitbook.io/tesa-user-manual/ (accessed on 1 March 2022).
- FastICA. Available online: http://research.ics.aalto.fi/ica/fastica/code/dlcode.shtml (accessed on 1 March 2021).
- Bell, A.J.; Sejnowski, T.J. An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Sullivan, C.; Thomson, R.H.; Rose, N.S.; Bailey, N.W.; Fitzgerald, P.B.; Farzan, F.; Hernandez-Pavon, J.C. Analysing Concurrent Transcranial Magnetic Stimulation and Electroencephalographic Data: A Review and Introduction to the Open-Source TESA Software. Neuroimage 2017, 147, 934–951. [Google Scholar] [CrossRef]
- Lehmann, D.; Skrandies, W. Reference-Free Identification of Components of Checkerboard-Evoked Multichannel Potential Fields. Electroencephalogr. Clin. Neurophysiol. 1980, 48, 609–621. [Google Scholar] [CrossRef]
- Esser, S.K.; Huber, R.; Massimini, M.; Peterson, M.J.; Ferrarelli, F.; Tononi, G. A Direct Demonstration of Cortical LTP in Humans: A Combined TMS/EEG Study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Fecchio, M.; Pigorini, A.; Comanducci, A.; Sarasso, S.; Casarotto, S.; Premoli, I.; Derchi, C.-C.; Mazza, A.; Russo, S.; Resta, F.; et al. The Spectral Features of EEG Responses to Transcranial Magnetic Stimulation of the Primary Motor Cortex Depend on the Amplitude of the Motor Evoked Potentials. PLoS ONE 2017, 12, e0184910. [Google Scholar] [CrossRef]
- Casarotto, S.; Canali, P.; Rosanova, M.; Pigorini, A.; Fecchio, M.; Mariotti, M.; Lucca, A.; Colombo, C.; Benedetti, F.; Massimini, M. Assessing the Effects of Electroconvulsive Therapy on Cortical Excitability by Means of Transcranial Magnetic Stimulation and Electroencephalography. Brain Topogr. 2013, 26, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Oostenveld, R.; van Wingerden, M.; Battaglia, F.; Pennartz, C.M.A. An Improved Index of Phase-Synchronization for Electrophysiological Data in the Presence of Volume-Conduction, Noise and Sample-Size Bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Ortiz, E.; Stingl, K.; Münßinger, J.; Braun, C.; Preissl, H.; Belardinelli, P. Weighted Phase Lag Index and Graph Analysis: Preliminary Investigation of Functional Connectivity during Resting State in Children. Comput. Math. Methods Med. 2012, 2012, 186353. [Google Scholar] [CrossRef]
- Imperatori, L.S.; Betta, M.; Cecchetti, L.; Canales-Johnson, A.; Ricciardi, E.; Siclari, F.; Pietrini, P.; Chennu, S.; Bernardi, G. EEG Functional Connectivity Metrics wPLI and wSMI Account for Distinct Types of Brain Functional Interactions. Sci. Rep. 2019, 9, 8894. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective Dynamics of “Small-World” Networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.T.; Amin, H.U.; Izhar, L.I.; Saad, M.N.M.; Abdul Rahman, M.; Malik, A.S.; Tang, T.B. Exploring EEG Effective Connectivity Network in Estimating Influence of Color on Emotion and Memory. Front. Neuroinform. 2019, 13, 66. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Wang, Y.; Nelissen, N.; Vandenbulcke, M.; Dhollander, T.; Sunaert, S.; Dupont, P. The Associative-Semantic Network for Words and Pictures: Effective Connectivity and Graph Analysis. Brain Lang. 2013, 127, 264–272. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Mandl, R.C.W.; Stam, C.J.; Kahn, R.S.; Hulshoff Pol, H.E. Aberrant Frontal and Temporal Complex Network Structure in Schizophrenia: A Graph Theoretical Analysis. J. Neurosci. 2010, 30, 15915–15926. [Google Scholar] [CrossRef]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring Phase-Amplitude Coupling between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG Data Analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Mosher, J.C.; Leahy, R.M.; Lewis, P.S. EEG and MEG: Forward Solutions for Inverse Methods. IEEE Trans. Biomed. Eng. 1999, 46, 245–259. [Google Scholar] [CrossRef]
- Engemann, D.A.; Gramfort, A. Automated Model Selection in Covariance Estimation and Spatial Whitening of MEG and EEG Signals. Neuroimage 2015, 108, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Threshold-Free Cluster Enhancement: Addressing Problems of Smoothing, Threshold Dependence and Localisation in Cluster Inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Rogasch, N.C.; Zipser, C.; Darmani, G.; Mutanen, T.P.; Biabani, M.; Zrenner, C.; Desideri, D.; Belardinelli, P.; Müller-Dahlhaus, F.; Ziemann, U. The Effects of NMDA Receptor Blockade on TMS-Evoked EEG Potentials from Prefrontal and Parietal Cortex. Sci. Rep. 2020, 10, 3168. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, Z.J.; Farzan, F.; Barr, M.S.; Maller, J.J.; Chen, R.; Fitzgerald, P.B. Long-Interval Cortical Inhibition from the Dorsolateral Prefrontal Cortex: A TMS-EEG Study. Neuropsychopharmacology 2008, 33, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of Cortical Effective Connectivity during Sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014; ISBN 9780262319560. [Google Scholar]
- Vallesi, A.; Del Felice, A.; Capizzi, M.; Tafuro, A.; Formaggio, E.; Bisiacchi, P.; Masiero, S.; Ambrosini, E. Natural Oscillation Frequencies in the Two Lateral Prefrontal Cortices Induced by Transcranial Magnetic Stimulation. Neuroimage 2021, 227, 117655. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Daskalakis, Z.J.; Fitzgerald, P.B. Cortical Inhibition of Distinct Mechanisms in the Dorsolateral Prefrontal Cortex Is Related to Working Memory Performance: A TMS–EEG Study. Cortex 2015, 64, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Brignani, D.; Manganotti, P.; Rossini, P.M.; Miniussi, C. Modulation of Cortical Oscillatory Activity during Transcranial Magnetic Stimulation. Hum. Brain Mapp. 2008, 29, 603–612. [Google Scholar] [CrossRef]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006; ISBN 9780199863716. [Google Scholar]
- Watrous, A.J.; Tandon, N.; Conner, C.R.; Pieters, T.; Ekstrom, A.D. Frequency-Specific Network Connectivity Increases Underlie Accurate Spatiotemporal Memory Retrieval. Nat. Neurosci. 2013, 16, 349–356. [Google Scholar] [CrossRef]
| Demographic Data and TMS Parameters for This Study | |
|---|---|
| Sample size (numbers of males/females) | 28 (15/13) |
| Age (years old) | 33.9 ± 11.0 (mean ± S.D.) |
| RMT (%MSO) | 58.2 ± 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, M.; Wada, M.; Zomorrodi, R.; Taniguchi, K.; Li, X.; Honda, S.; Tobari, Y.; Mimura, Y.; Nakajima, S.; Kitahata, R.; et al. Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition. Biosensors 2022, 12, 814. https://doi.org/10.3390/bios12100814
Takano M, Wada M, Zomorrodi R, Taniguchi K, Li X, Honda S, Tobari Y, Mimura Y, Nakajima S, Kitahata R, et al. Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition. Biosensors. 2022; 12(10):814. https://doi.org/10.3390/bios12100814
Chicago/Turabian StyleTakano, Mayuko, Masataka Wada, Reza Zomorrodi, Keita Taniguchi, Xuemei Li, Shiori Honda, Yui Tobari, Yu Mimura, Shinichiro Nakajima, Ryosuke Kitahata, and et al. 2022. "Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition" Biosensors 12, no. 10: 814. https://doi.org/10.3390/bios12100814
APA StyleTakano, M., Wada, M., Zomorrodi, R., Taniguchi, K., Li, X., Honda, S., Tobari, Y., Mimura, Y., Nakajima, S., Kitahata, R., Mimura, M., Daskalakis, Z. J., Blumberger, D. M., & Noda, Y. (2022). Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition. Biosensors, 12(10), 814. https://doi.org/10.3390/bios12100814






