Cervical Imaging in the Low Resource Setting: A Review
Abstract
1. Introduction
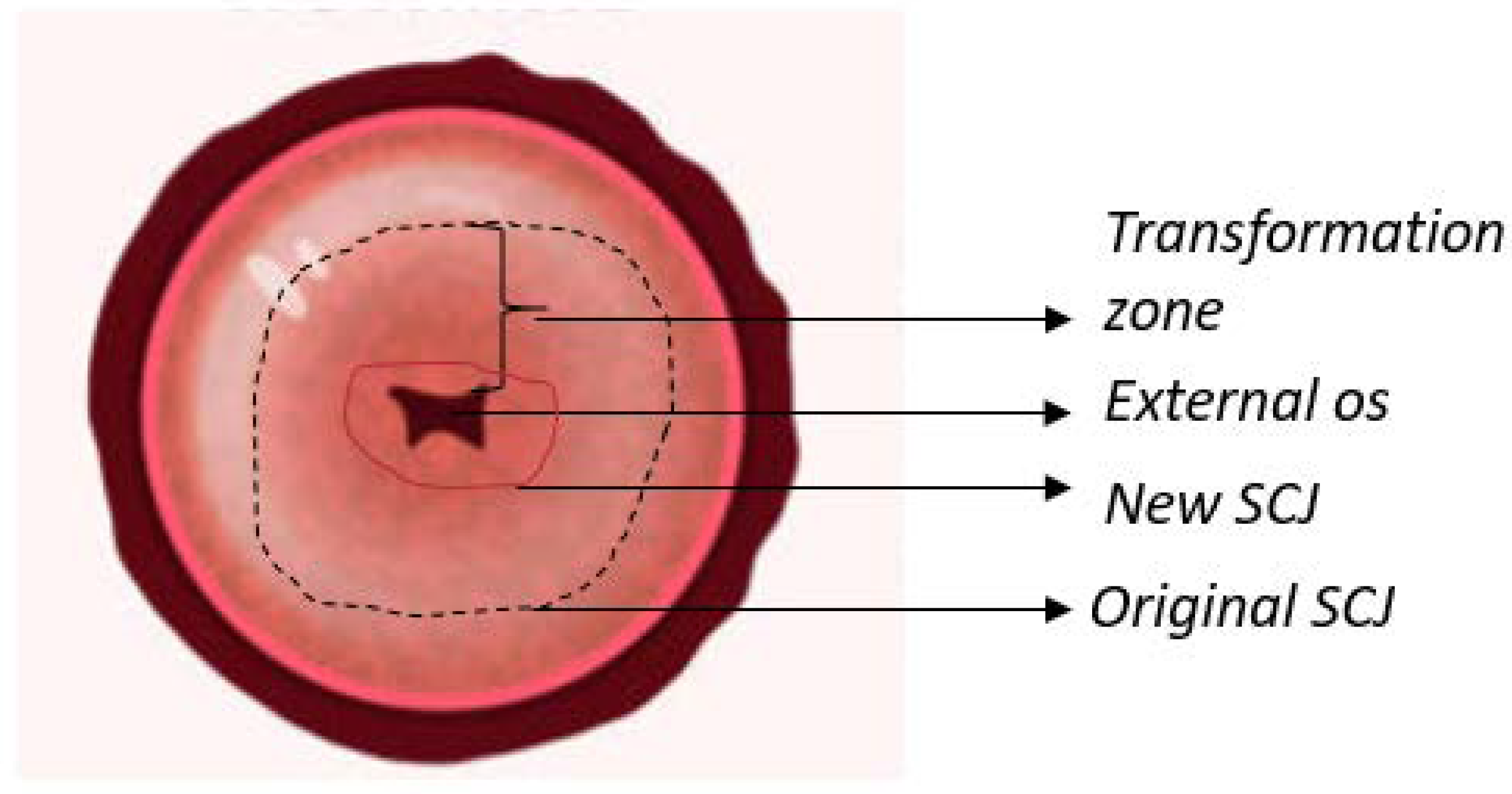
1.1. Anatomy of The Cervix
1.2. Disease Progression
1.3. Cervical Testing and Treatment
1.3.1. HPV DNA Testing, Cytology, Colposcopy, and Biopsy
1.3.2. Visual Inspection
1.3.3. Treatments
1.4. HPV Vaccines
2. Limits of Cervical Screening in the Low Resource Setting
3. Cervical Imaging Targeted for Neoplastic Detection
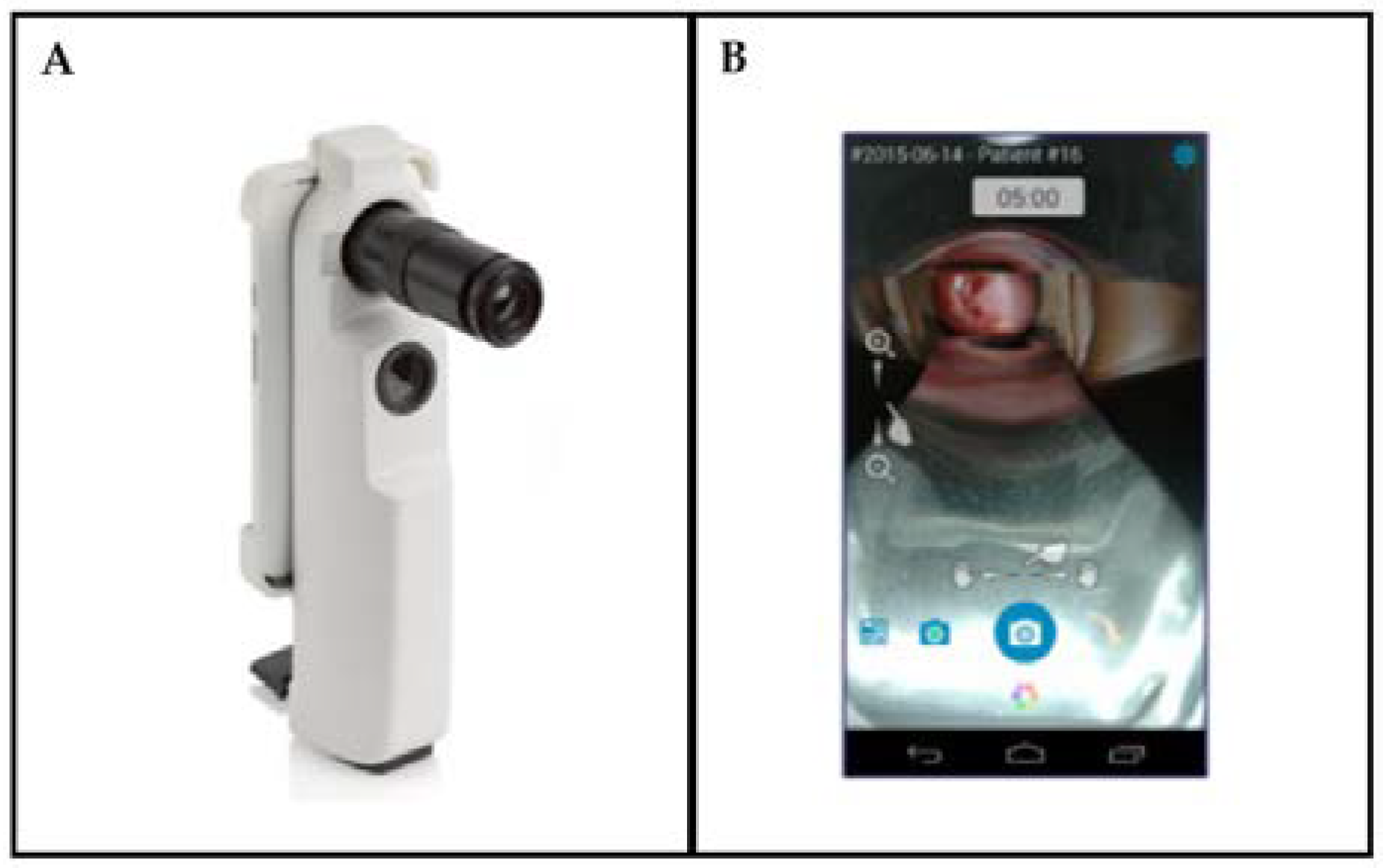
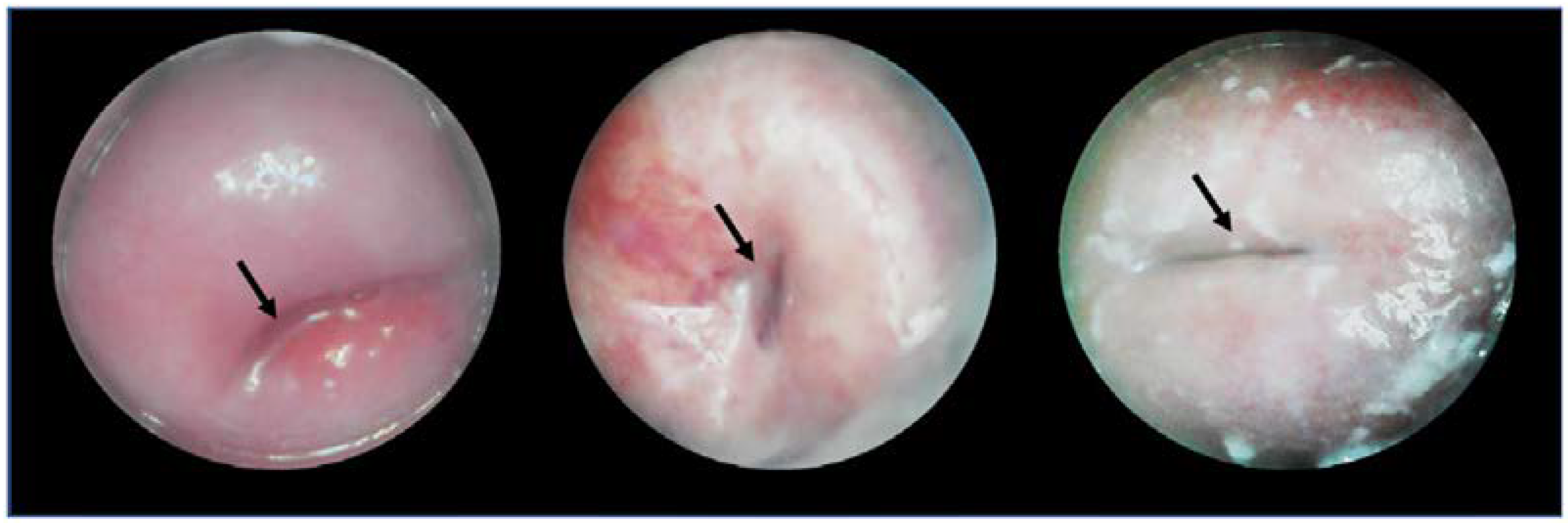
3.1. Callascope
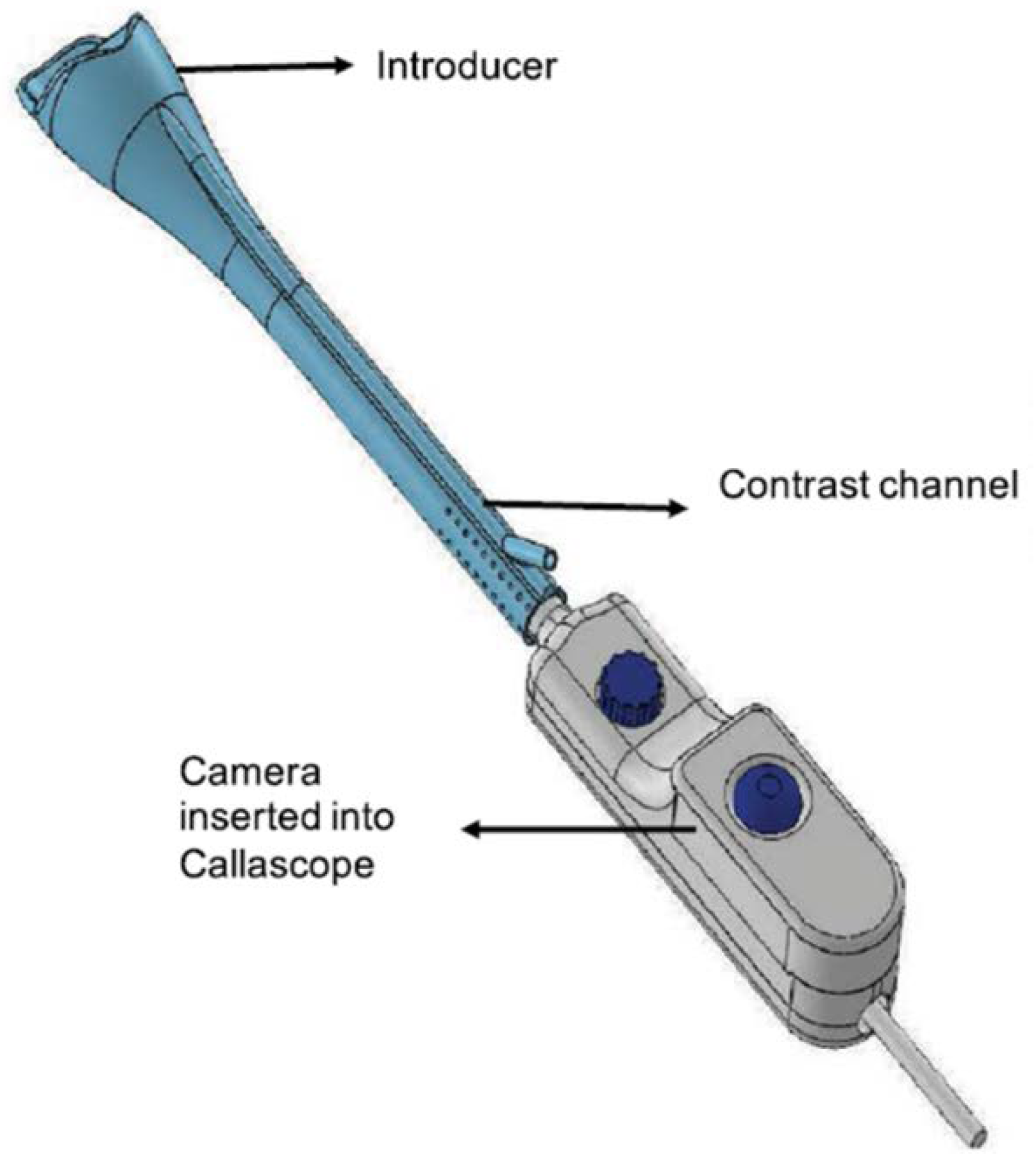
3.1.1. Device
3.1.2. Clinical Testing
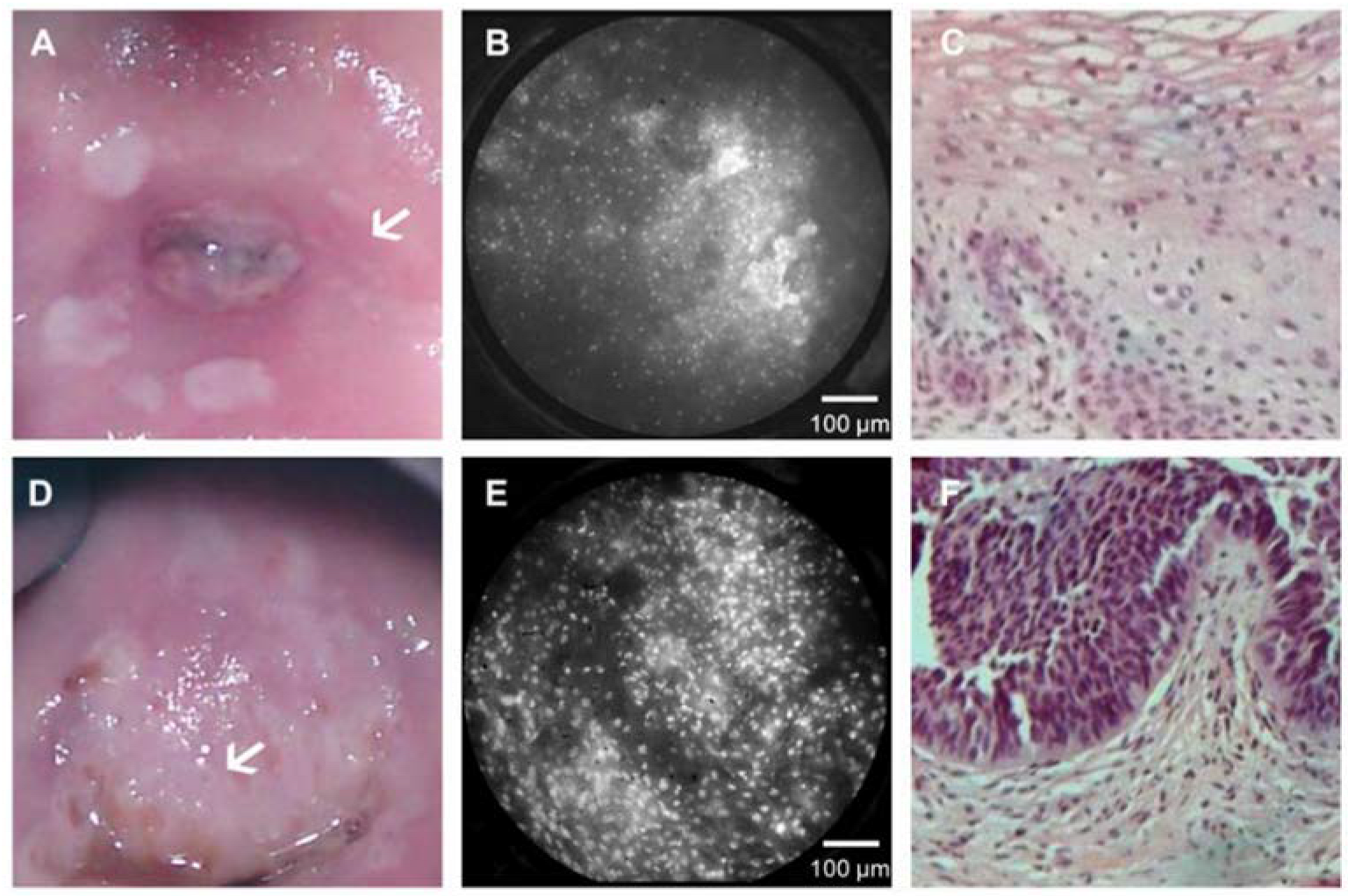
3.2. High-Resolution Microendoscope (HRME)
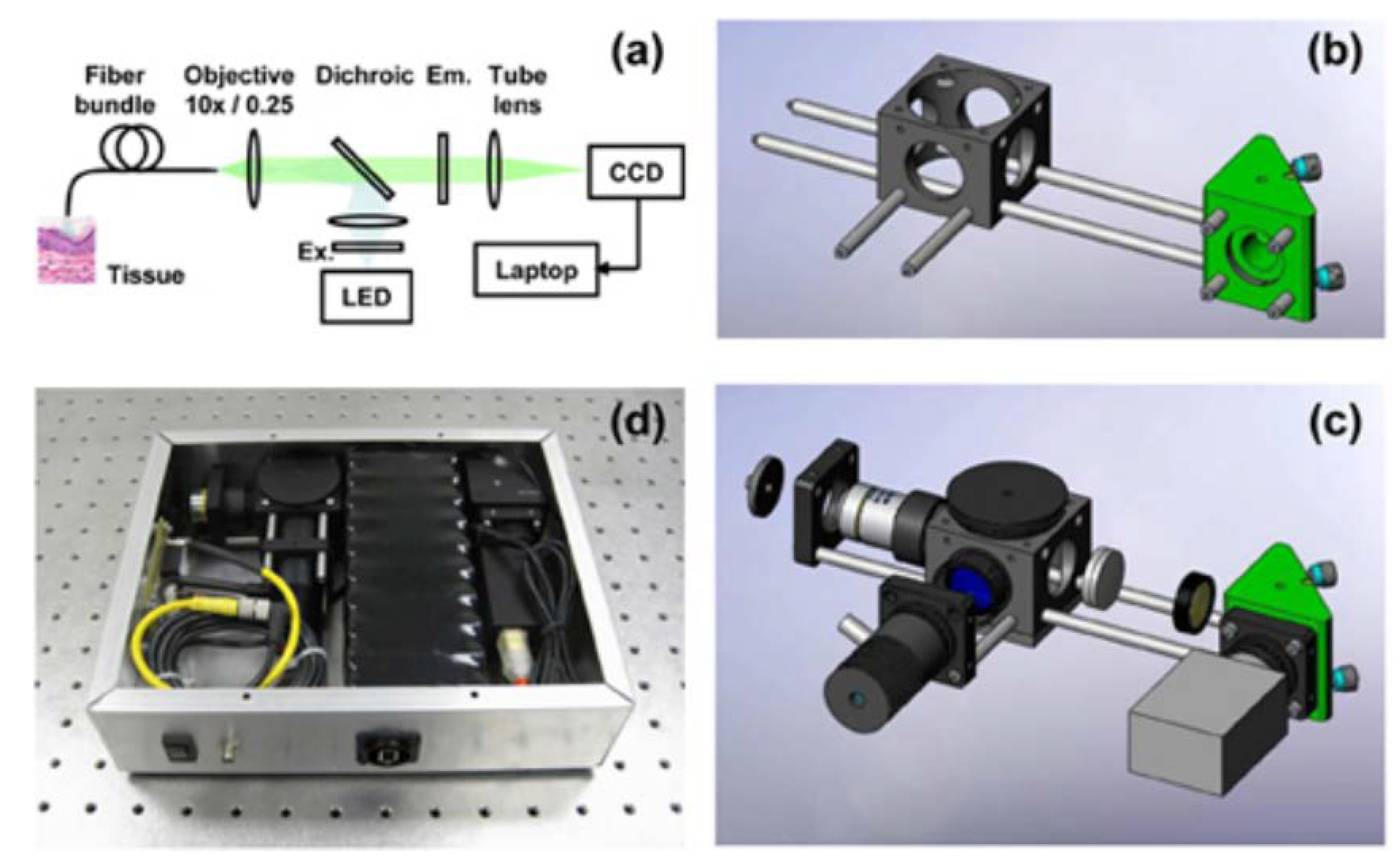
3.2.1. Device
3.2.2. Clinical Testing
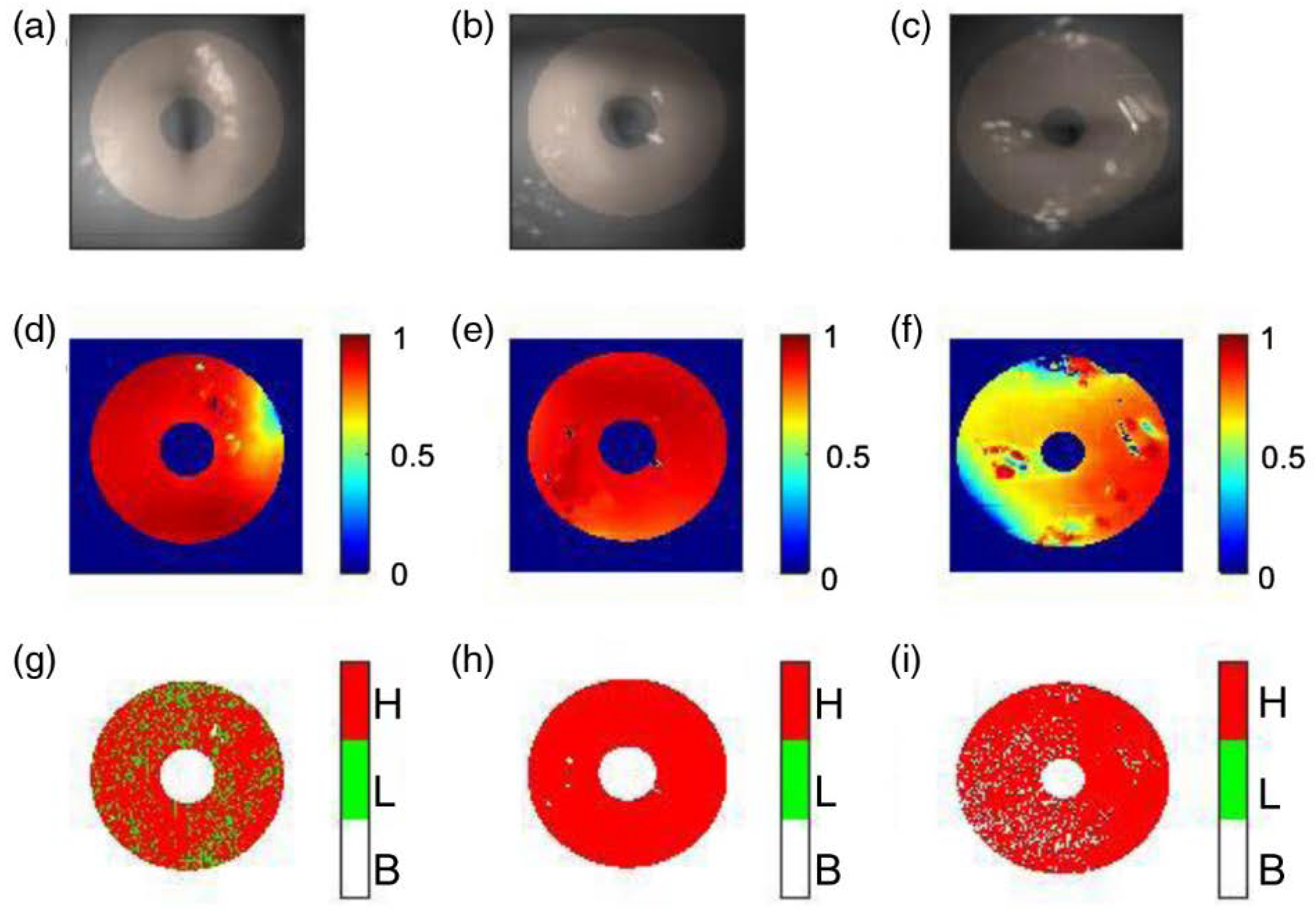
3.3. Snapshot Mueller Matrix Polarimeter
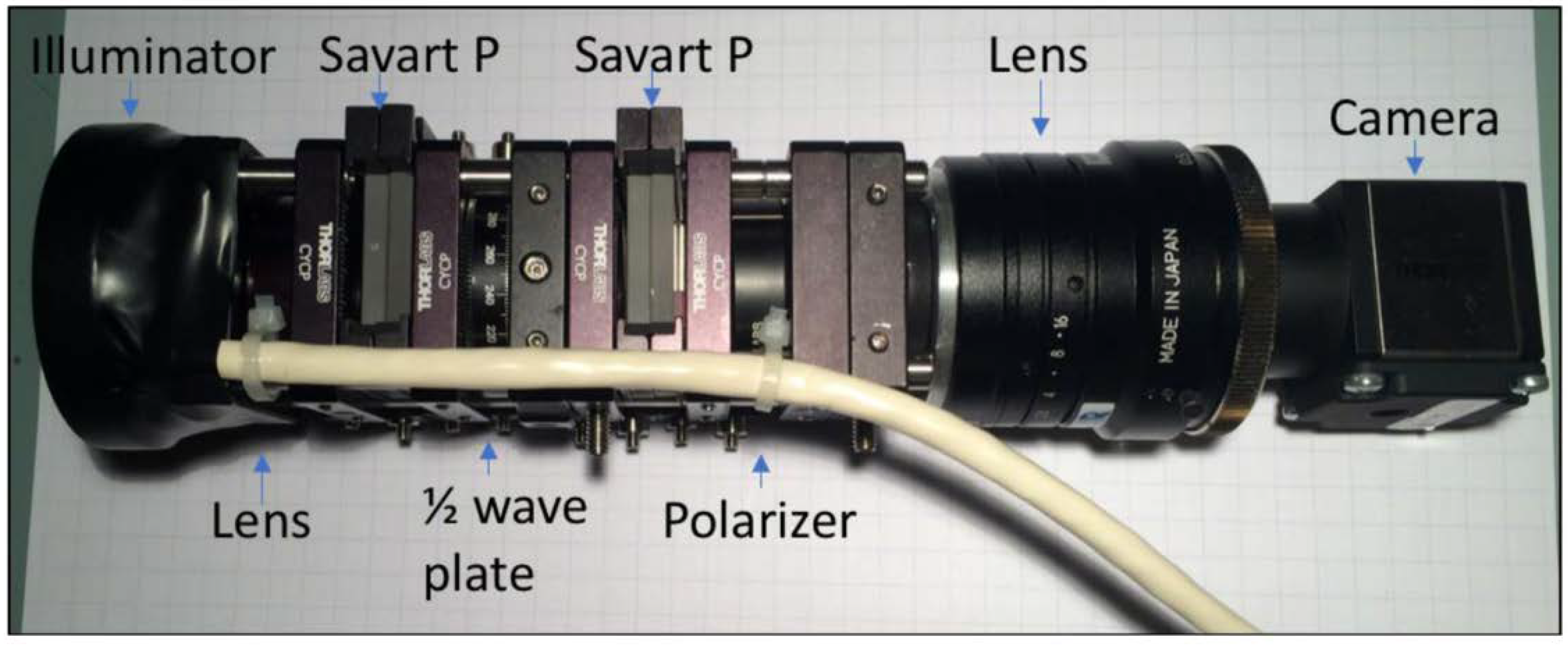
3.3.1. Device
3.3.2. Clinical Testing
3.4. Enhanced Visual Assessment (EVA) System
3.4.1. Device
3.4.2. Clinical Testing
3.5. Gynocular
3.5.1. Device
3.5.2. Clinical Testing
3.6. Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ashford, L.C.Y. Preventing Cervical Cancer Worldwide; Population Reference Bureau: Washington, DC, USA, 2005. [Google Scholar]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.C.M.; et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef]
- Novikova, T. Optical techniques for cervical neoplasia detection. Beilstein J. Nanotechnol. 2017, 8, 1844–1862. [Google Scholar] [CrossRef]
- Søfteland, S.; Sebitloane, M.H.; Taylor, M.; Roald, B.B.; Holmen, S.; Galappaththi-Arachchige, H.N.; Gundersen, S.G.; Kjetland, E.F. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int. J. Gynecol. Obstet. 2021, 153, 190–199. [Google Scholar] [CrossRef]
- Devine, C.; Viswanathan, C.; Faria, S.; Marcal, L.; Sagebiel, T.L. Imaging and Staging of Cervical Cancer. Semin. Ultrasound CT MRI 2019, 40, 280–286. [Google Scholar] [CrossRef]
- Fadare, O.; Roma, A.A. Normal Anatomy of the Uterine Cervix. In Atlas of Uterine Pathology; Fadare, O., Roma, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 193–196. [Google Scholar]
- Aspden, R.M. Collagen Organisation in the Cervix and its Relation to Mechanical Function. Coll. Relat. Res. 1988, 8, 103–112. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Lowy, D.R.; Solomon, D.; Hildesheim, A.; Schiller, J.T.; Schiffman, M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer 2008, 113, 1980–1993. [Google Scholar] [CrossRef]
- Mirkovic, J.; Howitt, B.E.; Roncarati, P.; Demoulin, S.; Suarez-Carmona, M.; Hubert, P.; McKeon, F.D.; Xian, W.; Li, A.; Delvenne, P.; et al. Carcinogenic HPV infection in the cervical squamo-columnar junction. J. Pathol. 2015, 236, 265–271. [Google Scholar] [CrossRef]
- Safaeian, M.; Solomon, D.; Castle, P.E. Cervical cancer prevention—Cervical screening: Science in evolution. Obstet. Gynecol. Clin. N. Am. 2007, 34, 739–760. [Google Scholar] [CrossRef]
- Sahebali, S.; Van den Eynden, G.; Murta, E.; Michelin, M.; Cusumano, P.; Petignat, P.; Bogers, J.-P. Stromal issues in cervical cancer: A review of the role and function of basement membrane, stroma, immune response and angiogenesis in cervical cancer development. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2010, 19, 204–215. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ménard, S.; Forti, S.; Tagliabue, E. New insights into the role of extracellular matrix during tumor onset and progression. J. Cell Physiol. 2002, 192, 259–267. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent Advances in Chemoprevention of Cancer. Science 1997, 278, 1073. [Google Scholar] [CrossRef]
- Ingber, D.E. Cancer as a disease of epithelial–mesenchymal interactions and extracellular matrix regulation. Differentiation 2002, 70, 547–560. [Google Scholar] [CrossRef]
- Arifler, D.; Pavlova, I.; Gillenwater, A.; Richards-Kortum, R. Light Scattering from Collagen Fiber Networks: Micro-Optical Properties of Normal and Neoplastic Stroma. Biophys. J. 2007, 92, 3260–3274. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Available online: https://apps.who.int/iris/bitstream/handle/10665/94830/9789241548694_eng.pdf;jsessionid=3538E6098C159F912E419FB7F0CFC1B9?sequence=1 (accessed on 25 January 2022).
- Stoler, M.H.; Schiffman, M.; for the Atypical Squamous Cells of Undetermined Significance–Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver Reproducibility of Cervical Cytologic and Histologic InterpretationsRealistic Estimates From the ASCUS-LSIL Triage Study. JAMA 2001, 285, 1500–1505. [Google Scholar] [CrossRef]
- Dabeski, D.; Duvlis, S.; Basheska, N.; Antovska, V.; Stojovski, M.; Trajanova, M.; Dimitrov, G.; Dabeski, A.; Gureva-Gjorgievska, N. Comparison Between HPV DNA Testing and HPV E6/E7 MRNA Testing in Women with Squamous Cell Abnormalities of the Uterine Cervix. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2019, 40, 51–58. [Google Scholar] [CrossRef]
- Mitchell, M.F.; Schottenfeld, D.; Tortolero-Luna, G.; Cantor, S.B.; Richards-Kortum, R. Colposcopy for the diagnosis of squamous intraepithelial lesions: A meta-analysis. Obstet. Gynecol. 1998, 91, 626–631. [Google Scholar] [CrossRef]
- Orfanoudaki, I.M.; Kappou, D.; Sifakis, S. Recent advances in optical imaging for cervical cancer detection. Arch. Gynecol. Obstet. 2011, 284, 1197–1208. [Google Scholar] [CrossRef]
- Ho, D.S. Development of Optical Technologies for Comprehensive Screening of Cervical Epithelial Health. Ph.D. Thesis, Duke University, Ann Arbor, MI, USA, 2019. [Google Scholar]
- Adamopoulou, M.; Kalkani, E.; Charvalos, E.; Avgoustidis, D.; Haidopoulos, D.; Yapijakis, C. Comparison of Cytology, Colposcopy, HPV Typing and Biomarker Analysis in Cervical Neoplasia. Anticancer. Res. 2009, 29, 3401. [Google Scholar]
- Sellors, J.W.; Nieminen, P.; Vesterinen, E.; Paavonen, J. Observer Variability in the Scoring of Colpophotographs. Obstet. Gynecol. 1990, 76, 1006–1008. [Google Scholar]
- Sritipsukho, P.; Thaweekul, Y. Accuracy of visual inspection with acetic acid (VIA) for cervical cancer screening: A systematic review. J. Med. Assoc. Thai. 2010, 93 (Suppl. 7), S254–S261. [Google Scholar]
- Arbyn, M.; Sankaranarayanan, R.; Muwonge, R.; Keita, N.; Dolo, A.; Mbalawa, C.G.; Nouhou, H.; Sakande, B.; Wesley, R.; Somanathan, T.; et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int. J. Cancer 2008, 123, 153–160. [Google Scholar] [CrossRef]
- Catarino, R.; Schäfer, S.; Vassilakos, P.; Petignat, P.; Arbyn, M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: A meta-analysis. BJOG 2018, 125, 545–553. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Nessa, A.; Esmy, P.O.; Dangou, J.-M. Visual inspection methods for cervical cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 221–232. [Google Scholar] [CrossRef]
- Raifu, A.O.; El-Zein, M.; Sangwa-Lugoma, G.; Ramanakumar, A.; Walter, S.D.; Franco, E.L.; For the Congo Screening Study Group. Determinants of Cervical Cancer Screening Accuracy for Visual Inspection with Acetic Acid (VIA) and Lugol’s Iodine (VILI) Performed by Nurse and Physician. PLoS ONE 2017, 12, e0170631. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, X.; Shao, H.; Liu, Q.; Tou, J.; Zhang, Y.; Luo, Q.; Xiang, Q. VIA/VILI is more suitable for cervical cancer prevention in Chinese poverty-stricken region: A health economic evaluation. BMC Public Health 2017, 17, 118. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E.; Centers for Disease Control and Prevention (CDC). Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. Available online: https://www.cdc.gov/mmwr/pdf/wk/mm6411.pdf#page=12 (accessed on 20 January 2022).
- Rosenblum, H.G.; Lewis, R.M.; Gargano, J.W.; Querec, T.D.; Unger, E.R.; Markowitz, L.E. Declines in Prevalence of Human Papillomavirus Vaccine-Type Infection Among Females after Introduction of Vaccine—United States, 2003–2018. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 415–420. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7012a2.htm?s_cid=mm7012a2_w (accessed on 20 January 2022). [CrossRef]
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine 2017, 35, 5753–5755. [Google Scholar] [CrossRef]
- Nikumbh, D.B.; Nikumbh, R.D.; Kanthikar, S.N. Limitations of cytological cervical cancer screening (Papanicolaou test) regarding technical and cultural aspect in rural India. South Asian J. Cancer 2016, 5, 79. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S.; Mehrotra, R.; Sodhani, P. Cervical Cancer Screening in Resource-Constrained Countries: Current Status and Future Directions. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 1461–1467. [Google Scholar] [CrossRef]
- Cervical Cancer Screening in Low-Resource Settings. In Committee Opinion No. 624; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2015; pp. 526–528.
- Gallay, C.; Girardet, A.; Viviano, M.; Catarino, R.; Benski, A.-C.; Tran, P.L.; Ecabert, C.; Thiran, J.-P.; Vassilakos, P.; Petignat, P. Cervical cancer screening in low-resource settings: A smartphone image application as an alternative to colposcopy. Int. J. Women’s Health 2017, 9, 455–461. [Google Scholar] [CrossRef]
- Asiedu, M.N.; Agudogo, J.S.; Dotson, M.E.; Skerrett, E.; Krieger, M.S.; Lam, C.T.; Agyei, D.; Amewu, J.; Asah-Opoku, K.; Huchko, M.; et al. A novel speculum-free imaging strategy for visualization of the internal female lower reproductive system. Sci. Rep. 2020, 10, 16570. [Google Scholar] [CrossRef]
- Asiedu, M.N.; Agudogo, J.; Krieger, M.S.; Miros, R.; Proeschold-Bell, R.J.; Schmitt, J.W.; Ramanujam, N. Design and preliminary analysis of a vaginal inserter for speculum-free cervical cancer screening. PLoS ONE 2017, 12, e0177782. [Google Scholar]
- Asiedu, M.N.; Simhal, A.; Lam, C.T.; Mueller, J.; Chaudhary, U.; Schmitt, J.W.; Sapiro, G.; Ramanujam, N. Image processing and machine learning techniques to automate diagnosis of Lugol’s iodine cervigrams for a low-cost point-of-care digital colposcope. In Proceedings of the Optics and Biophotonics in Low-Resource Settings IV, San Francisco, CA, USA, 27–28 January 2018; p. 1048508. [Google Scholar]
- Asiedu, M.N.; Simhal, A.; Chaudhary, U.; Mueller, J.L.; Lam, C.T.; Schmitt, J.W.; Venegas, G.; Sapiro, G.; Ramanujam, N. Development of algorithms for automated detection of cervical pre-cancers with a low-cost, point-of-care, pocket colposcope. IEEE Trans. Biomed. Eng. 2018, 66, 2306–2318. [Google Scholar] [CrossRef]
- Quinn, M.K.; Bubi, T.C.; Pierce, M.C.; Kayembe, M.K.; Ramogola-Masire, D.; Richards-Kortum, R. High-resolution microendoscopy for the detection of cervical neoplasia in low-resource settings. PLoS ONE 2012, 7, e44924. [Google Scholar] [CrossRef]
- Pierce, M.; Yu, D.; Richards-Kortum, R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J. Vis. Exp. JoVE 2011, 47, e2306. [Google Scholar] [CrossRef]
- Quang, T.; Schwarz, R.A.; Dawsey, S.M.; Tan, M.C.; Patel, K.; Yu, X.; Wang, G.; Zhang, F.; Xu, H.; Anandasabapathy, S.; et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia. Gastrointest. Endosc. 2016, 84, 834–841. [Google Scholar] [CrossRef]
- Parra, S.; Carranza, E.; Coole, J.; Hunt, B.; Smith, C.; Keahey, P.; Maza, M.; Schmeler, K.; Richards-Kortum, R. Development of Low-Cost Point-of-Care Technologies for Cervical Cancer Prevention Based on a Single-Board Computer. IEEE J. Transl. Eng. Health Med. 2020, 8, 4300210. [Google Scholar] [CrossRef]
- Hunt, B.; Fregnani, J.H.T.G.; Schwarz, R.A.; Pantano, N.; Tesoni, S.; Possati-Resende, J.C.; Antoniazzi, M.; de Oliveira Fonseca, B.; de Macêdo Matsushita, G.; Scapulatempo-Neto, C.; et al. Diagnosing Cervical Neoplasia in Rural Brazil Using a Mobile Van Equipped with In Vivo Microscopy: A Cluster-Randomized Community Trial. Cancer Prev. Res. 2018, 11, 359. [Google Scholar] [CrossRef]
- Parra, S.G.; Rodriguez, A.M.; Cherry, K.D.; Schwarz, R.A.; Gowen, R.M.; Guerra, L.B.; Milbourne, A.M.; Toscano, P.A.; Fisher-Hoch, S.P.; Schmeler, K.M.; et al. Low-cost, high-resolution imaging for detecting cervical precancer in medically-underserved areas of Texas. Gynecol. Oncol. 2019, 154, 558–564. [Google Scholar] [CrossRef]
- Grant, B.D.; Fregnani, J.H.; Possati Resende, J.C.; Scapulatempo-Neto, C.; Matsushita, G.M.; Mauad, E.C.; Quang, T.; Stoler, M.H.; Castle, P.E.; Schmeler, K.M.; et al. High-resolution microendoscopy: A point-of-care diagnostic for cervical dysplasia in low-resource settings. Eur. J. Cancer Prev. 2017, 26, 63–70. [Google Scholar] [CrossRef]
- Hunt, B.; Fregnani, J.H.T.G.; Brenes, D.; Schwarz, R.A.; Salcedo, M.P.; Possati-Resende, J.C.; Antoniazzi, M.; de Oliveira Fonseca, B.; Santana, I.V.V.; de Macêdo Matsushita, G.; et al. Cervical lesion assessment using real-time microendoscopy image analysis in Brazil: The CLARA study. Int. J. Cancer 2021, 149, 431–441. [Google Scholar] [CrossRef]
- Vila, P.M.; Park, C.W.; Pierce, M.C.; Goldstein, G.H.; Levy, L.; Gurudutt, V.V.; Polydorides, A.D.; Godbold, J.H.; Teng, M.S.; Genden, E.M.; et al. Discrimination of Benign and Neoplastic Mucosa with a High-Resolution Microendoscope (HRME) in Head and Neck Cancer. Ann. Surg. Oncol. 2012, 19, 3534–3539. [Google Scholar] [CrossRef]
- Muldoon, T.J.; Roblyer, D.; Williams, M.D.; Stepanek, V.M.; Richards-Kortum, R.; Gillenwater, A.M. Noninvasive imaging of oral neoplasia with a high-resolution fiber-optic microendoscope. Head Neck 2012, 34, 305–312. [Google Scholar] [CrossRef]
- Vila, P.M.; Thekkek, N.; Richards-Kortum, R.; Anandasabapathy, S. Use of in vivo real-time optical imaging for esophageal neoplasia. Mt. Sinai J. Med. 2011, 78, 894–904. [Google Scholar] [CrossRef]
- Pierce, M.C.; Schwarz, R.A.; Bhattar, V.S.; Mondrik, S.; Williams, M.D.; Lee, J.J.; Richards-Kortum, R.; Gillenwater, A.M. Accuracy of in vivo multimodal optical imaging for detection of oral neoplasia. Cancer Prev. Res. (Phila.) 2012, 5, 801–809. [Google Scholar] [CrossRef]
- Gonzalez, M.; Montejo, K.A.; Krupp, K.; Srinivas, V.; DeHoog, E.; Madhivanan, P.; Ramella-Roman, J.C. Design and implementation of a portable colposcope Mueller matrix polarimeter. J. Biomed. Opt. 2020, 25, 116006. [Google Scholar] [CrossRef]
- Ramella-Roman, J.C.; Gonzalez, M.; Chue-Sang, J.; Montejo, K.; Krup, K.; Srinivas, V.; DeHoog, E.; Madhivanan, P. Development and characterization of a snapshot Mueller matrix polarimeter for the determination of cervical cancer risk in the low resource setting. In Proceedings of the Society of Photo-Optical Instrumentation Engineers Biomedical Optics, San Francisco, CA, USA, 27 January–1 February 2018; p. 12. [Google Scholar]
- Peterson, C.W.; Rose, D.; Mink, J.; Levitz, D. Real-Time Monitoring and Evaluation of a Visual-Based Cervical Cancer Screening Program Using a Decision Support Job Aid. Diagnostics 2016, 6, 20. [Google Scholar] [CrossRef]
- Mayoore, J.; Matt, H.; Liming, H.; Yau, B.-O.; Cary, C.; Benjamin, W.; David, L. Characterization of cervigram image sharpness using multiple self-referenced measurements and random forest classifiers. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, San Francisco, CA, USA, 27 January–1 February 2018. [Google Scholar]
- Marta, M.; Sonia, C.; Octavio, V.; Bruce, S.K.; Amit, S.; David, L. Mobile colposcopy in urban and underserved suburban areas in Baja California. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, San Francisco, CA, USA, 13–18 February 2018. [Google Scholar]
- Matti, R.; Gupta, V.; D’Sa, D.K.; Sebag, C.; Peterson, C.W.; Levitz, D. Introduction of Mobile Colposcopy as a Primary Screening Tool for Different Socioeconomic Populations in Urban India. Pan. Asian J. Obstet. Gynecol. 2019, 2, 4–11. [Google Scholar]
- Ngonzi, J.; Bajunirwe, F.; Wistrand, C.; Mayanja, R.; Altman, D.; Thorsell, M.; Shemer, E. Agreement of Colposcope and Gynocular in Assessment of Cervical Lesions by Swede Score: A Randomized, Crossover Pilot Trial. J. Low. Genit. Tract Dis. 2013, 17, 372–377. [Google Scholar] [CrossRef]
- Nessa, A.; Roy, J.S.; Chowdhury, M.A.; Khanam, Q.; Afroz, R.; Wistrand, C.; Thuresson, M.; Thorsell, M.; Shemer, I.; Wikström Shemer, E.A. Evaluation of the accuracy in detecting cervical lesions by nurses versus doctors using a stationary colposcope and Gynocular in a low-resource setting. BMJ Open 2014, 4, e005313. [Google Scholar] [CrossRef]
- Nessa, A.; Wistrand, C.; Begum, S.A.; Thuresson, M.; Shemer, I.; Thorsell, M.; Shemer, E.A.W. Evaluation of stationary colposcope and the Gynocular, by the Swede score systematic colposcopic system in VIA positive women: A crossover randomized trial. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 339–345. [Google Scholar] [CrossRef]
- Kallner, H.K.; Persson, M.; Thuresson, M.; Altman, D.; Shemer, I.; Thorsell, M.; Shemer, E.A.W. Diagnostic Colposcopic Accuracy by the Gynocular and a Stationary Colposcope. Int. J. Technol. Assess. Health Care 2015, 31, 181–187. [Google Scholar] [CrossRef]
- Basu, P.; Banerjee, D.; Mittal, S.; Mandal, R.; Ghosh, I.; Das, P.; Muwonge, R.; Biswas, J. Evaluation of a compact, rechargeable, magnifying device to triage VIA and HPV positive women in a cervical cancer screening program in rural India. Cancer Causes Control 2016, 27, 1253–1259. [Google Scholar] [CrossRef]
- Srinivas, V.; Nishimura, H.M.; Jayakrishna, P.; Krupp, K.; Madhivanan, P.; Madhunapantula, S.V. Evaluating the feasibility of utilizing Gynocular-triage-to-diagnose application with VIA (Visual inspection with Acetic acid) in community cervical cancer screening programs in rural Mysore, India. Indian J. Cancer 2021, 58, 409–416. [Google Scholar]
- Singh, N.; Negi, N.; Sharma, S.; Singh, T. Diagnostic Role of Portable Colposcope (Gynocular) for Diagnosis of Pre-Invasive Lesions of Cervix. Indian J. Gynecol. Oncol. 2020, 18, 69. [Google Scholar] [CrossRef]

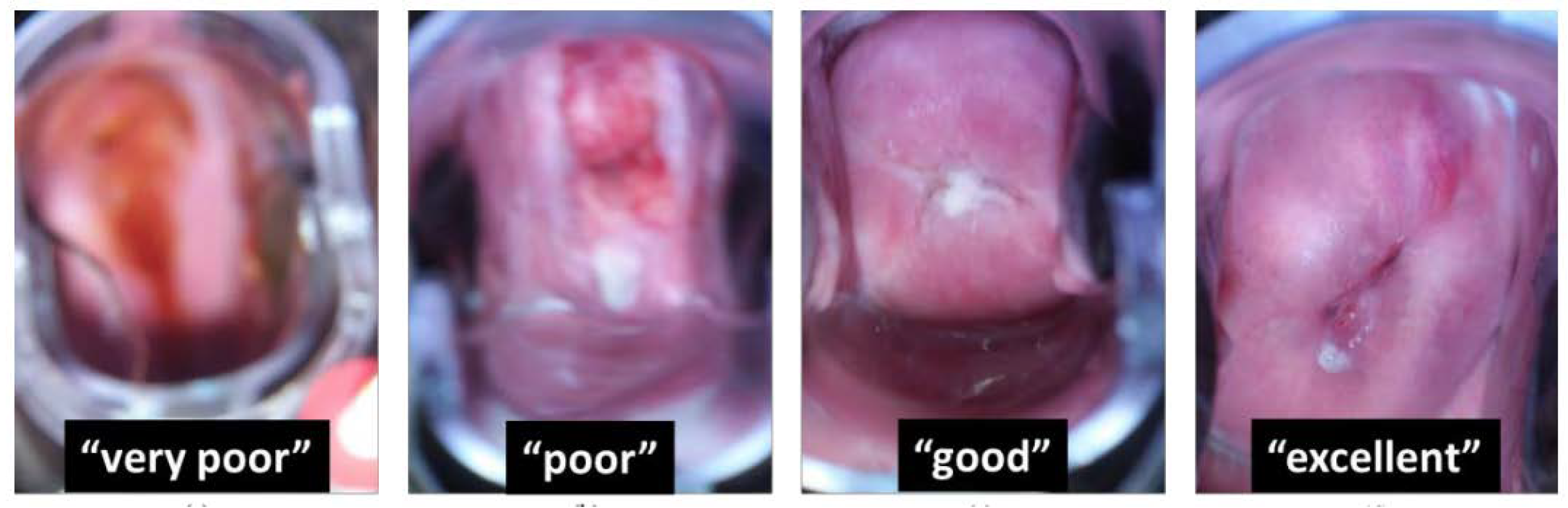
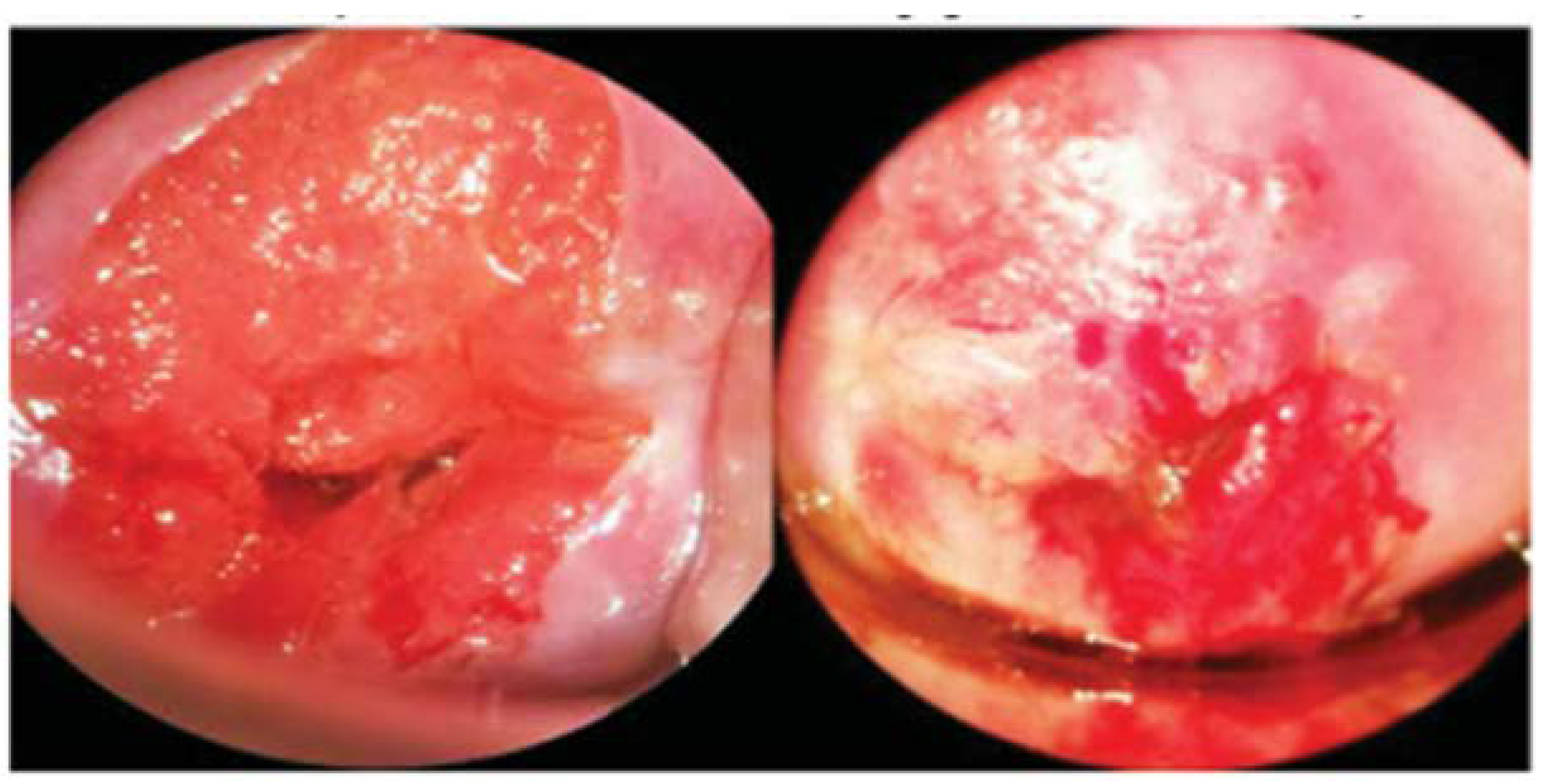
| View of at Least Cervical Quadrants | Callascope | |
|---|---|---|
| U.S. | Ghana | |
| [%] | ||
| 2 | 89 | 84 |
| 3 | 72 | 71 |
| 4 | 50 | 44 |
| Device | Company | FOV | Weight | Power | Cost (Dollars) | Portable? | Magnification | Illumination | Can It Be Mounted? | Need Speculum? | Software Included? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Callascope | Duke University | 30 mm | - | PC | - | Yes | 4× | White ring LED | No | No | Yes |
| HRME | Rice University | 720 microns | 2.3 kg | PC | 2450 | Yes | 10× | 455 nm LED | No | Yes | Yes |
| snapshot Mueller matrix polarimeter | FIU | 30 mm | - | PC | 2000 | Yes | none | (4) 633 nm LEDs | Yes | Yes | Yes |
| EVA | Mobile ODT | - | 605 g | Battery | 8200 | Yes | 4×, 16× | 3 W (3.6 V) LED | Yes | Yes | Yes |
| Gynocular | Gynius | 20–40 mm | 480 g | Battery | 3000 | Yes | 5×, 8×, 12× | 3 W (3.6 V) LED | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, M.; Boonya-Ananta, T.; Madhivanan, P.; Ramella-Roman, J.C. Cervical Imaging in the Low Resource Setting: A Review. Biosensors 2022, 12, 786. https://doi.org/10.3390/bios12100786
Gonzalez M, Boonya-Ananta T, Madhivanan P, Ramella-Roman JC. Cervical Imaging in the Low Resource Setting: A Review. Biosensors. 2022; 12(10):786. https://doi.org/10.3390/bios12100786
Chicago/Turabian StyleGonzalez, Mariacarla, Tananant Boonya-Ananta, Purnima Madhivanan, and Jessica C. Ramella-Roman. 2022. "Cervical Imaging in the Low Resource Setting: A Review" Biosensors 12, no. 10: 786. https://doi.org/10.3390/bios12100786
APA StyleGonzalez, M., Boonya-Ananta, T., Madhivanan, P., & Ramella-Roman, J. C. (2022). Cervical Imaging in the Low Resource Setting: A Review. Biosensors, 12(10), 786. https://doi.org/10.3390/bios12100786







