High-Sensitivity PtSe2 Surface Plasmon Resonance Biosensor Based on Metal-Si-Metal Waveguide Structure
Abstract
:1. Introduction
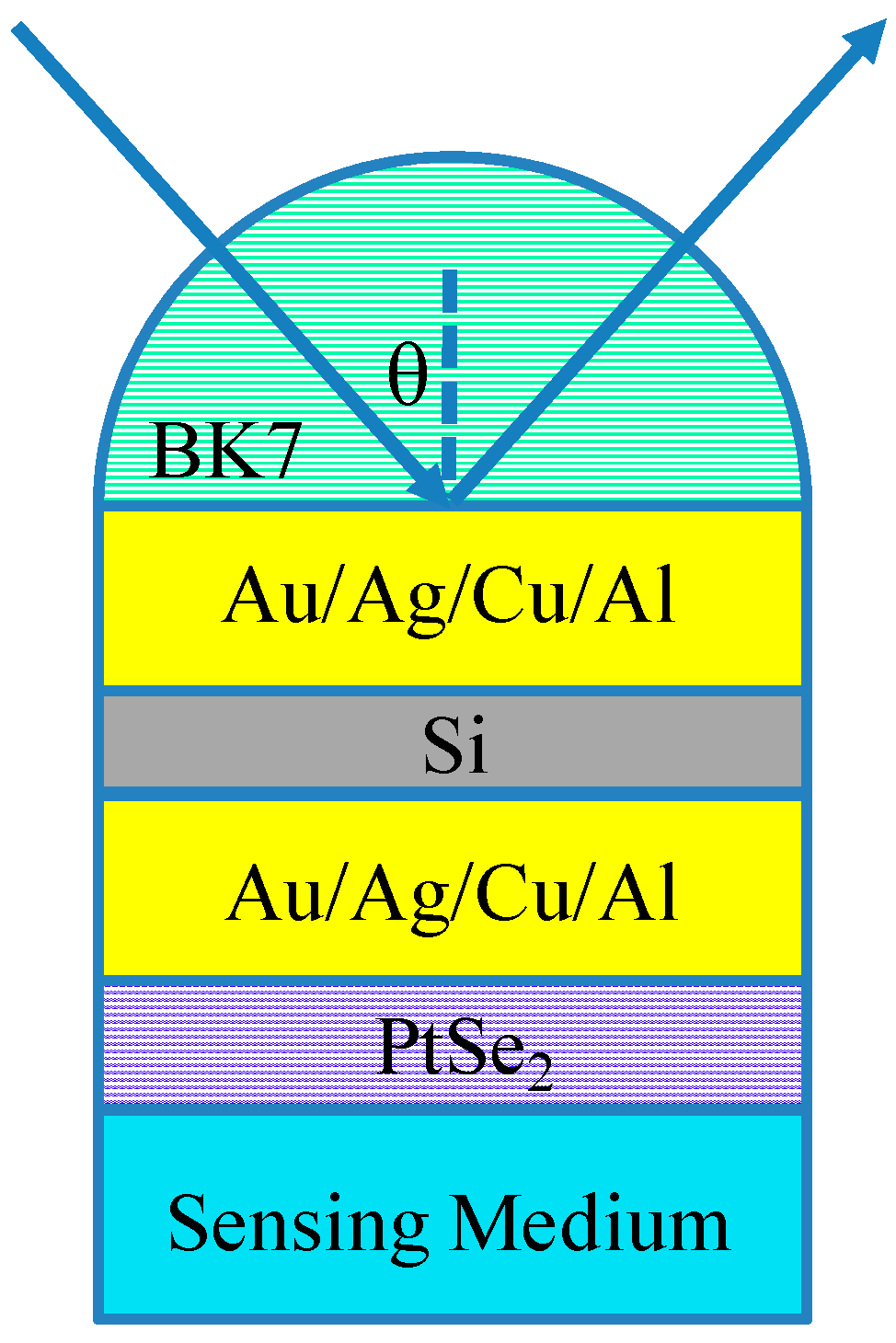
2. Calculation Models and Methods
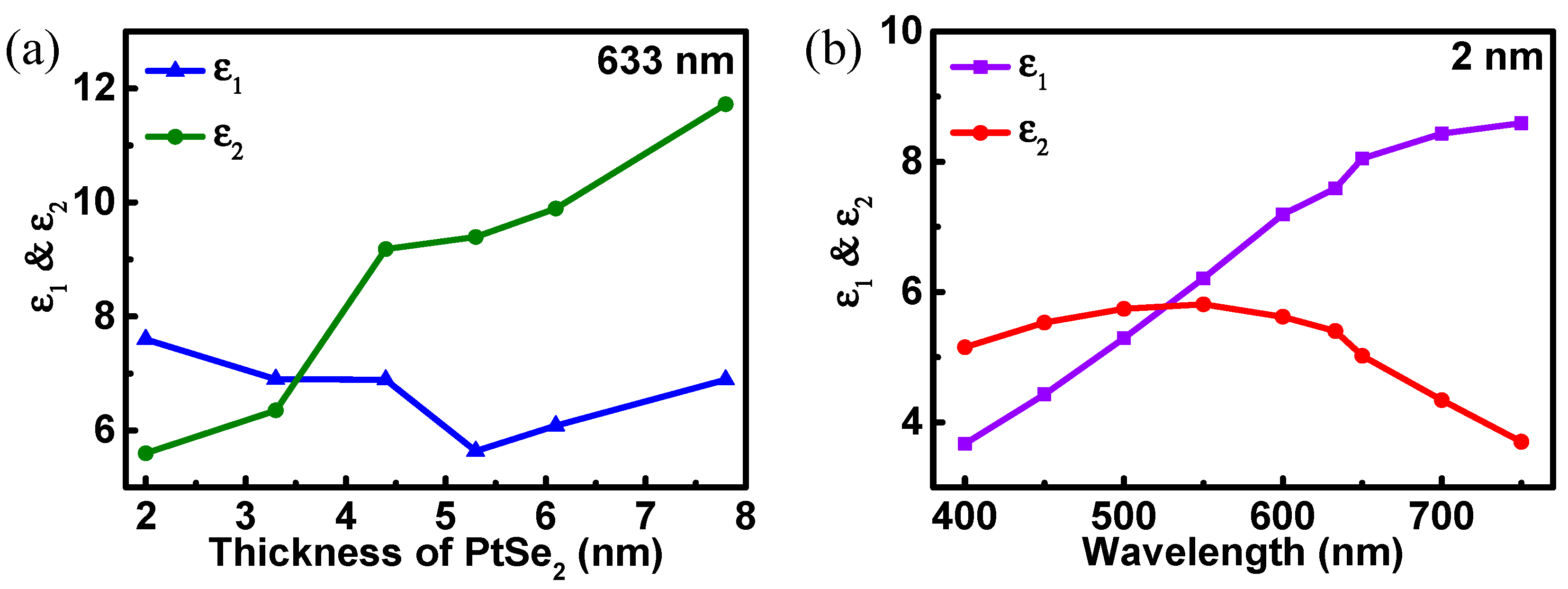
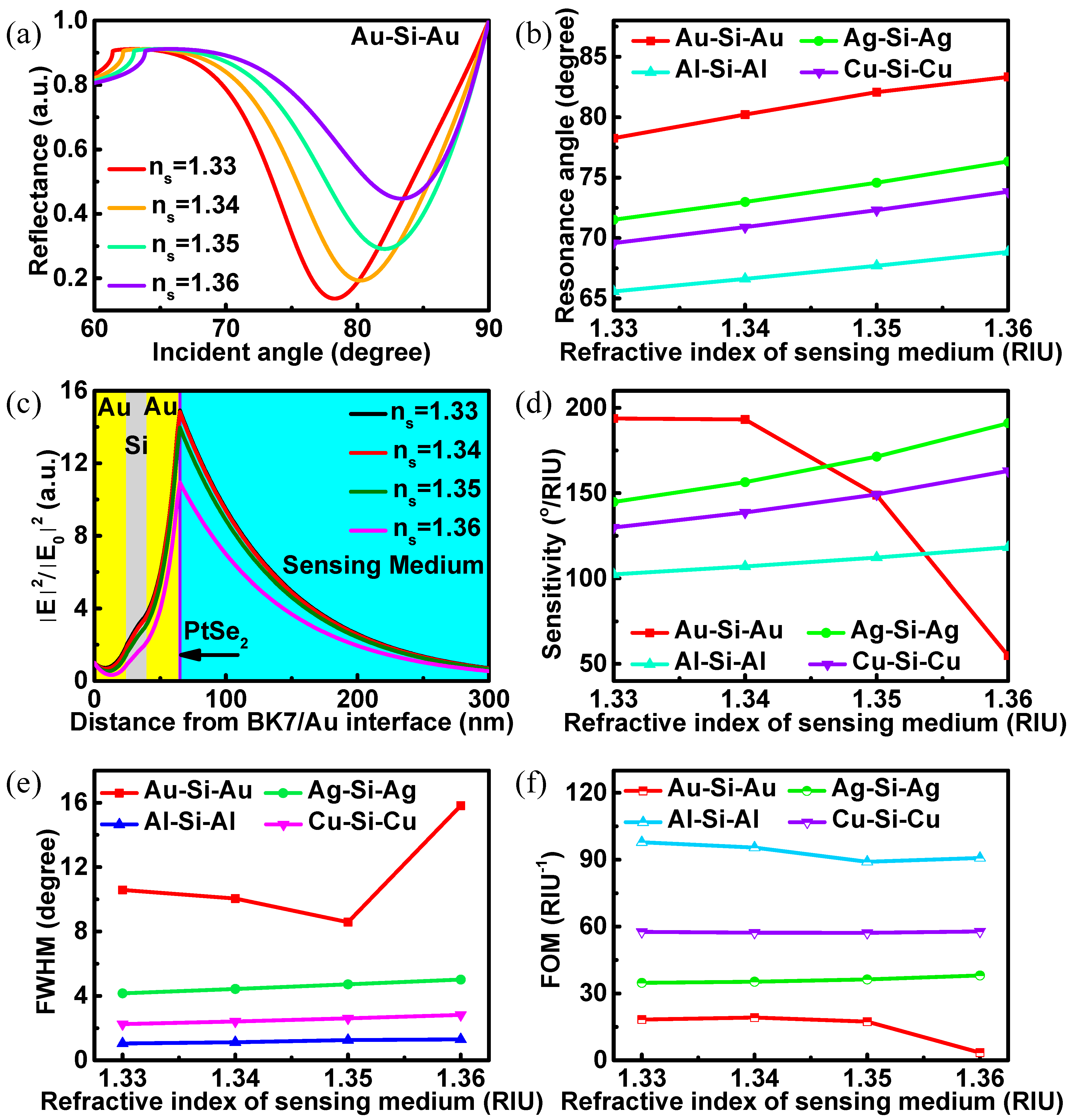
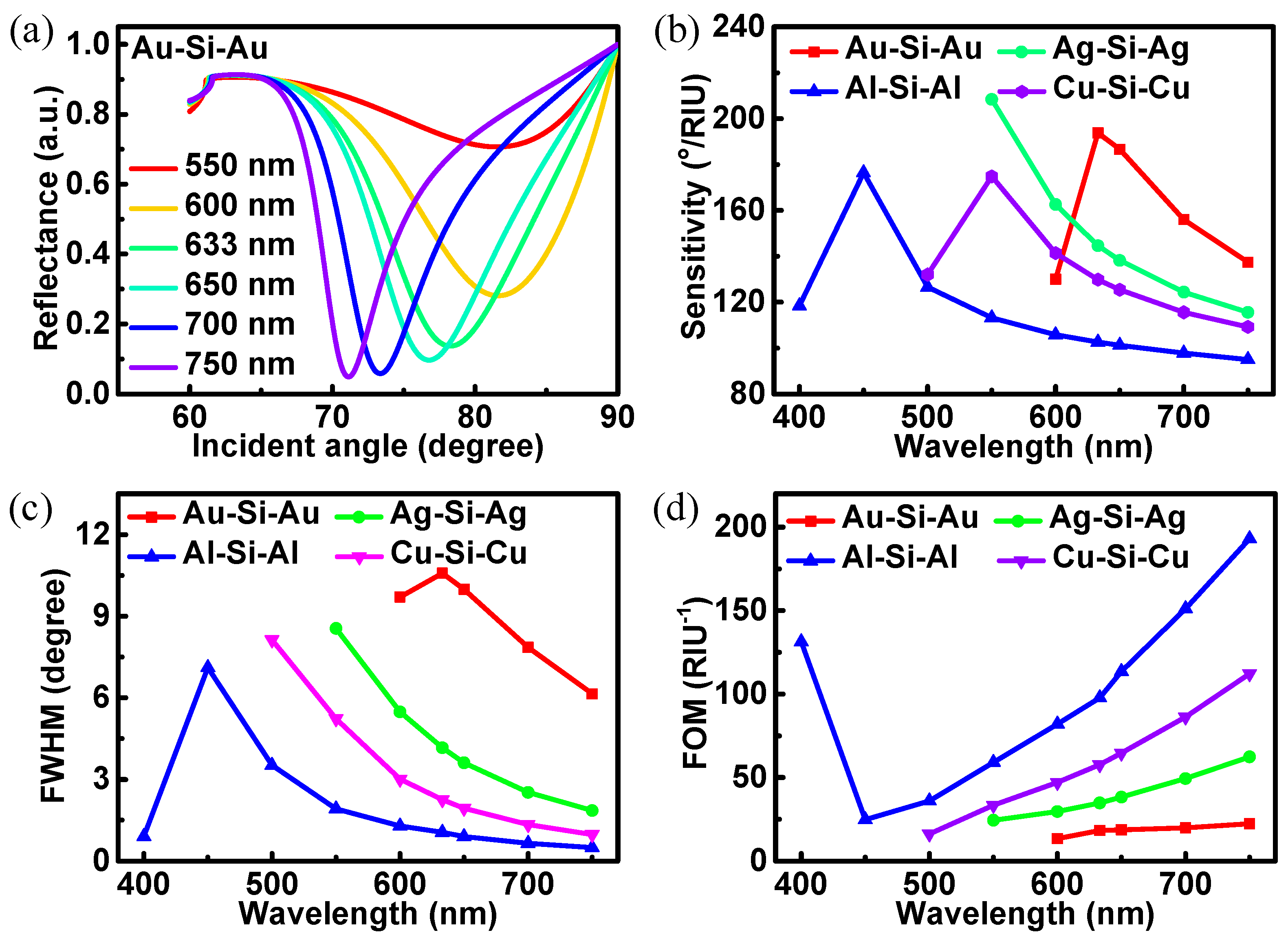
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. A Hadron. Nucl. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Qu, J.; Dinh, X.-Q.; Qian, J.; He, S.; Coquet, P.; Yong, K.-T. Two-Dimensional Transition Metal Dichalcogenide Enhanced Phase-Sensitive Plasmonic Biosensors: Theoretical Insight. J. Phys. Chem. C 2017, 121, 6282–6289. [Google Scholar] [CrossRef]
- Mansouri, M.; Fathi, F.; Jalili, R.; Shoeibie, S.; Dastmalchi, S.; Khataee, A.; Rashidi, M.R. SPR enhanced DNA biosensor for sensitive detection of donkey meat adulteration. Food Chem. 2020, 331, 127163. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Qian, Y.; Zhang, T.; Zheng, L.; Fu, L. Quantification of shellfish major allergen tropomyosin by SPR biosensor with gold patterned Biochips. Food Control 2020, 107, 106547. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Manclus, J.J.; Montoya, A.; Lechuga, L.M. Multi-analyte SPR immunoassays for environmental biosensing of pesticides. Anal. Bioanal. Chem. 2007, 387, 1449–1458. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.A.R.; Qureshi, M.Z.; Masson, J.F. Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors 2020, 10, 184. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Peeters, B.; Daems, D.; van der Donck, T.; Delport, F.; Lammertyn, J. Real-Time FO-SPR Monitoring of Solid-Phase DNAzyme Cleavage Activity for Cutting-Edge Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 6759–6768. [Google Scholar] [CrossRef]
- Wong, C.L.; Ho, H.P.; Suen, Y.K.; Kong, S.K.; Chen, Q.L.; Yuan, W.; Wu, S.Y. Real-time protein biosensor arrays based on surface plasmon resonance differential phase imaging. Biosens. Bioelectron. 2008, 24, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, H.; Zhu, A.; Qiu, F. A Label-Free and Anti-Interference Dual-Channel SPR Fiber Optic Sensor With Self-Compensation for Biomarker Detection. IEEE Trans. Instrum. Meas. 2021, 70, 1–7. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Mou, S.F.; Rahman, M.M.; Rana, M.M.; Islam, M.R.; Mehedi, I.M.; Mahmud, M.A.P.; Kouzani, A.Z. Design and Numerical Analysis of a Graphene-Coated SPR Biosensor for Rapid Detection of the Novel Coronavirus. Sensors 2021, 21, 3491. [Google Scholar] [CrossRef] [PubMed]
- Das, C.M.; Guo, Y.; Kang, L.; Poenar, D.P.; Xiong, J.; Ramaswamy, Y.; Martinez-Martin, D.; Yong, K.T. Improving the Sensitivity of SPR Sensors with Au–Ag alloys and 2D Materials—A Simulation-Based Approach. Adv. Theor. Simul. 2021, 4, 2100292. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Liu, K. Chemical and structural stability of 2D layered materials. 2D Mater. 2019, 6, 042001. [Google Scholar] [CrossRef]
- Ma, B.; Bianco, A. Recent Advances in 2D Material-Mediated Immuno-Combined Cancer Therapy. Small 2021, 17, e2102557. [Google Scholar] [CrossRef]
- Chiu, N.F.; Tai, M.J.; Nurrohman, D.T.; Lin, T.L.; Wang, Y.H.; Chen, C.Y. Immunoassay-Amplified Responses Using a Functionalized MoS2-Based SPR Biosensor to Detect PAPP-A2 in Maternal Serum Samples to Screen for Fetal Down’s Syndrome. Int. J. Nanomed. 2021, 16, 2715–2733. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rana, M.M.; Rahman, M.S.; Anower, M.S.; Mollah, M.A.; Paul, A.K. Sensitivity enhancement of SPR biosensors employing heterostructure of PtSe2 and 2D materials. Opt. Mater. 2020, 107, 110123. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, S.; Lin, C. Sensitivity Improvement of a Surface Plasmon Resonance Sensor Based on Two-Dimensional Materials Hybrid Structure in Visible Region: A Theoretical Study. Sensors 2020, 20, 2445. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, L.; Zhang, Z.; Tsang, Y.H.; Luo, L.; Lee, J.H. High-performance broadband heterojunction photodetectors based on multilayered PtSe2 directly grown on a Si substrate. Nanoscale 2018, 10, 15285–15293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Yao, W.; Song, S.; Sun, J.T.; Pan, J.; Ren, X.; Li, C.; Okunishi, E.; Wang, Y.Q.; et al. Monolayer PtSe(2), a New Semiconducting Transition-Metal-Dichalcogenide, Epitaxially Grown by Direct Selenization of Pt. Nano Lett. 2015, 15, 4013–4018. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, M.; Zhang, H.; Huang, H.; Arita, M.; Sun, Z.; Duan, W.; Wu, Y.; Zhou, S. Experimental evidence for type-II Dirac semimetal in PtSe2. Phy. Rev. B 2017, 96, 125102. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Ye, Z.; Yang, L.; Gou, L.; Wang, Z. Recent progress in Van der Waals 2D PtSe2. Nanotechnology 2021, 32, 412001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiao, J.; Yu, Z.; Yu, P.; Xu, K.; Lau, S.P.; Zhou, W.; Liu, Z.; Wang, X.; Ji, W.; et al. High-Electron-Mobility and Air-STable 2D Layered PtSe2 FETs. Adv. Mater. 2017, 29, 1604230. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.-J.; Fan, Q.; Huang, Z.-F.; Lu, Y.-C.; Xie, C.; Wu, C.-Y.; Luo, L.-B. A high-performance near-infrared light photovoltaic detector based on a multilayered PtSe2/Ge heterojunction. J. Mater. Chem. C 2019, 7, 5019–5027. [Google Scholar] [CrossRef]
- Sajjad, M.; Montes, E.; Singh, N.; Schwingenschlögl, U. Superior Gas Sensing Properties of Monolayer PtSe2. Adv. Mater. Interfaces 2017, 4, 1600911. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Wang, H.; Saeed, M.; Cai, H. Sensitivity Enhancement of a Surface Plasmon Resonance Sensor with Platinum Diselenide. Sensors 2019, 20, 131. [Google Scholar] [CrossRef] [Green Version]
- Konopsky, V.N.; Alieva, E.V. Imaging biosensor based on planar optical waveguide. Opt. Laser Technol. 2019, 115, 171–175. [Google Scholar] [CrossRef]
- Walter, J.G.; Eilers, A.; Alwis, L.S.M.; Roth, B.W.; Bremer, K. SPR Biosensor Based on Polymer Multi-Mode Optical Waveguide and Nanoparticle Signal Enhancement. Sensors 2020, 20, 2889. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, L.Y. Subtle Application of Electrical Field-Induced Lossy Mode Resonance to Enhance Performance of Optical Planar Waveguide Biosensor. Biosensors 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Barshilia, D.; Chau, L.K.; Chang, G.E. Low-cost planar waveguide-based optofluidic sensor for real-time refractive index sensing. Opt. Express 2020, 28, 27337–27345. [Google Scholar] [CrossRef]
- Meshginqalam, B.; Barvestani, J. Aluminum and phosphorene based ultrasensitive SPR biosensor. Opt. Mater. 2018, 86, 119–125. [Google Scholar] [CrossRef]
- Vahed, H.; Nadri, C. Sensitivity enhancement of SPR optical biosensor based on Graphene–MoS2 structure with nanocomposite layer. Opt. Mater. 2019, 88, 161–166. [Google Scholar] [CrossRef]
- Wu, L.; Guo, J.; Xu, H.; Dai, X.; Xiang, Y. Ultrasensitive biosensors based on long-range surface plasmon polariton and dielectric waveguide modes. Photonics Res. 2016, 4, 262–266. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, D.; Yan, X.-Q.; Ren, M.; Zhao, X.; Liu, F.; Sun, R.; Li, X.; Li, Z.; Chen, S.; et al. Optical properties of chemical vapor deposition-grown PtSe2 characterized by spectroscopic ellipsometry. 2D Mater. 2019, 6, 035011. [Google Scholar] [CrossRef]
- Rahman, M.S.; Anower, M.S.; Abdulrazak, L.F. Modeling of a fiber optic SPR biosensor employing Tin Selenide (SnSe) allotropes. Results Phys. 2019, 15, 102623. [Google Scholar] [CrossRef]
- You, Q.; Shan, Y.; Gan, S.; Zhao, Y.; Dai, X.; Xiang, Y. Giant and controllable Goos-Hänchen shifts based on surface plasmon resonance with graphene-MoS2 heterostructure. Opt. Mater. Express 2018, 8, 3036–3048. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Chen, G.; Shen, J.; Li, C.; Tang, T. A High-Sensitivity SPR Sensor with Bimetal/Silicon/Two-Dimensional Material Structure: A Theoretical Analysis. Photonics 2021, 8, 270. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, X.; Chen, K.; Lv, Z.; Pu, X.; Tang, C.; Cai, P. Ultranarrow Dual-Band Perfect Absorption in Visible and Near-infrared Regimes Based on Three-Dimensional Metamaterials for Ultrahigh-Sensitivity Sensing. J. Light. Technol. 2021, 39, 7217–7222. [Google Scholar] [CrossRef]
- Chen, J.; Nie, H.; Tang, C.; Cui, Y.; Yan, B.; Zhang, Z.; Kong, Y.; Xu, Z.; Cai, P. Highly sensitive refractive-index sensor based on strong magnetic resonance in metamaterials. Appl. Phys. Express 2019, 12, 52015. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Xu, Y.; Qi, Y.; Zhang, L.; Yang, H.; Yi, Z. A novel plasmonic refractive index sensor based on gold/silicon complementary grating structure. Chin. Phys. B 2021, 30, 24207. [Google Scholar] [CrossRef]
- Moznuzzaman, M.; Islam, M.R.; Khan, I. Effect of layer thickness variation on sensitivity: An SPR based sensor for formalin detection. Sens. Bio-Sens. Res. 2021, 32, 100419. [Google Scholar] [CrossRef]
- Mostufa, S.; Paul, A.K.; Chakrabarti, K. Detection of hemoglobin in blood and urine glucose level samples using a graphene-coated SPR based biosensor. OSA Contin. 2021, 4, 2164–2176. [Google Scholar] [CrossRef]


| Au (m) | Ag (m) | Al (m) | Cu (m) | |
|---|---|---|---|---|
| λc | 8.9342 × 10−6 | 1.7614 × 10−5 | 2.4511 × 10−5 | 4.0852 × 10−5 |
| λp | 1.6826 × 10−7 | 1.4541 × 10−7 | 1.0657 × 10−7 | 1.3617 × 10−7 |
| PtSe2 Thickness (nm) | S (°/RIU) | θmin (degree) | ∆θ (degree) | Rmin (a.u.) | FWHM (degree) | FOM (RIU−1) | ||
|---|---|---|---|---|---|---|---|---|
| Au | Without Si and PtSe2 | 0 | 136.8 | 70.53 | 0.684 | 6.6 × 10−8 | 3.79 | 36.12 |
| Without Si and with PtSe2 | 2 | 156.6 | 72.92 | 0.783 | 0.1263 | 6.32 | 24.78 | |
| With Si andPtSe2 | 2 | 193.8 | 78.26 | 0.969 | 0.1368 | 10.58 | 18.31 | |
| 3.3 | 144.2 | 79.73 | 0.721 | 0.3259 | 9.37 | 15.39 | ||
| 4.4 | 80.6 | 79.45 | 0.403 | 0.5209 | 16.15 | 4.99 | ||
| 5.3 | 68.2 | 78.92 | 0.341 | 0.5809 | 17.04 | 4.00 | ||
| 6.1 | 51.0 | 78.61 | 0.2550 | 0.6443 | 18.01 | 2.83 | ||
| 7.8 | 35.2 | 77.39 | 0.176 | 0.7350 | 20.21 | 1.74 | ||
| Ag | Without Si and PtSe2 | 0 | 116.0 | 67.64 | 0.580 | 0.0059 | 1.28 | 90.63 |
| Without Si and with PtSe2 | 2 | 127.4 | 69.25 | 0.637 | 0.1776 | 2.46 | 51.84 | |
| With Si andPtSe2 | 2 | 144.8 | 71.51 | 0.724 | 0.1365 | 4.16 | 34.78 | |
| 3.3 | 156.2 | 73.02 | 0.781 | 0.3045 | 4.87 | 32.06 | ||
| 4.4 | 158.4 | 74.61 | 0.792 | 0.4949 | 12.44 | 12.74 | ||
| 5.3 | 150.4 | 75.18 | 0.752 | 0.5741 | 14.05 | 10.71 | ||
| 6.1 | 134.6 | 76.14 | 0.673 | 0.6382 | 15.36 | 8.76 | ||
| 7.8 | 85.2 | 77.01 | 0.426 | 0.7553 | 17.46 | 4.88 | ||
| Al | Without Si and PtSe2 | 0 | 95.4 | 64.37 | 0.477 | 0.2542 | 0.17 | 570.89 |
| Without Si and with PtSe2 | 2 | 100.2 | 65.21 | 0.501 | 0.6658 | 0.89 | 112.58 | |
| With Si andPtSe2 | 2 | 102.4 | 65.58 | 0.512 | 0.6846 | 1.05 | 97.80 | |
| 3.3 | 106.6 | 66.26 | 0.533 | 0.7842 | 2.05 | 51.80 | ||
| 4.4 | 112.2 | 67.06 | 0.561 | 0.8492 | 4.40 | 25.49 | ||
| 5.3 | 115.6 | 67.57 | 0.578 | 0.8737 | 7.03 | 16.45 | ||
| 6.1 | 120.4 | 68.28 | 0.602 | 0.8874 | 10.81 | 11.14 | ||
| 7.8 | 132.8 | 70.27 | 0.664 | 0.9112 | 17.23 | 7.71 | ||
| Cu | Without Si and PtSe2 | 0 | 109.6 | 66.69 | 0.548 | 0.1143 | 0.55 | 200.14 |
| Without Si and with PtSe2 | 2 | 119.0 | 68.06 | 0.595 | 0.1953 | 1.47 | 80.68 | |
| With Si andPtSe2 | 2 | 129.8 | 69.58 | 0.649 | 0.1535 | 2.25 | 57.65 | |
| 3.3 | 139.4 | 70.80 | 0.697 | 0.3569 | 2.52 | 55.33 | ||
| 4.4 | 148.6 | 72.21 | 0.743 | 0.5537 | 9.04 | 16.45 | ||
| 5.3 | 150.0 | 72.89 | 0.750 | 0.6312 | 11.34 | 13.22 | ||
| 6.1 | 149.8 | 74.02 | 0.749 | 0.6858 | 13.48 | 11.12 | ||
| 7.8 | 116.2 | 75.99 | 0.581 | 0.7886 | 16.35 | 7.11 |
| Biosensor Structure | Incident Wavelength (nm) | Sensitivity (°/RIU) | Reference |
|---|---|---|---|
| BK7-Au/Ag-PtSe2 | 633 | 162 (Ag film) 165 (Au film) | [26] |
| BK7-Au/Ag-PtSe2-2D materials | 633 | 194 (Ag/PtSe2/WS2) 187 (Au/PtSe2/WS2) | [17] |
| BAK1-ZnO-silver-PtSe2-graphene | 633 | 155.33 | [41] |
| BK7-Au-PtSe2-graphene | 633 | 200 | [42] |
| BK7-metal-Si-metal-PtSe2 | 633 | 193.8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Shu, Y.; Chen, W.; Zhao, Y.; Li, J. High-Sensitivity PtSe2 Surface Plasmon Resonance Biosensor Based on Metal-Si-Metal Waveguide Structure. Biosensors 2022, 12, 27. https://doi.org/10.3390/bios12010027
Lin Z, Shu Y, Chen W, Zhao Y, Li J. High-Sensitivity PtSe2 Surface Plasmon Resonance Biosensor Based on Metal-Si-Metal Waveguide Structure. Biosensors. 2022; 12(1):27. https://doi.org/10.3390/bios12010027
Chicago/Turabian StyleLin, Zhitao, Yiqing Shu, Weicheng Chen, Yang Zhao, and Jianqing Li. 2022. "High-Sensitivity PtSe2 Surface Plasmon Resonance Biosensor Based on Metal-Si-Metal Waveguide Structure" Biosensors 12, no. 1: 27. https://doi.org/10.3390/bios12010027
APA StyleLin, Z., Shu, Y., Chen, W., Zhao, Y., & Li, J. (2022). High-Sensitivity PtSe2 Surface Plasmon Resonance Biosensor Based on Metal-Si-Metal Waveguide Structure. Biosensors, 12(1), 27. https://doi.org/10.3390/bios12010027





