An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue
Abstract
:1. Introduction
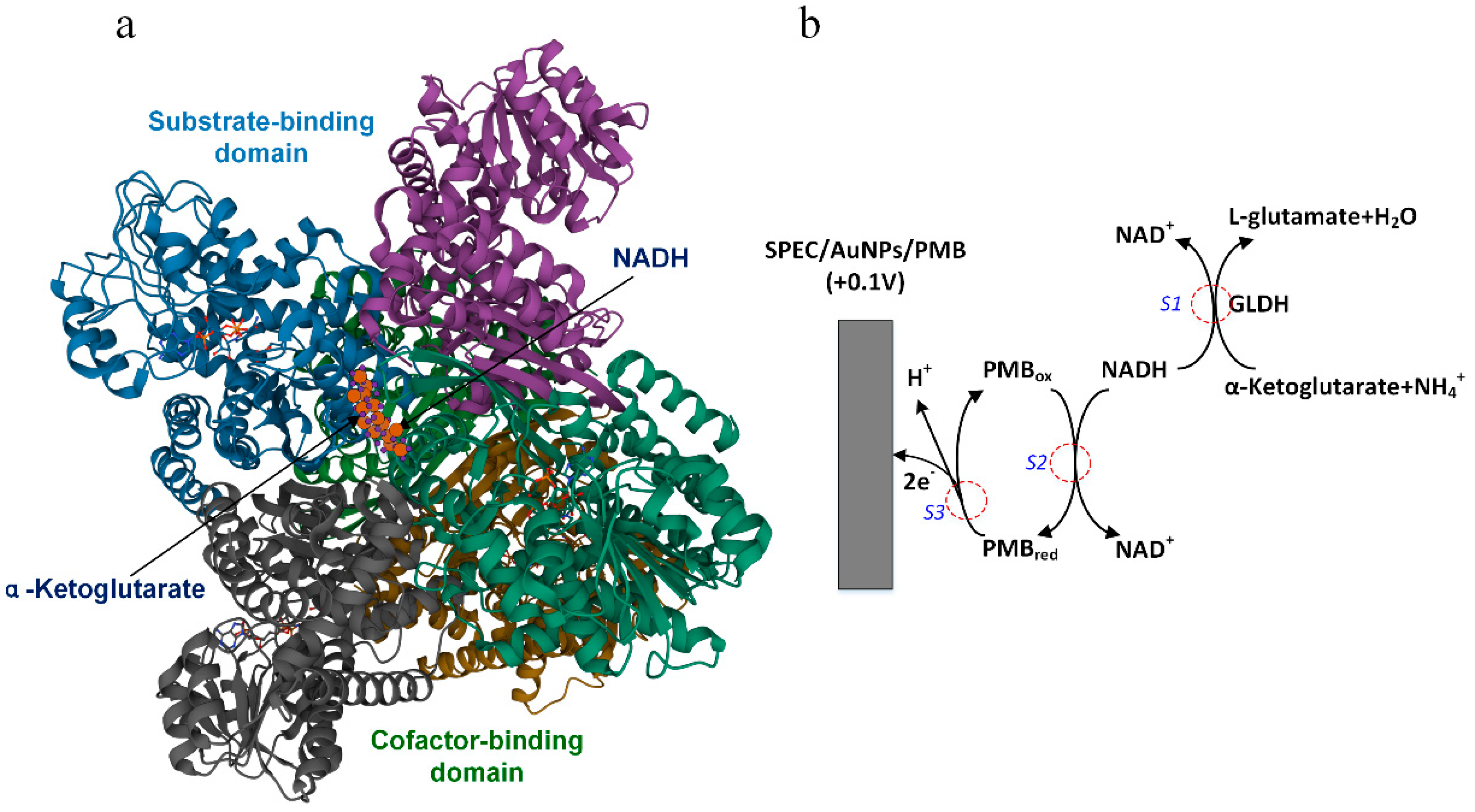
2. Principle of Ammonia Nitrogen Detection
3. Experimental
3.1. Reagents
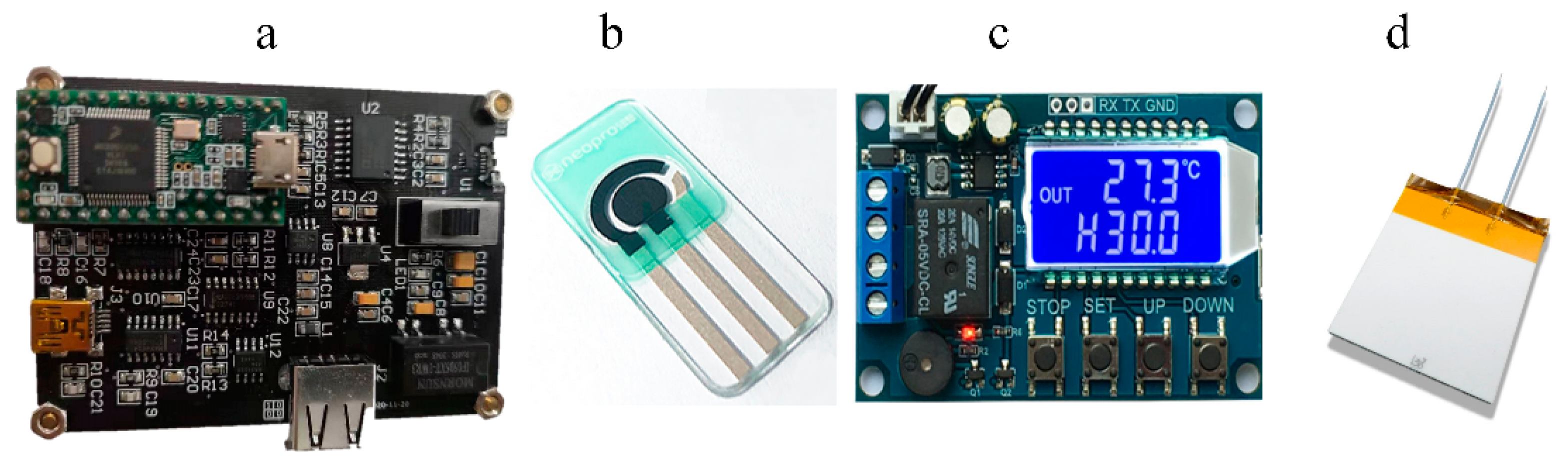
3.2. Instrumentation
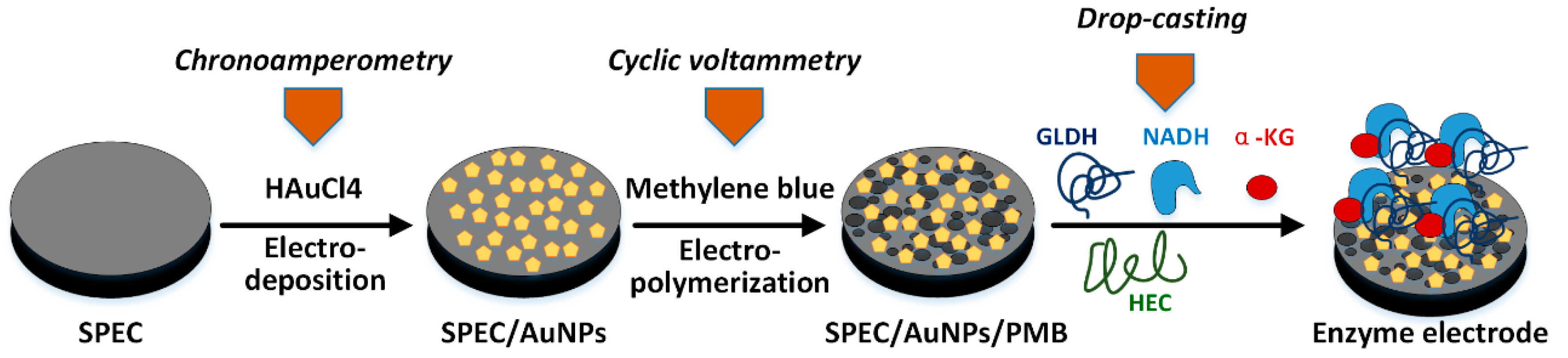
3.3. Construction of Biosensor
3.4. pH Compensation
3.5. Optimization of Biosensor Responses
4. Results and Discussion
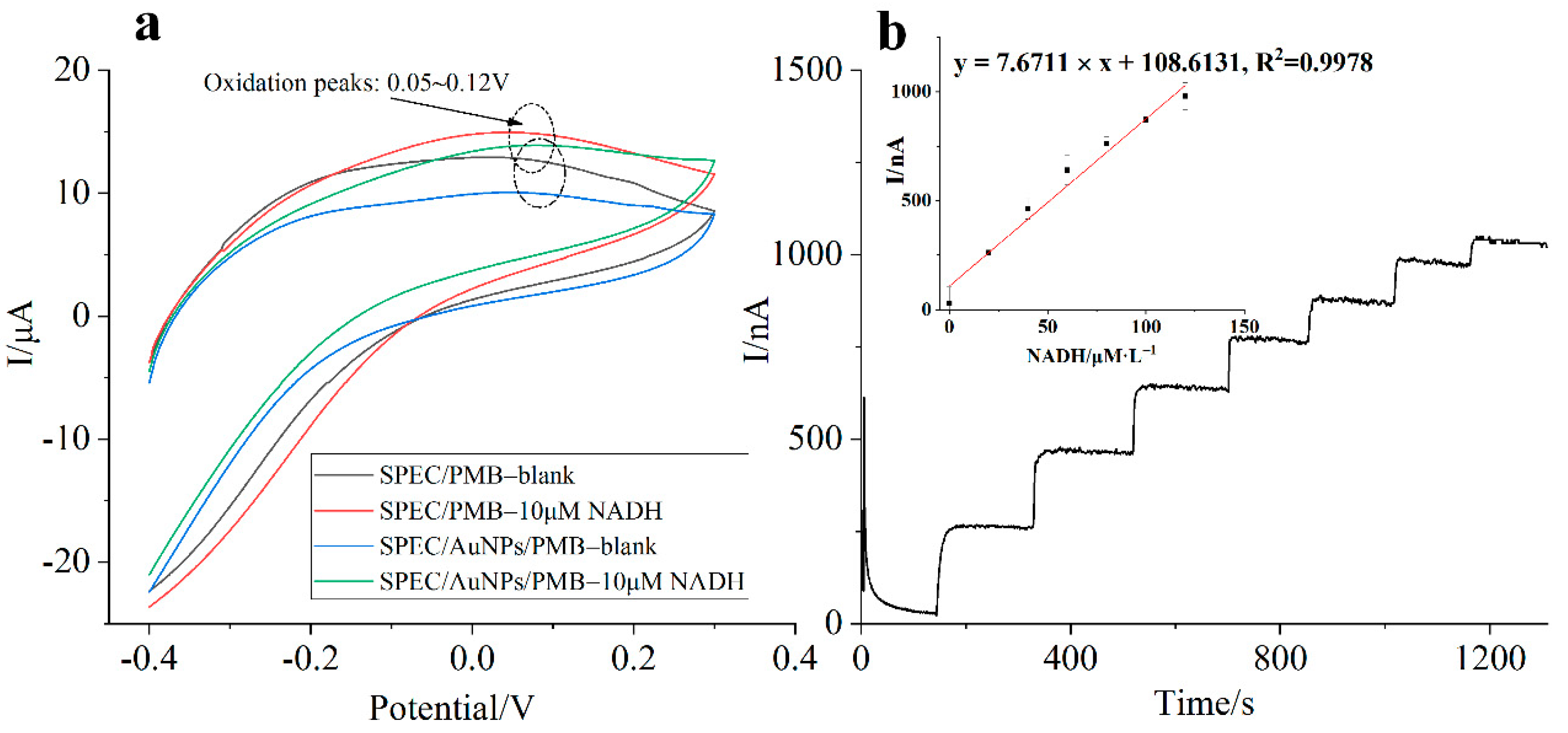
4.1. Electro-Polymerization of Methylene Blue (MB) Monomer
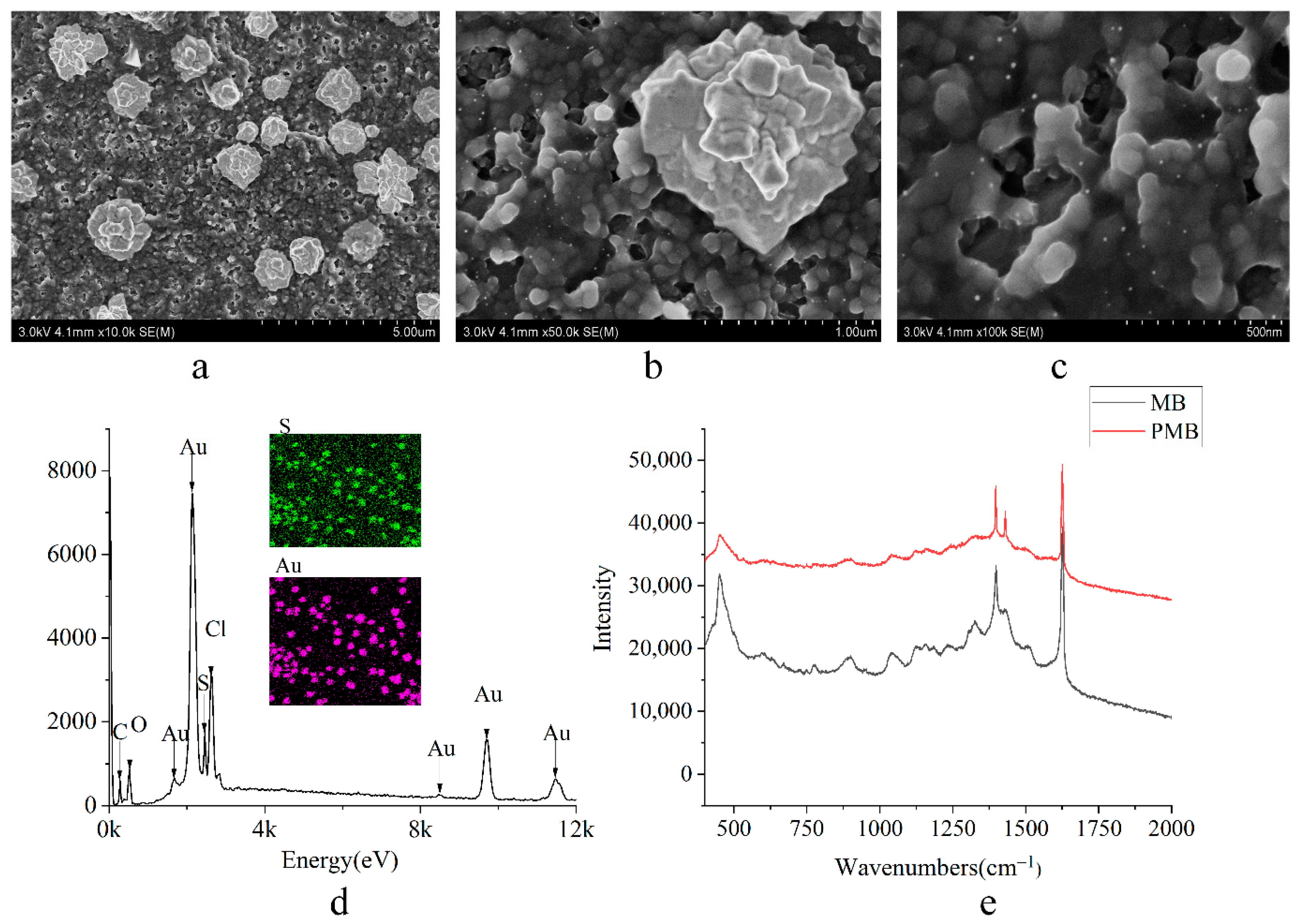
4.2. Characterization of Ammonia Nitrogen Enzyme Electrode
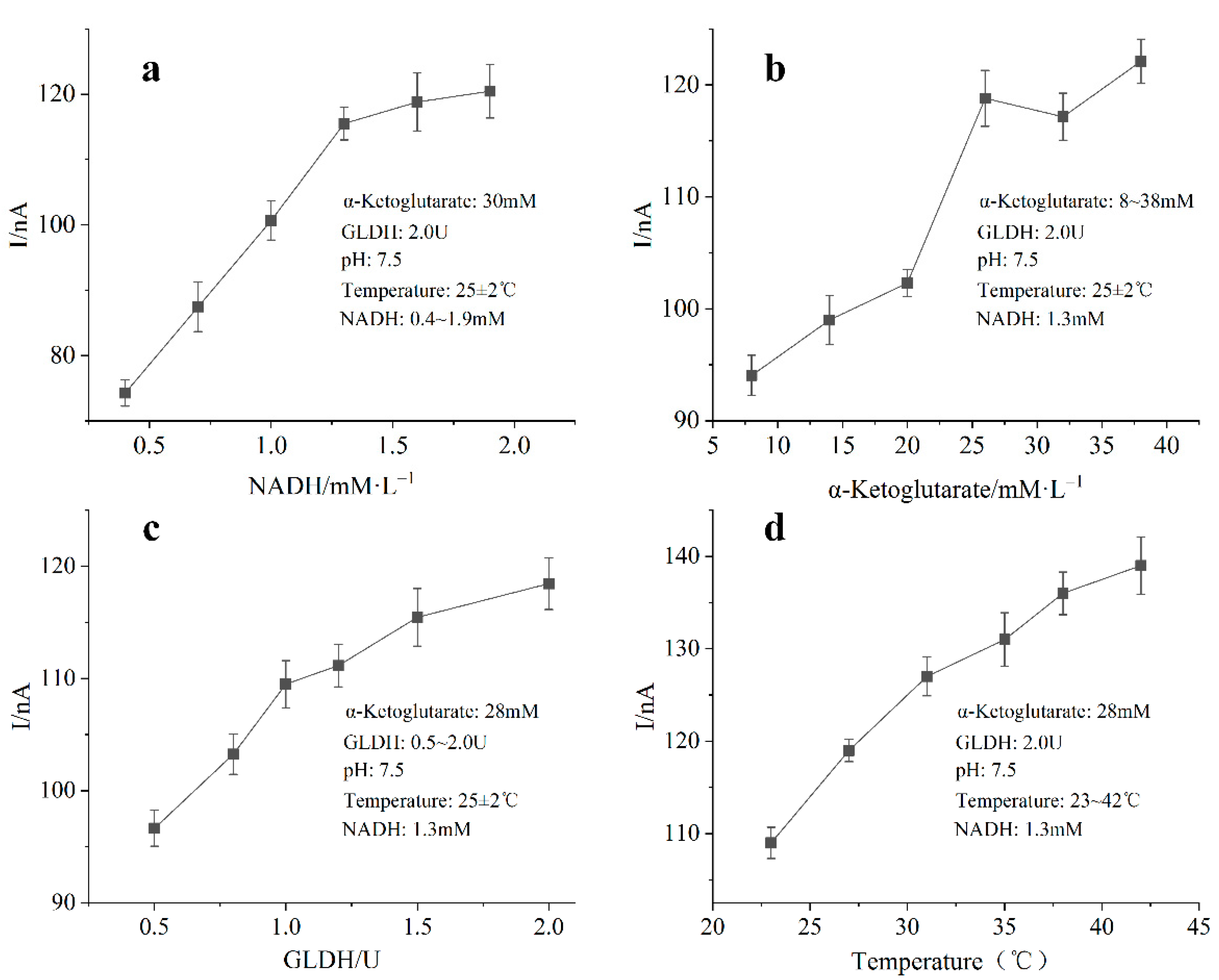
4.3. Optimization of Substrate Composition, Enzyme Loading and Temperature
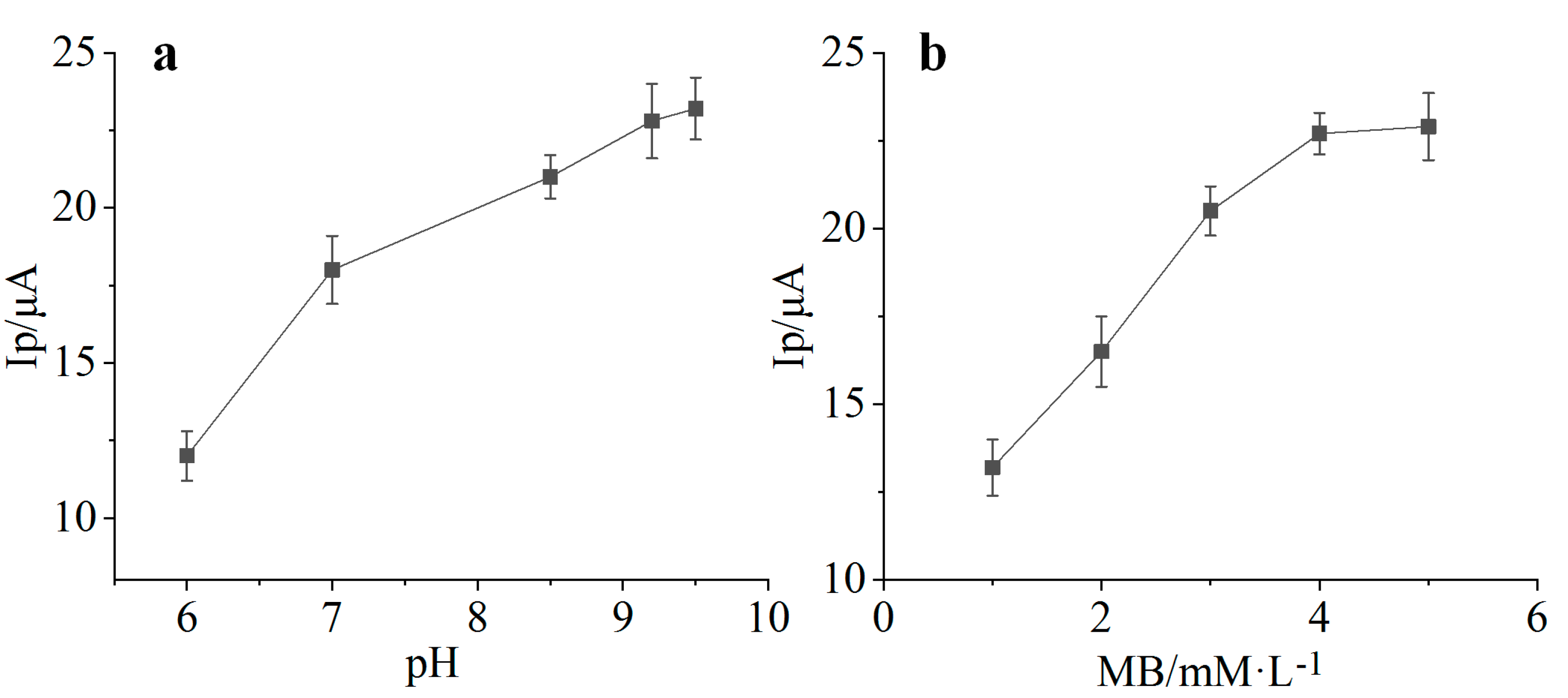
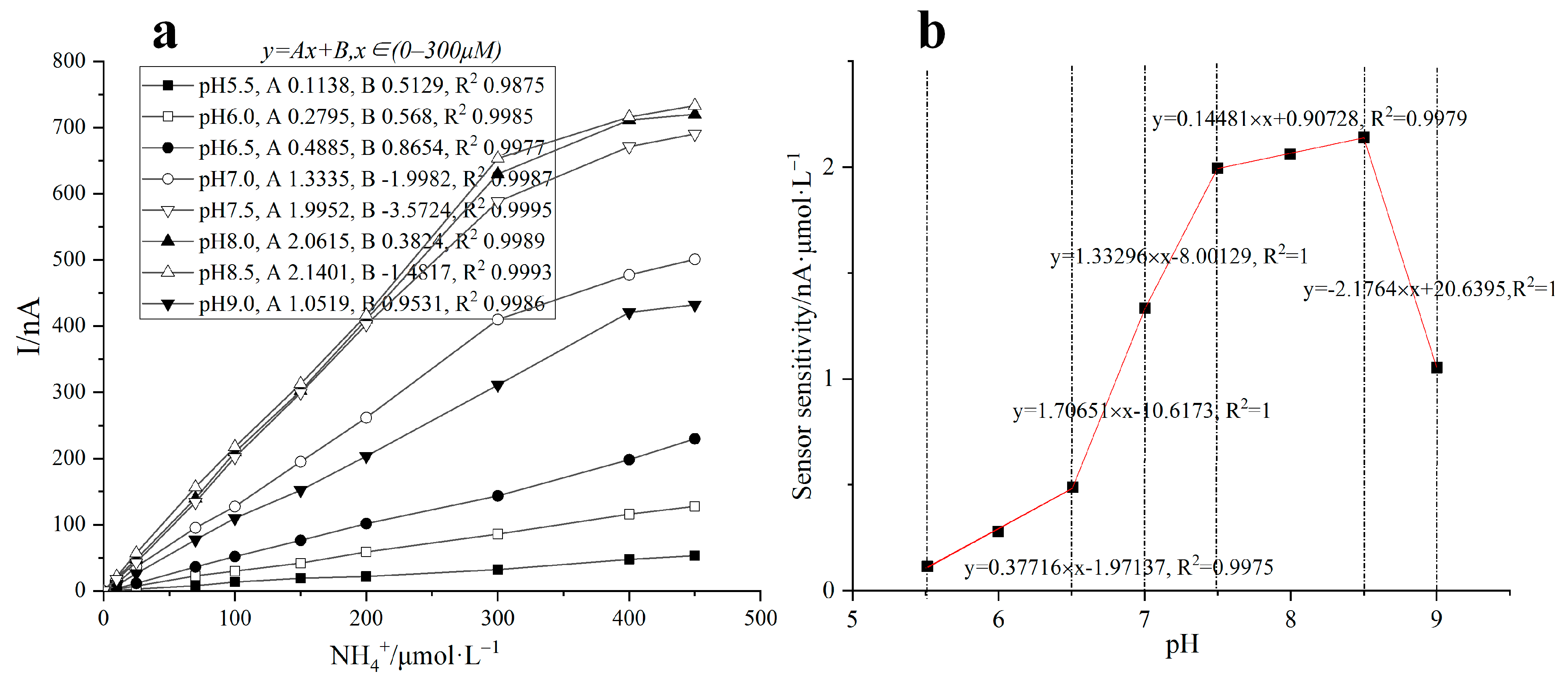
4.4. Effect of pH on the Response of Ammonia Nitrogen Biosensor
4.5. Reproducibility, Repeatability, Long-Term Stability, Anti-Interference and Actual Sample Detection of Biosensor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boardman, G.D.; Starbuck, S.M.; Hudgins, D.B.; Li, X.; Kuhn, D.D. Toxicity of ammonia to three marine fish and three marine invertebrates. Environ. Toxicol. 2004, 19, 134–142. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Guo, Y.F.; Yu, X.P.; Deng, T.L. Ammonia nitrogen speciation analysis in aquatic environments. Adv. Biol. Sci. Res. 2017, 3, 339–341. [Google Scholar]
- Li, D.L.; Xu, X.B.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.H.; Chun, H.; Kim, Y.S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. A ratiometric fluorescence sensor for the detection of ammonia in water. Sens. Actuators B 2014, 190, 768–774. [Google Scholar] [CrossRef]
- Hu, H.Z.; Liang, Y.; Li, S.; Guo, Q.; Wu, C.C. A modified o-phthalaldehyde fluorometric analytical method for ultratrace ammonium in natural waters using EDTA-NaOH as buffer. J. Anal. Methods Chem. 2014, 2014, 728068. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Pan, Y.M.; Guo, Q.; Hu, H.Z.; Wu, C.C.; Zhang, Q. A novel analytical method for trace ammonium in freshwater and seawater using 4-methoxyphthalaldehyde as fluorescent reagent. J. Anal. Methods Chem. 2015, 2015, 387207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, Z.; Pan, Z.L.; Li, D.L. Development and characterization of a highly sensitive fluorometric transducer for ultra-low aqueous ammonia nitrogen measurements in aquaculture. Comput. Electron. Agric. 2018, 150, 364–373. [Google Scholar] [CrossRef]
- Tai, H.J.; Ding, Q.S.; Zeng, L.H.; Liu, S.Y.; Li, D.L. An intelligent ammonia sensor based on multi-parameter for aquaculture. Sens. Lett. 2013, 11, 1022–1029. [Google Scholar] [CrossRef]
- Liu, W.Y.; Zheng, W.; Sun, Z.D.; Jiang, H.; Wang, X.R.; Chen, X.Q.; Wang, J.T. Online method of measuring ammonia-nitrogen in the ocean using three-electrode combined probe. J. Tianjin Univ. 2011, 34, 133–136. [Google Scholar]
- Choosang, J.; Numnuam, A.; Thavarungkul, P.; Kanatharana, P.; Radu, T.; Ullah, S.; Radu, A. Simultaneous detection of ammonium and nitrate in environmental samples using on ion-selective electrode and comparison with portable colorimetric assays. Sensors 2018, 18, 3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, Y.T.; Han, C.H.; Ye, Y.; Zhang, X.; Huang, Y.F.; Xing, L.; Zhou, Y.F.; Qin, H.W. An all-solid-state ammonium ion-selective electrode based on polyaniline as transducer and poly (o-phenylenediamine) as sensitive membrane. Int. J. Electrochem. Sci. 2016, 11, 9928–9940. [Google Scholar] [CrossRef]
- Dave, U.C.; Ingale, D.V.; Venkatesh, K.; Bayineni, V.K.; Kadeppagari, R. Multiwalled carbonnanotubes enhance the response and sensitivity of the ammonium biosensor based on alanine dehydrogenase. J. Electroanal. Chem. 2017, 784, 102–108. [Google Scholar] [CrossRef]
- Tan, L.L.; Musa, A.; Lee, Y.H. Determination of ammonium ion using a reagentless amperometric biosensor based on immobilized alanine dehydrogenase. Sensors 2011, 11, 9344–9360. [Google Scholar] [CrossRef] [Green Version]
- Vulcu, A.; Grosan, C.; Muresan, L.M.; Pruneanu, S.; Olenic, L. Modified gold electrodes based on thiocytosine/guanine-gold nanoparticles for uric and ascorbic acid determination. Electrochim. Acta 2013, 88, 839–846. [Google Scholar] [CrossRef]
- Piano, M.; Serban, S.; Biddle, N.; Pittson, R.; Drago, G.A.; Hart, J.P. A flow injection system, comprising a biosensor based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt coated with glucose dehydrogenase, for the measurement of glucose. Anal. Biochem. 2010, 396, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.E.; Ahmad, M.; Abdullah, J.; Sidek, H.; Heng, L.Y.; Karuppiah, N. Biosensor based on glutamate dehydrogenase immobilized in chitosan for the determination of ammonium in water samples. Anal. Biochem. 2009, 388, 28–32. [Google Scholar] [CrossRef]
- Azmi, N.E.; Abdullah, J.; Ahmad, M.; Sidek, H.; Heng, L.Y.; Rahman, S.A. An optical based biosensor for the determination of ammonium in aqueous environment. Am. J. Anal. Chem. 2012, 3, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screenprinted electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J. Disposable Screen Printed Electrochemical Sensors: Tools for Environmental Monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wring, S.A.; Hart, J.P. Chemically modified, carbon-based electrodes and their application as electrochemical sensors for the analysis of biologically important compounds: A review. Analyst 1992, 117, 1215–1229. [Google Scholar] [CrossRef]
- Yang, L.; Landick, B.; Raman, S. A regulatory NADH/NAD+ Redox biosensor for bacteria. ACS Synth. Biol. 2019, 8, 264–273. [Google Scholar]
- Kwan, R.C.H.; Hon, P.Y.T.; Renneberg, R. Amperometric determination of ammonium with bienzyme/poly(carbamoyl) sulfonate hydrogel-based biosensor. Sens. Actuators B 2005, 107, 616–622. [Google Scholar] [CrossRef]
- Tan, L.L.; Ahmad, M.; Heng, L.Y. An amperometric biosensor based on alanine dehydrogenase for the determination of low level of ammonium ion in water. J. Sens. 2011, 2011, 980709. [Google Scholar]
- Vulcu, A.; Olenic, L.; Pruneanu, S.; Berghian-Grosan, C.; Muresan, L.M. The reductive amination of α-ketoisocaproic acid using a leucine dehydrogenase modified gold electrode. Part. Sci. Technol. 2015, 2015, 1051685. [Google Scholar] [CrossRef]
- Tan, L.L.; Ahmad, M.; Heng, L.Y.; Seng, T.C. The effect of multilayer gold nanoparticles on the electrochemical response of ammonium ion biosensor based on alanine dehydrogenase enzyme. J. Sens. 2011, 2011, 754171. [Google Scholar]
- Jeong, H.; Park, J.; Kim, H. Determination of NH4+ in environmental water with interfering substances using the modified Nessler method. J. Chem. 2013, 2013, 359217. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.P.; Abass, A.K.; Cowell, D.C.; Chappell, A. Development of a disposable amperometric NH4+ biosensor based on a chemically modified screen-printed carbon electrode coated with glutamate dehydrogenase, 2-oxoglutarate, and NADH. Electroanalysis 1999, 11, 406. [Google Scholar] [CrossRef]
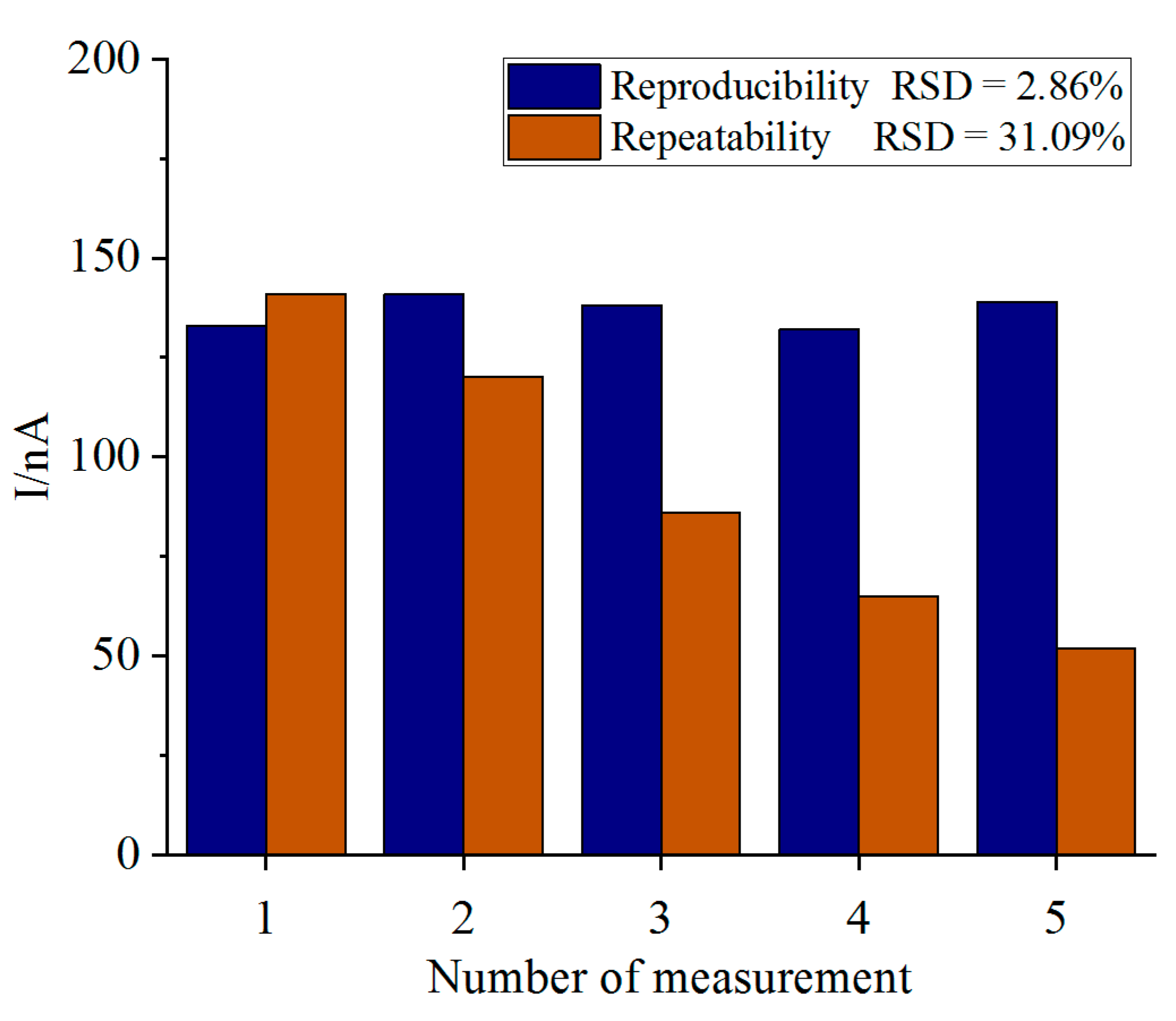
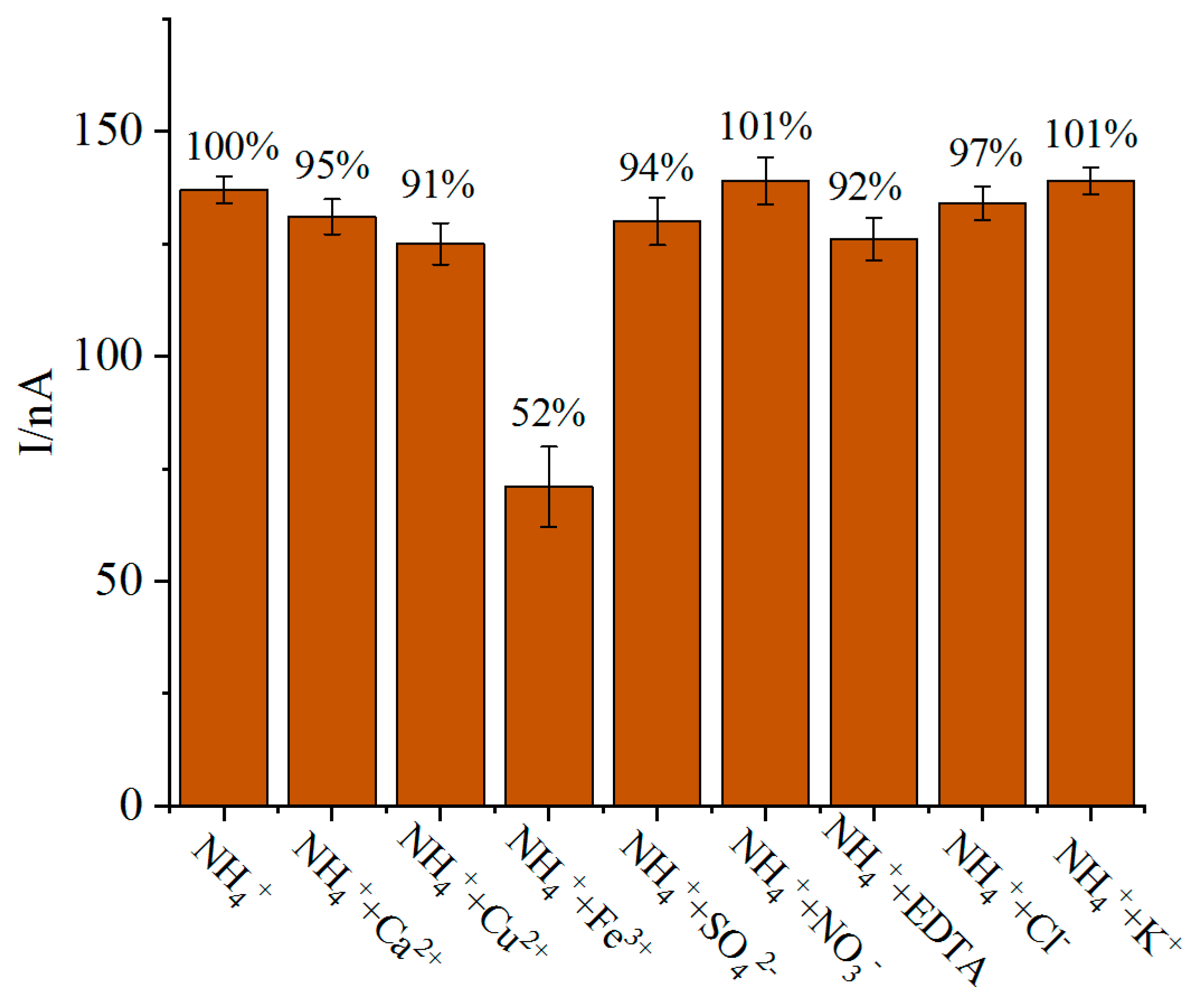
| Day | Current Difference (nA) | Retained Signal (%) | RSD |
|---|---|---|---|
| 1 | 591.25 ± 24.78 | 100 | 2.15% |
| 7 | 578.54 ± 36.89 | 97.85 | 3.27% |
| 14 | 572.98 ± 28.94 | 96.91 | 2.59% |
| 21 | 552.05 ± 37.89 | 93.37 | 3.52% |
| 28 | 538.69 ± 67.02 | 91.11 | 6.38% |
| 42 | 514.51 ± 55.79 | 87.02 | 5.56% |
| Sample. No | Spiked Concentration Using Nessler’s Method (μM) | Spiked Concentration Using Biosensor (μM) | Recovery against Nessler’s Method (%) | T-Value |
|---|---|---|---|---|
| 1 | 22.75 ± 0.10 | 24.10 ± 1.07 | 105.92% | 3.47 |
| 2 | 39.09 ± 0.06 | 36.63 ± 1.87 | 93.71% | 3.62 |
| 3 | 58.79 ± 0.18 | 61.31 ± 3.49 | 104.29% | 1.99 |
| 4 | 110.63 ± 2.02 | 107.90 ± 5.95 | 97.53% | 1.27 |
| 5 | 157.79 ± 2.64 | 152.11 ± 5.23 | 96.40% | 3.01 |
| Methods | Oxidation Potential (V) | Range (μM) | PH Compensation and Temperature Control | References |
|---|---|---|---|---|
| SPEC/AuNPs/PMB@GLDH | 0.1 | 0.65~300 | Yes | This work |
| Silver epoxy-carbon WE@ f-MWCNTs + AlaDH | 0.25 | 50~500 × 103 | No | [14] |
| SPEC@AlaDH | 0.55 | 10 × 103~100 × 103 | No | [15] |
| SPEC/AuNPs/2BME@AlaDH | 0.55 | 100~500 | No | [27] |
| Au/TC/AuNPs@LeuDH | 0.8 | 20 × 103~60 × 103 | No | [26] |
| SPEC/Meldola’s Blue@GLDH | 0.05 | 2~25 | No | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, T.; Li, Z.; Xu, X.; Zhang, X.; Li, D. An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue. Biosensors 2021, 11, 335. https://doi.org/10.3390/bios11090335
Wang C, Wang T, Li Z, Xu X, Zhang X, Li D. An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue. Biosensors. 2021; 11(9):335. https://doi.org/10.3390/bios11090335
Chicago/Turabian StyleWang, Cong, Tan Wang, Zhen Li, Xianbao Xu, Xiaoshuan Zhang, and Daoliang Li. 2021. "An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue" Biosensors 11, no. 9: 335. https://doi.org/10.3390/bios11090335
APA StyleWang, C., Wang, T., Li, Z., Xu, X., Zhang, X., & Li, D. (2021). An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue. Biosensors, 11(9), 335. https://doi.org/10.3390/bios11090335








