An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination
Abstract
:1. Introduction
2. Materials and Methods
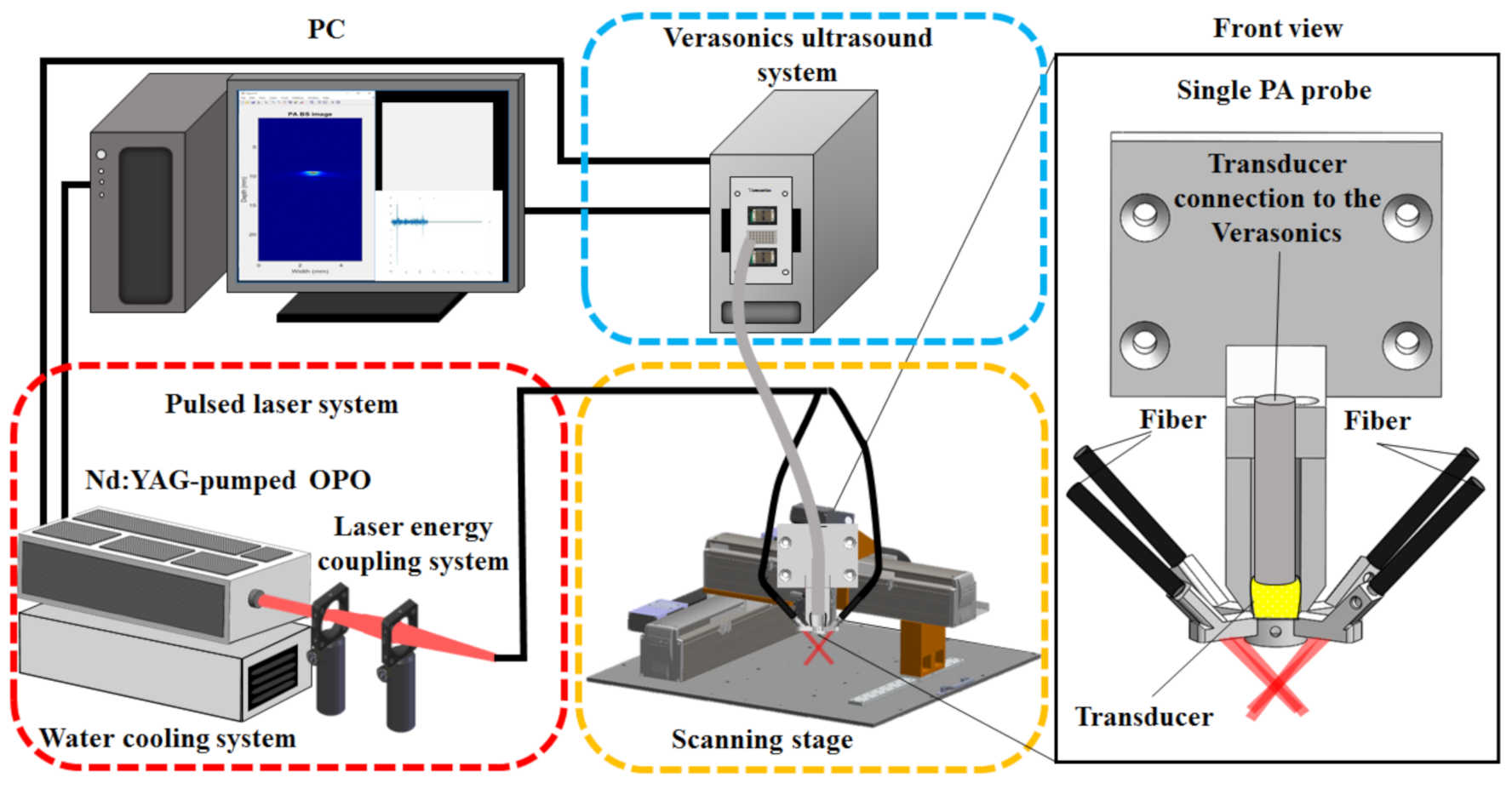
2.1. An Adjustable Dark-Field Acoustic-Resolution PAM (AR-PAM) Imaging System with Fiber Bundle-Based Illumination
2.2. Design of the AR-PAM Probe—Integration of the Fiber Bundle-Based Illumination System, US Transducer, and 3D-Printed Jacket
2.3. Testing the Imaging Performance of the Developed Adjustable AR-PAM System
2.4. Imaging of Blue and Red Inks Using the Developed PA Spectrum Technique
2.5. Imaging of a Hair Phantom to Assess the 3D Imaging Capability of the AR-PAM System
2.6. In Vitro Test of PA Imaging Using a Chicken Breast Phantom
2.7. In Vivo Vascular Mapping of Rat Ears and Functional Imaging of the Rat Brain with Electrical Stimulation
2.8. In Vivo Functional ICG-Based Pharmacokinetic Imaging of Rat Brains
3. Results
3.1. In Vitro Performance of the Developed AR-PAM Imaging System
3.2. Imaging Ink-Filled Tube, Hair, and Chicken Tissue Phantoms In Vitro
3.3. In Vivo Imaging of Blood Vessels in the Rat Ear
3.4. Evaluating Hemodynamic Changes in the SSS during Electrical Stimulation of the Left Forepaw
3.5. In Vivo Functional Imaging of Rat Brain Pharmacokinetics Following ICG Injection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hillman, E.M.C.; Amoozegar, C.B.; Wang, T.; McCaslin, A.F.H.; Bouchard, M.B.; Mansfield, J.; Levenson, R.M. In vivo optical imaging and dynamic contrast methods for biomedical research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4620–4643. [Google Scholar] [CrossRef] [Green Version]
- Balas, C. Review of biomedical optical imaging—A powerful, non-invasive, non-ionizing technology for improving in vivo diagnosis. Meas. Sci. Technol. 2009, 20, 104020. [Google Scholar] [CrossRef]
- Liao, L.-D.; Tsytsarev, V.; Delgado-Martínez, I.; Li, M.-L.; Erzurumlu, R.; Vipin, A.; Orellana, J.; Lin, Y.-R.; Lai, H.-Y.; Chen, Y.-Y.; et al. Neurovascular coupling: In vivo optical techniques for functional brain imaging. Biomed. Eng. Online 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paddock, S.W.; Eliceiri, K.W. Laser Scanning Confocal Microscopy: History, Applications, and Related Optical Sectioning Techniques. Methods Mol. Biol. 2014, 1075, 9–47. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.M.; Toca-Herrera, J.L. The new future of scanning probe microscopy: Combining atomic force microscopy with other surface-sensitive techniques, optical microscopy and fluorescence techniques. Nanoscale 2009, 1, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Yamada, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef] [Green Version]
- Applegate, M.B.; Istfan, R.E.; Spink, S.; Tank, A.; Roblyer, D. Recent advances in high speed diffuse optical imaging in biomedicine. APL Photonics 2020, 5, 040802. [Google Scholar] [CrossRef] [Green Version]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, L.V. Sensitivity of photoacoustic microscopy. Photoacoustics 2014, 2, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, L. Tutorial on Photoacoustic Microscopy and Computed Tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lin, J.; Wang, T.; Chen, X.; Huang, P. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics 2016, 6, 2394–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2018, 31, e1805875. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef]
- Liao, L.-D.; Lin, C.-T.; Shih, Y.-Y.I.; Duong, T.; Lai, H.-Y.; Wang, P.-H.; Wu, R.; Tsang, S.; Chang, J.-Y.; Li, M.-L.; et al. Transcranial Imaging of Functional Cerebral Hemodynamic Changes in Single Blood Vessels using in vivo Photoacoustic Microscopy. Br. J. Pharmacol. 2012, 32, 938–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, H.; Wang, Y.; Jhang, D.-F.; Chu, T.-S.; Tsao, C.-H.; Tsai, C.-H.; Giamundo, S.; Chen, Y.-Y.; Liao, K.-W.; Chuang, C.-C.; et al. Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications. Micromachines 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.-D.; Li, M.-L.; Lai, H.-Y.; Shih, Y.-Y.I.; Lo, Y.-C.; Tsang, S.; Chao, P.C.-P.; Lin, C.-T.; Jaw, F.-S.; Chen, Y.-Y. Imaging brain hemodynamic changes during rat forepaw electrical stimulation using functional photoacoustic microscopy. NeuroImage 2010, 52, 562–570. [Google Scholar] [CrossRef]
- Liao, L.-D.; Liu, Y.-H.; Lai, H.-Y.; Bandla, A.; Shih, Y.-Y.I.; Chen, Y.-Y.; Thakor, N.V. Rescue of cortical neurovascular functions during the hyperacute phase of ischemia by peripheral sensory stimulation. Neurobiol. Dis. 2015, 75, 53–63. [Google Scholar] [CrossRef]
- Bandla, A.; Liao, L.-D.; Chan, S.J.; Ling, J.M.; Liu, Y.-H.; Shih, Y.-Y.I.; Pan, H.-C.; Wong, P.T.-H.; Lai, H.-Y.; King, N.K.K.; et al. Simultaneous functional photoacoustic microscopy and electrocorticography reveal the impact of rtPA on dynamic neurovascular functions after cerebral ischemia. Br. J. Pharmacol. 2017, 38, 980–995. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Liao, L.-D.; Tan, S.S.H.; Kwon, K.Y.; Ling, J.M.; Bandla, A.; Shih, Y.-Y.I.; Tan, E.T.W.; Li, W.; Ng, W.H.; et al. Assessment of neurovascular dynamics during transient ischemic attack by the novel integration of micro-electrocorticography electrode array with functional photoacoustic microscopy. Neurobiol. Dis. 2015, 82, 455–465. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Chu, C.-H.; Cheng, S.-H.; Liao, L.-D.; Chu, T.-S.; Chen, N.-T.; Paldino, A.; Hsia, Y.; Chen, C.-T.; Lo, L.-W. Annealing-modulated nanoscintillators for nonconventional X-ray activation of comprehensive photodynamic effects in deep cancer theranostics. Theranostics 2020, 10, 6758–6773. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; De Liao, L.; Thakor, N.V.; Tan, M.C. Nanoparticles for Molecular Imaging. J. Biomed. Nanotechnol. 2014, 10, 2641–2676. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.-Q.; An, J.; Cheng, K.; Hou, X.-L.; Zhang, X.-S.; Song, X.-L.; Huang, K.-C.; Chen, W.; Liu, B.; et al. A near-infrared light-controlled smart nanocarrier with reversible polypeptide-engineered valve for targeted fluorescence-photoacoustic bimodal imaging-guided chemo-photothermal therapy. Theranostics 2019, 9, 7666–7679. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Liao, L.; Bandla, A.; Ling, J.M.; Liu, Y.-H.; Thakor, N.; Bazan, G.C.; Liu, B. Encapsulated Conjugated Oligomer Nanoparticles for Real-Time Photoacoustic Sentinel Lymph Node Imaging and Targeted Photothermal Therapy. Small 2016, 12, 4873–4880. [Google Scholar] [CrossRef]
- Cai, X.; Bandla, A.; Chuan, C.K.; Magarajah, G.; Liao, L.-D.; Teh, D.B.L.; Kennedy, B.K.; Thakor, N.V.; Liu, B. Identifying glioblastoma margins using dual-targeted organic nanoparticles for efficient in vivo fluorescence image-guided photothermal therapy. Mater. Horiz. 2018, 6, 311–317. [Google Scholar] [CrossRef]
- Sheng, Y.; Liao, L.-D.; Bandla, A.; Liu, Y.-H.; Yuan, J.; Thakor, N.; Tan, M.C. Enhanced near-infrared photoacoustic imaging of silica-coated rare-earth doped nanoparticles. Mater. Sci. Eng. C 2017, 70, 340–346. [Google Scholar] [CrossRef]
- Geng, J.; Liao, L.-D.; Qin, W.; Tang, B.Z.; Thakor, N.; Liu, B. Fluorogens with Aggregation Induced Emission: Ideal Photoacoustic Contrast Reagents Due to Intramolecular Rotation. J. Nanosci. Nanotechnol. 2015, 15, 1864–1868. [Google Scholar] [CrossRef]
- Razansky, D.; Bühler, A.; Ntziachristos, V. Volumetric real-time multispectral optoacoustic tomography of biomarkers. Nat. Protoc. 2011, 6, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Pramanik, M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. J. Biomed. Opt. 2016, 22, 041006. [Google Scholar] [CrossRef]
- Grinvald, A.; Frostig, R.D.; Lieke, E.; Hildesheim, R. Optical imaging of neuronal activity. Physiol. Rev. 1988, 68, 1285–1366. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.J.; Ponticorvo, A.; Konecky, S.D.; Cui, H.; Rice, T.B.; Choi, B.; Durkin, A.J.; Tromberg, B.J. Visible spatial frequency domain imaging with a digital light microprojector. J. Biomed. Opt. 2013, 18, 096007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.; Wang, B.; Ji, L.; Jiang, H. 4-D Photoacoustic Tomography. Sci. Rep. 2013, 3, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norat, P.; Soldozy, S.; Elsarrag, M.; Sokolowski, J.; Yaǧmurlu, K.; Park, M.S.; Tvrdik, P.; Kalani, M.Y.S. Application of Indocyanine Green Videoangiography in Aneurysm Surgery: Evidence, Techniques, Practical Tips. Front. Surg. 2019, 6, 34. [Google Scholar] [CrossRef]
- Ovsepian, S.V.; Jiang, Y.; Sardella, T.C.; Malekzadeh-Najafabadi, J.; Burton, N.C.; Yu, X.; Ntziachristos, V. Visualizing cortical response to optogenetic stimulation and sensory inputs using multispectral handheld optoacoustic imaging. Photoacoustics 2020, 17, 100153. [Google Scholar] [CrossRef] [PubMed]
- Roston, S. The blood flow of the brain. Bull. Math. Biol. 1967, 29, 541–548. [Google Scholar] [CrossRef]
- Vienneau, E.; Liu, W.; Yao, J. Dual-view acoustic-resolution photoacoustic microscopy with enhanced resolution isotropy. Opt. Lett. 2018, 43, 4413–4416. [Google Scholar] [CrossRef]
- Omar, M.; Gateau, J.; Ntziachristos, V. Raster-scan optoacoustic mesoscopy in the 25–125 MHz range. Opt. Lett. 2013, 38, 2472–2474. [Google Scholar] [CrossRef]
- Omar, M.; Soliman, D.; Gateau, J.; Ntziachristos, V. Ultrawideband reflection-mode optoacoustic mesoscopy. Opt. Lett. 2014, 39, 3911–3914. [Google Scholar] [CrossRef]
- Moothanchery, M.; Dev, K.; Balasundaram, G.; Bi, R.; Olivo, M. Acoustic resolution photoacoustic microscopy based on microelectromechanical systems scanner. J. Biophotonics 2019, 13, e201960127. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Yang, J.-M.; Gao, L.S.; Maslov, K.; Wang, L.; Huang, C.-H.; Zou, J. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror. J. Biomed. Opt. 2012, 17, 080505. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jhang, D.-F.; Chu, T.-S.; Tsao, C.-H.; Tsai, C.-H.; Chuang, C.-C.; Ger, T.-R.; Chen, L.-T.; Chang, W.-S.W.; Liao, L.-D. An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination. Biosensors 2021, 11, 262. https://doi.org/10.3390/bios11080262
Wang Y, Jhang D-F, Chu T-S, Tsao C-H, Tsai C-H, Chuang C-C, Ger T-R, Chen L-T, Chang W-SW, Liao L-D. An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination. Biosensors. 2021; 11(8):262. https://doi.org/10.3390/bios11080262
Chicago/Turabian StyleWang, Yuhling, De-Fu Jhang, Tsung-Sheng Chu, Chia-Hui Tsao, Chia-Hua Tsai, Chiung-Cheng Chuang, Tzong-Rong Ger, Li-Tzong Chen, Wun-Shaing Wayne Chang, and Lun-De Liao. 2021. "An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination" Biosensors 11, no. 8: 262. https://doi.org/10.3390/bios11080262
APA StyleWang, Y., Jhang, D.-F., Chu, T.-S., Tsao, C.-H., Tsai, C.-H., Chuang, C.-C., Ger, T.-R., Chen, L.-T., Chang, W.-S. W., & Liao, L.-D. (2021). An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination. Biosensors, 11(8), 262. https://doi.org/10.3390/bios11080262






