Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors
Abstract
:1. Introduction
2. Materials and Methods
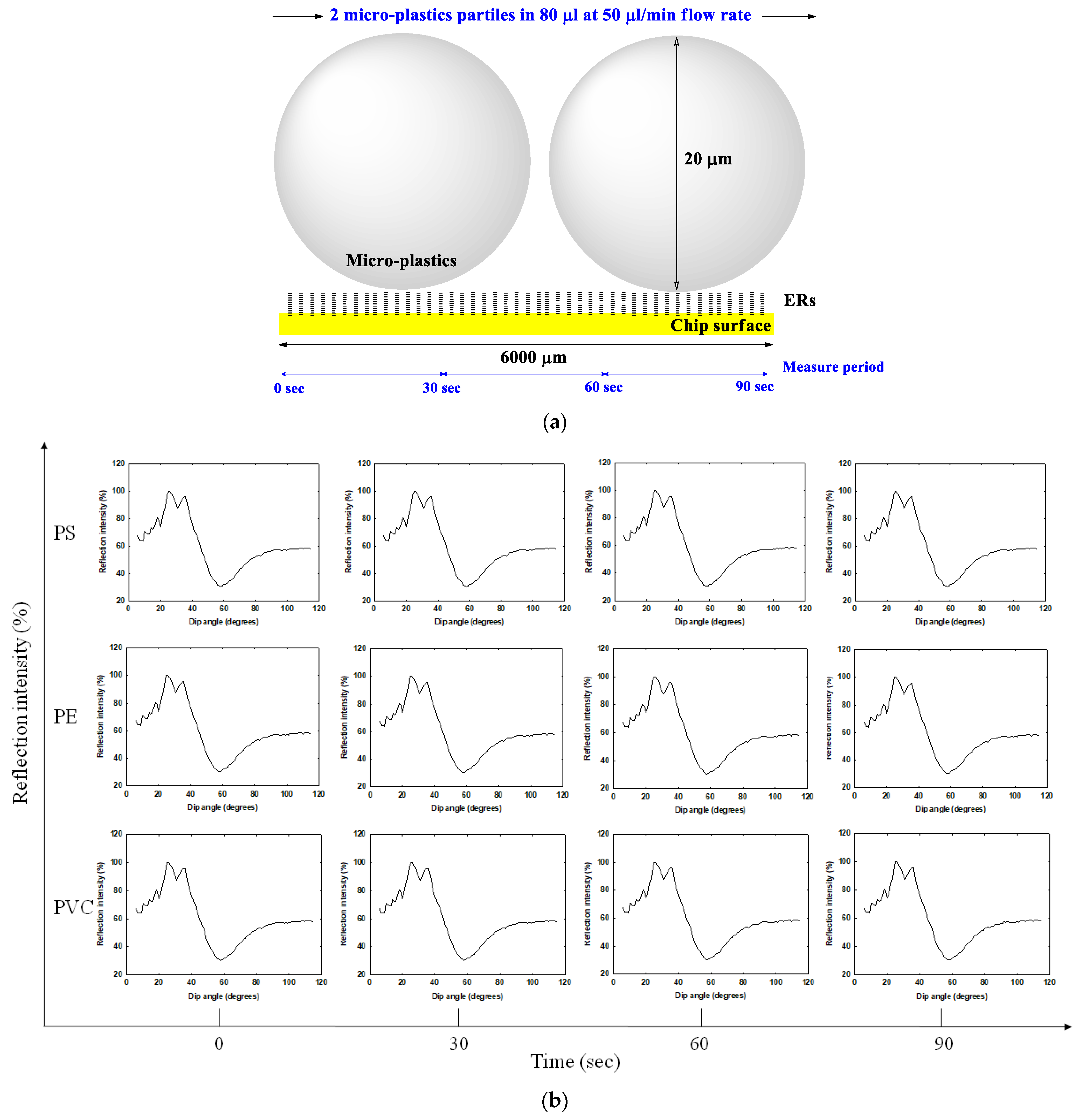
2.1. Preparation of 20-Micrometer Micro-Plastics
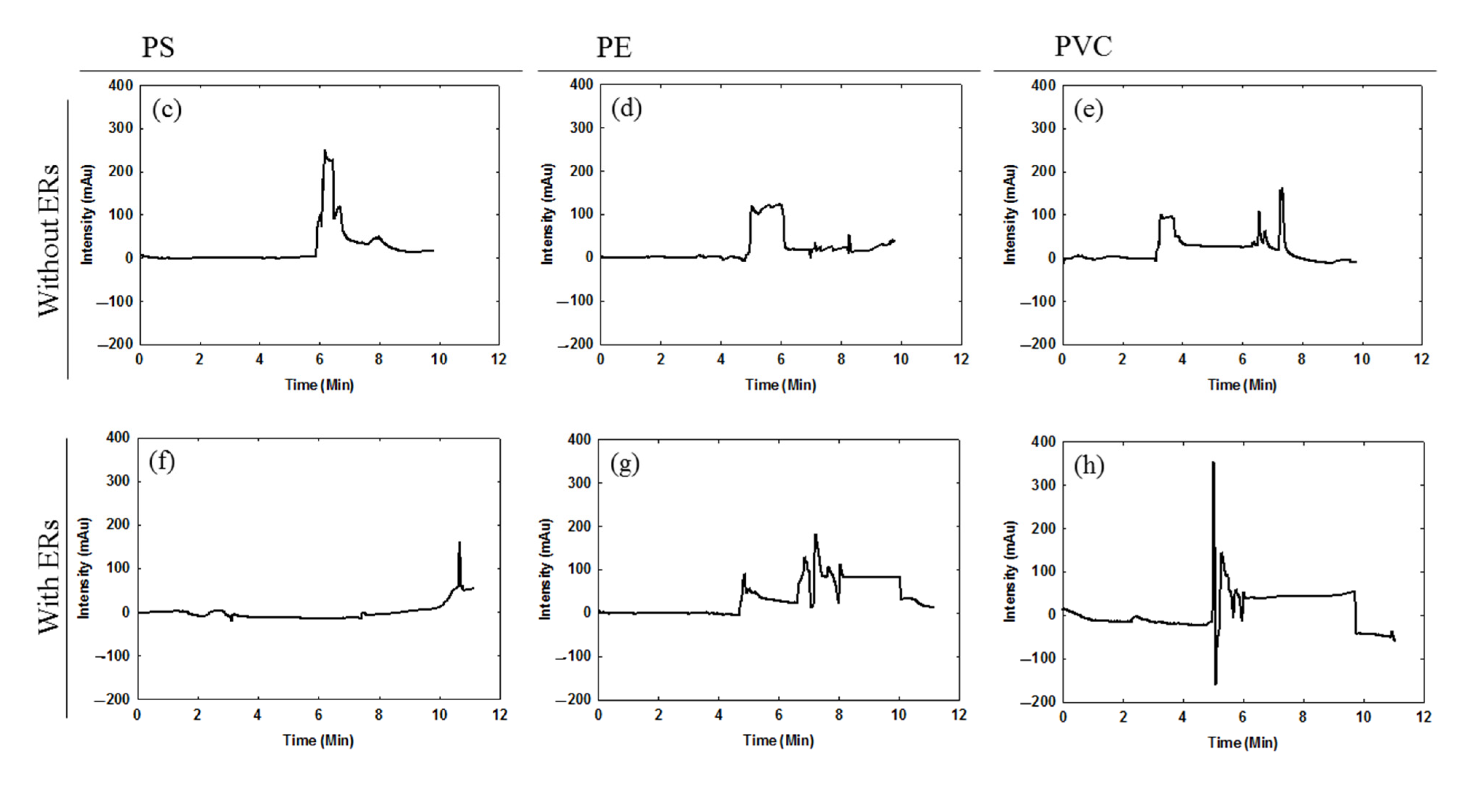
2.2. Chromatography of Micro-Plastics
2.3. Detection of Micro-Plastics on SPR
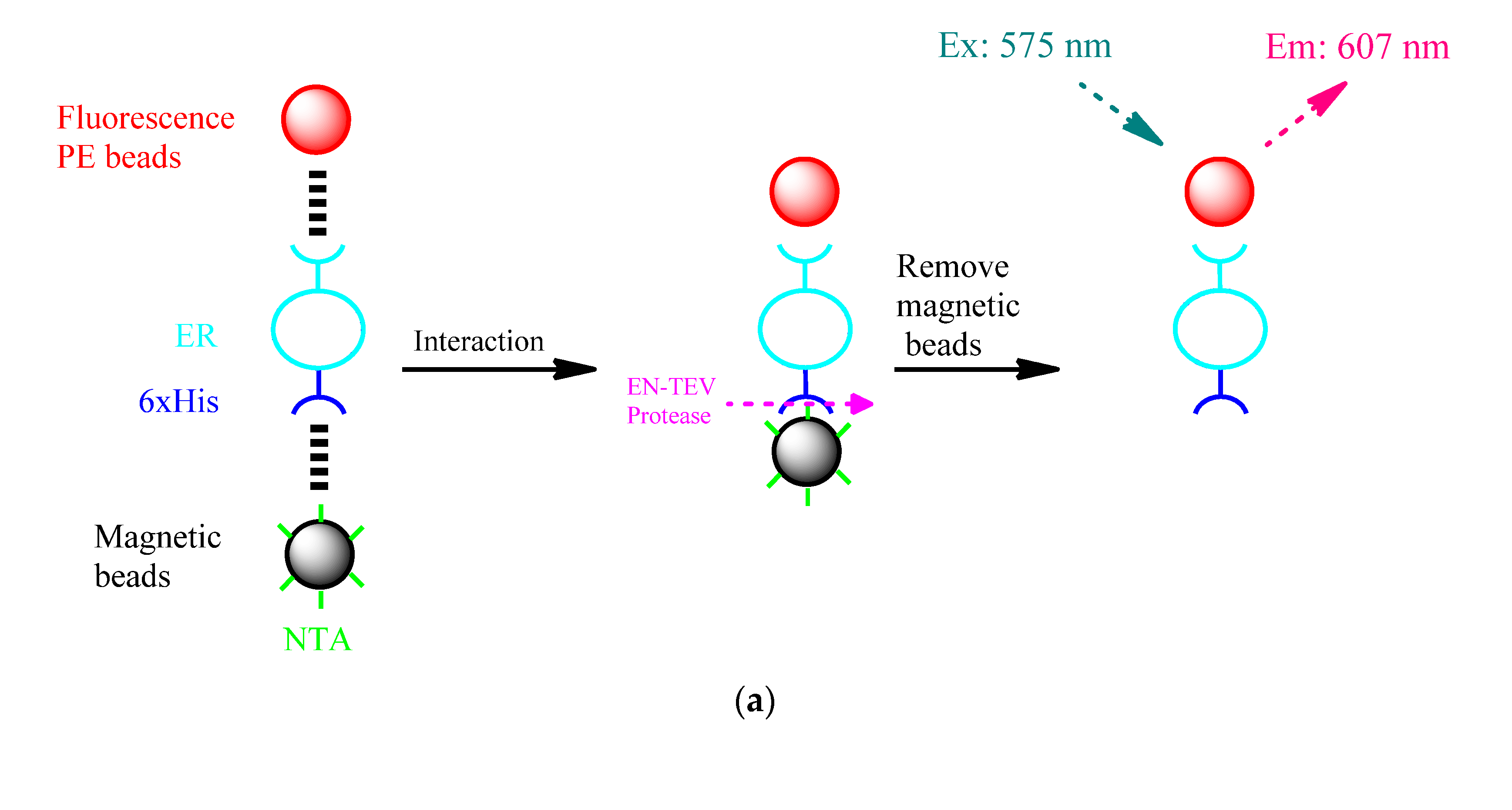
2.4. Magnetic Beads Verify the Interaction between ER and Micro-Plastics
3. Results
3.1. Preparation of 20-Micrometer Micro-Plastics
3.2. Using Liquid Chromatography to Observe the Interaction between Micro-Plastics and Estrogen Receptors
3.3. Micro-Plastics Movement Mode Speculation in SPR
3.4. Determination of Micro-Plastics Concentration by SPR
3.5. Measurement of the Interaction between ER and Micro-Plastics by SPR
3.6. Verification of the Interaction of Micro-Plastics on Estrogen Receptors by ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.O.; Abrantes, N.; Goncalves, F.J.M.; Nogueira, H.; Marques, J.C.; Goncalves, A.M.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K.A. Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef] [Green Version]
- Lechner, A.; Ramler, D. The discharge of certain amounts of industrial microplastic from a production plant into the River Danube is permitted by the Austrian legislation. Environ. Pollut. 2015, 200, 159–160. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicinska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Heddagaard, F.E.; Moller, P. Hazard assessment of small-size plastic particles: Is the conceptual framework of particle toxicology useful? Food Chem. Toxicol. 2020, 136, 111106. [Google Scholar] [CrossRef]
- Sunitha, T.G.; Monisha, V.; Sivanesan, S.; Vasanthy, M.; Prabhakaran, M.; Omine, K.; Sivasankar, V.; Darchen, A. Micro-plastic pollution along the Bay of Bengal coastal stretch of Tamil Nadu, South India. Sci. Total Environ. 2021, 756, 144073. [Google Scholar] [CrossRef]
- Winkler, A.; Nessi, A.; Antonioli, D.; Laus, M.; Santo, N.; Parolini, M.; Tremolada, P. Occurrence of microplastics in pellets from the common kingfisher (Alcedo atthis) along the Ticino River, North Italy. Environ. Sci. Pollut. Res. Int. 2020, 27, 41731–41739. [Google Scholar] [CrossRef]
- Enders, K.; Kappler, A.; Biniasch, O.; Feldens, P.; Stollberg, N.; Lange, X.; Fischer, D.; Eichhorn, K.J.; Pollehne, F.; Oberbeckmann, S.; et al. Tracing microplastics in aquatic environments based on sediment analogies. Sci. Rep. 2019, 9, 15207. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Isobe, A.; Buenaventura, N.T.; Chastain, S.; Chavanich, S.; Cozar, A.; DeLorenzo, M.; Hagmann, P.; Hinata, H.; Kozlovskii, N.; Lusher, A.L.; et al. An interlaboratory comparison exercise for the determination of microplastics in standard sample bottles. Mar. Pollut. Bull. 2019, 146, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Bao, L.J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. Int. 2018, 25, 11319–11332. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [Green Version]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.; Latif, U.; Feroz, S. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Harada, L.K.; Junior, W.B.; Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Junior, J.M.O.; Tubino, M.; Vila, M.; Balcao, V.M. Bacteriophage-Based Biosensing of Pseudomonas aeruginosa: An Integrated Approach for the Putative Real-Time Detection of Multi-Drug-Resistant Strains. Biosensors 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Benfenati, E.; van Cauwenberge, A.; Eidem, J.K.; Erratico, C.; Goksoyr, A.; Kloas, W.; Maggioni, S.; Mandich, A.; Urbatzka, R. Estrogenicity profile and estrogenic compounds determined in river sediments by chemical analysis, ELISA and yeast assays. Chemosphere 2008, 73, 1078–1089. [Google Scholar] [CrossRef]
- Lu, Y.; Peterson, J.R.; Luais, E.; Gooding, J.J.; Lee, N.A. Effects of Surface Epitope Coverage on the Sensitivity of Displacement Assays that Employ Modified Nanoparticles: Using Bisphenol A as a Model Analyte. Biosensors 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.L.; Quevedo, I.R.; He, D.; Basnet, M.; Tufenkji, N. Using the quartz crystal microbalance with dissipation monitoring to evaluate the size of nanoparticles deposited on surfaces. ACS Nano 2013, 7, 7833–7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, M.; Liang, L.; Wang, W.; Xie, J. Airborne particulate matter classification and concentration detection based on 3D printed virtual impactor and quartz crystal microbalance sensor. Sens. Actuators A Phys. 2016, 238, 379–388. [Google Scholar] [CrossRef]
- Kao, W.C.; Chen, Y.W.; Chu, C.H.; Chang, W.H.; Shiesh, S.C.; Wang, Y.L.; Lee, G.B. Detection of C-reactive protein on an integrated microfluidic system by utilizing field-effect transistors and aptamers. Biomicrofluidics 2017, 11, 044105. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [Green Version]
- Chau, Y.-F.C.; Chao, C.-T.C.; Chiang, H.-P.; Lim, C.M.; Voo, N.Y.; Mahadi, A.H. Plasmonic effects in composite metal nanostructures for sensing applications. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Chau, Y.-F.C.; Chao, C.-T.C.; Huang, H.J.; Anwar, U.; Lim, C.M.; Voo, N.Y.; Mahadi, A.H.; Kumara, N.T.R.N.; Chiang, H.P. Plasmonic perfect absorber based on metal nanorod arrays connected with veins. Results Phys. 2019, 15. [Google Scholar] [CrossRef]
- Chau, Y.-F.C.; Jiang, J.-C.; Chao, C.-T.C.; Chiang, H.-P.; Lim, C.M. Manipulating near field enhancement and optical spectrum in a pair-array of the cavity resonance based plasmonic nanoantennas. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Chau, Y.-F.C.; Chen, K.H.; Chiang, H.P.; Lim, C.M.; Huang, H.J.; Lai, C.H.; Kumara, N. Fabrication and Characterization of a Metallic-Dielectric Nanorod Array by Nanosphere Lithography for Plasmonic Sensing Application. Nanomaterials 2019, 9, 1691. [Google Scholar] [CrossRef] [Green Version]
- Chau, Y.-F.C.; Chao, C.-T.C.; Rao, J.-Y.; Chiang, H.-P.; Lim, C.M.; Lim, R.C.; Voo, N.Y. Tunable Optical Performances on a Periodic Array of Plasmonic Bowtie Nanoantennas with Hollow Cavities. Nanoscale Res. Lett 2016, 11, 411. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.-C.; Lin, W.-C.; Chen, C.-W.; Tsai, D.P.; Chiang, H.-P. Enhanced Sensitivity of Surface Plasmon Resonance Phase-Interrogation Biosensor by Using Silver Nanoparticles. Plasmonics 2010, 6, 29–34. [Google Scholar] [CrossRef]
- Mungroo, N.A.; Neethirajan, S. Biosensors for the Detection of Antibiotics in Poultry Industry—A Review. Biosensors 2014, 4, 472–493. [Google Scholar] [CrossRef] [Green Version]
- Ohno, K.; Azuma, Y.; Date, K.; Nakano, S.; Kobayashi, T.; Nagao, Y.; Yamada, T. Evaluation of styrene oligomers eluted from polystyrene for estrogenicity in estrogen receptor binding assay, reporter gene assay, and uterotrophic assay. Food Chem. Toxicol. 2003, 41, 131–141. [Google Scholar] [CrossRef]
- Kanako, S.; Fumiko, N.; Naoto, A. Several Environmental Pollutants Have Binding Affinities for Both Androgen Receptor and Estrogen Receptor α. J. Health Sci. 2001, 47, 495–501. [Google Scholar]
- Ohashi, A.; Kotera, H.; Hori, H.; Hibiya, M.; Watanabe, K.; Murakami, K.; Hasegawa, M.; Tomita, M.; Hiki, Y.; Sugiyama, S. Evaluation of endocrine disrupting activity of plasticizers in polyvinyl chloride tubes by estrogen receptor alpha binding assay. J. Artif. Organs 2005, 8, 252–256. [Google Scholar] [CrossRef]
- Tuoriniemi, J.; Moreira, B.; Safina, G. Determining Number Concentrations and Diameters of Polystyrene Particles by Measuring the Effective Refractive Index of Colloids Using Surface Plasmon Resonance. Langmuir 2016, 32, 10632–10640. [Google Scholar] [CrossRef] [PubMed]
- Hulme, E.C.; Trevethick, M.A. Ligand binding assays at equilibrium: Validation and interpretation. Br. J. Pharmacol. 2010, 161, 1219–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastritis, P.L.; Bonvin, A.M. On the binding affinity of macromolecular interactions: Daring to ask why proteins interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef]







| Compound | PS | PE | PVC | PE/Magnetic Beads | DEHP |
|---|---|---|---|---|---|
| Bmax | 100.41 | 34.65 | 110.57 | 96,243.94 | 79.52 |
| KD (nM) | 0.19 | 0.29 | 3.32 | 3.51 | 1921.07 |
| kd (nM) | 0.05 | 0.14 | 0.09 | 0.28 | 1000.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-J.; Narasimha, G.V.; Chen, Y.-C.; Chen, J.-K.; Dong, G.-C. Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors. Biosensors 2021, 11, 219. https://doi.org/10.3390/bios11070219
Huang C-J, Narasimha GV, Chen Y-C, Chen J-K, Dong G-C. Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors. Biosensors. 2021; 11(7):219. https://doi.org/10.3390/bios11070219
Chicago/Turabian StyleHuang, Chen-Ji, Gudivada Vijaya Narasimha, Yu-Cheng Chen, Jen-Kun Chen, and Guo-Chung Dong. 2021. "Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors" Biosensors 11, no. 7: 219. https://doi.org/10.3390/bios11070219
APA StyleHuang, C.-J., Narasimha, G. V., Chen, Y.-C., Chen, J.-K., & Dong, G.-C. (2021). Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors. Biosensors, 11(7), 219. https://doi.org/10.3390/bios11070219







