Biomedical Applications of Electromagnetic Detection: A Brief Review
Abstract
:1. Introduction
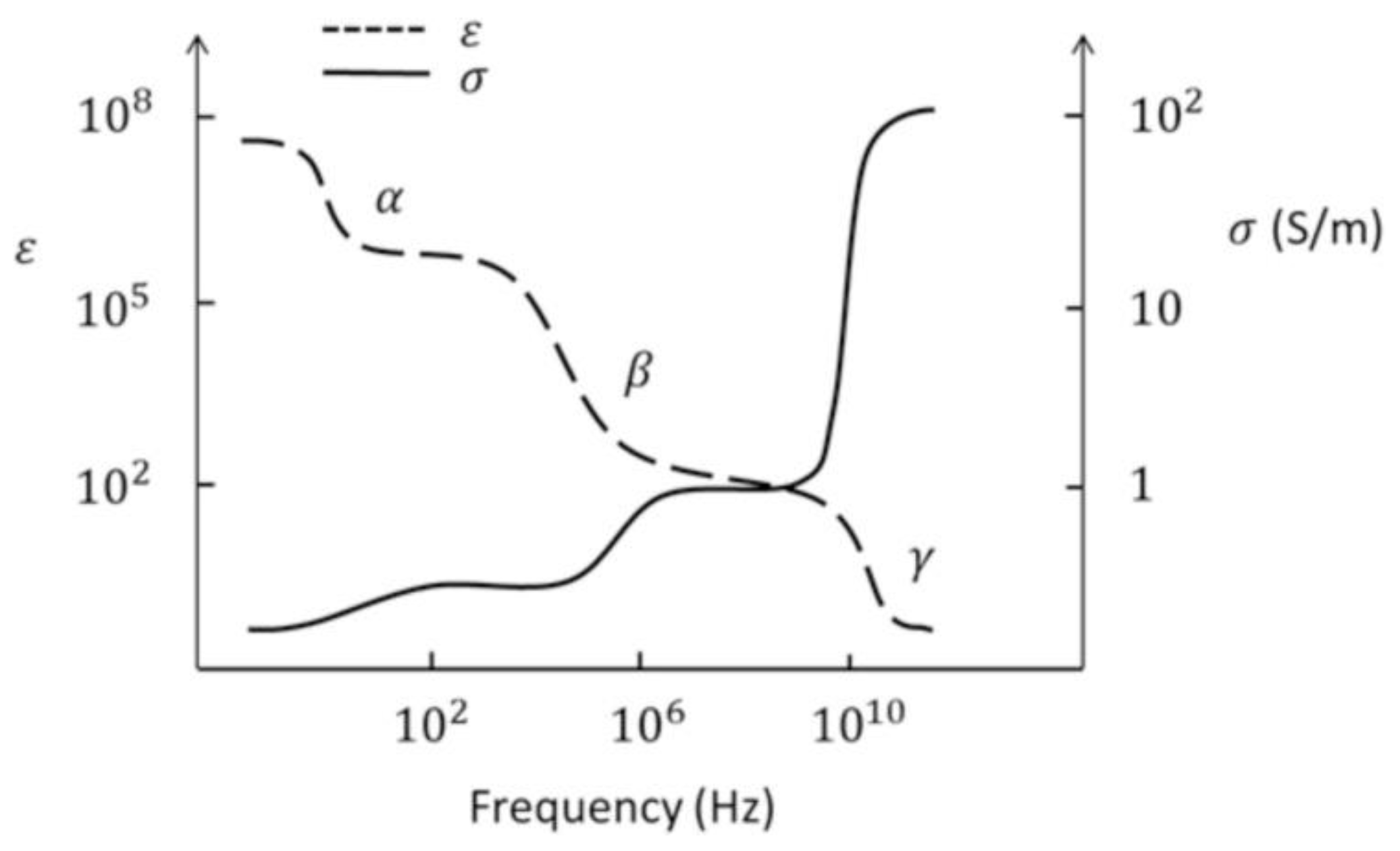
2. Electromagnetic Biological Effect and Mechanism
2.1. Biological Thermal Effects
2.2. Biological Non-Thermal Effects
2.2.1. Coherent Oscillation Theory
2.2.2. Ion Transmembrane Cyclotron Resonance Theory
2.2.3. Free Radical Mechanism Theory
2.3. Cumulative Effects
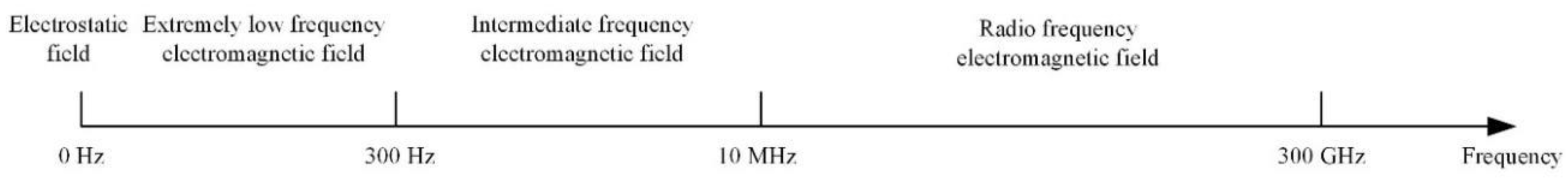
3. Application of Electromagnetic Detection Technology at Different Frequencies
3.1. High-Voltage Electrostatic Field (0 Hz)
3.1.1. Seed Germination and Growth
3.1.2. Food Thawing
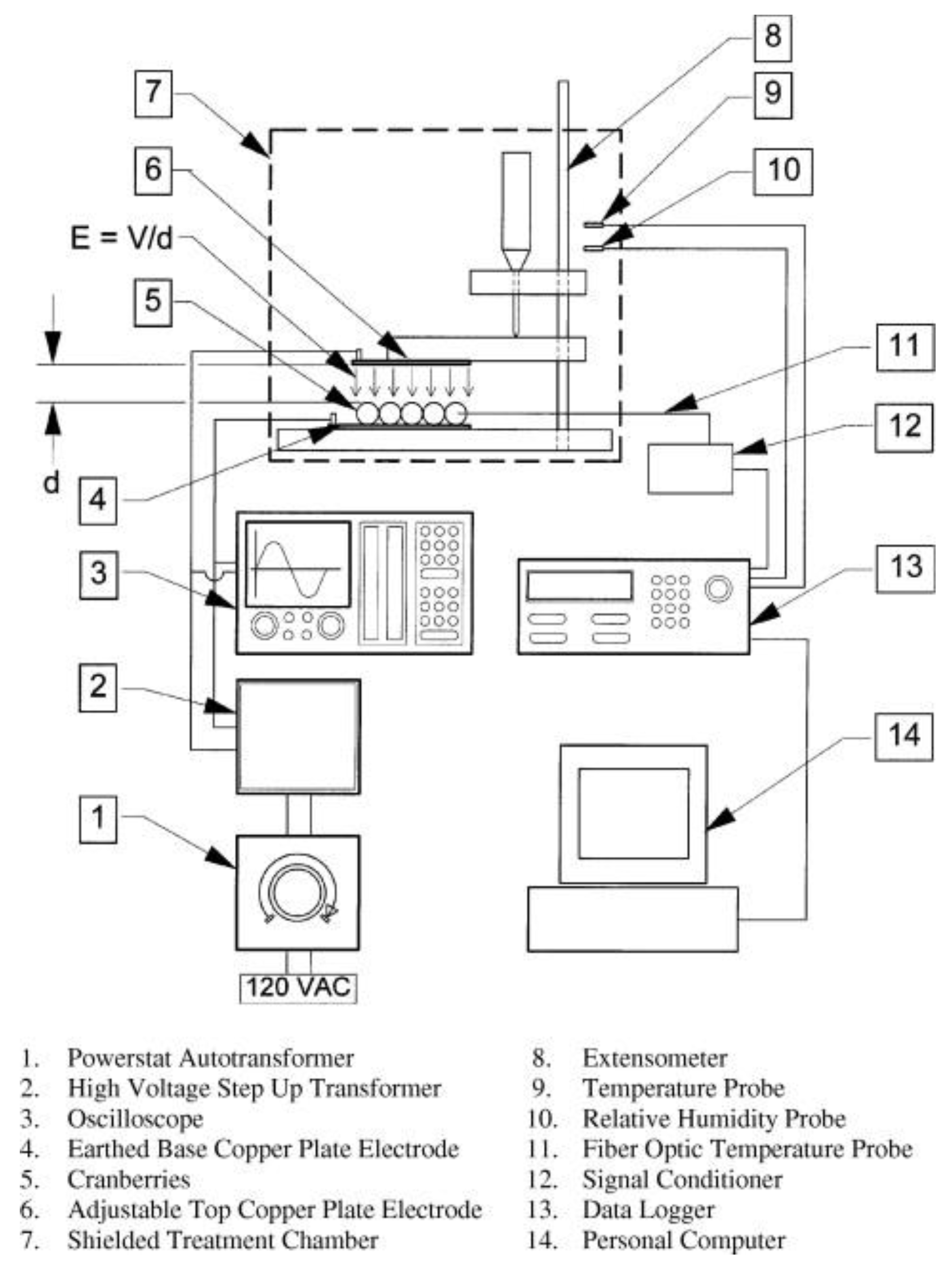
3.1.3. Food Preservation
3.2. Extremely Low Frequency Electromagnetic Field (0–300 Hz)
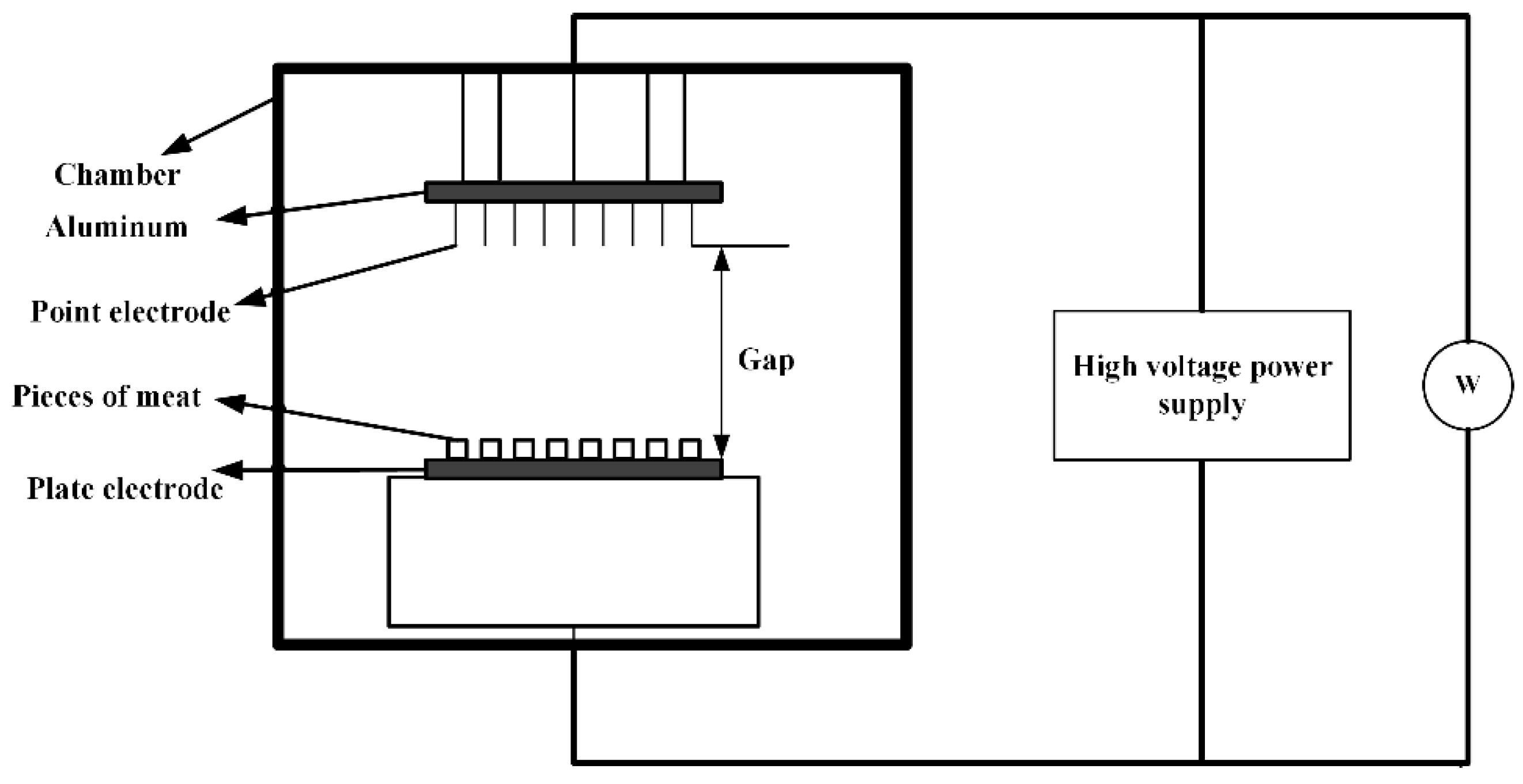
3.2.1. Cancer Treatment
- Induce the level of ROS in tumor cells to increase, which in turn damages DNA, proteins, and membrane lipids;
- Produce selective cytotoxicity to tumor cells and achieve an anti-tumor effect by improving cellular immunity;
- Directly damage the DNA chain to cause chromosome aberrations and inhibit tumor growth;
- Promote tumor cell apoptosis and cell cycle arrest.
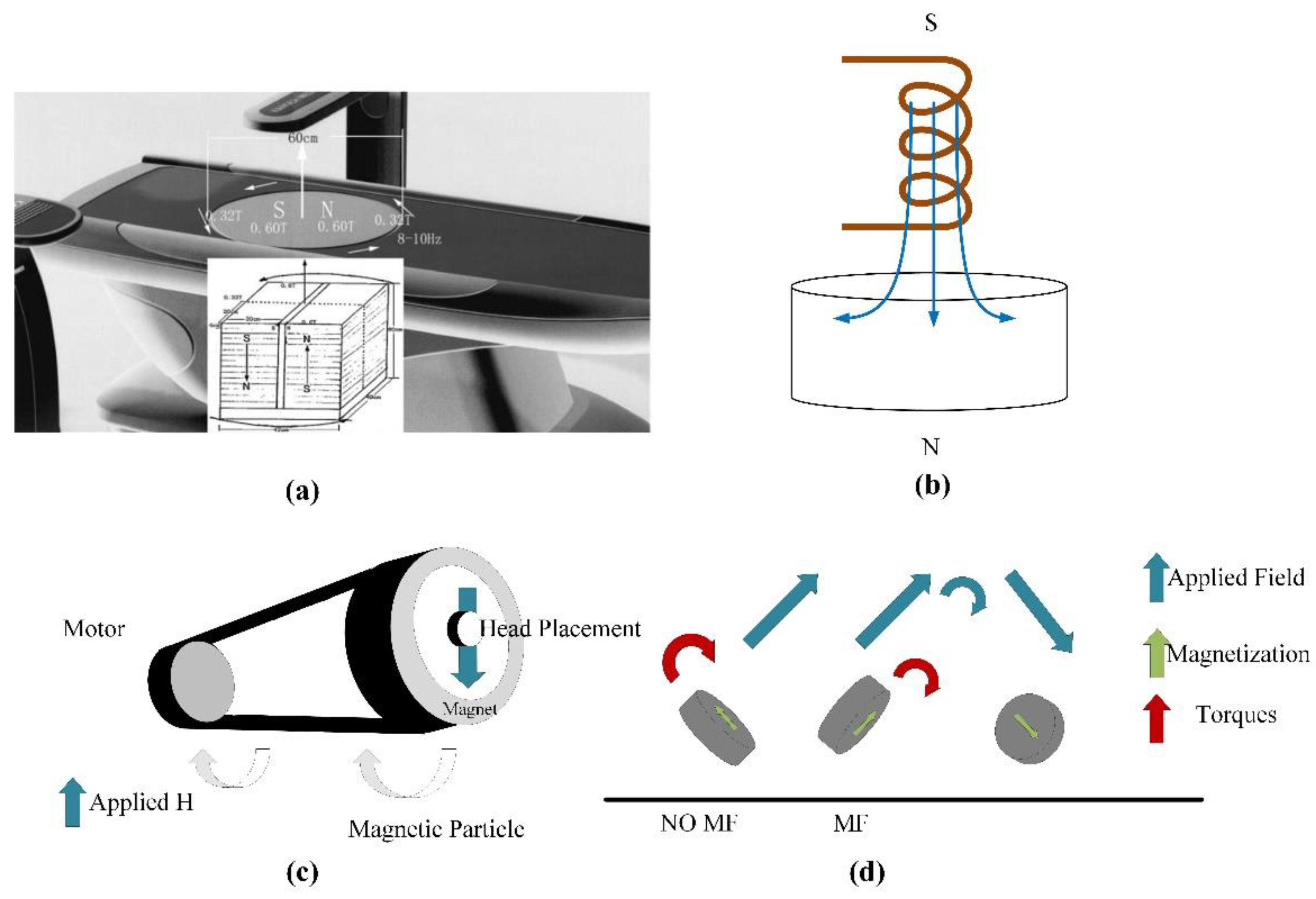
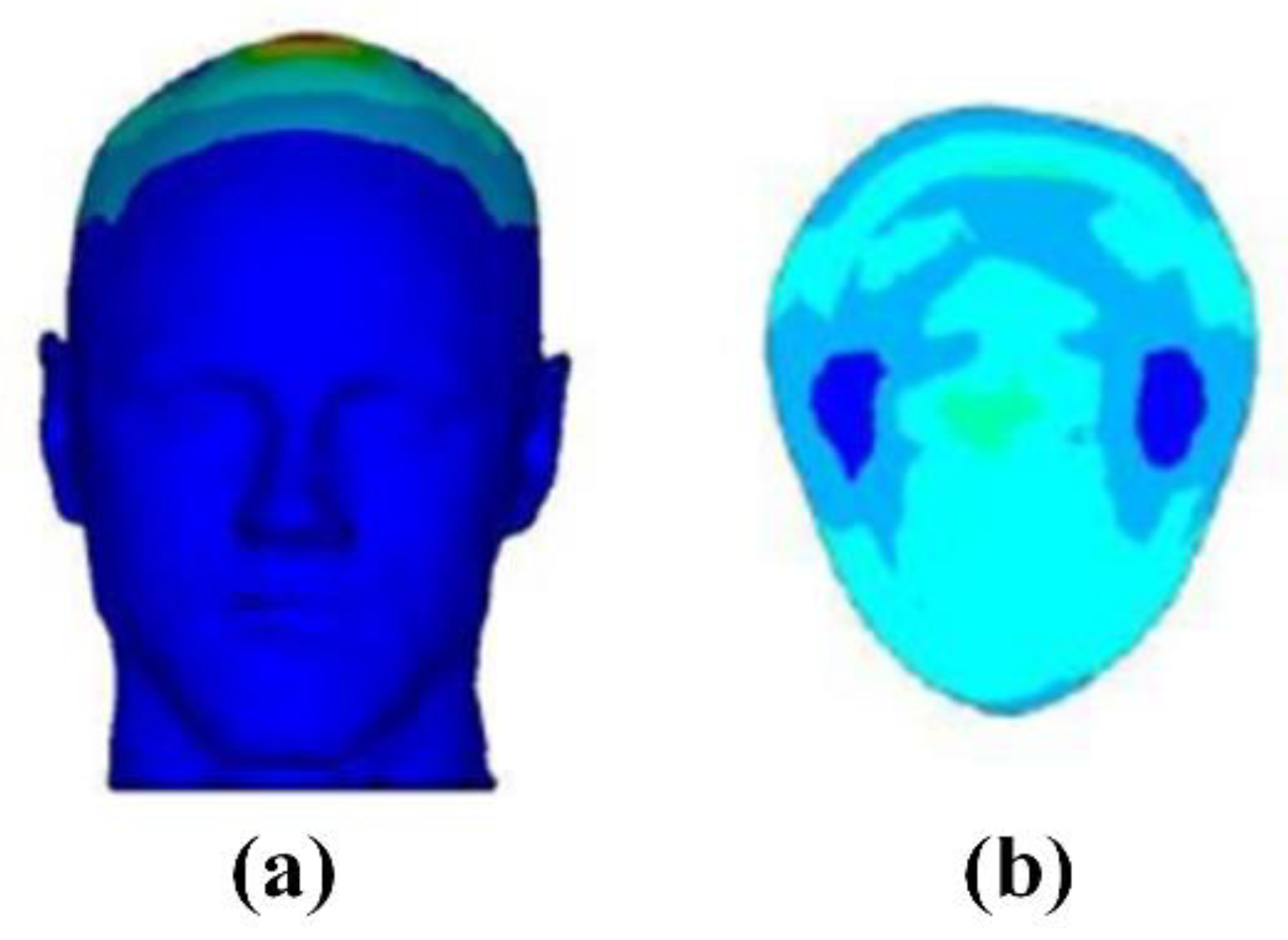
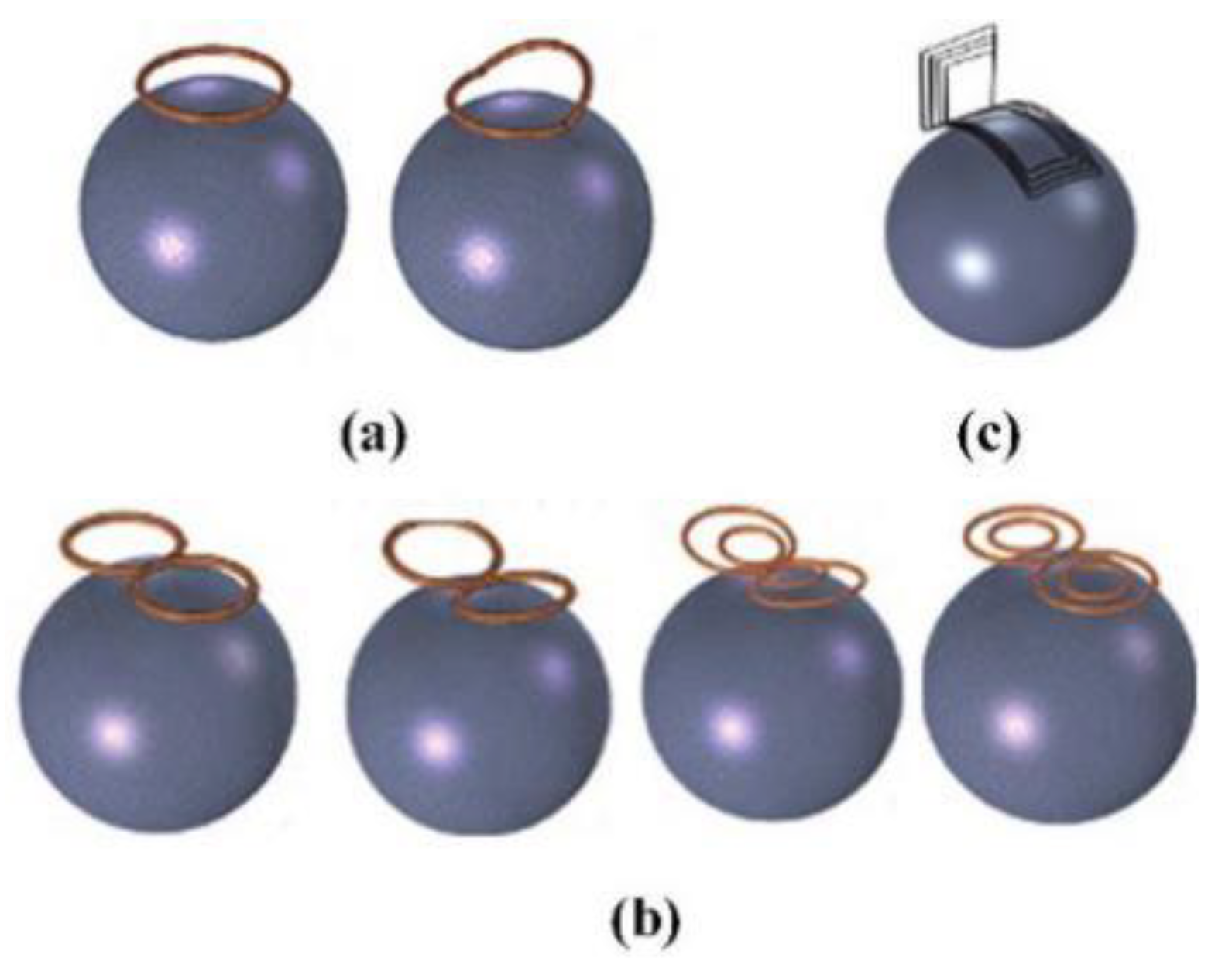
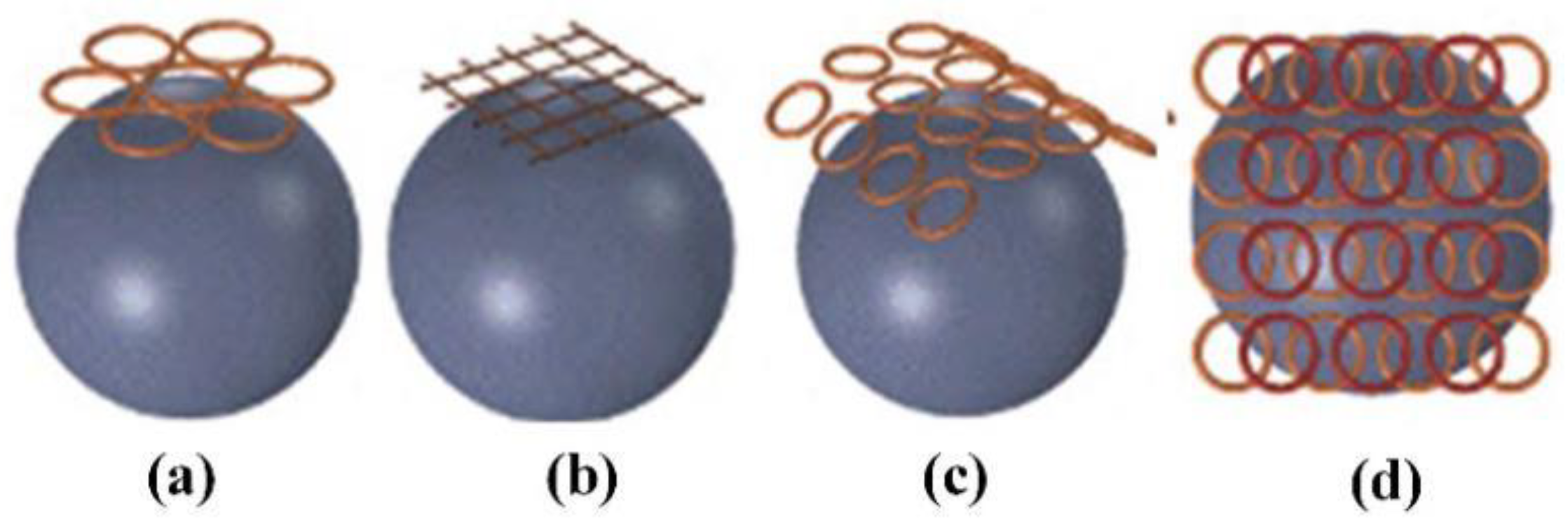
3.2.2. Transcranial Magnetic Stimulation (TMS)
- (1)
- Circular coil
- (2)
- Figure-of-Eight coil
- (3)
- H-shaped coil
- (4)
- Halo coil
3.3. Intermediate Frequency Electromagnetic Field (300 Hz–10 MHz)
3.3.1. Food Quality Inspection
3.3.2. Spatial Position Measurement
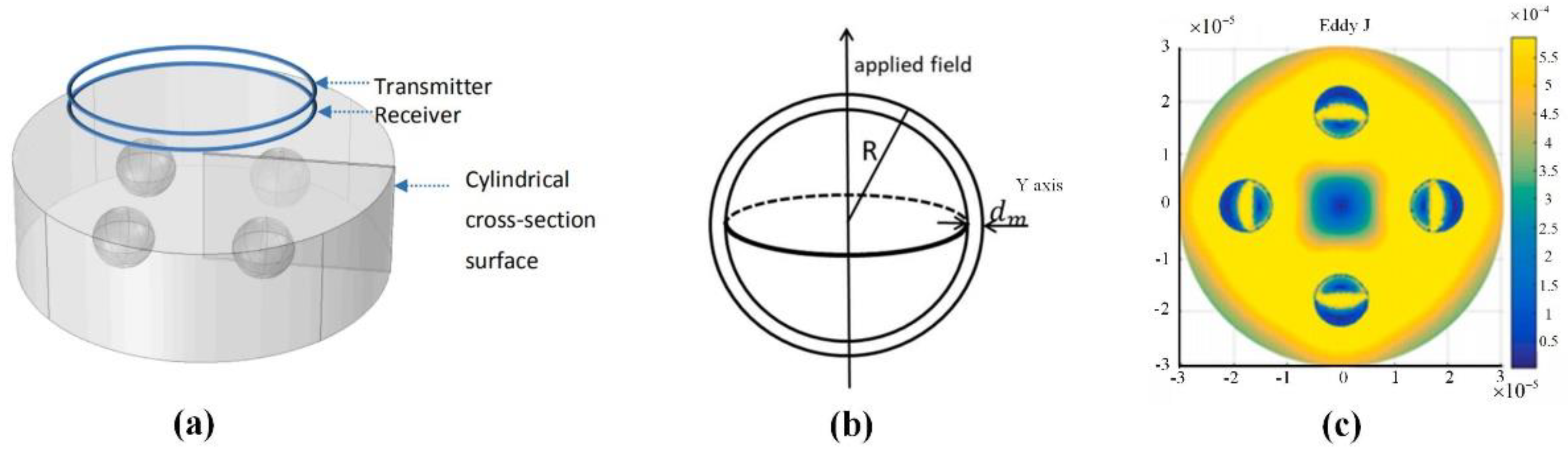
3.4. Radio Frequency Electromagnetic Field (10 MHz–300 GHz)
3.4.1. Disease Detection Based on MRI
3.4.2. Disease Detection Based on Microwave
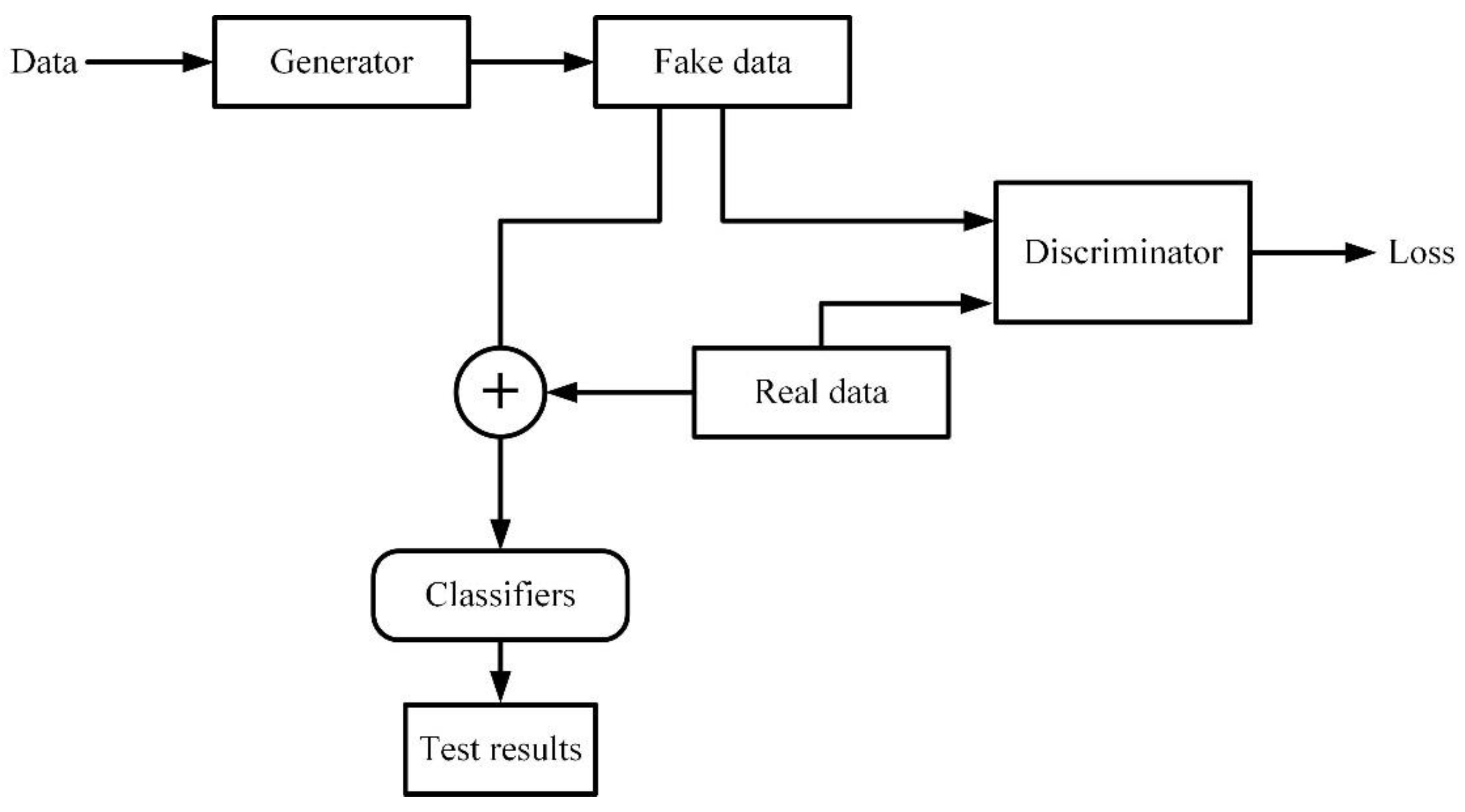
4. Application of Machine Learning Technology in Electromagnetic Medical Images
4.1. Application of Machine Learning in Microwave Breast Cancer Images
4.2. Application of Machine Learning in MRI Prostate Cancer Images
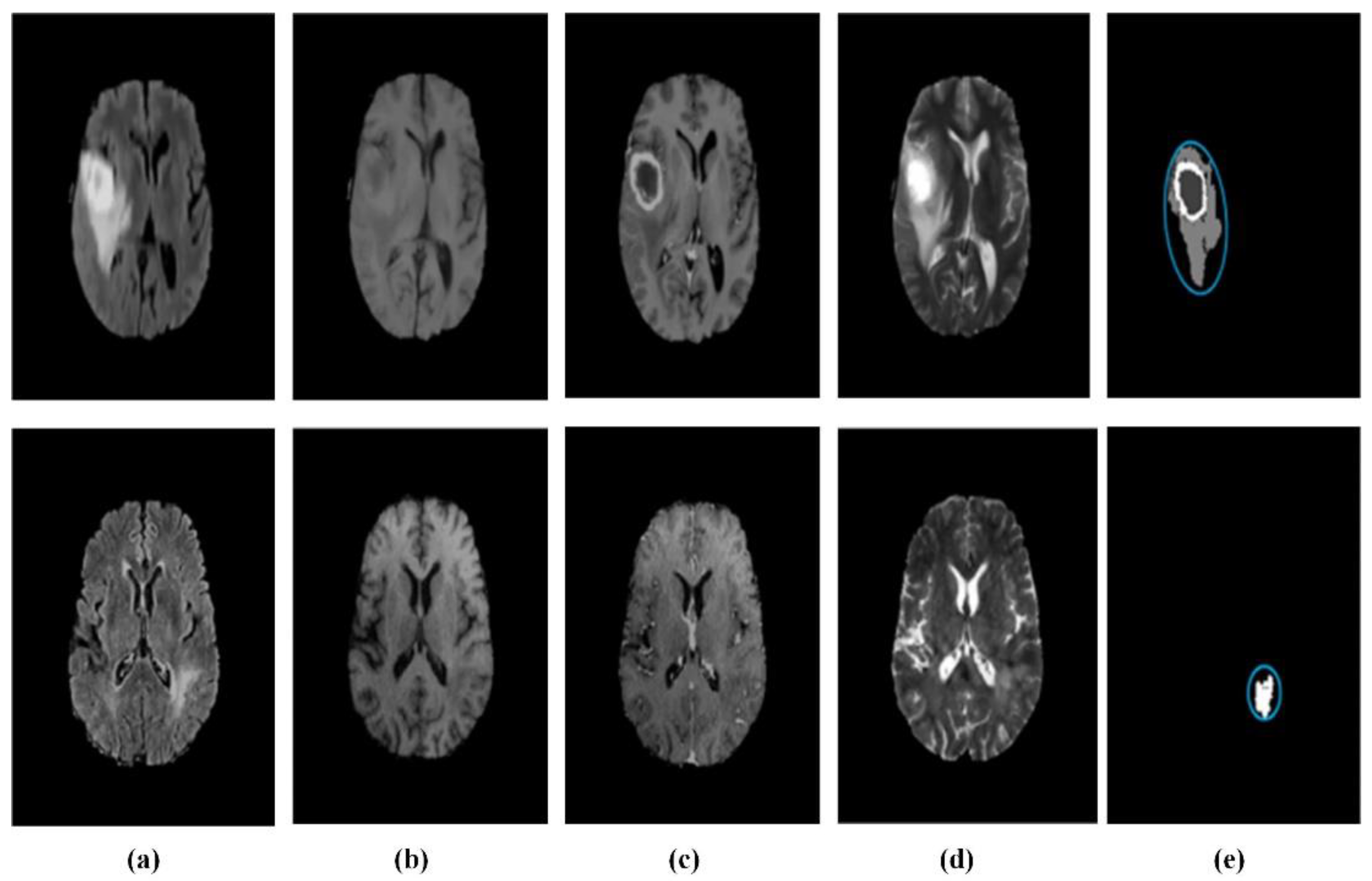
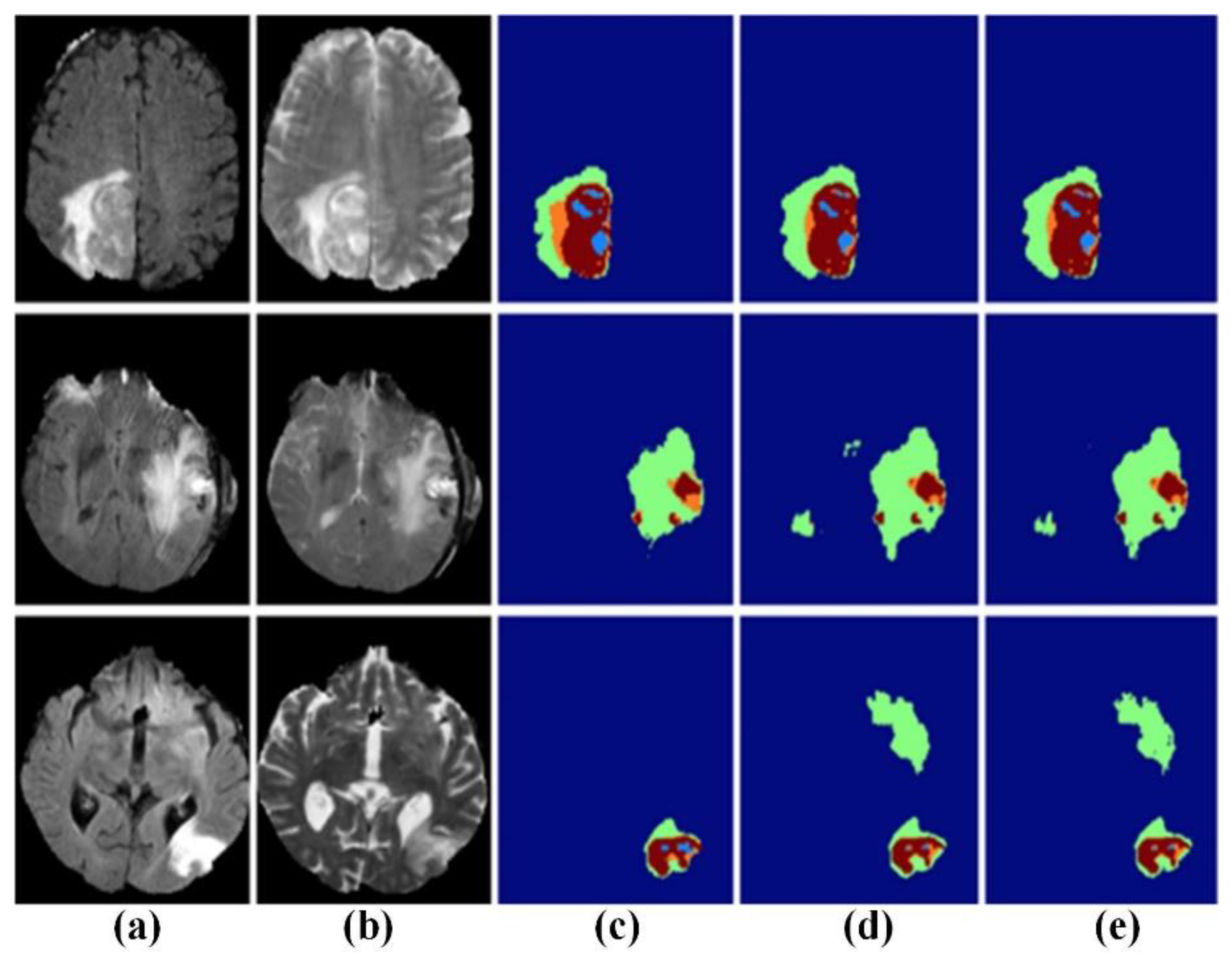
4.3. Application of Machine Learning in MRI Brain Tumor Segmentation Image
| Reference | Dataset | Evaluation Index DSC | ||
|---|---|---|---|---|
| Intact Tumor | Core Tumor | Enhance Tumor | ||
| [141] | BraTS 2013 | 0.88 | 0.83 | 0.77 |
| [35] | BraTS 2013 | 0.86 | 0.75 | 0.73 |
| [145] | BraTS 2014 | 0.83 | 0.75 | 0.77 |
| [146] | BraTS 2016 | 0.87 | 0.75 | 0.71 |
| [147] | BraTS 2017 | 0.89 | 0.76 | 0.81 |
5. Future Research Directions
- Integration and intelligentization
- 2.
- Multi-sensor fusion technology
- 3.
- Portability
- 4.
- Mechanism Research
- 5.
- Networked detection
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulazeez, A.T. Progress in utilisation of graphene for electrochemical biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar]
- Zhang, C.; Song, H.Z.; Guo, W.L.; Wu, H.; Xu, X.; Yan, S.C. Multi-index detection electrochemical biosensor based on graphene aerogel/platinum nanoparticle hybrid materials. J. Bionanosci. 2016, 10, 495–500. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lee, Y.C.; Lai, Y.H.; Lim, J.C.; Huang, N.T.; Lin, C.T.; Huang, J.J. Review of integrated optical biosensors for point-of-care applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Martynko, E.; Kirsanov, D. Application of chemometrics in biosensing: A brief review. Biosensors 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.C.; Comeau, L.A. Use of High-frequency noninvasive electromagnetic biosensors to detect ocean acidification effects on shellfish behavior. J. Shellfish Res. 2019, 38, 811–818. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, Y.; Lee, K.; Krishnan, V.; Pelled, G.; Gilad, A.A.; Choi, J. Regulation of electromagnetic perceptive gene using ferromagnetic particles for the external control of calcium ion transport. Biomolecules 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Edge, R.; Henbest, K.; Timmel, C.R.; Hore, P.J.; Gast, P. Magnetic field effect on singlet oxygen production in a biochemical system. Chem. Commun. 2005, 2, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, e1700068. [Google Scholar] [CrossRef]
- Wang, H.W.; Fu, Z.H.; Wang, Y.; Tai, H.M.; Chen, W.L. On-site calibration of air-coil sensor for transient electromagnetic exploration. Geophys. Prospect. 2019, 67, 1595–1610. [Google Scholar] [CrossRef]
- McCully, K.S. Environmental pollution, oxidative stress and thioretinaco ozonide: Effects of glyphosate, fluoride and electromagnetic fields on mitochondrial dysfunction in carcinogenesis, atherogenesis and aging. Ann. Clin. Lab. Sci. 2020, 50, 408–411. [Google Scholar] [PubMed]
- Wang, X. Study on biological effect protection of electromagnetic radiation shielding fabric. Adv. Mater. Res. 2011, 1445, 999–1002. [Google Scholar] [CrossRef]
- Kalantaryan, V.P.; Hakobyan, S.N.; Vardevanyan, P.O. Effect of weak electromagnetic waves on thermal properties of biomacromolecule water solutions. J. Contemp. Phys. 2018, 53, 175–178. [Google Scholar] [CrossRef]
- Vanderstraeten, J.; Verschaeve, L. Biological effects of radiofrequency fields: Testing a paradigm shift in dosimetry. Environ. Res. 2020, 184, 109387. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Meike, M. Manmade electromagnetic fields and oxidative stress—Biological effects and consequences for health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef]
- Ince-Yilmaz, O.; Erol, T.; Kara, K.; Dogan, M.; Erdem, U. Impacts of high voltage electric field (HVEF) applications on germination and seedling growth of seed (Tritcum aestivum L.) with analysis by fourier transform infrared (FTIR) Spectroscopy. Fresenius Environ. Bull. 2018, 27, 5153–5162. [Google Scholar]
- Li, D.P.; Jia, S.L.; Zhang, L.T.; Wang, Z.Y.; Pan, J.F.; Zhu, B.W.; Luo, Y.K. Effect of using a high voltage electrostatic field on microbial communities, degradation of adenosine triphosphate, and water loss when thawing lightly-salted, frozen common carp (Cyprinus carpio). J. Food Eng. 2017, 212, 226–233. [Google Scholar] [CrossRef]
- Han, Z.W.; Fu, J.; Fang, Y.Q.; Zhang, J.Q.; Niu, S.C.; Ren, L.Q. Anti-adhesive property of maize leaf surface related with temperature and humidity. J. Bionic Eng. 2017, 14, 540–548. [Google Scholar] [CrossRef]
- Sabouni, A.; Honrath, M.; Khamechi, M. Thermal effects in the brain during transcranial magnetic stimulation. IEEE Magn. Lett. 2017, 8, 1509603. [Google Scholar] [CrossRef]
- Van den Brink, J.S. Thermal effects associated with RF exposures in diagnostic MRI: Overview of existing and emerging concepts of protection. Concepts Magn. Reson. Part B 2019, 2019, 9618680. [Google Scholar] [CrossRef] [Green Version]
- Campi, T.; Cruciani, S.; De Santis, V.; Palandrani, F.; Maradei, F.; Feliziani, M. Induced effects in a pacemaker equipped with a wireless power transfer charging system. IEEE Trans. Magn. 2017, 53, 9401704. [Google Scholar] [CrossRef]
- Sehatbakhsh, S.; Kushnir, A.; Kabach, M.; Kolek, M.; Chait, R.; Ghumman, W. A case of electromagnetic interference between HeartMate 3 LVAD and implantable cardioverter defibrillator. Pace-Pacing Clin. Electrophysiol. 2018, 41, 218–220. [Google Scholar] [CrossRef]
- Berkelmann, L.; Bader, A.; Meshksar, S.; Dierks, A.; Majernik, G.H.; Krauss, J.K.; Schwabe, K.; Manteyffel, D.; Ngezahayo, A. Tumour-treating fields (TTFields): Investigations on the mechanism of action by electromagnetic exposure of cells in telophase/cytokinesis. Sci. Rep. 2019, 9, 7362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raco, V.; Bauer, R.; Norim, S.; Gharabaghi, A. Cumulative effects of single TMS pulses during beta-tACS are stimulation intensity-dependent. Brain Stimul. 2017, 10, 1055–1060. [Google Scholar] [CrossRef]
- Bilgin, H.M.; Celik, F.; Gem, M.; Akpolat, V.; Yildiz, I.; Ekinci, A.; Ozerdem, M.S.; Tunik, S. Effects of local vibration and pulsed electromagnetic field on bone fracture: A comparative study. Bioelectromagnetics 2017, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Israel, M.; Zaryabova, V.; Ivanova, M. Electromagnetic field occupational exposure: Non-thermal vs. thermal effects. Electromagn. Biol. Med. 2013, 32, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jukka, J.; Anne, H.; Timo, T.; Jonne, N. Review of possible modulation-dependent biological effects of radiofrequency fields. Bioelectromagnetics 2011, 32, 511–534. [Google Scholar]
- Goyal, R.; Vafai, K. Electromagnetic field-induced thermal management of biological materials. Numer. Heat Transf. Part A Appl. 2017, 72, 275–290. [Google Scholar] [CrossRef]
- Donald, I. Biological effects of microwave radiation. J. Air Pollut. Control Assoc. 2012, 24, 122–127. [Google Scholar]
- Frohlich, H. What are non-thermal electric biological effects. Bioelectromanetics 1982, 3, 45–46. [Google Scholar] [CrossRef]
- Geesink, J.H.; Meijer, D.K.F. Bio-solition model that predicts non-thermal electromagnetic frequency bands, that either stabilize or destabilize living cells. Electromagn. Biol. Med. 2017, 36, 357–378. [Google Scholar] [CrossRef]
- Lebedeva, N.N.; Sulimov, A.V.; Sulimova, O.P.; Kotrovskaya, T.I.; Gailus, T. Cellular phone electromagnetic field effects on bioelectric activity of human brain. Crit. Rev. Biomed. Eng. 2000, 28, 323–337. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Lokajícek, M.; Kozubek, S.; Prokĕs, K. A new concept of cumulative biological effect. Strahlentherapie 1981, 157, 41–45. [Google Scholar] [PubMed]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C.A. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans. Med. Imaging 2016, 35, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, B.; Zhang, W.J. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- He, X.L.; Liu, R.; Nirasawa, S.; Zheng, D.J.; Liu, H.J. Effect of high voltage electrostatic field treatment on thawing characteristics and post-thawing quality of frozen pork tenderloin meat. J. Food Eng. 2013, 115, 245–250. [Google Scholar] [CrossRef]
- Heieh, C.W.; Ko, W.C. Effect of high—Voltage electrostatic field on quality of carrot juice during refrigeration. LWT-Food Sci. Technol. 2008, 41, 1752–1757. [Google Scholar]
- Wu, Y.; Zhu, J.; Li, Y.; Li, M. Effect of high-voltage electrostatic field on inorganic nitrogen uptake by cucumber plants. Trans. Aasabe 2016, 59, 25–29. [Google Scholar]
- Krueger, A.P.; Kotaka, S.; Andriese, P.C. Some observations on the physiological effects of gaseous ions. Int. J. Biometeorol. 1962, 6, 33–48. [Google Scholar] [CrossRef]
- Palanimuthu, V.; Rajkumar, P.; Orsat, V.; Gariepy, Y.; Raghavan, G.S.V. Improving cranberry shelf-life using high voltage electric field treatment. J. Food Eng. 2009, 90, 365–371. [Google Scholar] [CrossRef]
- Sidaway, G. Influence of electrostatic fields on seed germination. Nature 1966, 211, 303. [Google Scholar] [CrossRef]
- Dhayal, M.; Lee, S.Y.; Park, S.U. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Huang, R.; Sukprakarn, S.; Phavaphutanon, L. A comparison of electric field treatments to hydropriming on cucumber seed germination enhancement. Nat. Sci. 2006, 40, 559–565. [Google Scholar]
- Li, M.Q.; Wu, Y.Y.; Zhang, M.M.; Zhu, J.Y. High-voltage electrostatic fields increase nitrogen uptake and improve growth of tomato seedlings. Can. J. Plant Sci. 2018, 98, 93–106. [Google Scholar] [CrossRef]
- Shao, C.Y.; Wang, D.C.; Tang, X.; Zhao, L.J.; Li, Y. Stimulating effects of magnetized arc plasma of different intensities on the germination of old spinach seeds. Math. Comput. Model. 2013, 58, 808–812. [Google Scholar] [CrossRef]
- Xu, W.Q.; Song, Z.Q.; Luan, X.Y.; Ding, C.J.; Cao, Z.Y.; Ma, X.H. Biological effects of high-voltage electric field treatment of naked oat seeds. Appl. Sci. 2019, 9, 3829. [Google Scholar] [CrossRef] [Green Version]
- Kiatgamjorn, P.; Khan-ngern, W.; Nitta, S. The comparison of electric field intensity affects to the bean sprouts growing. In Proceedings of the Asia-Pacific Conference on Environmental Electromagnetics, Hangzhou, China, 4–7 November 2003; pp. 142–147. [Google Scholar]
- Isobe, S.; Ishida, N.; Koizumi, M.; Kano, H.; Hazlewood, C.F. Effect of electric field on physical states of cell-associated water in germinating morning glory seeds observed by 1 H-NMR. Bichim. Biophys. Acta. Gen. Subj. 1999, 1426, 17–31. [Google Scholar] [CrossRef]
- Li, F.F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.W.; Xia, X.F.; Kong, B.H. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its structural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Cai, L.Y.; Cao, M.J.; Regenstein, J.; Cao, A.L. Recent advances in food thawing technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 953–970. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.W.; Lai, C.H.; Lee, C.H.; Ko, W.C. Effects of high-voltage electrostatic fields on the quality of tilapia meat during refrigeration. J. Food Sci. 2011, 76, 312–317. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Jia, G.L.; Tatsumi, E.; Liu, H.J. Effect of corona wind, current, electric field and energy consumption on the reduction of the thawing time during the high-voltage electrostatic-field (HVEF) treatment process. Innov. Food Sci. Emerg. Technol. 2016, 34, 135–140. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of combined high voltage electrostatic with air blast freezing on quality attributes of lamb meat. J. Food Process Eng. 2018, 41, e12811. [Google Scholar] [CrossRef]
- Li, D.P.; Jia, S.L.; Zhang, L.T.; Li, Q.Z.; Pan, J.F.; Zhu, B.W.; Prinyawiwatkul, W.; Luo, Y.K. Post-thawing quality changes of common carp (Cyprinus carpio) cubes treated by high voltage electrostatic field (HVEF) during chilled storage. Innov. Food Sci. Emerg. Technol. 2017, 42, 25–32. [Google Scholar] [CrossRef]
- Rahbari, M.; Hamdami, N.; Mirzaei, H.; Jafari, S.M. Investigation of the histological and textural properties of chicken breast thawed by high voltage electric field. J. Food Process Eng. 2020, 43, e13543. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Shafiekhani, S.; Mandal, R.; Singh, A.P.; Kenari, R.E. Effect of high voltage electrostatic field thawing on the functional and physicochemical properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2019, 56, 102191. [Google Scholar] [CrossRef]
- Mousakhani-Ganjeh, A.; Hamdami, N.; Soltanizadeh, N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares). J. Food Eng. 2015, 156, 39–44. [Google Scholar] [CrossRef]
- Jia, G.L.; Sha, K.; Feng, X.D.; Liu, H.J. Post-thawing metabolite profile and amino acid oxidation of thawed pork tenderloin by HVEF-A short communication. Food Chem. 2019, 291, 16–21. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Yin, J.J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Zhao, R.; Hao, J.; Xue, J.; Liu, H.; Li, L. Effect of high-voltage electrostatic pretreatment on the antioxidant system in stored green mature tomatoes. J. Sci. Food Agric. 2011, 91, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Kao, N.Y.; Tu, Y.F.; Sridhar, K.; Tsai, P.J. Effect of a high voltage electrostatic field (HVEF) on the shelf-life of fresh-cut broccoli (Brassica oleracea var. italica). LWT-Food Sci. Technol. 2019, 116, 108532. [Google Scholar] [CrossRef]
- Takaki, K.; Hayashi, N.; Wang, D.; Ohshima, T. High-voltage technologies for agriculture and food processing. J. Phys. D Appl. Phys. 2019, 52, 473001. [Google Scholar] [CrossRef]
- Ko, W.C.; Shi, H.Z.; Chang, C.K.; Huang, Y.H.; Chen, Y.A.; Hsieh, C.W. Effect of adjustable parallel high voltage on biochemical indicators and actomyosin Ca2+-ATPase from tilapia (Orechromis niloticus). LWT-Food Sci. Technol. 2016, 69, 417–423. [Google Scholar] [CrossRef]
- Christopher, L.; Alan, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by a alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.Z.; Du, L.L.; Mou, Y.B.; Xu, Z.J.; Weng, L.H.; Du, Y.W.; Zhu, Y.N.; Hou, Y.Y.; Wang, T.T. Effect of low frequency magnetic fields on melanoma: Tumor inhibition and immune modulation. BioMed Cent. 2013, 13, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Jang, Y.W.; Hyung, K.E.; Lee, D.K.; Hyun, K.H.; Jeong, S.H.; Min, K.H.; Kang, W.; Jeong, J.H.; Park, S.Y. Extremely Low-frequency electromagnetic field exposure enhances inflammatory response and inhibits effect of antioxidant in RAW 264.7 cells. Bioelectromagnetics 2017, 38, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Simko, M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 2007, 14, 1141–1152. [Google Scholar] [CrossRef]
- Wang, T.T.; Nie, Y.Z.; Zhao, S.L.; Han, Y.; Du, Y.W.; Hou, Y.Y. Involvement of midkine expression in the inhibitory effects of low-frequency magnetic fields on cancer cells. Bioelectromagnetics 2011, 32, 443–452. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Xu, Q.Y.; Shi, G.P.; Li, X.J.; Li, X.J.; Ji, J.J.; Zhang, D.Y.; Wang, Y.P.; Wang, T.T.; et al. LF-MF inhibits iron metabolism and suppresses lung cancer through activation of P53-miR-34a-E2F1/E2F3 pathway. Sci. Rep. 2017, 7, 749. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xue, Y.; Zhang, Y. Effects of 0.4 T rotating magnetic field exposure on density, strength, calcium and metabolism of rat thigh bones. Bioelectromagnetics 2006, 27, 1–9. [Google Scholar] [CrossRef]
- Du, S.H.; Li, J.X.; Du, C.H.; Huang, Z.M.; Chen, G.N.; Yan, W.Q. Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget 2017, 8, 9410–9424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Muroski, M.E.; Peteit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.L. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Sisken, B.F.; Kanje, M.; Lundborg, G.; Herbst, E.; Kurtz, W. Stimulation of rat sciatic nerve regeneration with pulsed electromagnetic fields. Brain Res. 1989, 485, 309–316. [Google Scholar] [CrossRef]
- Sunkari, V.G.; Aranovitch, B.; Portwood, N.; Nikoshkov, A. Effects of a low-intensity electromagnetic field on fibroblast migration and proliferation. Electromagn. Biol. Med. 2011, 30, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhang, X.; Xu, Z.J.; He, R.X. Effects of low-intensity electromagnetic fields on the proliferation and differentiation of cultured mouse bone marrow stromal cells. Phys. Ther. 2012, 92, 1208–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, P.; Soejima, K.; Ito, G. Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide 2002, 7, 18–23. [Google Scholar] [CrossRef]
- Liu, H.X.; Lees, P.; Abbott, J.; Bee, J.A. Pulsed electromagnetic fields preserve proteoglycan composition of extracellular matrix in embryonic chick sternal cartilage. Bichim. Biophys. Acta Gen. Subj. 1997, 1336, 303–314. [Google Scholar] [CrossRef]
- Rastogi, P.; Lee, E.G.; Hadimani, R.L.; Jiles, D.C. Transcranial magnetic stimulation: Development of a novel deep-brain triple-Halo Coil. IEEE Magn. Lett. 2019, 10, 3102205. [Google Scholar] [CrossRef]
- George, M.S.; Taylor, J.J.; Short, E.B. The expanding evidence base for rTMS treatment of depression. Curr. Opin. Psychiatry 2013, 26, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.S.; Aiken, E.E.; Kelley, M.E.; Burch, W.; Waterman, L.; Holtzheimer, P.E. Synchronized transcranial magnetic stimulation for posttraumatic stress disorder and comorbid major depression. Brain Stimul. 2019, 12, 1335–1337. [Google Scholar] [CrossRef]
- Jiang, C.G.; Zhang, T.; Yue, F.G.; Yi, M.L.; Gao, D. Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biochem. Biophys. 2013, 67, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.; Kalender, G.; O’neill, J. Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J. Psychiatry Neurosci. JPN 2019, 44, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblhuber, F.; Sreiner, K.; Fuchs, D. Treatment of patients with geriatric depression with repetitive transcranial magnetic stimulation. J. Neural Transm. 2019, 126, 1105–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.D.; Lisanby, S.H.; Peterchev, A.V. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zucca, M.; Bottauscio, O.; Chiampi, M.; Zilberti, L. Operator safety and field focality in aluminum shielded transcranial magnetic stimulation. IEEE Trans. Magn. 2017, 53, 5100704. [Google Scholar] [CrossRef]
- Colak-Atmaca, M.; Ciftci-Kavalioglu, B.; Aldan, M.A.; Argun, T.; Atmaca, M.M.; Gul, G.; Soysal, A. Investigation of the effects of continuous theta burst transcranial magnetic stimulation in patients with migraine. Neurol. Sci. Neurophysiol. 2018, 35, 177–182. [Google Scholar] [CrossRef]
- Shimizu, T.; Hosomi, K.; Maruo, T.; Goto, Y.; Yokoe, M.; Kageyama, Y.; Shimokawa, T.; Yoshimine, T.; Saitoh, Y. Efficacy of deep rTMS for neuropathic pain in the lower limbs: A randomized, double-blind crossover trial of an H-coil and figure-8 coil. J. Neurosurg. 2017, 127, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.P.; Wang, L. Research progress of transcranial magnetic for posttraumatic stress disorder. Prog. Biochem. Biophys. 2020, 47, 319–334. [Google Scholar]
- Lu, M.; Ueno, S. Computational study toward deep transcranial magnetic stimulation using coaxial circular coils. IEEE Trans. BioMed Eng. 2015, 62, 2911–2919. [Google Scholar] [CrossRef]
- Samoudi, A.M.; Emmeric, T.; Luc, M.; Wout, J.; Toshiyuki, S. Deep transcranial magnetic stimulation: Improved coil design and assessment of the induced fields using MIDA model. BioMed Res. Int. 2018, 2018, 7061420. [Google Scholar] [CrossRef]
- Wei, X.L.; Li, Y.; Lu, M.L.; Wang, J.; Yi, G.S. Comprehensive survey on improved focality and penetration depth of transcranial magnetic stimulation employing multi-coil arrays. Int. J. Environ. Res. Public Health 2017, 14, 1388. [Google Scholar] [CrossRef] [Green Version]
- Li, J.T.; Cao, H.; Zhao, Z.; Zheng, M.J.; Liu, Y.H.; He, J.X.; Sun, Y.; Rem, Z.Y. A study on the multi-channel TMS device. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Sun, Z.B.; Liang, L.M.; Li, J.K.; Liu, X.Y.; Sun, J.; Zou, X.B.; Zou, M.; Guo, Z.M. Rapid detection of Atlantic salmon multi-quality based on impedance properties. Food Sci. Nutr. 2020, 8, 862–869. [Google Scholar] [CrossRef]
- Schwan, A.; Herman, P. Electrical properties of tissue and cell suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar] [PubMed]
- Aalhus, J.L.; Larsen, I.L.; Dubeski, P.L.; Jeremiah, L.E. Improved beef tenderness using a modified on-line carcass suspension method with or without low voltage electrical stimulation. Can. J. Anim. Sci. 2000, 80, 51–58. [Google Scholar] [CrossRef]
- Swatland, H.J. Observations on rheological, electrical, and optical changes during rigor development in pork and beef. J. Anim. Sci. 1997, 75, 975–985. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.D.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance and circuit parameters: An analysis for tracking fruit ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Castro-Giraldez, M.; Botella, P.; Toldra, F.; Fito, P. Low-frequency dielectric spectrum to determine pork meat quality. Innov. Food Sci. Emerg. Technol. 2010, 11, 376–386. [Google Scholar] [CrossRef]
- Kim, J.; Abbasi, M.A.; Kim, T.; Park, K.D.; Cho, S. Lock-in amplifier-based impedance detection of tissue type using a monopolar injection needle. Sensors 2019, 19, 4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.W.; Yin, W.L.; Lu, M.Y. Bio-impedance spectroscopy for frozen-thaw of bio-samples: Non-contact inductive measurement and finite element (FE) based cell modelling. J. Food Eng. 2020, 272, 109784. [Google Scholar] [CrossRef]
- Tang, J.W.; Lu, M.Y.; Xie, Y.D.; Yin, W.L. A Novel Efficient FEM Thin shell model for bio-impedance analysis. Biosensors 2020, 10, 69. [Google Scholar] [CrossRef]
- Yin, W.L.; Tang, J.W.; Lu, M.Y.; Xu, H.Y.; Huang, R.C.; Zhao, Q.; Zhang, Z.J.; Peyton, A. An equivalent-effect phenomenon in eddy current non-destructive testing of thin structures. IEEE Access 2019, 7, 70296–70307. [Google Scholar] [CrossRef]
- Li, M.F.; Hansen, C.; Rose, G. A simulator for advanced analysis of a 5-DOF EM tracking systems in use for image-guided surgery. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2217–2229. [Google Scholar] [CrossRef]
- Kim, W.; Song, J.; Park, F.C. Closed-form position and orientation estimation for a three-axis electromagnetic tracking system. IEEE Trans. Ind. Electron. 2018, 65, 4331–4337. [Google Scholar] [CrossRef]
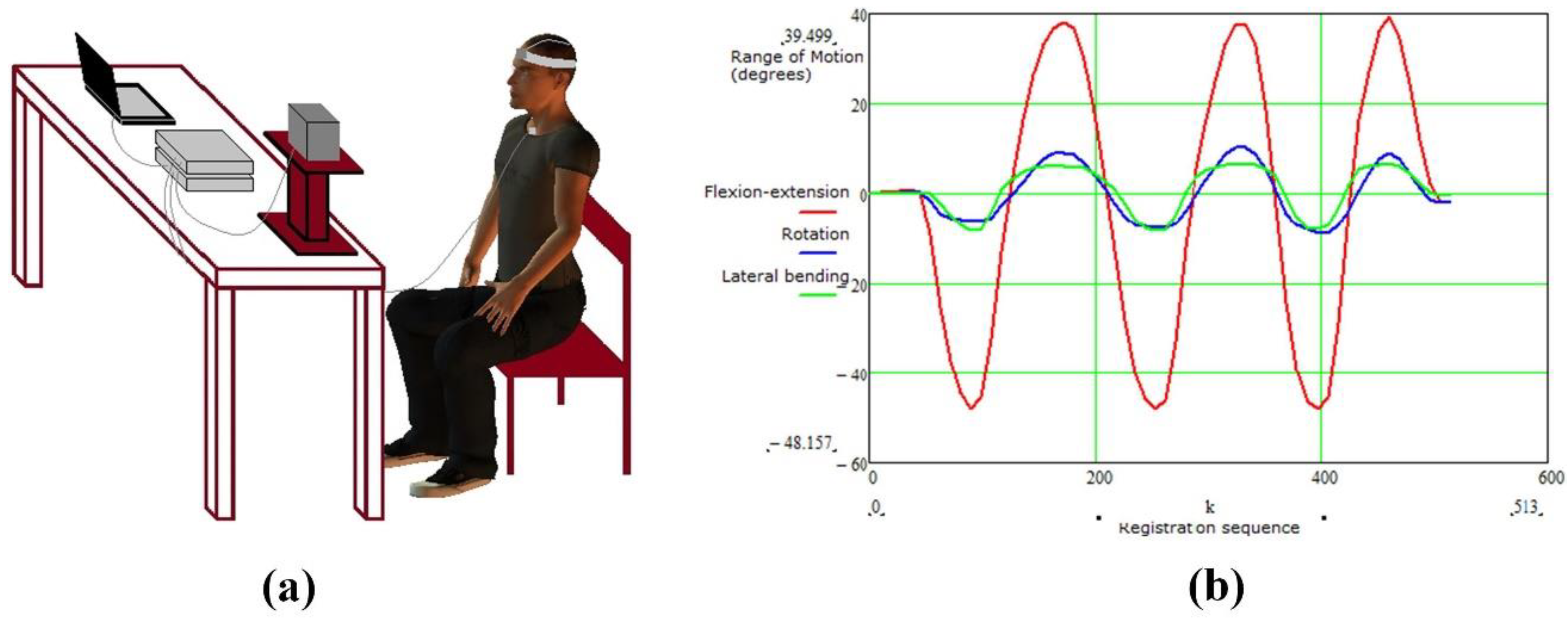
- Lemmers, G.P.G.; Heijmans, M.W.M.; Scafoglieri, A.; Buyl, R.; Staal, J.B.; Schmitt, M.A.; Cattrysse, E. Three-dimensional kinematics of the cervical spine using an electromagnetic tracking device. Differences between healthy subjects and subjects with nonspecific neck pain and the effect of age. Clin. Biomech. 2018, 54, 111–117. [Google Scholar] [CrossRef] [PubMed]
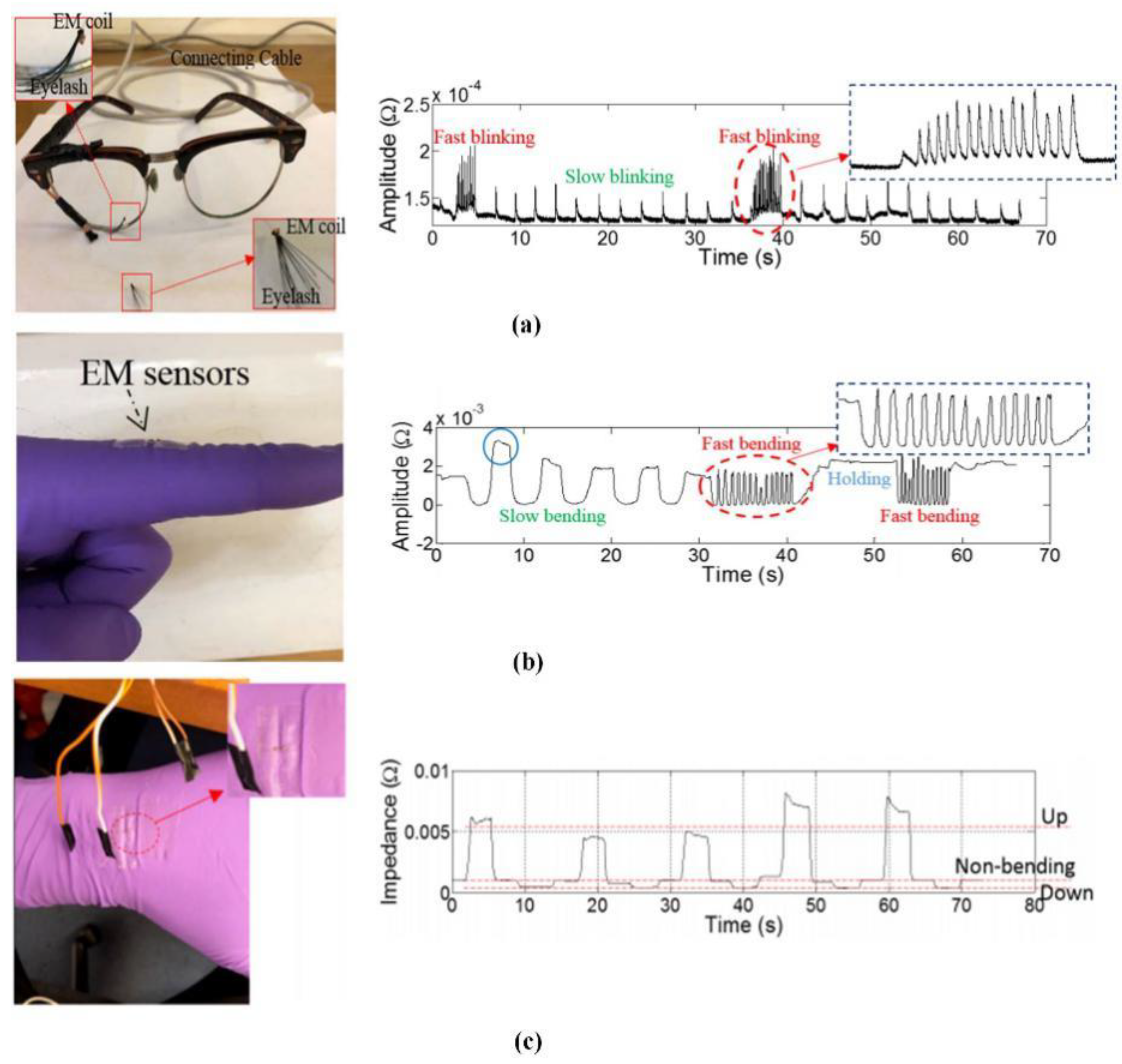
- Xie, Y.D.; Lu, M.Y.; Yin, W.L.; Xu, H.Y.; Zhu, S.; Tang, J.W.; Chen, L.M.; Ran, Q.Y.; Zhang, Y.N.; Qu, Z.G. Novel wearable sensors for biomechanical movement monitoring based on electromagnetic sensing techniques. IEEE Sens. J. 2020, 20, 1019–1027. [Google Scholar] [CrossRef]
- Robinson, D.A. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans. Biomed. Eng. 1963, 10, 137–145. [Google Scholar] [PubMed]
- Remmel, R.S. An inexpensive eye movement monitor using the scleral search coil technique. IEEE Trans. Biomed. Eng. 1984, 31, 388–390. [Google Scholar] [CrossRef]
- Remmel, R.S. Use of an electromagnetic eye movement monitor for easy measurement of arm movements. IEEE Trans. Biomed. Eng. 2006, 53, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, T.G.; Dobson, J. Theoretical evaluation of cell membrane ion channel activation by applied magnetic fields. Eur. Biophys. J. 2000, 29, 455–456. [Google Scholar] [CrossRef]
- Ito, D.; Numano, T.; Mizuhara, K.; Takamoto, K.; Onishi, T.; Nishijo, H. A new technique for MR elastography of the supraspinatus muscle: A gradient-echo type multi-echo sequence. Magn. Reson. Imaging 2016, 34, 1181–1188. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.L.; Huang, L.; Bian, S.T. Recent progress in wearable biosensors: From healthcare monitoring to sports analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
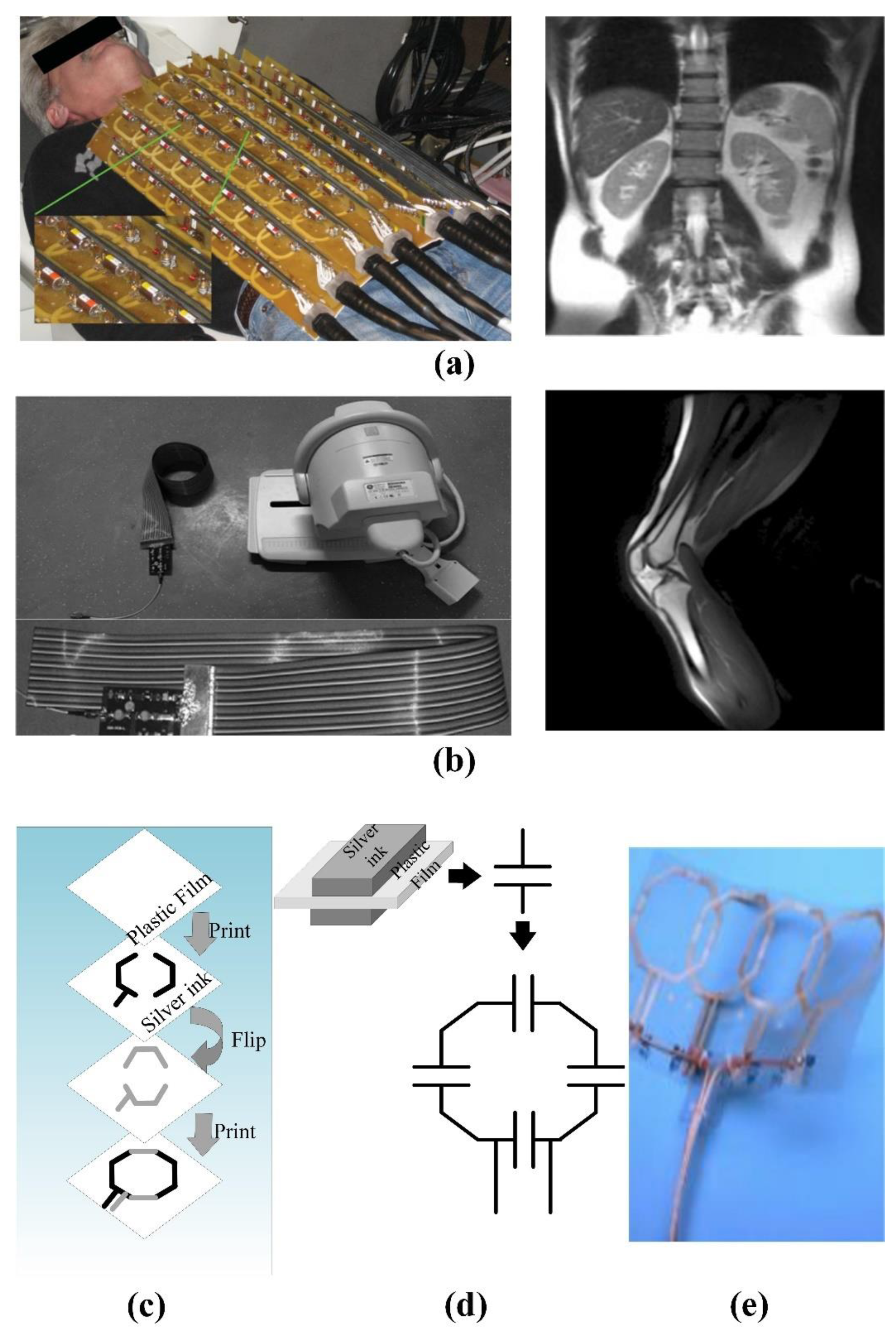
- He, X.W.; Yuan, R.B.; Li, B.K.; Hou, Y.Q. The design of an open MRI 4-channel receive-only phased array knee coil. Appl. Magn. Reson. 2016, 47, 499–510. [Google Scholar] [CrossRef]
- Fujita, H.; Zheng, T.H.; Yang, X.Y.; Finnerty, M.J.; Handa, S. RF surface receive array coils: The art of an LC circuit. J. Magn. Reson. Imageing 2013, 38, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.J.; Giaquinto, R.O.; Piel, J.E.; Rohling, K.W.; Marinelli, L.; Blezek, D.J.; Fiveland, E.W.; Darrow, R.D.; Foo, T.K.F. 128-channel body MRI with a flexible high-density receiver-coil array. J. Mgan. Reson. Imaging 2008, 28, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Nordmeter-Massner, J.A.; De Zanche, N.; Pruessmann, K.P. Mechanically adjustable coil array for wrist MRI. Magn. Reson. Med. 2009, 61, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Nordmeter-Massner, J.A.; De Zanche, N.; Pruessmann, K.P. Stretchable coil arrays: Application to knee imaging under varying flexion angles. Magn. Reson. Med. 2012, 67, 872–879. [Google Scholar] [CrossRef]
- Jia, F.; Yuan, H.Y.; Zhou, D.G.; Zhang, J.; Wang, X.Y.; Fang, J. Knee MRI under varying flexion angles utilizing a flexible flat cable antenna. NMR Biomed. 2015, 28, 460–467. [Google Scholar] [CrossRef]
- Corea, J.R.; Flynn, A.M.; Lechene, B.; Scott, G.; Reed, G.D.; Shin, P.J.; Lustig, M.; Arias, A.C. Screen-printed flexible MRI receive coils. Nat. Commun. 2016, 7, 10839. [Google Scholar] [CrossRef] [Green Version]
- Zidane, M.A.; Amar, H.; Rouane, A. Study of two constraints impacting measurements of human glycemia using a microwave sensor. Biosensors 2021, 11, 83. [Google Scholar] [CrossRef]
- Wang, L.L. Microwave sensors for breast cancer detection. Sensors 2018, 18, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassi, M.; Caruso, M.; Khan, M.S.; Bevilacqua, A.; Capobianco, A.D.; Neviani, A. An integrated microwave imaging radar with planar antennas for breast cancer detection. IEEE Trans. Microw. Theory 2013, 61, 2108–2118. [Google Scholar] [CrossRef]
- Yang, Z.K.; Cheng, C.C.; Wang, Z.B.; Yang, H.W. Detection of breast cancer using ultra-wide band beamforming algorithm. Mod. Phys. Lett. B 2017, 31, 1750091. [Google Scholar] [CrossRef]
- Rappaport, C. A dispersive microwave model for human breast tissue suitable for FDTD computation. IEEE Antennas Wirel. Propag. Lett. 2007, 6, 179–181. [Google Scholar] [CrossRef]
- Singer, C.F.; Tan, Y.Y.; Muhr, D.; Rappaport, C.; Gschwantler-Kaulich, D.; Grimm, C.; Polterauer, S.; Pfeiler, G.; Berger, A.; Tea, M.K.M. Association between family history, mutation locations, and prevalence of BRCA1 or 2 mutations in ovarian cancer patients. Cancer Med. 2019, 8, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, B.; Abbosh, A.; Ireland, D. Circular antenna array for brain imaging systems. In Proceedings of the IEEE International Symposium on Antennas and Propagation, Chicago, IL, USA, 8–14 July 2012; pp. 1125–1126. [Google Scholar]
- Ireland, D.; Bialkowski, K.; Abbosh, A. Microwave imaging for brain stroke detection using Born iterative method. IET Microw. Antennas Propag. 2013, 7, 909–915. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.R.; Zhang, B.; Lei, M.Y.; Cui, W.G.; Guo, Y.Z. A channel-projection mixed-scale convolutional neural network for motor imagery EEG decoding. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1170–1180. [Google Scholar] [CrossRef]
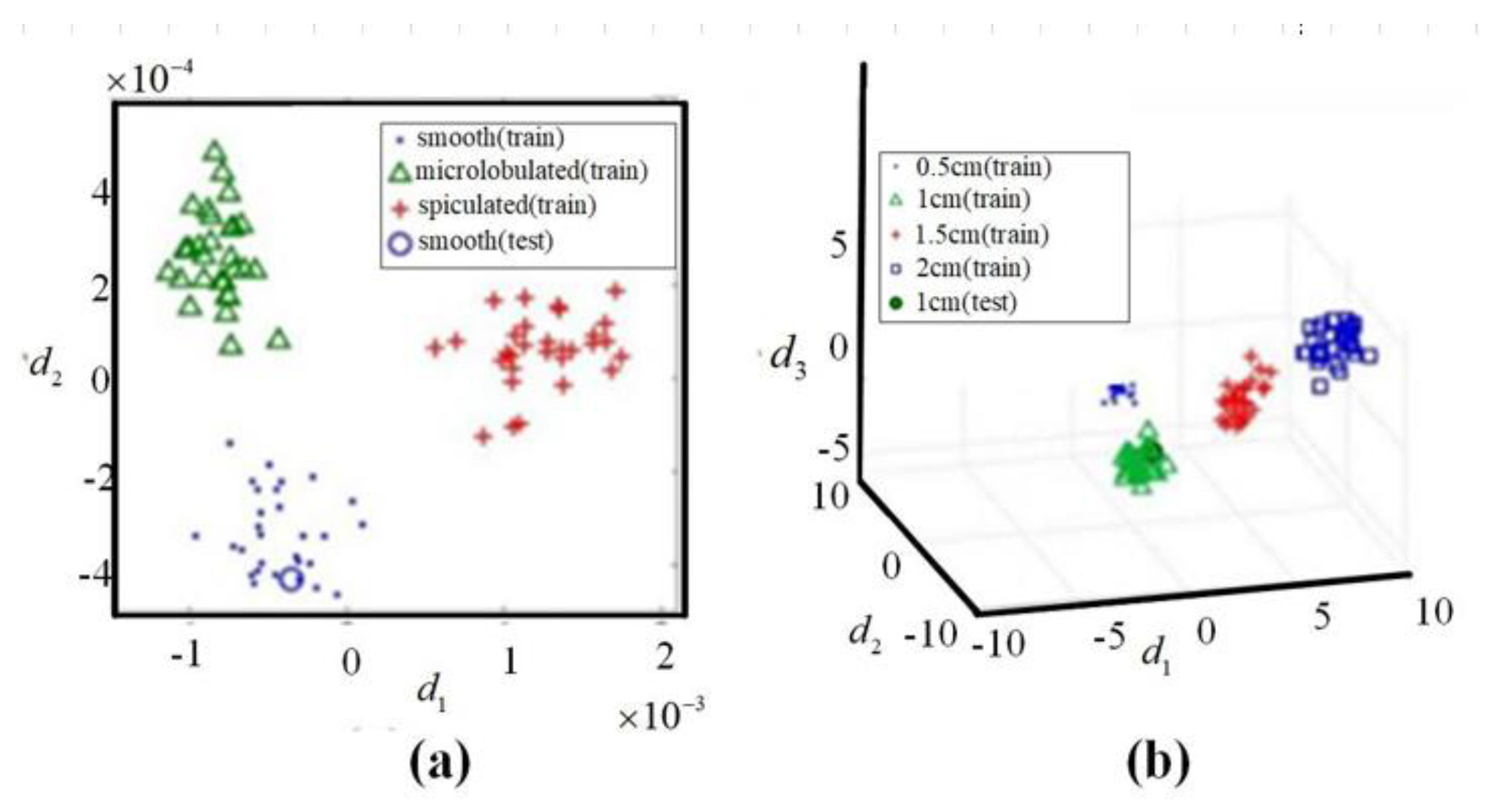
- Song, H.C.; Li, Y.P.; Men, A.C. Microwave breast cancer detection using time-frequency representations. Med Biol. Eng. Comput. J. Int. Fed. Med Biol. Eng. 2018, 56, 571–582. [Google Scholar] [CrossRef]
- Davis, S.K.; Van Veen, B.D.; Hagness, S.C.; Kelcz, F. Breast tumor characterization based on ultrawideband microwave backscatter. IEEE Trans. Biomed. Eng. 2008, 55, 237–246. [Google Scholar] [CrossRef]
- Conceicao, R.C.; Mediros, H.; O’Halloran, M.; Rodriguez-Herrera, D.; Flores-Tapia, D.; Pistorius, S. SVM-based classification of breast tumour phantoms using a UWB radar prototype system. In Proceedings of the URSI General Aassembly and Scientific Symposium, Beijing, China, 16–23 August 2014. [Google Scholar]
- Alshehri, S.A.; Khatun, S.; Jantan, A.B.; Abdullah, R.S.A.R.; Mahmood, R.; Awang, Z. Experimental breast tumor detection using NN-based UWB imaging. Prog. Electromagn. Res. PIER 2011, 111, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.; Ohalloran, M.; Jones, E.; Glavin, M. Support Vector Machine-Based Ultrawideband Breast Cancer Detection System. J. Electromagn. Waves Appl. 2011, 25, 1807–1816. [Google Scholar] [CrossRef]
- Santorelli, A.; Porter, E.; Kirshin, E.; Liu, Y.J.; Popovic, M. Investigation of classifiers for tumor detection with an experimental time-domain breast screening system. Prog. Electromagn. Res. PIER 2014, 144, 45–47. [Google Scholar] [CrossRef] [Green Version]
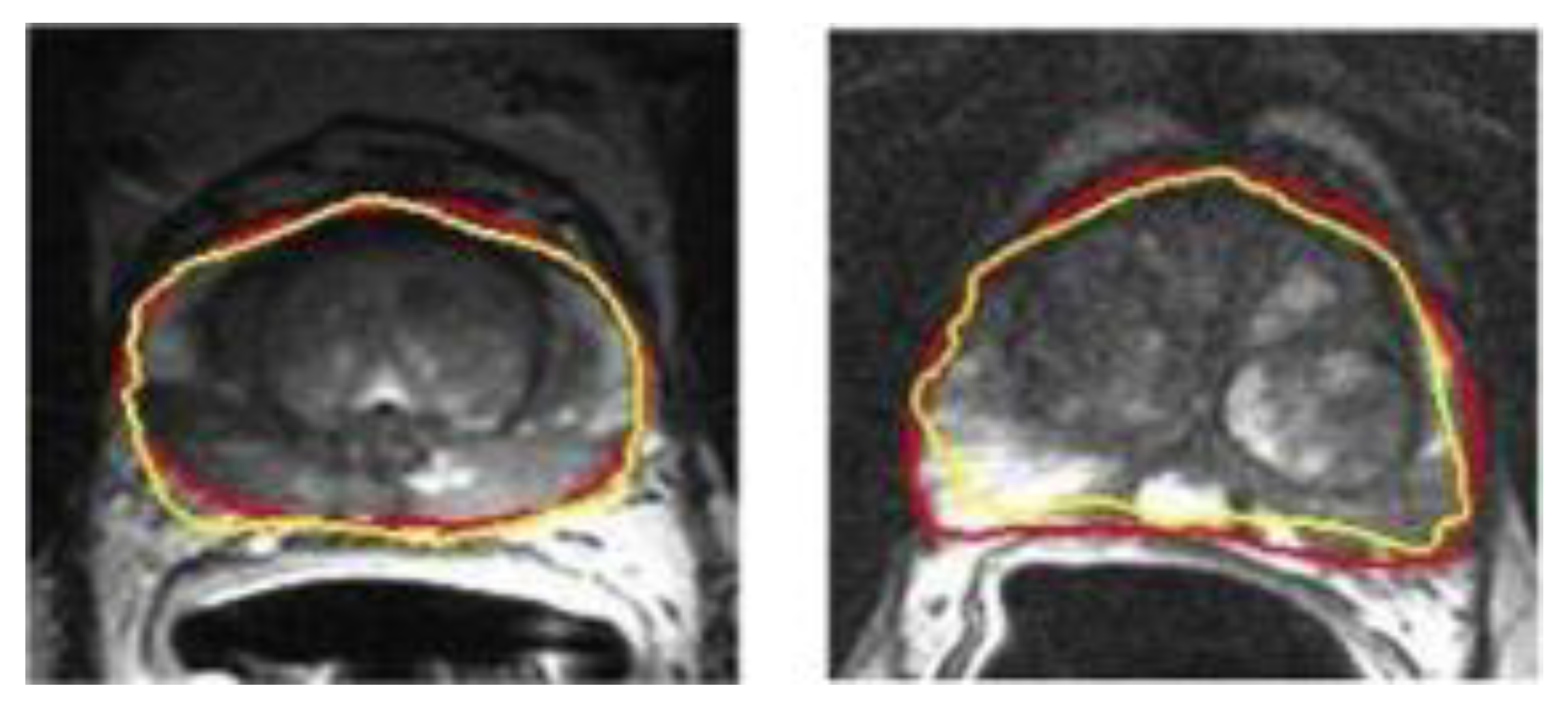
- Sun, Y.; Reynolds, H.M.; Parameswaran, B.; Wraith, D.; Finnegan, M.; Williams, S.; Haworth, A. Multiparametric MRI and radiomics in prostate cancer: A review. Australas. Phys. Eng. Sci. Med. 2019, 42, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, X.Y.; Kim, J.; Khadra, M.; Fulham, M.; Feng, D.G. A propagation-DNN: Deep combination learning of multi-level features for MR prostate segmentation. Comput. Methods Programs Biomed. 2019, 170, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Alkadi, R.; Taher, F.; El-baz, A.; Werghi, N. A deep learning-based approach for the detection and localization of prostate cancer in T2 magnetic resonance images. J. Digit. Imaging 2019, 32, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Mcgarry, S.; Hurrell, S.L.; Iczkowksi, K.A.; Hall, W.; Kaczmarowski, A.L.; Banerjee, A.; Keuter, T.; Jacobsohn, K.; Bukowy, J.D.; Nevalainen, M.T.; et al. Radio-pathomic maps of epithelium and lumen density predict the location of high-grade prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Esteva, A.; Kuprel, B.; Novoa, R.; Ko, J.; Swetter, S.; Blau, H.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
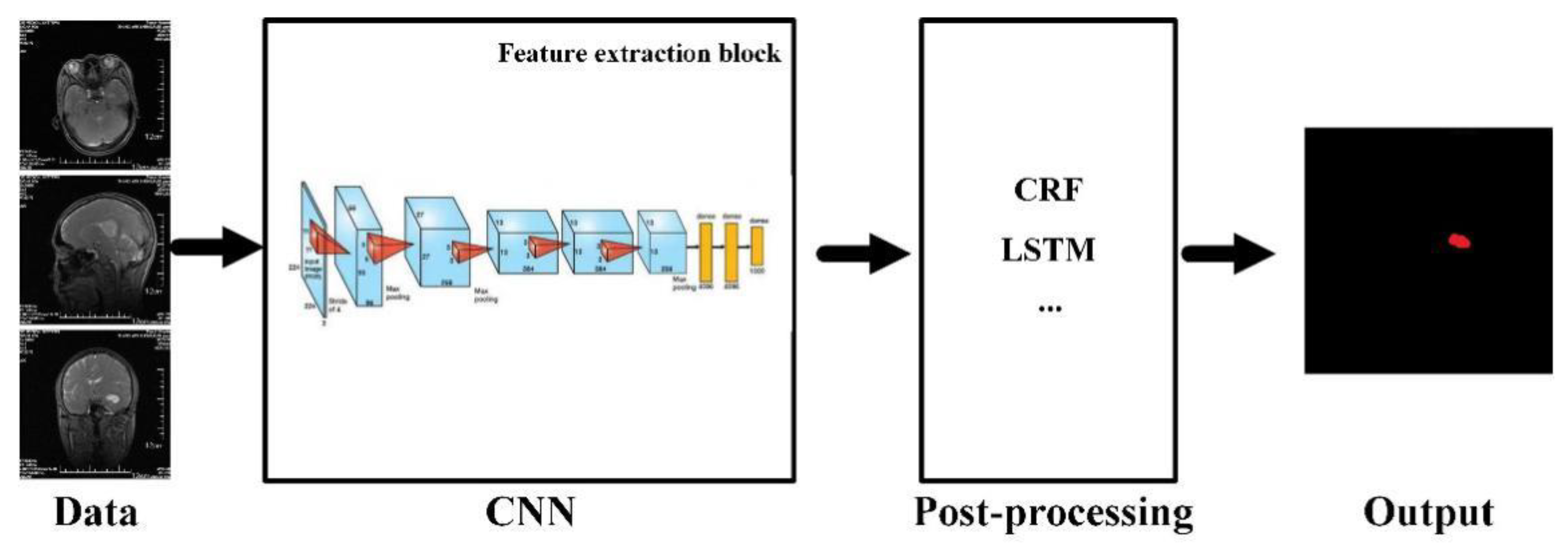
- Xue, Y.; Xu, T.; Zhang, H.; Long, L.R.; Huang, X.L. Segan: Adversarial network with multi-scale L1 loss for medical image segmentation. Neuroinformatics 2018, 16, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dou, Q.; Yu, L.Q.; Qin, J.; Heng, P.A. VoxRes Net: Deep voxelwise residual networks for brain segmentation from 3D MR images. Neuroimage 2018, 170, 446–455. [Google Scholar] [CrossRef]
- Zikic, D.; Ioannou, Y.; Brown, M.; Criminisi, A. Segmentation of brain tumor tissues with convolutional neural networks. In Proceedings of the MICCAI-BRATS, Boston, MA, USA, 9 September 2014; pp. 36–39. [Google Scholar]
- Dvorak, P.; Menze, B. Local structure prediction with convolutional neural networks for multimodal brain tumor segmentation. In Proceedings of the International MICCAI Workshop on Medical Computer Vision, Munich, Germany, 9 October 2016; Springer: Berlin/Heidelberg, Germany, 2015; pp. 59–71. [Google Scholar]
- Randhawa, R.S.; Modi, A.; Jain, P.; Warier, P. Improving boundary classification for brain tumor segmentation and longitudinal disease progression. In Proceedings of the 2nd International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Athens, Greece, 9 October 2016; pp. 65–74. [Google Scholar]
- Ben Naceur, M.; Saouli, R.; Akil, M.; Kachouri, R. Fully automatic brain tumor segmentation using end-to-end incremental deep neural networks in MRI images. Comput. Methods Programs Biomed. 2018, 166, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.P.; Yang, J.; Qiao, Y. Brain MRI segmentation with patch-based CNN approach. In Proceedings of the 35th Chinese Control Conference, Chengdu, China, 27–29 July 2016; pp. 7026–7031. [Google Scholar]
- Kamnitsas, K.; Ferrante, E.; Parisot, S.; Ledig, C.; Nori, A.V.; Criminisi, A.; Rueckert, D.; Glocker, B. DeepMedic for brain tumor segmentation. In Proceedings of the 2nd International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Athens, Greece, 9 October 2016; pp. 138–149. [Google Scholar]
- Casamitjana, A.; Puch, S.; Aduriz, A.; Vilaplana, V. 3D convolutional neural networks for brain tumor segmentation: A comparison of multi-resolution architectures. In Proceedings of the 2nd International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Athens, Greece, 9 October 2016; pp. 150–161. [Google Scholar]
- Kamnitsas, K.; Ledig, C.; Newcombe, V.F.J.; Simpson, J.P.; Kane, A.D.; Menon, D.K.; Rueckert, D.; Glocker, B. Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med. Image Anal. 2017, 36, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wu, Y.; Dsouza, A.M.; Abidin, A.Z.; Wismüller, A.; Xu, C.L. MRI tumor segmentation with densely connected 3D CNN. In Proceedings of the Medical Imaging 2018: Image Processing, Houston, TX, USA, 11–13 February 2018. [Google Scholar]
- Havaei, M.; Davy, A.; Warde-Farley, D.; Biard, A.; Courville, A.; Bengio, Y.; Pal, C.; Jodoin, P.M.; Larochelle, H. Brain tumor segmentation with deep neural networks. Med. Image Anal. 2017, 35, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Anwar, S.M.; Majid, M. Brain tumor segmentation using cascaded deep convolutional neural network. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Seogwipo, Korea, 11–15 July 2017. [Google Scholar]
- Gao, X.; Deng, F.; Yue, X.H. Data augmentation in fault diagnosis based on the Wasserstein generative adversarial network with gradient penalty. Neurocomputing 2020, 396, 487–494. [Google Scholar] [CrossRef]
- Moreno-Barea, F.J.; Jerez, J.M.; Franco, L. Improving classification accuracy using data augmentation on small data sets. Expert Syst. Appl. 2020, 161, 113696. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.Y.; Yu, J.H. Brain tumor segmentation using an adversarial network. In Proceedings of the 3rd International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Quebec City, QC, Canada, 9 September 2018; pp. 123–132. [Google Scholar]
- Rezaei, M.; Harmuth, K.; Gierke, W.; Kellermeier, T.; Fischer, M.; Yang, H.; Meinel, C. A conditional adversarial network for semantic segmentation of brain tumor. In Proceedings of the 3rd International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic, Quebec City, QC, Canada, 9 September 2018; pp. 241–252. [Google Scholar]
- Rezaei, M.; Yang, H.J.; Meinel, C. Voxel-GAN: Adversarial framework for learning imbalanced brain tumor segmentation. In Proceedings of the 4th International Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Granada, Spain, 16 September 2018. [Google Scholar]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent advances in MXene nanocomposite-based biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent progress in manufacturing techniques of printed and flexible sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Song, J.S.; Zhang, J. Integration of nanomaterials and bioluminescence resonance energy transfer techniques for sensing biomolecules. Biosensors 2019, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L. Early diagnosis of breast cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [PubMed]
- Karamfard, S.S.; Asl, B.M. 2-stage delay-multiply-and-sum beamforming for breast cancer detection using microwave imaging. In Proceedings of the Iranian Conference on Electrical Engineering (ICEE 2017), Tehran, Iran, 2–4 May 2017; pp. 101–106. [Google Scholar]

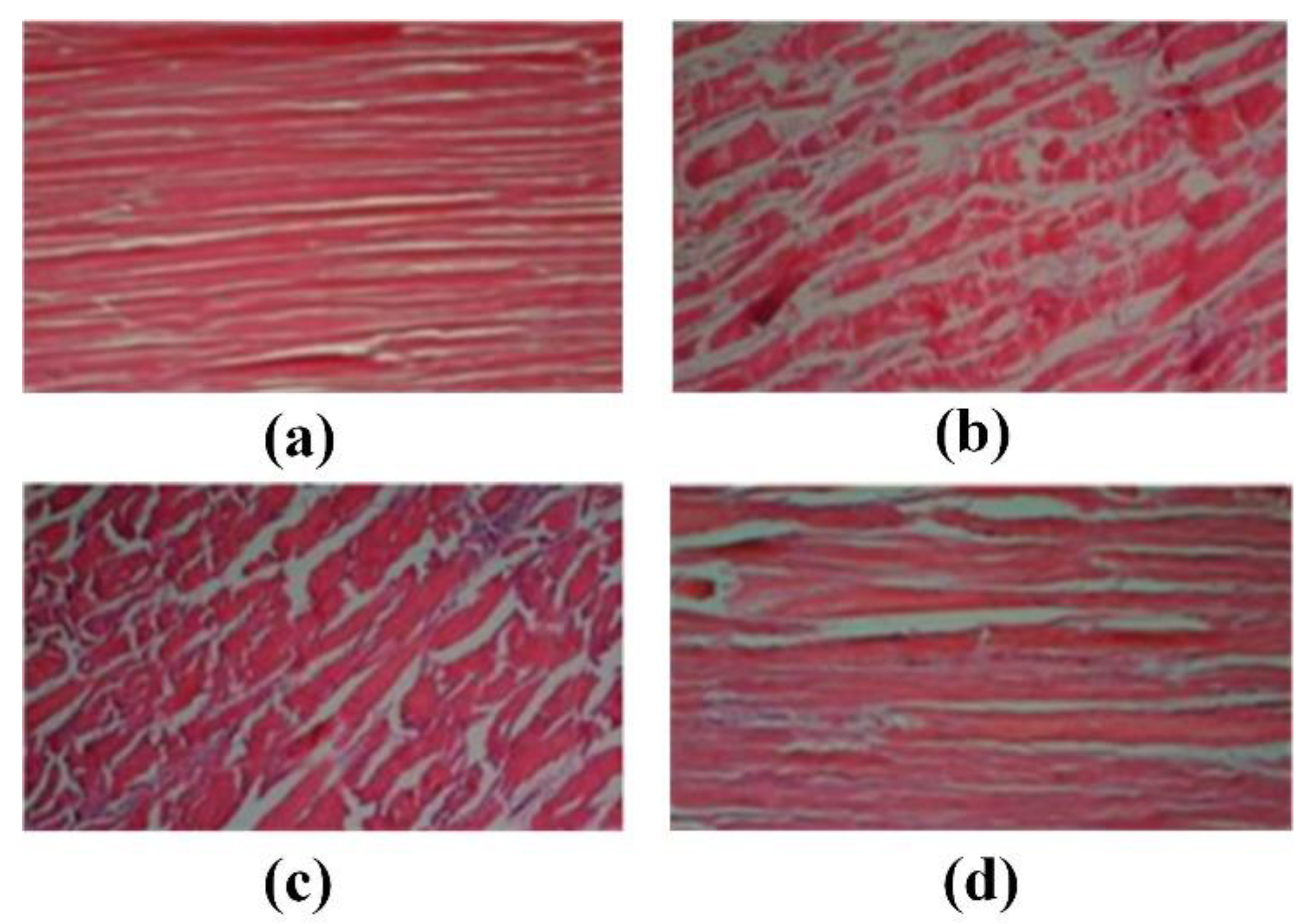


| Electromagnetic Biological Effect | Application | References |
|---|---|---|
| Thermal effects | Seed sterilization and inactivation | [15] |
| Food storage | [16] | |
| High frequency electric knife | [17] | |
| Transcranial magnetic stimulation | [18] | |
| NMR | [19] | |
| Non-thermal effects | Cardiac pacing | [20] |
| Cardiac defibrillation | [21] | |
| Tumor treatment | [22] | |
| Cumulative effects | Transcranial magnetic stimulation | [23] |
| Fracture healing | [24] |
| Material | Sensor Structure Parameters | Thaw Effect | Reference |
|---|---|---|---|
| Beef | 4 cm × 4 cm × 2 cm, Space of electrodes: 4 cm, 10 kv | The thawing time decreases as the number of electrodes increases | [39] |
| Tuna | 2 cm × 4 cm × 4 cm, number of electrodes: 16, 5–14 kv | Thawing time is significantly reduced | [38] |
| Pork | 5 cm × 5 cm × 1 cm, Space of electrodes: 5 cm, −10 kv | The pH and tenderness do not present obvious variation from normal air thawing | [40] |
| Coil Model | Advantages | Disadvantages |
|---|---|---|
| Halo | Increase the electromagnetic penetration depth | The required the current is relatively large |
| HCA | Improve the penetration depth | Poor deep focality |
| HTC | Better deep focality | Compared with HCA, the strength of the induced electric field is reduced |
| HFA | The electric field strength and penetration depth increase on the side | The focality is poor and the attenuation rate is increased |
| HAD | Compared with HFA, the strength and penetration are larger | The focality at the gray and white areas is poor |
| THC | High flexibility | The superficial electric field strength is high |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Xu, L.; Xie, Y. Biomedical Applications of Electromagnetic Detection: A Brief Review. Biosensors 2021, 11, 225. https://doi.org/10.3390/bios11070225
Huang P, Xu L, Xie Y. Biomedical Applications of Electromagnetic Detection: A Brief Review. Biosensors. 2021; 11(7):225. https://doi.org/10.3390/bios11070225
Chicago/Turabian StyleHuang, Pu, Lijun Xu, and Yuedong Xie. 2021. "Biomedical Applications of Electromagnetic Detection: A Brief Review" Biosensors 11, no. 7: 225. https://doi.org/10.3390/bios11070225
APA StyleHuang, P., Xu, L., & Xie, Y. (2021). Biomedical Applications of Electromagnetic Detection: A Brief Review. Biosensors, 11(7), 225. https://doi.org/10.3390/bios11070225






