Whole Cell Recognition of Staphylococcus aureus Using Biomimetic SPR Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
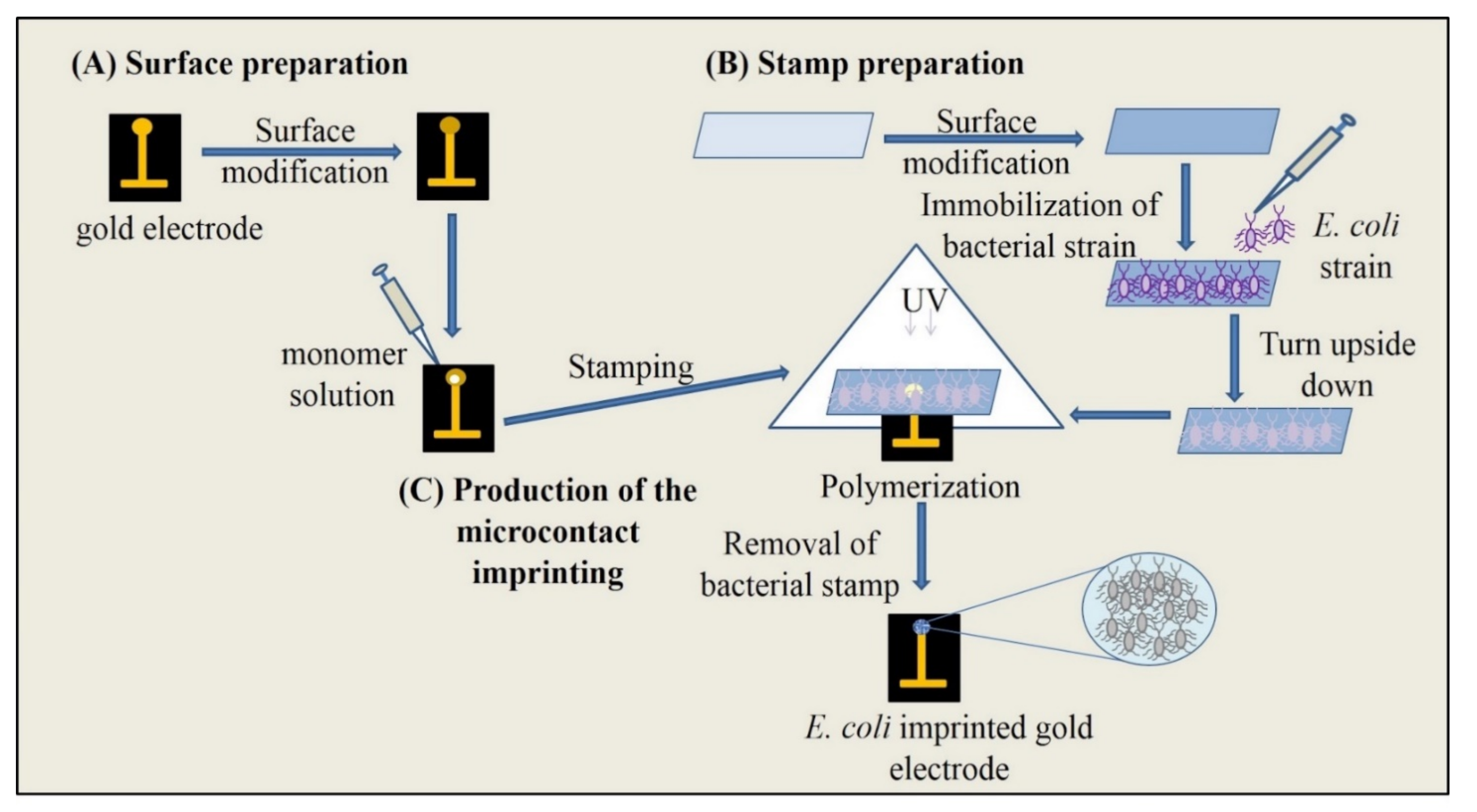
2.3. Microcontact Imprinting
2.3.1. Functionalization of Glass Slides
2.3.2. Surface Modification of SPR Chips
2.3.3. Preparation of S. aureus Imprinted SPR Chips
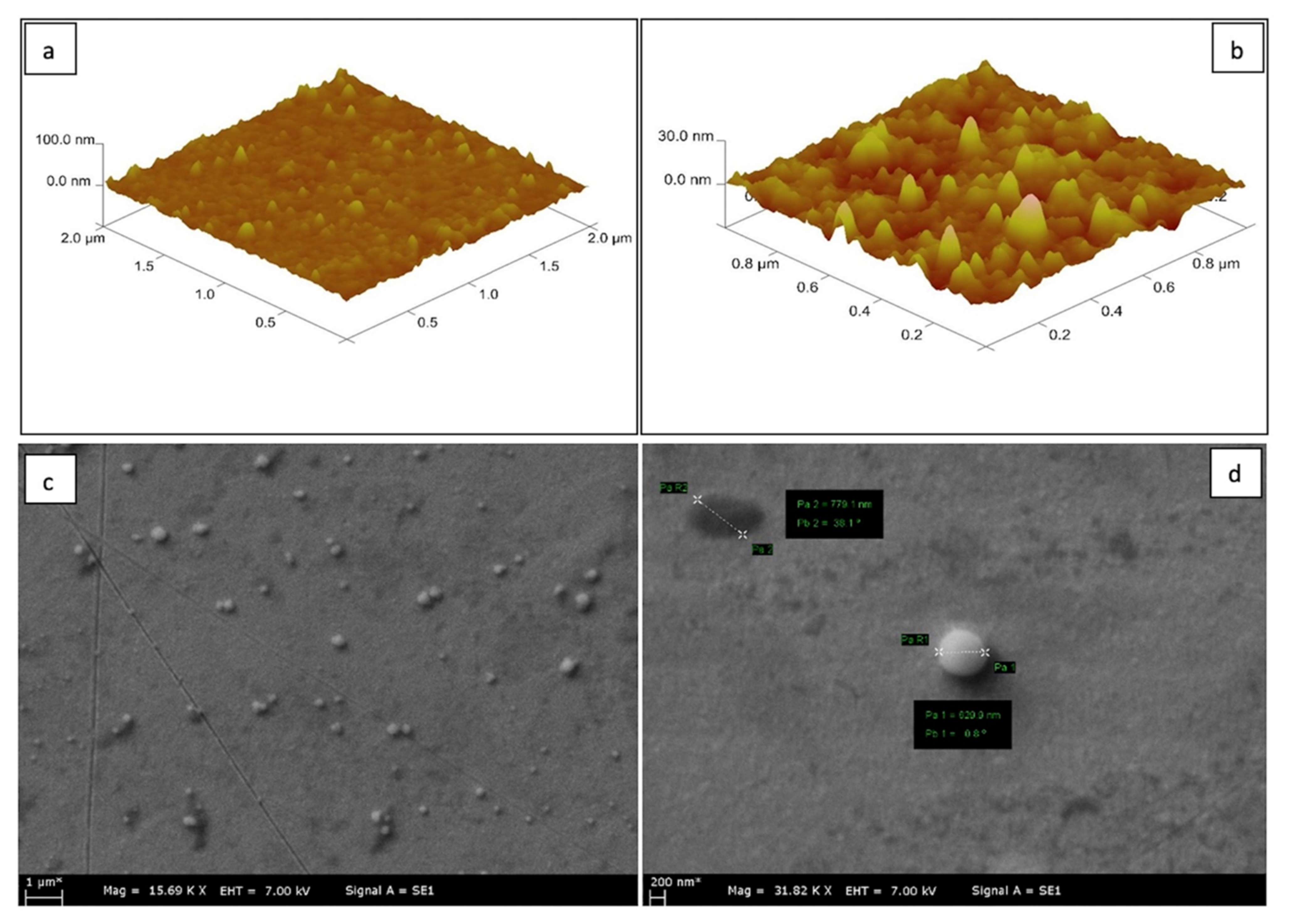
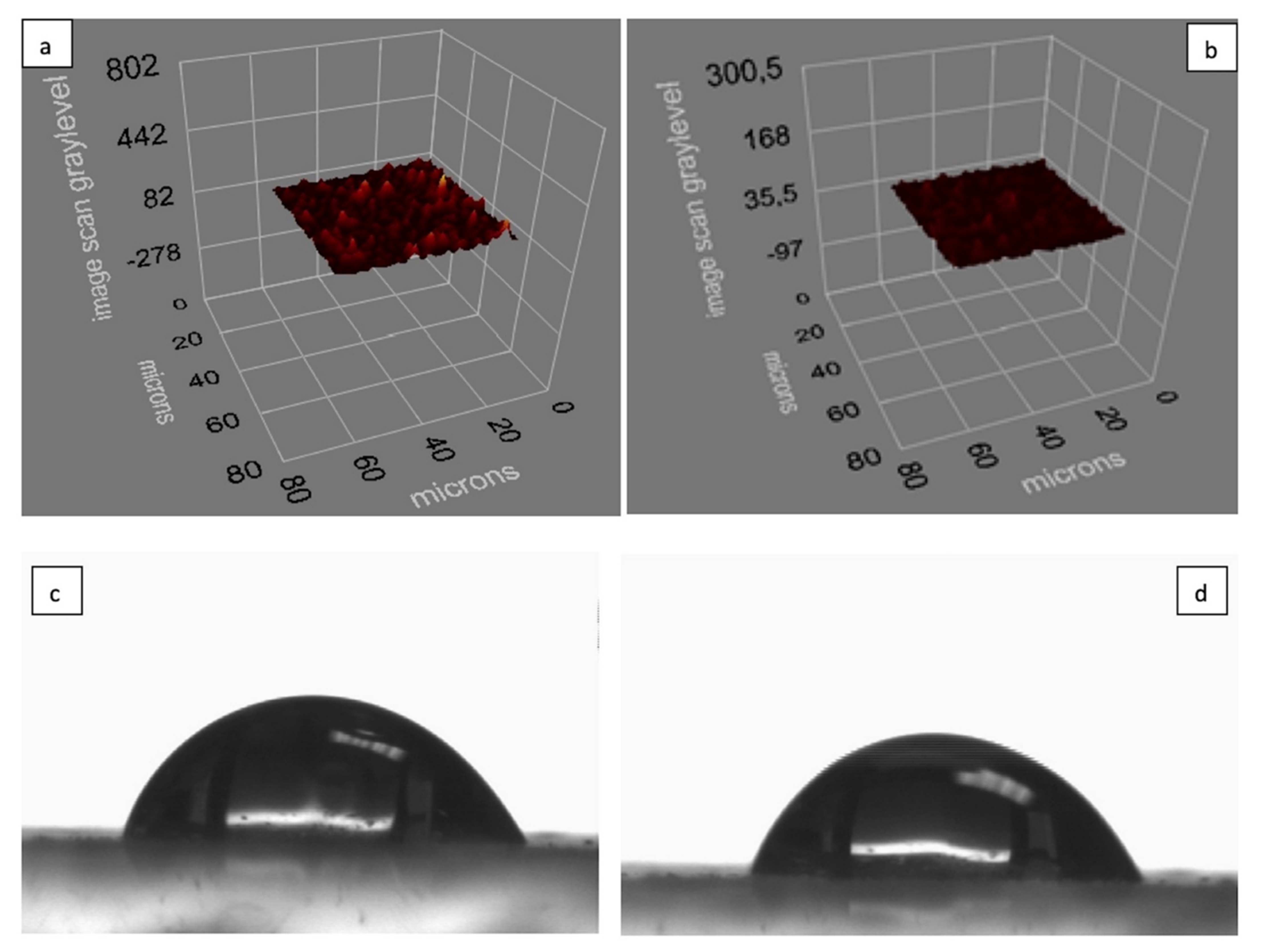
2.4. Characterization of SPR Chips
2.5. Real-Time Detection of S. aureus
2.6. Selectivity of the Sensing
2.7. Applicability and Reusability Testing
3. Results and Discussion
3.1. Characterization of SPR Chips
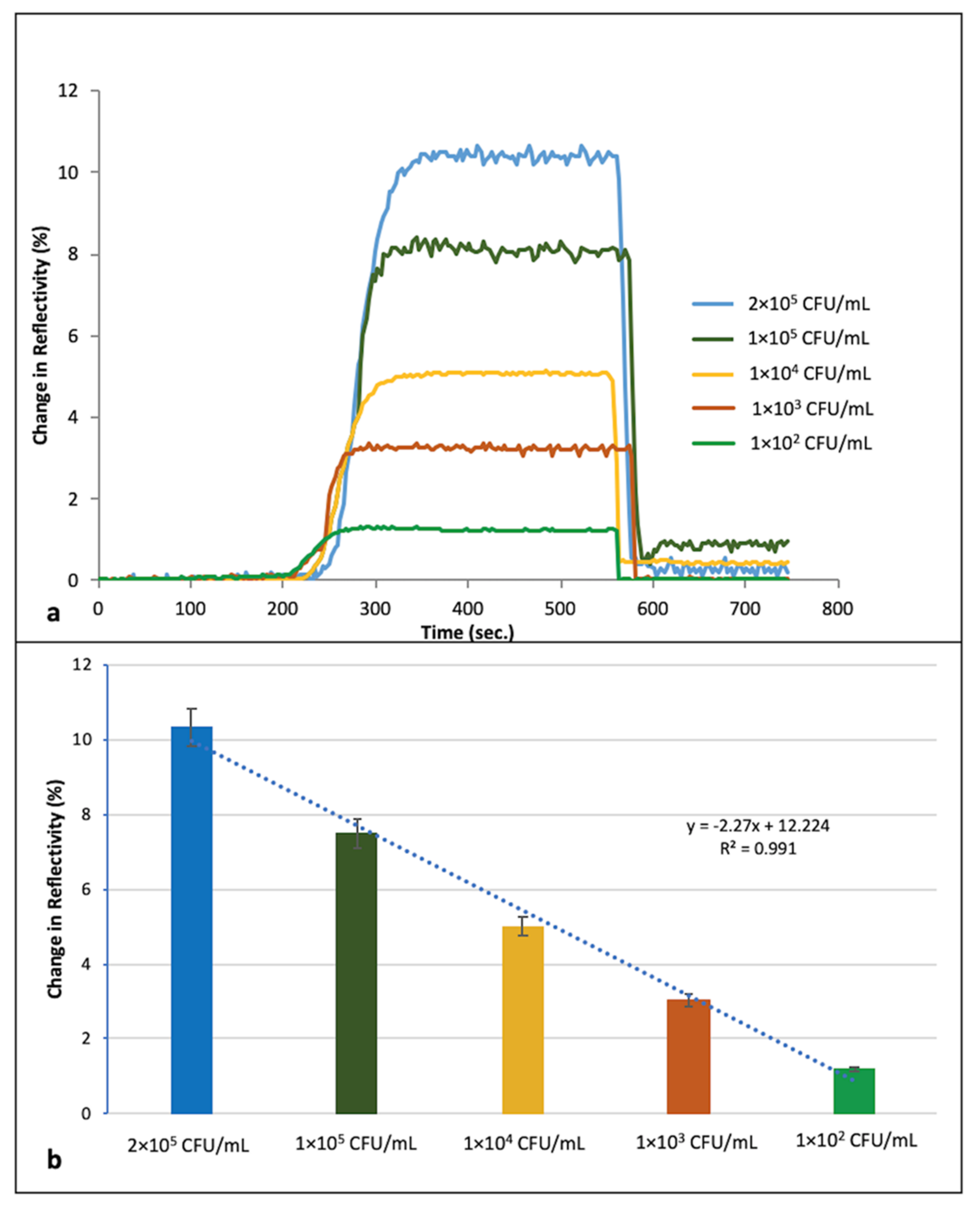
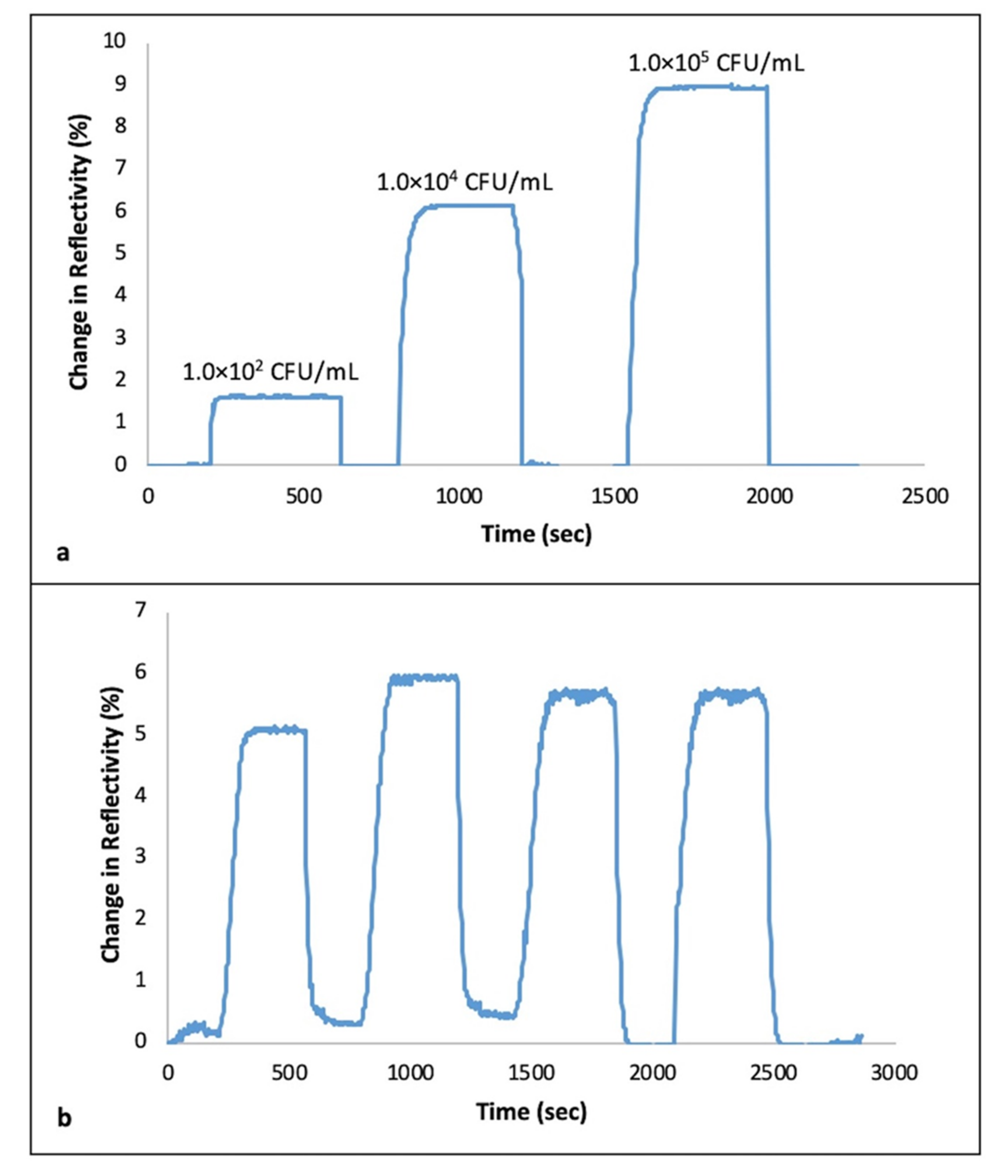
3.2. Real Time Detection of S. aureus
3.3. Selectivity of the Sensing
3.4. Real Sample Experiments and Reusability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Idil, N.; Bilkay, I.S. Application of RAPD-PCR for determining the clonality of methicillin resistant Staphylococcus aureus isolated from different hospitals. Braz. Arch. Biol. Technol. 2014, 57, 548–553. [Google Scholar] [CrossRef]
- Katsarou, I.; Paraskevopoulou, N.M.; Papadimitriou-Olivgeris, M.; Giormezis, N.; Militsopoulou, M.; Kolonitsiou, F.; Marangos, M.; Anastassiou, E.D.; Spiliopoulou, I. Fatality of Staphylococcus aureus infections in a Greek university hospital: Role of inappropriate empiric treatment, methicillin resistance, and toxin genes’ presence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [PubMed]
- Suaifan, G.A.R.Y.; Alhogail, S.; Zourob, M. Rapid and low-cost biosensor for the detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kozitsina, A.N.; Svalova, T.S.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K.Z. Sensors based on bio and biomimetic receptors in medical diagnostic, environment, and food analysis. Biosensors 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Perçin, I.; Idil, N.; Bakhshpour, M.; Yılmaz, E.; Mattiasson, B.; Denizli, A. Microcontact imprinted plasmonic nanosensors: Powerful tools in the detection of Salmonella paratyphi. Sensors 2017, 17, 1375. [Google Scholar] [CrossRef]
- Khateb, H.; Klös, G.; Meyer, R.L.; Sutherland, D.S. Development of a label-free LSPR-apta sensor for Staphylococcus aureus detection. ACS Appl. Bio Mater. 2020, 3, 3066–3077. [Google Scholar] [CrossRef]
- Yilmaz, E.; Majidi, D.; Ozgur, E.; Denizli, A. Whole cell imprinting based Escherichia coli sensors: A study for SPR and QCM. Sens. Actuators B Chem. 2015, 209, 714–721. [Google Scholar]
- Taylor, A.D.; Ladd, J.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar]
- Nishimura, T.; Hifumi, E.; Fujii, T.; Niimi, Y.; Egashira, N.; Shimizu, K.; Uda, T. Measurement of Helicobacter pylori Using Anti Its Urease Monoclonal Antibody by Surface Plasmon Resonance. Electrochemistry 2000, 68, 916–919. [Google Scholar] [CrossRef]
- Oh, B.K.; Kim, Y.K.; Park, K.W.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1497–1504. [Google Scholar] [CrossRef]
- Oh, B.K.; Lee, W.; Kim, Y.K.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor using self-assembled protein G for the detection of Salmonella paratyphi. J. Biotechnol. 2004, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Kim, Y.K.; Lee, W.; Bae, Y.M.; Lee, W.H.; Choi, J.W. Immunosensor for detection of Legionella pneumophila using surface plasmon resonance. Biosens. Bioelectron. 2003, 18, 605–611. [Google Scholar] [CrossRef]
- Oh, B.K.; Lee, W.; Lee, W.H.; Choi, J.W. Nano-scale probe fabrication using self-assembly technique and application to detection of Escherichia coli O157:H7. Biotechnol. Bioprocess Eng. 2003, 8, 227–232. [Google Scholar] [CrossRef]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. A mixed self-assembled monolayer-based surface plasmon immunosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2006, 21, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Lee, W.; Chun, B.S.; Bae, Y.M.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor for the detection of Yersinia enterocolitica. Colloids Surf. A Physicochem. Eng. Asp. 2005, 257–258, 369–374. [Google Scholar] [CrossRef]
- Hu, J.; Fu, K.; Bohn, P.W. Whole-Cell Pseudomonas aeruginosa Localized Surface Plasmon Resonance Aptasensor. Anal. Chem. 2018, 90, 2326–2332. [Google Scholar] [CrossRef]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. Mono and dithiol surfaces on surface plasmon resonance biosensors for detection of Staphylococcus aureus. Sens. Actuators B Chem. 2006, 114, 192–198. [Google Scholar] [CrossRef]
- Jyoung, J.Y.; Hong, S.H.; Lee, W.; Choi, J.W. Immunosensor for the detection of Vibrio cholerae O1 using surface plasmon resonance. Biosens. Bioelectron. 2006, 21, 2315–2319. [Google Scholar] [CrossRef]
- Dickert, F.L.; Hayden, O. Bioimprinting of polymers and sol-gel phases. Selective detection of yeasts with imprinted polymers. Anal. Chem. 2002, 74, 1302–1306. [Google Scholar] [CrossRef]
- Hayden, O.; Haderspöck, C.; Dickert, F.L. Microorganism detection with selective bioimprinted polymers and sol-gels. Int. J. Bio-Chromatography 2001, 6, 303–311. [Google Scholar]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly imprinted polymers for cell recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef]
- Idil, N.; Mattiasson, B. Imprinting of microorganisms for biosensor applications. Sensors 2017, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole cell based microcontact imprinted capacitive biosensor for the detection of Escherichia coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef]
- Tokonami, S.; Saimatsu, K.; Nakadoi, Y.; Furuta, M.; Shiigi, H.; Nagaoka, T. Vertical immobilization of viable bacilliform bacteria into polypyrrole films. Anal. Sci. 2012, 28, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Bole, A.L.; Manesiotis, P. Advanced materials for the recognition and capture of whole cells and microorganisms. Adv. Mater. 2016, 28, 5349–5366. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Starosvetsky, J.; Cheruti, U.; Armon, R. Whole cell imprinting in sol-gel thin films for bacterial recognition in liquids: Macromolecular fingerprinting. Int. J. Mol. Sci. 2010, 11, 1236–1252. [Google Scholar] [CrossRef]
- Poller, A.M.; Spieker, E.; Lieberzeit, P.A.; Preininger, C. Surface imprints: Advantageous application of ready2use materials for bacterial quartz-crystal microbalance sensors. ACS Appl. Mater. Interfaces 2017, 9, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Thomas, J.L.; Li, M.H.; Shih, C.P.; Jan, J.S.; Lin, H.Y. Recognition of Rhodobacter sphaeroides by microcontact-imprinted poly(ethylene-co-vinyl alcohol). Colloids Surfaces B Biointerfaces 2015, 135, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Zhang, H.; Guo, Y.; Li, J.; Nie, H.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Rapid and selective recognition of Vibrio parahaemolyticus assisted by perfluorinated alkoxysilane modified molecularly imprinted polymer film. RSC Adv. 2020, 10, 14305–14312. [Google Scholar] [CrossRef]
- Ertürk, G.; Berillo, D.; Hedström, M.; Mattiasson, B. Microcontact-BSA imprinted capacitive biosensor for real-time, sensitive and selective detection of BSA. Biotechnol. Rep. 2014, 3, 65–72. [Google Scholar] [CrossRef]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly imprinted polymers for the selective recognition of microorganisms. Biotechnol. Adv. 2020, 45, 107640. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef]
- Mahadhy, A.; Ståhl-Wernersson, E.; Mattiasson, B.; Hedström, M. Rapid detection of mecA gene of methicillin-resistant Staphylococcus aureus by a novel, label-free real-time capacitive biosensor. Biotechnol. Rep. 2020, 28, e00568. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Chen, W.J.; Li, M.H.; Shih, C.P.; Lin, H.Y. Fabrication of bacteria-imprinted polymer coated electrodes for microbial fuel cells. ACS Sustain. Chem. Eng. 2015, 3, 1190–1196. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, D.; Shao, J.; Sun, X. Magnetic molecularly imprinted polymer nanoparticles based electrochemical sensor for the measurement of Gram-negative bacterial quorum signaling molecules (N-acyl-homoserine-lactones). Biosens. Bioelectron. 2016, 75, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.R.; Aires Cardoso, A.R.; Sales, M.G.F.; Merino, S.; Tomás, J.M.; Rius, F.X.; Riu, J. Artificial receptors for the electrochemical detection of bacterial flagellar filaments from Proteus mirabilis. Sens. Actuators B Chem. 2017, 244, 732–741. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC Trends Anal. Chem. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Ertürk Bergdahl, G.; Andersson, T.; Allhorn, M.; Yngman, S.; Timm, R.; Lood, R. In Vivo Detection and Absolute Quantification of a Secreted Bacterial Factor from Skin Using Molecularly Imprinted Polymers in a Surface Plasmon Resonance Biosensor for Improved Diagnostic Abilities. ACS Sens. 2019, 4, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, W.; Carlquist, M.; Ye, L. Boronic Acid Modified Polymer Nanoparticles for Enhanced Bacterial Deactivation. ChemBioChem 2019, 20, 2991–2995. [Google Scholar] [CrossRef]
- Medina Rangel, P.X.; Moroni, E.; Merlier, F.; Gheber, L.A.; Vago, R.; Tse Sum Bui, B.; Haupt, K. Chemical Antibody Mimics Inhibit Cadherin-Mediated Cell–Cell Adhesion: A Promising Strategy for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 2816–2822. [Google Scholar] [CrossRef]
- Mahajan, R.; Rouhi, M.; Shinde, S.; Bedwell, T.; Incel, A.; Mavliutova, L.; Piletsky, S.; Nicholls, I.A.; Sellergren, B. Highly Efficient Synthesis and Assay of Protein-Imprinted Nanogels by Using Magnetic Templates. Angew. Chem. Int. Ed. 2019, 58, 727–730. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Jenik, M.; Seifner, A.; Krassnig, S.; Seidler, K.; Lieberzeit, P.A.; Dickert, F.L.; Jungbauer, C. Sensors for bioanalytes by imprinting-Polymers mimicking both biological receptors and the corresponding bioparticles. Biosens. Bioelectron. 2009, 25, 9–14. [Google Scholar] [CrossRef]
- Kai, E.; Ikebukuro, K.; Hoshina, S.; Watanabe, H.; Karube, I. Detection of PCR products of Escherichia coli O157:H7 in human stool samples using surface plasmon resonance (SPR). FEMS Immunol. Med. Microbiol. 2000, 29, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Nawattanapaiboon, K.; Kiatpathomchai, W.; Santanirand, P.; Vongsakulyanon, A.; Amarit, R.; Somboonkaew, A.; Sutapun, B.; Srikhirin, T. SPR-DNA array for detection of methicillin-resistant Staphylococcus aureus (MRSA) in combination with loop-mediated isothermal amplification. Biosens. Bioelectron. 2015, 74, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Naimushin, A.N.; Soelberg, S.D.; Nguyen, D.K.; Dunlap, L.; Bartholomew, D.; Elkind, J.; Melendez, J.; Furlong, C.E. Detection of Staphylococcus aureus enterotoxin B at femtomolar levels with a miniature integrated two-channel surface plasmon resonance (SPR) sensor. Biosens. Bioelectron. 2002, 17, 573–584. [Google Scholar] [CrossRef]
- Chen, L.; Deng, L.; Liu, L.; Peng, Z. Immunomagnetic separation and MS/SPR end-detection combined procedure for rapid detection of Staphylococcus aureus and protein A. Biosens. Bioelectron. 2007, 22, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Sorokulova, I.B.; Vodyanoy, V.J.; Simonian, A.L. Lytic phage as a specific and selective probe for detection of Staphylococcus aureus-A surface plasmon resonance spectroscopic study. Biosens. Bioelectron. 2007, 22, 948–955. [Google Scholar] [CrossRef]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Surface plasmon resonance detection of E. coli and methicillin-resistant S. aureus using bacteriophages. Biosens. Bioelectron. 2012, 37, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bezdekova, J.; Zemankova, K.; Hutarova, J.; Kociova, S.; Smerkova, K.; Adam, V.; Vaculovicova, M. Magnetic molecularly imprinted polymers used for selective isolation and detection of Staphylococcus aureus. Food Chem. 2020, 321, 126673. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Bakhshpour, M.; Perçin, I.; Mattiasson, B. Molecular imprinting-based sensing platorm for recognition of microorganisms. In Molecular Imprinting-Based Sensing Platform for Recognition of Microorganisms; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–307. [Google Scholar]
- Mattiasson, B.; Ertürk, G. Why using molecularly imprinted polymers in connection with biosensors. Sensors 2017, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Mattiasson, B. Molecular imprintinhg techniques used for the preparation of biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Aslıyüce, S.; Mattiasson, B.; Denizli, A. Combined protein A imprinting and cryogelation for production of spherical affinity material. Biomed. Chromatogr. 2019, 33, e4605. [Google Scholar] [CrossRef] [PubMed]

| Reflectance Change, ΔR | Reflectance Change, ΔR | Selectivity Coefficient, k | Selectivity Coefficient, k | Relative Selectivity Coefficient, k, k’ | |
|---|---|---|---|---|---|
| Bacterial strains | Imprinted | Non-imprinted | Imprinted | Non-imprinted | |
| S. aureus | 5.08 | 0.15 | - | - | - |
| B. subtilis | 0.07 | 0.11 | 47.47 | 1.36 | 34.81 |
| E. coli | 0.10 | 0.10 | 50.80 | 1.50 | 33.86 |
| S. paratyphi | 0.11 | 0.10 | 72.57 | 1.50 | 48.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idil, N.; Bakhshpour, M.; Perçin, I.; Mattiasson, B. Whole Cell Recognition of Staphylococcus aureus Using Biomimetic SPR Sensors. Biosensors 2021, 11, 140. https://doi.org/10.3390/bios11050140
Idil N, Bakhshpour M, Perçin I, Mattiasson B. Whole Cell Recognition of Staphylococcus aureus Using Biomimetic SPR Sensors. Biosensors. 2021; 11(5):140. https://doi.org/10.3390/bios11050140
Chicago/Turabian StyleIdil, Neslihan, Monireh Bakhshpour, Işık Perçin, and Bo Mattiasson. 2021. "Whole Cell Recognition of Staphylococcus aureus Using Biomimetic SPR Sensors" Biosensors 11, no. 5: 140. https://doi.org/10.3390/bios11050140
APA StyleIdil, N., Bakhshpour, M., Perçin, I., & Mattiasson, B. (2021). Whole Cell Recognition of Staphylococcus aureus Using Biomimetic SPR Sensors. Biosensors, 11(5), 140. https://doi.org/10.3390/bios11050140




