Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future
Abstract
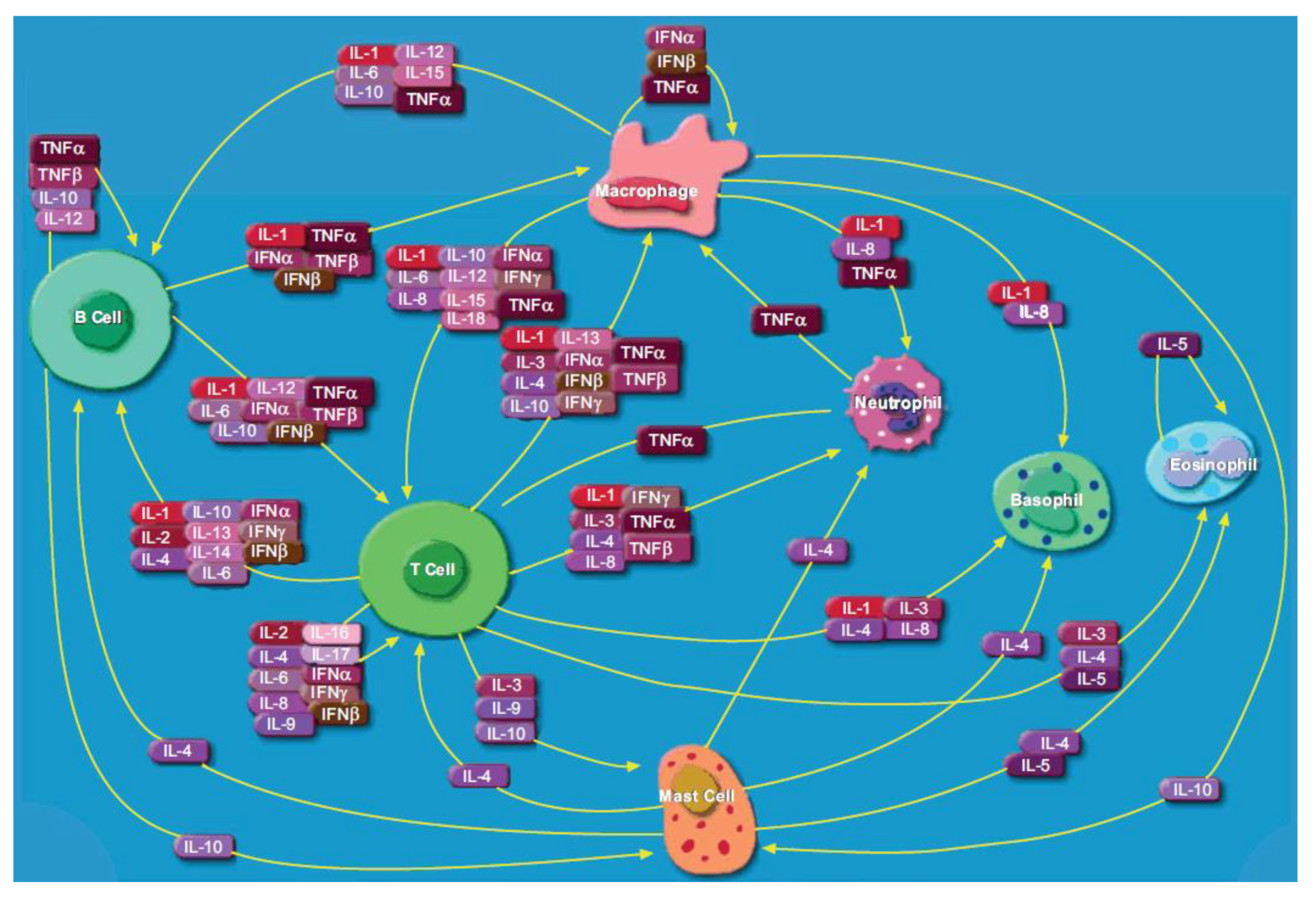
1. Introduction to Cytokines
1.1. Cytokine Expression in Infection, Cancer, Autoimmunity and Neurodegeneration
1.2. Important Cytokines as Potential Biomarkers
1.3. Techniques for Cytokine Detection and Quantification
2. Brief Overview of Electrochemical Detection
2.1. Electrochemical Biosensors for Cytokine Detection
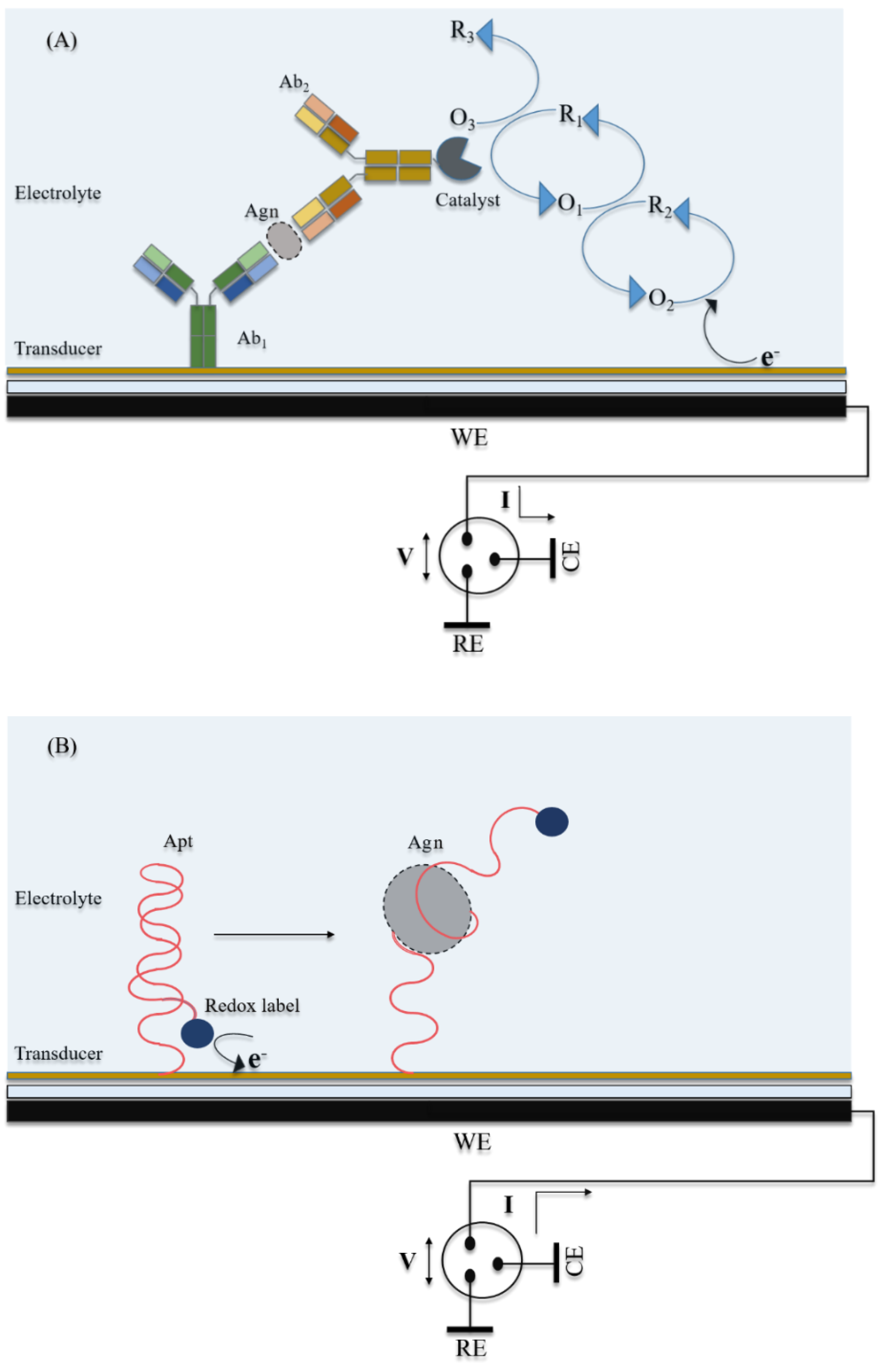
2.1.1. Aptasensors
| Sl. No. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [94] | SPGE/PPyNPs/AuNPs/Apt | [Fe(CN)6]4−/3− | Anti-IL-6 Apt | 6-mercaptohexanol | SPGE+ Py+ LiClO4 → polymerization: multipulse amperometry SPGE/PPyNPs+ HAuCl4+ H2SO4 → Au3+ reduction, NP formation: cyclic voltammetry SPGE/PPyNPs/AuNPs + Apt (thiolated): Au-S chemistry, multipulse amperometry SPGE/PPyNPs/AuNPs/Apt + MCH: Au-S chemistry, multipulse amperometry |
| 2. [105] | Apt/AuNP/Au electrode | [Fe(CN)6]4−/3− | Anti-IL-6 Apt | HS-(CH2)11(OCH2CH2)3OH | Au electrode/AuNP+ Aptamer(thiolated): Au-S chemistry EG3+ Au electrode/AuNP/Aptamer: Au-S chemistry |
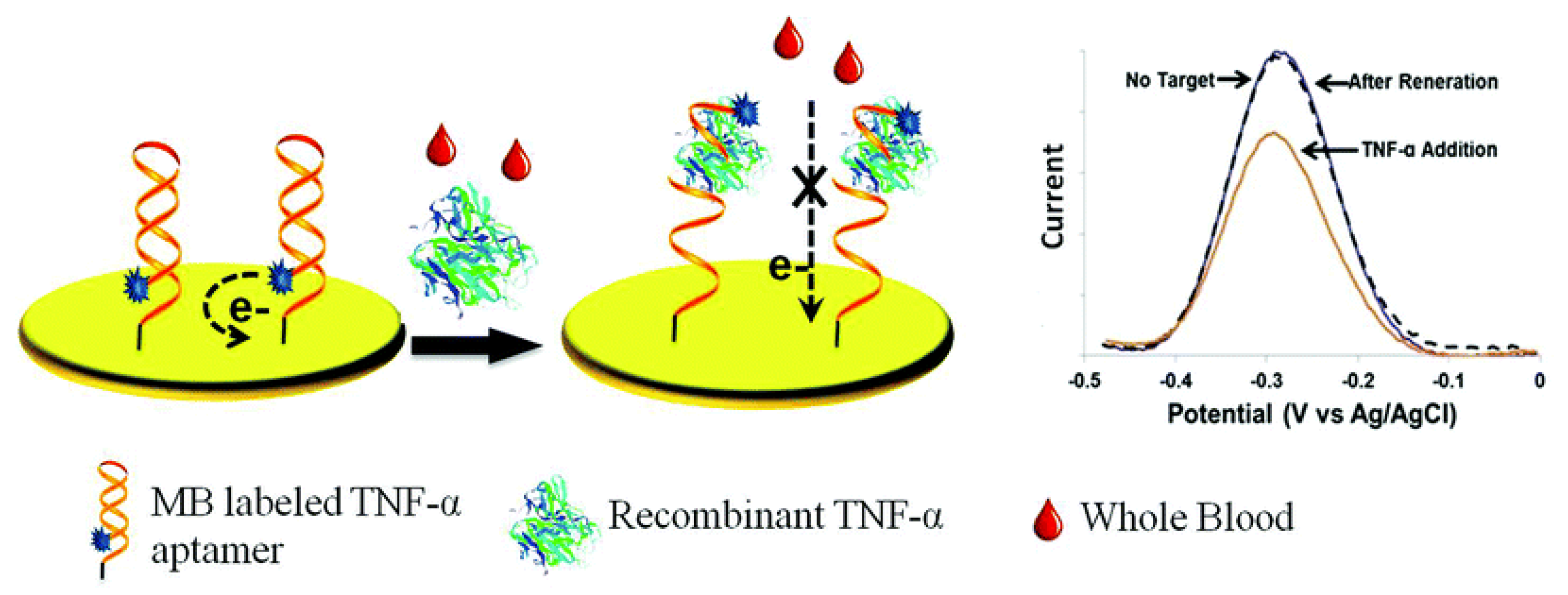
| 3. [106] | Au/Apt-MB | MB (ox/red) | Anti-TNFα Apt | 6-mercapto-1-hexanol | 28-mer RNA Apt nucleotides are phosphorothiolated to protect from RNase 5’ NH2-Apt-(CH2)6SH 3’ + MB-NHS → 5’ MB-Apt-(CH2)6SH 3’ or Apt-MB Apt-(CH2)6S-S-(CH2)6-OH 3’ + TCEP+ H2O → Apt-(CH2)6SH+ HS-(CH2)6-OH + TCEP = O 5’ MB-Apt-(CH2)6SH 3’ + Au electrode: Au-S chemistry |
| 4. [107] | Au/Apt-MB | MB (ox/red) | Anti-IFNγ Apt | 6-mercapto-1-hexanol | 3’ NH2-(CH2)6-Apt+ MB-NHS → 3’ MB-NH-(CH2)6-Apt or MB-Apt 5’ OH-(CH2)6-S-S-(CH2)6-Apt+ TCEP → 5’ HS-(CH2)6-Apt+ OH-(CH2)6-SH+ TCEP = O 5’ SH-Apt-MB 3’+ Au electrode: Au-S chemistry |
| 5. [108] | Au/capture probe Recognition probe (Apt) H1-Bt H2-Bt SAv-ALP | 1-naphthol (red → ox) | Anti-IFNγ Apt | 6-mercapto-1-hexanol | Au+ 5’ SH-capture probe: immobilization with Au-S chemistry Recognition probe+ H1-Bt: hairpin opening, partial hybridization Partially hybridized Bt-H1-recognition probe+ H2-Bt: hairpin opening, partial hybridization; resulting in a cascade of successive hybridization events Bt-SAv: affinity bonding 1-naphthyl phosphate (1-NPP) converted to 1-naphthol (1-NP) by ALP |
| 6. [109] | Au/Apt | [Fe(CN)6]4−/3− | Anti-IFNγ Apt | β-mercaptoethanol | Deactivation of RNase in solutions with diethyl pyrocarbonate (DEPC) Au electrode+ 5’ SH-Apt (DNA/RNA)+ dithiothreitol+ pentanethiol/MgCl2/PBS: Apt immobilization, interspaced with pentanethiol |
| 7. [110] | Au/Apt-MB | MB(ox/red) | Anti-TGF-β1 Apt | 6-mercapto-1-hexanol | MB-NHS+ 5’ NH2-Apt: covalent linkage; MB-Apt formation Au+ 3’ SH-Apt: Au-S chemistry 5’ NH2-Apt-SH 3’ (DNA) has a phosphorothioated backbone on 5’ adenine & cytosine nucleotides, to resist nuclease degradation |
| Sl. no. Ref. | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [94] | EIS | 0.33 pg/mL | 1 pg/mL–15 μg/mL | BSA, CEA, MUC1, MUC4, MUC16 | +IL-6/30 min | IL-6 in PBS Spiked serum | (6 assays) RSD = 3.42% | - | - |
| 2. [105] | EIS | 0.02 pg/mL | 0.02–20 pg/mL | BSA | - | IL-6 in buffer Spiked artificial sweat | (5 assays, at [IL-6] = 0.02 pg/mL) RSD = 14.1% | After 2 weeks- 90% of initial signal retained for [IL-6] = 0.2 pg/mL | 90% of initial signal retained for [IL-6] = 0.02 pg/mL |
| 3. [106] | SWV | 58 pM | 58 pM–6 nM | - | Sensor equilibration/30 min +TNFα/15 min | rTNFα in spiked whole blood | - | Stable over 10 h | After 6 cycles & regeneration/urea, 90% of original signal retained |
| 4. [107] | SWV | 0.06 nM | 0.06–10 nM | IgG, anti-IgG, BSA | +IFNγ/15 min | rIFNγ in HEPES buffer IFNγ in RPM1 culture media IFNγ in RPM1/serum | - | - | Regeneration/urea/1min + rinsing/diH2O; sensor can be reused more than 10 or more times |
| 5. [108] | DPV | 0.3 nM | 0.5–300 nM | PDGF-BB, BSA, IgG, CEA, IL-6 | IFNγ+ recognition probe/2 h +Au/capture probe/1 h +H1-Bt, H2-Bt/90 min, rinsing/10 min +SAv-ALP/30 min, rinsing/20 min +1NPP/3 min | IFNγ in HEPES buffer IFNγ in RPM1 culture media IFNγ in RPM1/serum | - | After 2 weeks, no significant change in current response | Regeneration/NaOH/20 min + washing/diH2O/10 min; sensor can be reused more than 3 times |
| 6. [109] | EIS | 500 fM (RNA Apt) 1 pM (DNA Apt), 1.21 pM (DNA in FBS) | - | BSA, FBS | +IFNγ/30 min | IFNγ in PBS Spiked FBS | - | - | - |
| 7. [110] | SWV | 1 ng/mL | 1–200 ng/mL | IL-2, IFNγ, BSA, IgG, TGF-β2, TGF-β3 | (real-time detection at constant flow) | rTGF-β1 in cell culture media DMEM | - | - | - |
2.1.2. Enzyme-Labeled Immunosensors
| Sl. no. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
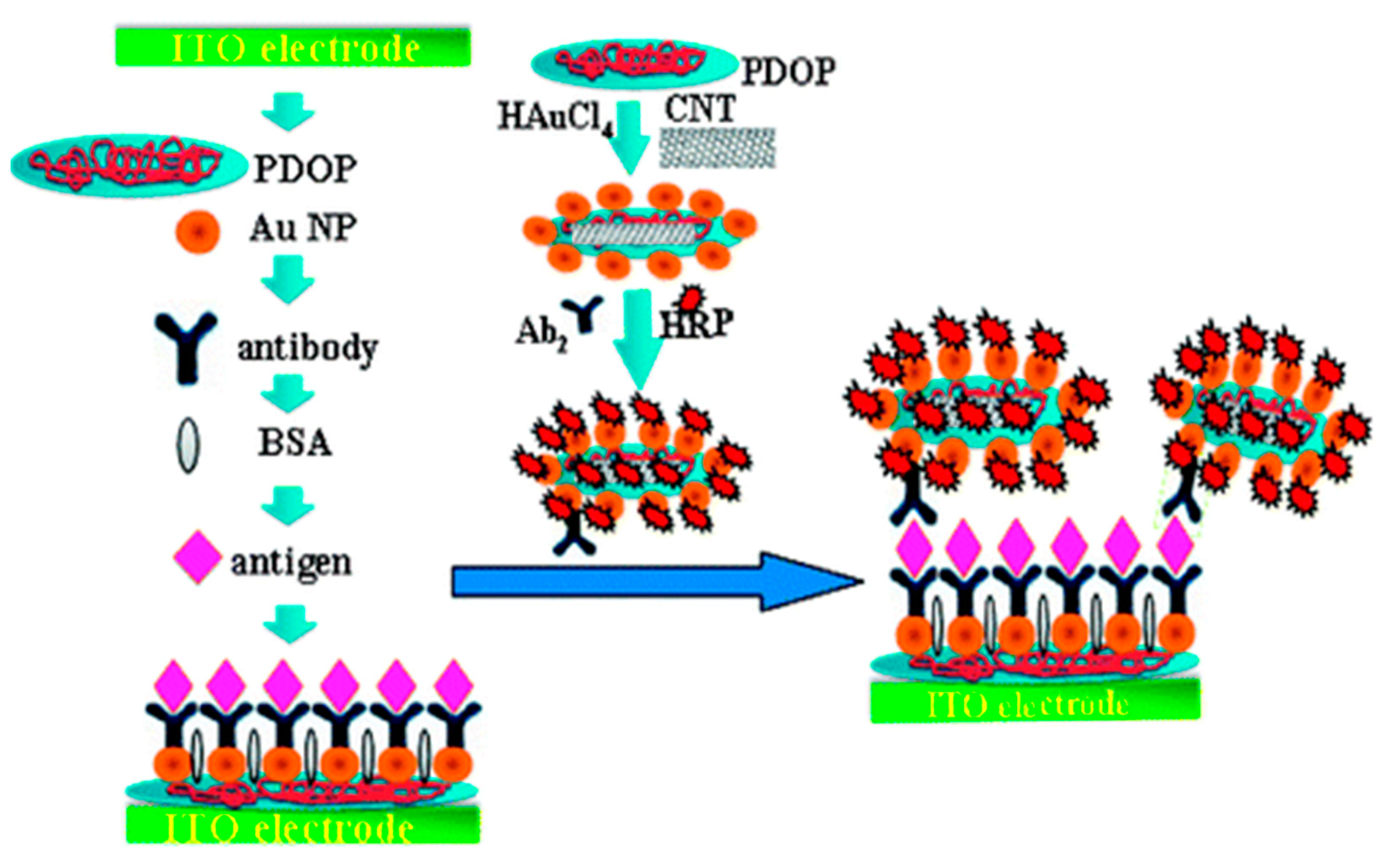
| 1. [111] | ITO/PDOP-AuNP/Ab1 HRP-Ab2-AuNP-PDOP@CNT | 1,2-phenylenediamine/2,2’-diaminoazobenzene H2O2/H2O | Anti-IL-6 Ab1, Ab2 | BSA | ITO + Dopamine + AuNP: polymerization of dopamine, AuNP absorption ITO/PDOP-AuNP + Ab1: physical absorption CNT + dopamine + HAuCl4: Polymerization of dopamine, formation & absorption of AuNP HRP+ Ab2-AuNP-PDOP@CN: Physical absorption |
| 2. [95] | PG/PDDA/GSH-AuNP/Ab1/BSA Bt-Ab2 SAv-HRP | Hydroquinone/benzoquinone H2O2/H2O | Anti-IL-6 Ab1, Ab2 | GSH + AuNP: Au-S chemistry GSH-AuNP + Ab1: amide bond formation with EDC/NHS catalyst Bt-Ab2+ SAv-HRP: affinity bonding | |
| 3. [113] | ITO/PDDA/AuNP/Ab1 AuNP-Ab2-Bt-SAv-HRP | Hydroquinone/benzoquinone H2O2/H2O | Anti-IFNγ Ab1, Ab2 | BSA | ITO electrode + PDDA: drop-casting ITO/PDDA(+ve) + AuNP(-ve): electrostatic adsorption ITO/PDDA/AuNP + Ab1: adsorption Citrate reduction of HAuCl4: AuNP formation HRP-Ab2+ AuNP: adsorption Bt+ SAv: affinity bonding |
| 4. [114] | SPCE-Phen-Ab1 Bt-Ab2 SAv-HRP | Hydroquinone/benzoquinone H2O2/H2O | Anti-IFNγ Ab1, Ab2 | BSA | p-aminobenzoic acid (p-ABA) + NaNO2/HCl → p-ABA diazonium salt SPCE + p-ABA diazonium salt: electrochemical reduction; grafting with CV SPCE-Phe-COOH + Ab1: amide bond formation with EDC/NHS catalyst |
| 5. [115] | Mb-Ab1 Bt-Ab2 SAv-HRP- SPCE | Hydroquinone/benzoquinone H2O2/H2O | Anti-TGF-β1 Ab1, Ab2 | Ethanolamine | MB-COOH+ Mix&Go polymer + Ab1: covalent immobilization SAv+ Bt: affinity bonding MB-Ab1-TGFβ-1-Ab2-HRP separated with magnet at the base of SPCE |
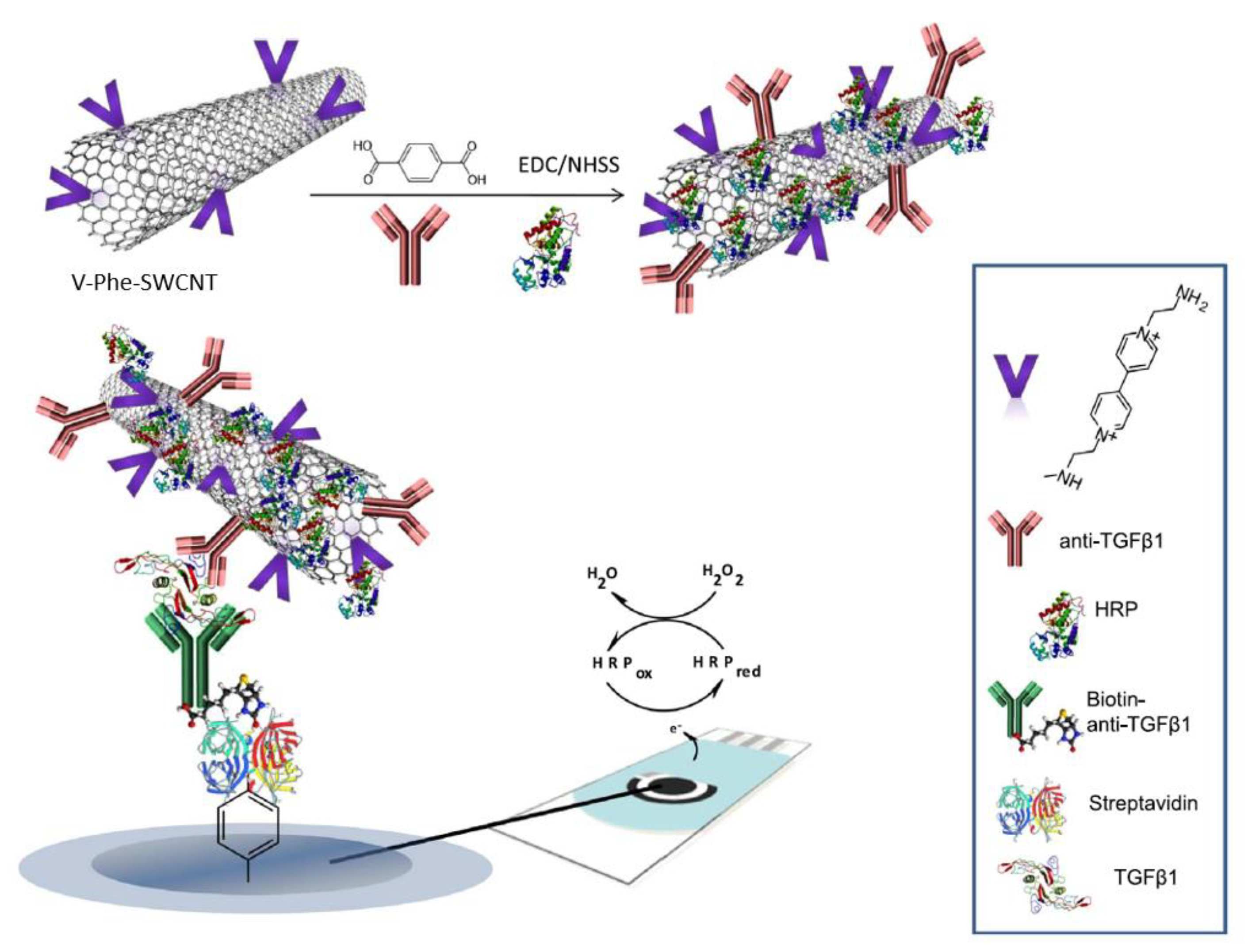
| 6. [116] | SPCE/SAv-Bt-Ab2 V-Phen-SWCNT(-HRP)-Ab1 | (C5H4N(CH2)2NH2)1+/2+ or V1+/2+ H2O2/H2O Hydroquinone/benzoquinone | Anti-TGF-β1 Ab1, Ab2 | Biotin | SWCNT + p-aminobenzoic acid (p-ABA) + isoamylnitrile/NMP → SWCNT-Phe-COOH; grafting 4,4’-bipyridine+ 2-bromoethylamine/CH3CN → 1-(3-aminoethyl)-4,4’-bipyridinium bromide ((C5H4N(CH2)2NH2)22+), a viologen V (C5H4N(CH2)2NH2)22+ + SWCNT-Phe-COOH → SWCNT-Phe-V; amide bond formation with DCC/HOBt catalyst SWCNT-Phe-V + Ab1, HRP: amide bond formation with EDC/NHSS catalyst SPCE+ p-ABA+ NaNO2/HCl → SPCE-Phe-COOH, diazotization followed by reduction, grafting with CV SPCE-Phe-COOH + SAv: amide bond formation with EDC/NHSS catalyst SAv + Bt: affinity bonding |
| 7. [117] | SPCE/MWCNT-alkyne-azide-IgG Ab1 Bt-Ab2 SAv-HRP | Hydroquinone/benzoquinone H2O2/H2O | Anti-TGF-β1 Ab1, Ab2 | Casein | MWCNT(-COOH gr.) + 11-azide-3,6,9-trixaundecan-1-amine: amide bond formation with EDC/NHS catalyst IgG+ NaIO4: oxidation of 1,2-diols of glycosylated Fc region of Ab, leading to alkyne formation MWCNT(-N3 gr.) + IgG(-C≡CH gr.): azide-alkyl cycloaddition into triazole, with ascorbic acid+ Cu(I) catalyst; click chemistry MWCNT-alkyne-azide-IgG drop-casted on SPCE |
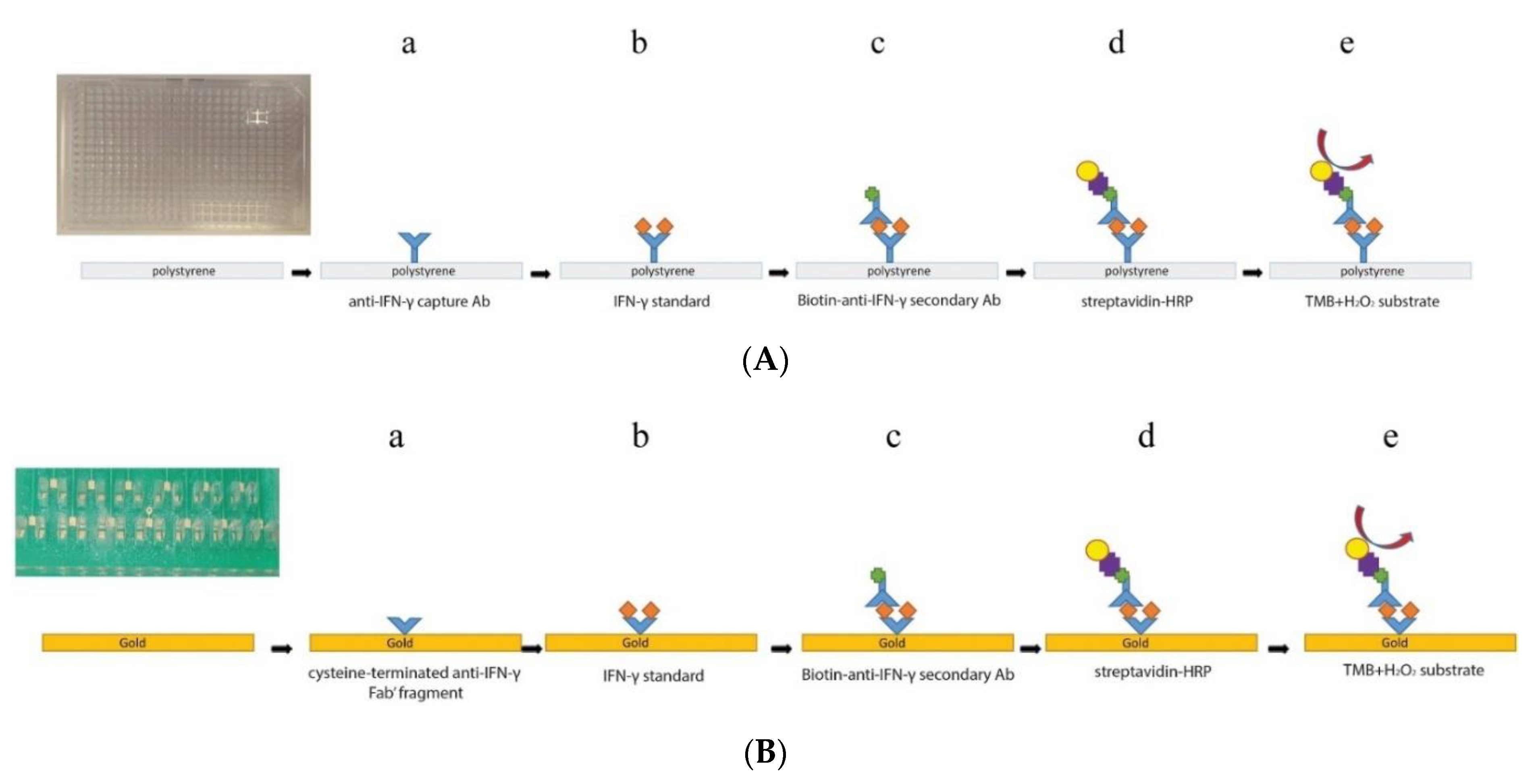
| 8. [118] | PMMA/PCB/Au/Fab1-cys Bt-Ab2 SAv-HRP | 3,3’,5,5’-tetramethylbenzidine (TMBox/TMBred) H2O2/H2O | Anti-IFNγ Fab1, Ab2 | BSA | PMMA/PCB containing Au plated microchannels+ Fab-cys: Au-S chemistry Bt+ SAv: affinity bonding TMB oxidation by HRP, coupled to H2O2 reduction |
| 9. [119] | GCE/PANA/Ab1 PSA/PAH/AuNP-Ab2-ALP | α-naphthol (1-NP) (red→ox) | Anti-TNFα Ab1, Ab2 | BSA | Styrene + acrylic acid + K2S2O8: polymerization into PSA spheres PSA+ PAH: functionalization PSA/PAH(+ve) + AuNP(-ve) colloid: electrostatic attraction Aniline + PAA: electropolymerization into PANA on GCE GCE/PANA + Ab1; amide bond formation with EDC/NHS catalyst α-naphthyl phosphate (1-NPP) converted to α-naphthol by ALP |
| 10. [120] | Si/SiO2/Au/PMMA/FNAB/Ab1 Bt-Ab2-AuN SAv-ALP-AuNP | 4-aminophenol (4AP)/quinoneimine(QI) | Anti-TNFα Ab1, Ab2 | StartingBlock T20 (PBS) blocking buffer | PMMA sheet: laser-engraved, with fluidic channels punched PMMA+ FNAB: attachment with amine bond formation PMMA/FNAB+ Ab1: attachment with amine bond formation 4-aminophenyl phosphate (4APP) converted to 4AP by ALP Comb-shaped Au electrodes fabricated on Si/SiO2 wafers |
| 11. [123] | SPCE/MWCNT-IgG Ab1 Bt-Ab2 SAv-ALP | 1-naphthol (red→ox) | Anti-IL-1β Ab1, Ab2 | Caesin | CuSO4: Cu(II)→Cu(I); electrochemical reduction MWCNT(-COOH gr.) + 11-azide-3,6,9-trixaun-decan-1-amine: amide bond formation IgG + NaIO4: oxidation of 1,2-diols of glycosylated Fc region of Ab, leading to alkyne formation MWCNT drop-casted on SPCE SPCE/MWCNT(-N3 gr.) + IgG(-C≡CH gr.): azide-alkyl cycloaddition into triazole, with Cu(I) catalyst; electro-click chemistry 1-naphthyl phosphate (pNPP) converted to 1-naphthol (pNP) by ALP |
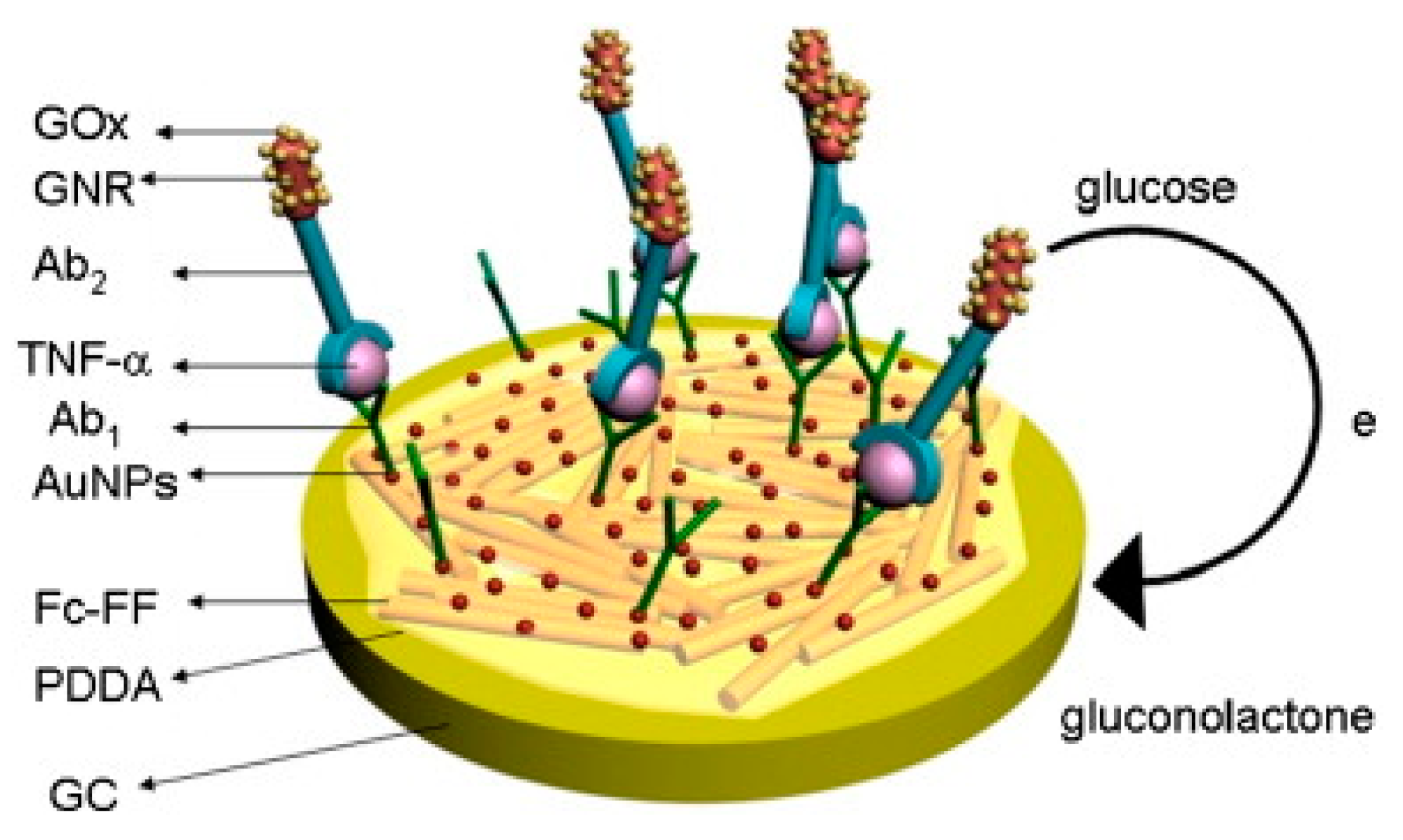
| 12. [124] | GCE/Fc-PNW/PDDA/AuNP/Ab1 GNR-GOx-Ab2 | Fe2+/3+ of Ferrocene Glucose/gluconolactone | Anti-TNFα Ab1, Ab2 | BSA | Boc-Phe-Phe-OH + H-Phe-OMe + HBTU/HOBT/CH2Cl2, Et3N → Boc-Phe-Phe-OMe; separated by column chromatography Boc-Phe-Phe-OMe + CF3COOH/CH2Cl2 → H-Phe-Phe-OMe H-Phe-Phe-OMe+ Et3N/CH2Cl2 + Fc-OBt → Fc- Phe-Phe-OMe Fc-Phe-Phe-OMe+ CF3CHOH/CH3OH → Fc-Phe-Phe-OH; self-assembly to Fc-PNW CTAB + HAuCl4+ NaBH4: Au reduction; + CTAB+ HAuCl4 + AgNO3+ C6H8O6: GNR formation GNR+ cysteine(SAM)+ glutaraldehyde + GOx, Ab2: covalent immobilization Fc-PNW coated with PDDA, AuNP & Ab1 adsorbed |
| Sl. no. Ref. | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [111] | CA | 1.0 pg/mL | 4–800 pg/mL | AFP, CEA, hIgG, L-cys, L-lys, Glucose | +IL-6/1h +HRP-Ab2-AuNP-PDOP@CNT/50 min | IL-6 in buffer Serum sample | Intra-assay precision (5 readings per run, at [IL-6] = 40 pg/mL) SD = 5.5% Inter-assay precision (5 sensor samples, at [IL-6] = 40 pg/mL) SD = 6.8% | After 30 days- 85.5% of initial signal retained | - |
| 2. [95] | Rotating disc amperometry | 10 pg/mL | 10–4000 pg/mL | - | +IL-6/1 h +Bt-Ab2/1 h +SAv-HRP/30 min | Recombinant human IL-6 (rhIL-6) in calf serum | Sensitivity: 1.6 nA/cm2 (pg/mL IL-6) | ||
| 3. [113] | DPV | 0.048 pg/mL | 0.1–10,000 pg/mL | BSA, AA, glucose, UA, IL-22 | +IFNγ/2 h +AuNP-Ab2-HRP/1 h | IFNγ in PBS Serum sample | (5 assays, at [IFNγ] = 0.1 ng/mL) RSD = 2.7% | After 2 weeks, 92% of initial signal retained | (disposable) |
| 4. [114] | CA | 1.6 pg/mL | 2.5–2000 pg/mL | AA, BSA, Glucose, Hb, hIgG, IL-1β, IL-6, IL-8, RANKL, TGF-β1, TNFα, UA | +IFNγ/60 min +Bt-Ab2/45 min +SAv-HRP/20 min | IFNγ in PBS Biological international standard (BIS) for IFNγ Saliva sample | (5 assays, same day, at [IFNγ] = 1 ng/mL) RSD = 1.8% (5 assays, different days, at [IFNγ] = 1 ng/mL) RSD = 2.6% | Within 40 days, retained signal acceptable | - |
| 5. [115] | CA | 10 pg/mL | 15–3000 pg/mL | AA’, UA, Creatinine, APN, IL-6, IL-8, TNFα | +TGF-β1/60 min +Bt-Ab2, BSA/60 min +SAv-HRP/20 min | TGF-β1 in ELISA standard solution Spiked urine | (5 assays, same day, at [TGF-β1] = 250 pg/mL) RSD = 3.9% (5 assays, different days, at [TGF-β1] = 250 pg/mL) RSD = 4.2% | Signal within control limits within 30 days | - |
| 6. [116] | CA | 0.95 pg/mL | 2.5–1000 pg/mL | AA’, APN, BSA, Cortisol, IgG, IL-1β, IL-6, IL-8, TNFα, UA | +TGF-β1/60 min +V-Phe-SWCNT(-HRP)-Ab1/60 min | TGF-β1 in ELISA standard solution Saliva sample | (5 assays, same day, at [TGF-β1] = 125 pg/mL) RSD = 3.1% (5 assays, different days, at [ TGF-β1] = 125 pg/mL) RSD = 7.2% | Signal within control limits for: (i) SPCE/SAv-Bt-Ab2, within 30 days; (ii) V-Phe-SWCNT(-HRP)-Ab1, within 14 days | - |
| 7. [117] | CA | 1.3 pg/mL | 5–200 pg/mL | APN, BSA, BR, CP, TNFα, GHRL, Hb, IL-6, IL-8, CRP, Chl, TGF-β2, TGF-β3, TGF-β5 | +TGF-β1, Bt-Ab2/60 min +SAv-HRP/20 min | TGF-β1 in ELISA standard solution Spiked human serum | (5 assays, same day, at [TGF-β1] = 125 pg/mL) RSD = 2.7% (5 assays, different days, at [TGF-β1] = 125 pg/mL) RSD = 2.5% | Signal within control limits within 40 days | - |
| 8. [118] | CA | 126.75 pg/mL | 15–1000 pg/mL | TREM-1 | +Fab1-cys/60 min + IFNγ standard+ Bt-Ab2/1 h +SAv-HRP/20 min +TMB, H2O2/20 min | IFNγ in ELISA standard solution Spiked human serum | - | - | - |
| 9. [119] | DPV | 0.01 ng/mL | 0.02–200 ng/mL | CEA, BSA, hIgG | +TNFα/1 h +PSA/PAH/AuNP-Ab2-ALP/1 h +pNPP/10 min | TNFα in buffer Human serum sample | Intra-assay precision (7 runs, at [TNFα] = 5 ng/mL) RSD=5.7% Inter-assay precision (7 assays, at [TNFα] = 5 ng/mL) RSD = 8.1% | After 1 month, 95% of initial response retained | - |
| 10. [120] | DPV | 66.8 pg/mL | 0.1–100 ng/mL | IL-2, IFNγ | +TNFα/20 min +Bt-Ab2/20 min +SAv-ALP/20 min +4APP/20 min | TNFα in spiked undiluted serum | - | Until 6 weeks, 96% of initial signal was retained, after which it dropped to 90% by 9th week | - |
| 11. [123] | DPV | 5.2 pg/mL | 1st slope: 10–200 pg/mL; 2nd slope: 200–1200 pg/mL | BR, TGF-β1, LEP, IL-8, IL-6, Hb, Chl, BSA, GHRL, TNFα | +IL-1β/1 h +Bt-Ab2/1 h +SAv-ALP/20 min +1-NPP/5 min | IL-1β standard solution Spiked saliva | (10 assays, same day, at [IL-1β] = 1 ng/mL) RSD = 5.2% (10 assays, different days, at [IL-1β] = 1 ng/mL) RSD = 6.2% | Signal within control limits within 10 days | (disposable) |
| 12. [124] | SWV | 2 pg/mL | 0.005–10 ng/mL | - | +TNFα/1 h +GNR-GOx-Ab2/1 h | TNFα in buffer Spiked serum | (6 assays, at [TNFα] = 0.1 ng/mL) RSD = 5.4% | - | - |
2.1.3. SAM-Enabled Impedimetric Immunosensors
| Sl. no. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [125] | Si/SiO2/SWCNT/AuNP/Ab | [Fe(CN)6]4−/3− | Anti-IL-6 Ab | BSA | Si/SiO2/SWCNT: prepared by ethanol chemical vapor condensation on Si/SiO2 wafer substrate Si/SiO2/SWCNT+ HAuCl4: AuNP formation by electrochemical deposition Si/SiO2/SWCNT/AuNP+ mercaptoacetic acid+ Ab: Au-S covalent bonding, amide bond formation with EDC/NHS catalyst |
| 2. [126] | Au/CMA/Ab | [Fe(CN)6]4−/3− | Anti-TNFα Ab | Ethanolamine | Au microelectrodes+ CMA: Diazotization of CMA in presence of NaNO3, HCl; electrodeposition on Au by CV Au/CMA+ Ab: covalent immobilization; amide bond formation with EDC/NHS catalyst |
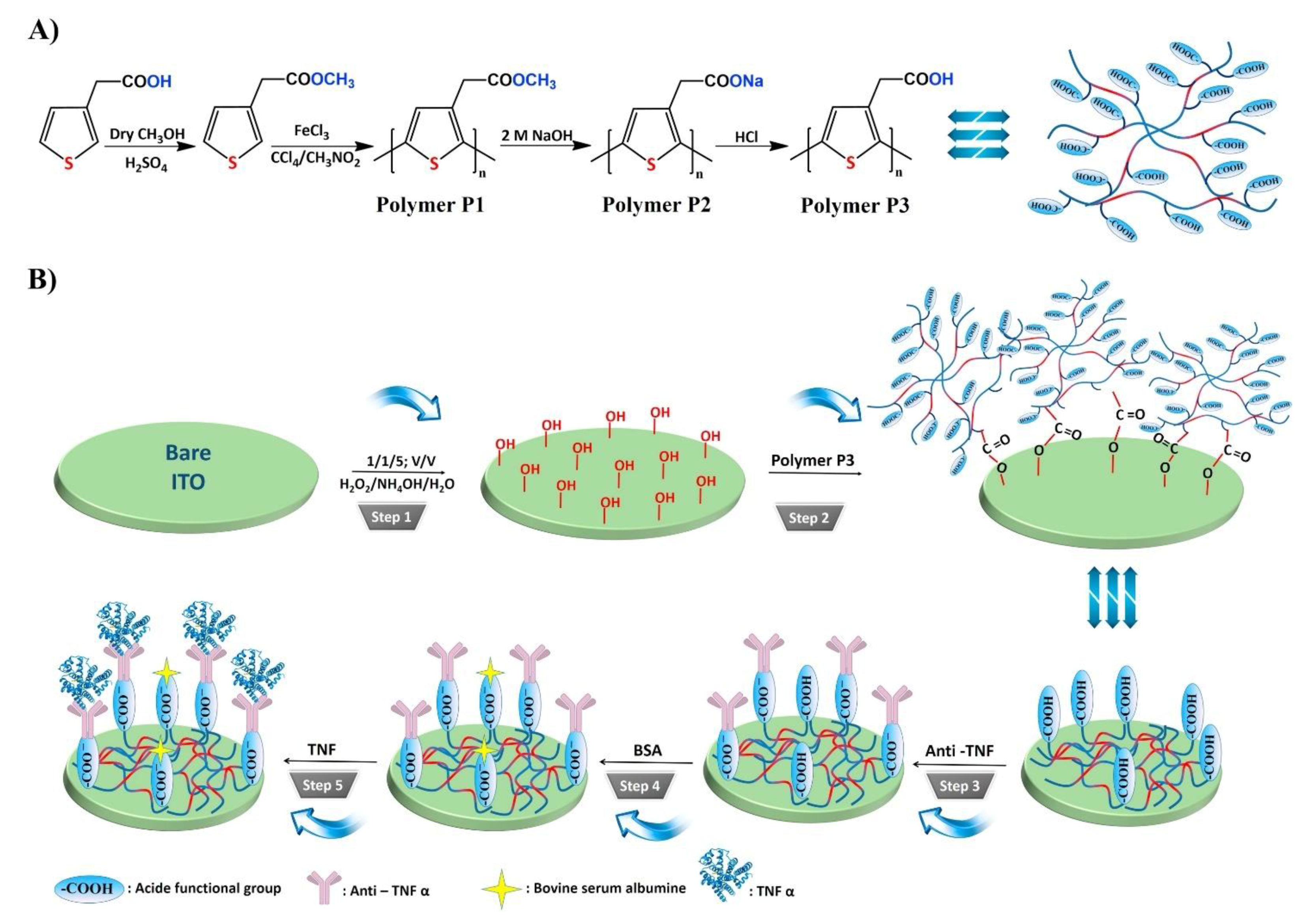
| 3. [96] | ITO/P3/Ab | [Fe(CN)6]4−/3− | Anti-TNFα Ab | BSA | ITO(-OH gr.) + P3(-COOH gr.): ester bond formation: SAM formation ITO/P3+ Ab: amide bond formation with EDC/NHS catalyst |
| 4. [127] | Au/NAC/Ab | - | Anti-IFNγ Ab | Ethanolamine | Au electrode+ acetylcysteine: SAM formation by Au-S chemistry Au/NAC+ Ab: amide bond formation with EDC/NHS catalyst |
| 5. [128] | Au/PEG/anti-TGF-β1 Ab, anti-HA Ab | [Fe(CN)6]4−/3− | Anti-TGF-β1 Ab | - | Cr-Au layered interdigited electrodes prepared with lithography + etching Au electrode+ COOH-PEG-SH: Au-S chemistry; SAM formation Au/PEG+ Ab: amide bond formation with EDC/NHS catalyst |
| 6. [130] | PI/Au-Ab | [Fe(CN)6]4−/3− | Anti-IL-10 Ab | - | Au layered on PI by soft lithography + etching Carboxyl diazonium reduced with CV, grafted on PI/Au electrode Au-COOH+ Ab: amide bond formation with EDC/NHS catalyst |
| 7. [131] | Si/HfO2/TESUD/Ab | - | Anti-IL-10 Ab | MeO-PEG-NH2 in triethylamine | HfO2 grown on Si substrate by atomic layer deposition Si/HfO2(-OH gr.) + TESUD: chemical vapor deposition, SAM formation PDMS stamp + Ab: physisorption Si/HfO2/TESUD(-CHO gr.) + PDMS/Ab(-NH2 gr.): microcontact printing; imine bond formation |
| 8. [132] | Si/Au/DSP/Ab/EA | [Fe(CN)6]4−/3− | Anti-TNFα Ab | Ethanolamine | Si/Ti/Au microelectrode arrays preparation: photolithography, etching Si/Au + DSP: DSP reduced with TCEP; immobilization with Au-S chemistry Si/Au/DSP + Ab: Amide bond formation Si/Au/DSP/Ab + EA: Amide bond formation |
| 9. [134] | Si/SiO2/Ti-Au/mcp | - | Anti-IL-8 mcp | Ethanolamine | Phage display selection of IL-8 binding Ab-mimetic capture protein mcp coding region sub-cloned in pET11 vector; expressed recombinant mcp purified Si/SiO2 layered with Ti-Au Au+ SH-(CH2)11-(OCH2CH2)6-OCH2-COOH (monothiol-alkane-PEG-acid): SAM formation with Au-S chemistry Au/SH-(CH2)11-(OCH2CH2)6-OCH2-COOH + mcp(-NH2): amide bond formation with EDC/NHS catalyst |
| 10. [135] | Si/SiO2/Ti:Au disc/Ab needle-shaped microelectrode | [Fe(CN)6]4−/3− | Anti-IL-6 Ab | 6-mercapto-1-hexanol | Si/SiO2/Ti:Au disc: lift-off lithography; reactive ion etching Ab + sulfo-LC-SPDP: amide bond formation Ab/sulfo-LC-SPDP + DTT + Au disc: Au-S chemistry |
| 11. [136] | AuNP-Ab2 Au/MUA/Ab1 Growth solution: HAuCl4 + ascorbic acid+ CTAB | [Fe(CN)6]4−/3− | Anti-IL-6 Ab1, Ab2 | BSA | AuNP: citrate reduction of HAuCl4 AuNP + Ab2: adsorption Au electrode + 11-MUA: Au-S chemistry Au/MUA+ Ab1: amide bond formation with EDC/NHS catalyst HAuCl4 + ascorbic acid + CTAB: growth of immobilized AuNP-Ab1 by reduction of HAuCl4 upon AuNP seeds; positively charged capping of AuNPs |
| Sl. no. [Ref.] | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [125] | EIS | 0.01 fg/mL | 0.01–100 fg/mL | Serum, glucose, cysteine, Epinephrine | - | IL-6 in buffer Spiked serum | - | After 1 month- initial Rct retained | - |
| 2. [126] | EIS | - | 1–15 pg/mL | rhIL-8 rhIL-1 | +TNFα/30 min | rTNFα in PBS Spiked artificial saliva Real human saliva sample | - | - | No significant change in signal after 3 subsequent detections |
| 3. [96] | EIS | 3.7 fg/mL | 0.01–2 pg/mL | Drugs- ampicillin, amoxicillin, erythromycin, clarithromycin, acetylsalicylic acid; Proteins- biotin, albumin; Biomarkers- SOX2, MAGE1, RACK1, HER2, VEGFR | 45 min | TNFα in PBS Human saliva sample Human serum sample | 80 electrodes used to draw 10 calibration plots; RSD of slopes = 2.5% | After 8 weeks, impedance decreased to 50% of initial value | Good response for 6 cycles |
| 4. [127] | EIS CA | 0.02 fg/mL | 0–12 pg/mL | IL-2 | - | rIFNγ in PBS | - | - | Removal of non-specifically adsorbed proteins/KCl solution, regeneration with: SAM wipeout/thioctic acid/potential pulses+ SAM reassembly; 10% repeatability |
| 5. [128] | EIS | 0.57 ng/mL | 1–1000 ng/mL | BSA | +TGF-β1, HA/30 min | TGF-β1 in PBS Serum sample | - | - | - |
| 6. [130] | EIS | - | 1–15 pg/mL | - | - | IL-10 in buffer | - | - | - |
| 7. [131] | EIS | 0.1 pg/mL | 0.1–20 pg/mL | TNFα, IL-1β | +IL-10/1 h | rh1L-10 in PBS | - | - | - |
| 8. [132] | EIS | ~57 fM | 1–100 pg/mL | IFNγ | +TNFα/15 min | TNFα in culture media | - | - | - |
| 9. [134] | EIS | 90 fg/mL | 0.0009–900 ng/mL | BSA | +IL-8/15 min | IL-8 in spiked horse serum | - | - | - |
| 10. [135] | DPV EIS | - - | - 0–60 pg/mL | BSA, Enterotoxin A | +IL-6/2.5 min (real-time detection) | IL-6 in PBS Spiked human serum | - | - | - |
| 11. [136] | SWV | 2 pg/mL | 0.005–50 ng/mL | hIgG, lysozyme, BSA, AFP | +IL-6/1 h +AuNP-Ab2/1 h +growth solution/1h | IL-6 in PBS | (6 assays, at [IL-6] = 10 ng/mL) RSD = 7.9% | - | - |
2.1.4. Heavy Metal Nanoparticle-Labeled Immunosensors
| Sl. no. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [97] | Ab2-AgNP-TiP Fe3O4-Ab1 | Ag0/1+ | Anti-IL-6 Ab1, Ab2 | BSA | AgNP + TiP: ion exchange AgNP-TiP + Ab2: covalent bond using mercapto or primary amino group of Ab2 Fe3O4 + Ab1: assembled with external magnet |
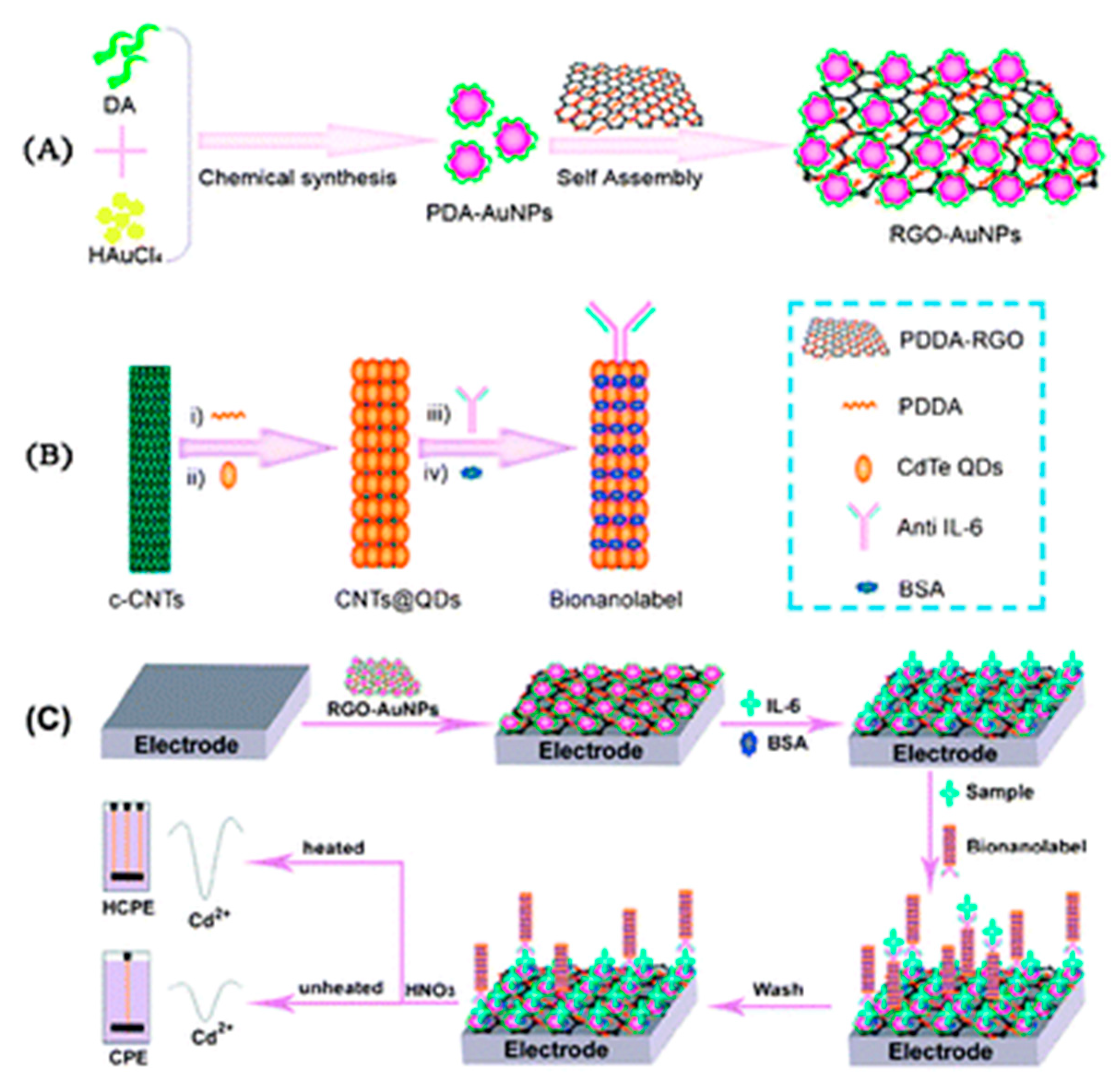
| 2. [137] | GCE/PDDA-RGO-AuNP-PDA/IL-6/BSA PDDA-CNT@CdTe QDs-Ab/BSA HCPE (working electrode) | Cd1+/2+ | Anti-IL-6 Ab | BSA | AuNP-PDA: by reduction of HAuCl4, oxidation and polymerization of dopamine (DA) PDDA-RGO + AuNP-PDA: Adsorption GCE/PDDA-RGO-AuNP-PDA + IL-6: Adsorption MPA capped CdTe QDs: prepared from MPA, CdCl2 & NaHTe CNT(-COOH gr.) + PDDA: covalent bonding PDDA-CNT + CdTe QDs (mercaptopropanoic acid capped): Adsorption PDDA-CNT@CdTe QDs + anti-IL-6 Ab: amide bond formation with EDC catalyst Competitive binding of Ab with IL-6 in sample vs. IL-6 in modified GCE; bound CdTe dissolved with HNO3, Cd2 + deposited, followed by stripping at HCPE; HCPE heated with high frequency AC at the deposition step |
| 3. [139] | Ab2/PS@PDA/AgNP HSPCE/GNR/Ab1 | Ag0/1+ | Anti-IL-6 Ab1, Ab2 | - | PS + DA (dopamine): Self polymerization of DA; adhesion of PDA to PS GNRs: prepared from GONRs (graphene oxide nanoribbons) reduced with N2H4 PS@PDA/AgNP: prepared from AgNO3 PS@PDA/AgNP + Ab2: amide bond formation with EDC/NHS catalyst HSPCE/GNR + Ab1: Adsorption; HSPCE heated with high frequency AC |
| 4. [140] | SiNP/PGMA/CdTe QD/Ab2 Au-PAB-Ab1 BFE | Cd1+/2+ | Anti-TNFα Ab1, Ab2 | BSA | SiO2 NP + APTES/toluene: SiO2-NH2 NP formation SiO2-NH2 NP + trimethylamine/toluene/BriBuBr: SiO2-Br NP formation SiO2-Br NP + dimethylformamide + GMA + CuBr: surface initiated atom transfer radical polymerization; SiNP/PGMA formation CdTe-S-CH2COOH + SiNP/PGMA (-(CH)2O gr.): ring-open reaction SiNP/PGMA/CdTe QD + Ab2: covalent immobilization with EDC/NHS catalyst |
| 5. [141] | MNP/PANI/Ab1 AuNP-Ab2-CdS NP SPCE | Cd1+/2+ | Anti-IFNγ Ab1, Ab2 | Casein/tris buffer for AuNP BSA for MNP | MNP + PANI: coating MNP/PANI + Ab1: adsorption AuNP + Ab2: adsorption CdCl2 + SHCH2COOH + Na2S → CdS NP capped with (-SCH2COOH) AuNP-Ab2 + 3’ SH-poly[A]-NH2 5’ linker + CdS NP: linkage with EDC/NHS catalyst; Au-S chemistry MNP-IFNγ separated magnetically; resuspended in HNO3 + Bi/acetate buffer |
| Sl. no. Ref. | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [97] | Electrodeposition followed by stripping with DPV | 0.1 pg/mL | 0.0005–10 ng/mL | TNF-α, hIgG, CEA | +IL-6/40 min +Ab2-AgNP-TiP/40 min | IL-6 in PBS Spiked serum | (2 assays) RSD = 8.0% | After 2 months- 94.2% of initial signal retained | - |
| 2. [137] | ASV | 0.033 pg/mL for HCPE | 0.1–100 pg/mL for HCPE | CEA, CRP, TNF-α, BSA | +IL-6, +CNT@CdTe QDs-Ab/40 min +dissolution of Cd2 + with HNO3/30 min | IL-6 in buffer Spiked serum | (5 assays, at [IL-6] = 10 pg/mL) RSD = 4.1% | After 1 week- >90% of initial signal retained | - |
| 3. [139] | Stripping SWV | 0.1 pg/mL | 0.001–1000 ng/mL | BSA, CEA, CTnI, IgG | +IL-6/50 min +Ab2/PS@PDA/AgNP/50 min | IL-6 in buffer Spiked serum | (5 assays) initial signal significantly retained at [IL-6] = 0.1 ng/mL | After 2 weeks- initial signal not significantly changed | - |
| 4. [140] | SWV | 3.0 pg/mL | 0.01–100 ng/mL | - | AuNP-PAB-Ab1 + TNFα/45 min +SiNP/PGMA/CdTe QD/Ab2/45 min | TNFα in buffer | Intra-assay precision (4 runs) CV=5.1% Inter-assay precision (4 assays) CV = 6.7% | After 2 weeks, no apparent change in signal | After 6 cycles & regeneration/glycine-HCl, 95.7% of initial signal retained |
| 5. [141] | SWASV | 0.4 pg/mL | 0.01–1 IU/mL | - | IFNγ + MNP/PANI/Ab1/20 min +BSA blocking/5 min +AuNP-Ab2-CdS NP/20 min +HNO3/10 min +Bi electrodeposition/10 min | IFNγ in PBS | - | - | - |
2.1.5. Redox-Labeled Immunosensors
| Sl. no. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [98] | GCE/GO-Ab1 CaCO3/Fc-PPN-Ab2 | Fe2+/3 + of Fc | Anti-IL-6 Ab1, Ab2 | BSA | CaCO3 + PDDA + PSS + Fc: Adsorption CaCO3/Fc-PPN + Ab2: Electrostatic Adsorption GCE/GO + Ab1: Amide bond formation with EDC/NHS catalyst |
| 2. [142] | CeO2/CS-PB-Ab2 GCE/CNT/PDDA/AuNP/Ab1 | PB (Fe2+/2+/Fe2+/3+/Fe3+/3+) Ce3+/4+ H2O2/H2O | Anti-TNFα Ab1, Ab2 | BSA | CeO2 NP/CS (+ve) + Fe(CN)63−: Adsorption CeO2/CS/Fe(CN)63− + FeCl2: PB formation CeO2/CS-PB + Ab2 + glutaraldehyde: imine bond formation; CS-Ab2 cross-linking AuNP: citrate reduction of HAuCl4 CNT(-COOH gr.) + PDDA(+ve) + AuNP(-ve) + Ab1: Adsorption |
| 3. [143] | GCE/K-CS-GA/Ab | [Fe(CN)6]4−/3− | Anti-TNFα Ab | BSA | CS (-NH2 gr.) + GA (-CHO gr.) + K3[Fe(CN)6]: crosslinking with imine bond formation; doping with K3[Fe(CN)6] N + Ab: physical or electrostatic adsorption |
| 4. [144] | GCE/ZnO NP/Ab | [Fe(CN)6]4−/3− | Anti-IFNγ Ab | BSA | Zn(NO3)2 + H2N2 → ZnO NP ZnO NP + Ab: Adsorption ZnO NP/Ab + GCE: drop-casting GCE/ZnO NP/Ab + Nafion: top-coating |
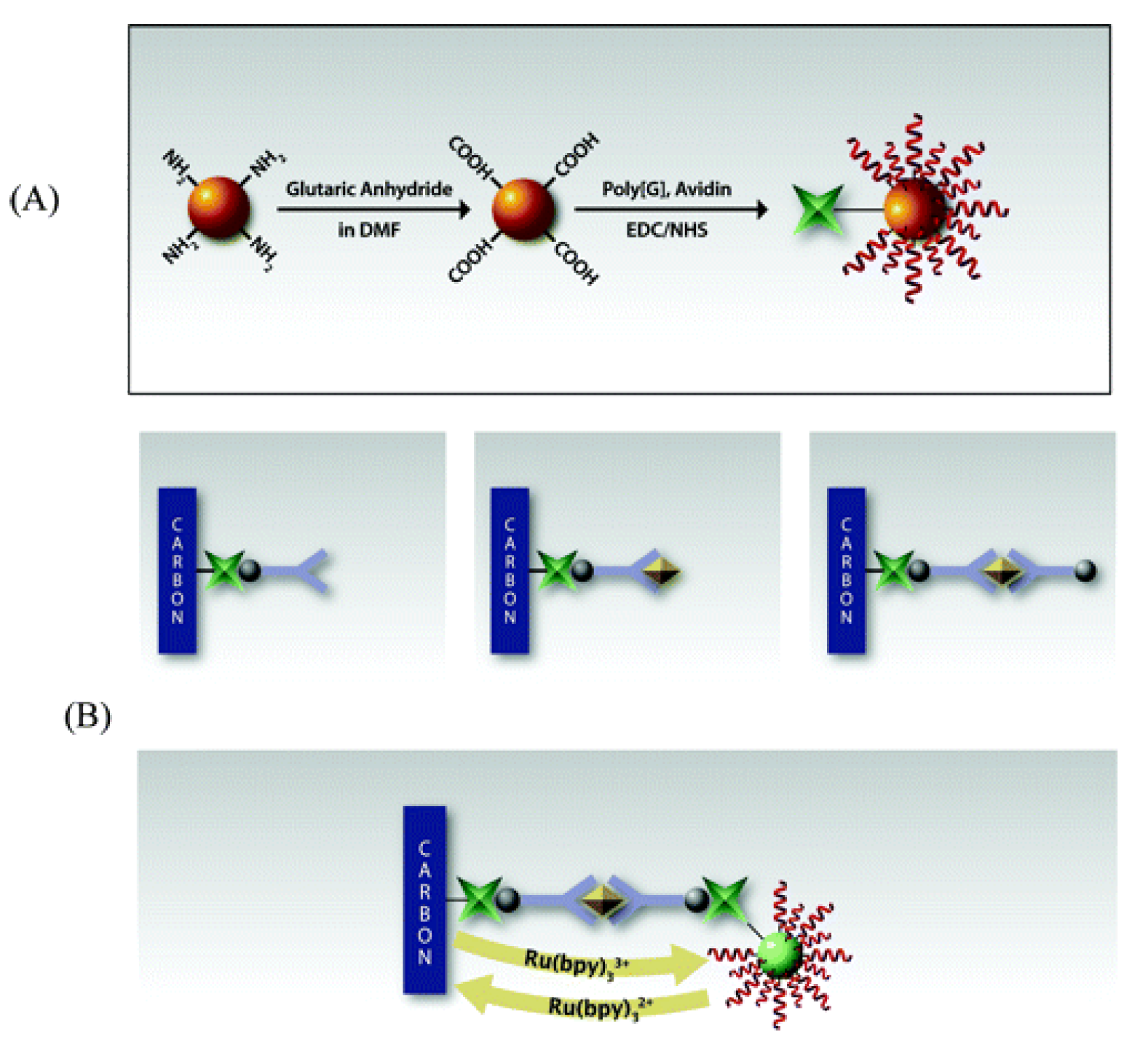
| 5. [145] | SPCE/Av/Bt-Ab1 Bt-Ab2 SiNP/poly[G]/Av | Guanine (irreversible oxidation) Tris(bipyridine)ruthenium(II) chloride or Ru(bpy)32+/3+ | Anti-TNFα Ab1, Ab2 | BSA | SiNP(-NH2 gr.) + glutaric anhydride + dimethylformamide: SiNP(-COOH gr.) SiNP(-COOH gr.) + Av, poly[G]: covalent immobilization with EDC/NHS catalyst Bt-Ab1 + Av/SPCE: affinity bonding |
| Sl. no. Ref. | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [98] | SWV | 1 pg/mL | 0.002–20 ng/mL | hIgG, Lysozyme, AFP, PSA | +IL-6/1 h +CaCO3/FC-PPN-Ab2/1 h | IL-6 in PBS Spiked serum | (6 assays) RSD = 5.8% | After 30 days- 90% of initial signal retained | 92.3% of initial signal retained after 6 regeneration cycles |
| 2. [142] | CA | 2 pg/mL | 0.005–5 ng/mL | PSA, AFP, CEA | +TNFα/1 h +CeO2/CS-PB-Ab2/1 h | TNFα in buffer | Intra-assay precision (5 runs, at [TNFα] = 1 ng/mL) RSD = 4.6% Inter-assay precision (5 assays, at [TNFα] = 1 ng/mL) RSD = 7.5% | After 2 weeks, negligible current variation | - |
| 3. [143] | CV | 10 pg/mL | 0.02–34 ng/mL | CA-125, CA-153, CA 19–9, AFP | +TNFα/1 h | TNFα in PBS Human serum sample | (5 assays, at [TNFα] = 1 ng/mL) RSD = 3.2% | - | - |
| 4. [144] | EIS | 0.12 pg/mL | 0.0001–0.1 ng/mL | - | +IFNγ/80 min | rIFNγ in PBS Spiked bovine serum | Intra-assay precision (6 runs, at [IFNγ] = 0.01ng/mL) CV = 4.1% Inter-assay precision (6 assays, at [IFNγ] = 0.01ng/mL) CV = 3.9% | After 60 days, no apparent change in Rct | - |
| 5. [145] | SWV | 2 pM | - | - | +TNFα/45 min + Bt-Ab2/45 min +SiNP/poly[G]/Av/45 min | TNFα in PBS | (6 runs, at [TNFα] = 1 ng/mL) RSD = 9.8% | - | - |
2.1.6. Magnetoimmunosensors
| Sl. no. [Ref.] | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
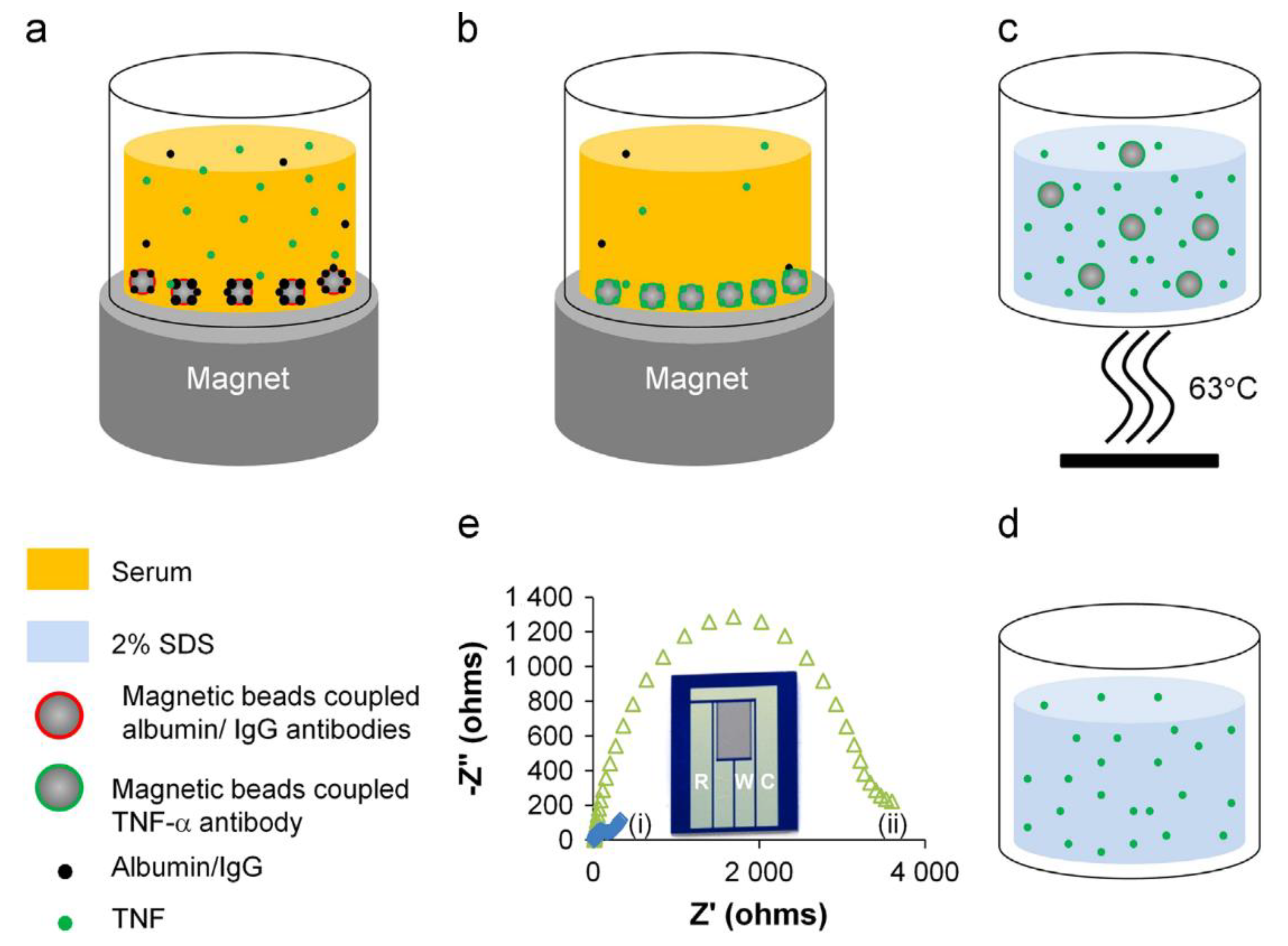
| 1. [99] | Mb/anti-albumin Ab Mb/anti-IgG Ab Mb/anti-TNFα Ab Si/SiO2/CSGM | [Fe(CN)6]4−/3− | Anti-TNFα Ab | - | Mb(-COOH gr.) + anti-(albumin/IgG/TNFα) Ab: amide bond formation with EDC/NHS catalyst Si/SiO2/CSGM preparation: photolithography TNFα elution with SDS in Tris |
| 2. [146] | Mb/Ab1 Bt-Ab2 SAv-HRP SPCE | Hydroquinone/Benzoquinone H2O2/H2O | Anti-TNFα Ab1, Ab2 | Ethanolamine | Mb(-COOH gr.) + Ab1, Mb(-COOH gr.) + ethanolamine, Ab2 + Bt, SAv + HRP : amide bond formation with EDC/NHS Mb/Ab1: magnetically captured onto SPCE |
| 3. [147] | SPGE (with magnetic bars) Mb/Protein G/Ab1 Bt-Ab2 SAv-ALP | 1-naphthol (1-NP) (red → ox) | Anti-TNFα Ab1, Ab2 | Rabbit IgG | Mb/Protein G + Ab1: affinity bonding Bt-Ab2 + SAv-ALP: affinity bonding Mb captured onto SPGE with magnetic bars 1-naphthyl phosphate (1-NPP) converted to 1-naphthol by ALP |
| Sl. no. [Ref.] | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [99] | EIS | 1 pg/mL | 1–1000 pg/mL | IL-2 | +Mb/anti-(albumin & IgG) Ab/1 h +Mb/anti-TNFα Ab/1 h +TNFα elute on CSGM | TNFα in PBS Spiked human serum | - | - | - |
| 2. [146] | CA | 2.0 pg/mL (standard solution) 5.8 pg/mL (spiked serum) | - | hIgG, BSA | +TNFα/1 h +Bt-Ab2/1 h +SAv-HRP/10 min | rTNFα in PBS-Tween20 Spiked human serum | (5 assays, same day) RSD = 5.7% (10 assays, different days) RSD = 7.5% | After 2 weeks, no significant decrease in S/N (signal/noise) ratio | - |
| 3. [147] | DPV | 0.044 ng/mL | - | - | Mb +TNFα +Bt-Ab2/2 h +SAv-Ab1/20 min +pNPP/5 min | TNFα in PBS-Tween20 | In array (8 assays) RSD = 4%; Batch-to-batch (24 assays) RSD = 6% | - | (disposable) |
2.1.7. Flow-Injection/Microfluidic Immunosensors
| Sl. no. [Ref.] | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [101] | HRP-Ab-AuNP/BSA/SPGE | Thionine (TH/TH(H+)) H2O2/H2O | Anti-IL-6 Ab | BSA | HRP-Ab + AuNP + BSA: entrapment, covalent bonding with glutaraldehyde |
| 2. [148] | Au/cysteamine/BDE/Ab | - | Anti-IL-6 Ab | 1-dodecanethiol | Au rods + cysteamine + BDE + Ab: Au-S bond formation; cross-linking of Ab and cysteamine with BDE |
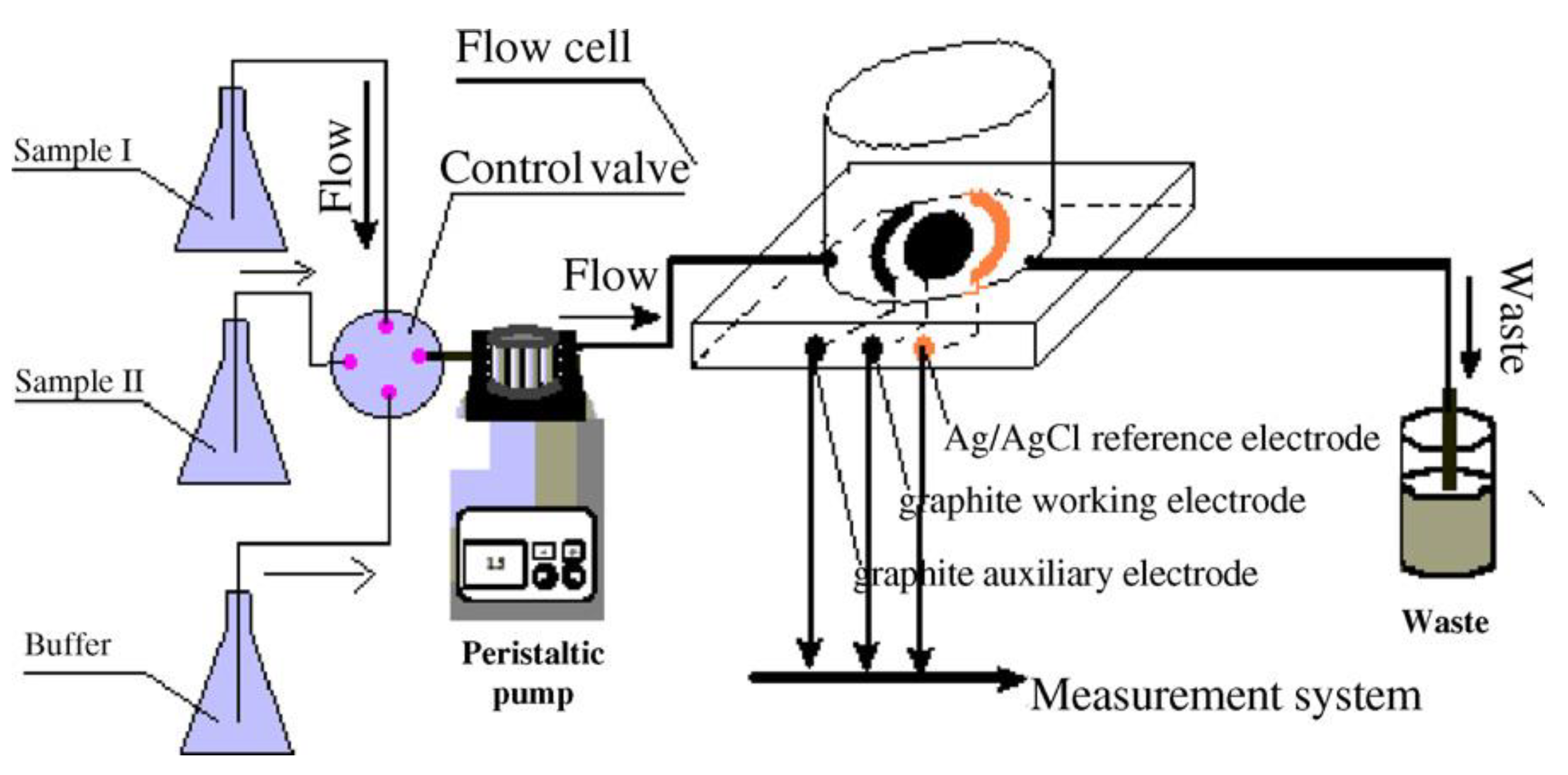
| 3. [149] | APCPG/Ab1 Au electrode Bt-Ab2 SAv-ALP Carrier stream: PBS with skim milk | p-aminophenol (pAP)/p-benzoquinoneimine (QI) | Anti-IL-6 Ab1, Ab2 | Skim milk | APCPG + glutaraldehyde + Ab1: imine bond formation Bt + SAv: Affinity bonding pAP converted from p-aminophenyl phosphate (pAPP) |
| 4. [150] | SPPE/PANI-G/Ab | [Fe(CN)6]4−/3− | Anti-IFNγ Ab | BSA | Paper (wax-printed) + aniline/H2SO4: Electropolymerization by CV PANI-G + Ab: amide bond formation with EDC/NHS catalyst |
| Sl. no. [Ref.] | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [101] | DPV | 1.0 ng/L | 5–100 ng/L | - | +IL-6/45 min +washing, enzymatic reaction & detection/~5 min | IL-6 in acetate buffer IL-6 in serum specimen | Intra-assay precision (5 runs, at [IL-6] = 50 ng/L) CV = 4.7% Inter-assay precision (5 assays, at [IL-6] = 50 ng/L) CV = 5.4% | After 10 days- 77.6% of initial signal retained | (one-time use, disposable biosensor) |
| 2. [148] | Potentiostatic detection | - | 0.5 fM–0.5 pM | IL-2 | 10 min | rIL-6 in PBS/Tween20/NaN3 | 30–40% | - | (sensor cannot be regenerated) |
| 3. [149] | CA | 0.41 pg/mL | - | - | 25 min = serum sample+ Bt-Ab2/5 min +washing/3 min +SAv-ALP/5 min +washing/3 min +pAPP/5 min +detection/2 min | IL-6 standard solution (ELISA kit) IL-6 in human serum sample | Intra-assay precision (5 runs, at [IL-6] = 200 pg/mL) CV = 2.74% Inter-assay precision (5 assays, at [IL-6] = 200 pg/mL) CV = 5.62% | - | sensor could be used for ≥ 100 determinations following regeneration with glycine-HCl desorption buffer+ PBS washing |
| 4. [150] | EIS | 3.4 pg/mL | 5–1000 pg/mL | BSA | +IFNγ/30 min | rIFNγ in PBS Spiked human serum (proteins precipitated with CCl3COOH) | RSD < 5% (5 assays) | After 2 weeks, 94% of initial Rct retained | (disposable) |
2.1.8. FET-Based Biosensors
| Sl. No. [Ref.] | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
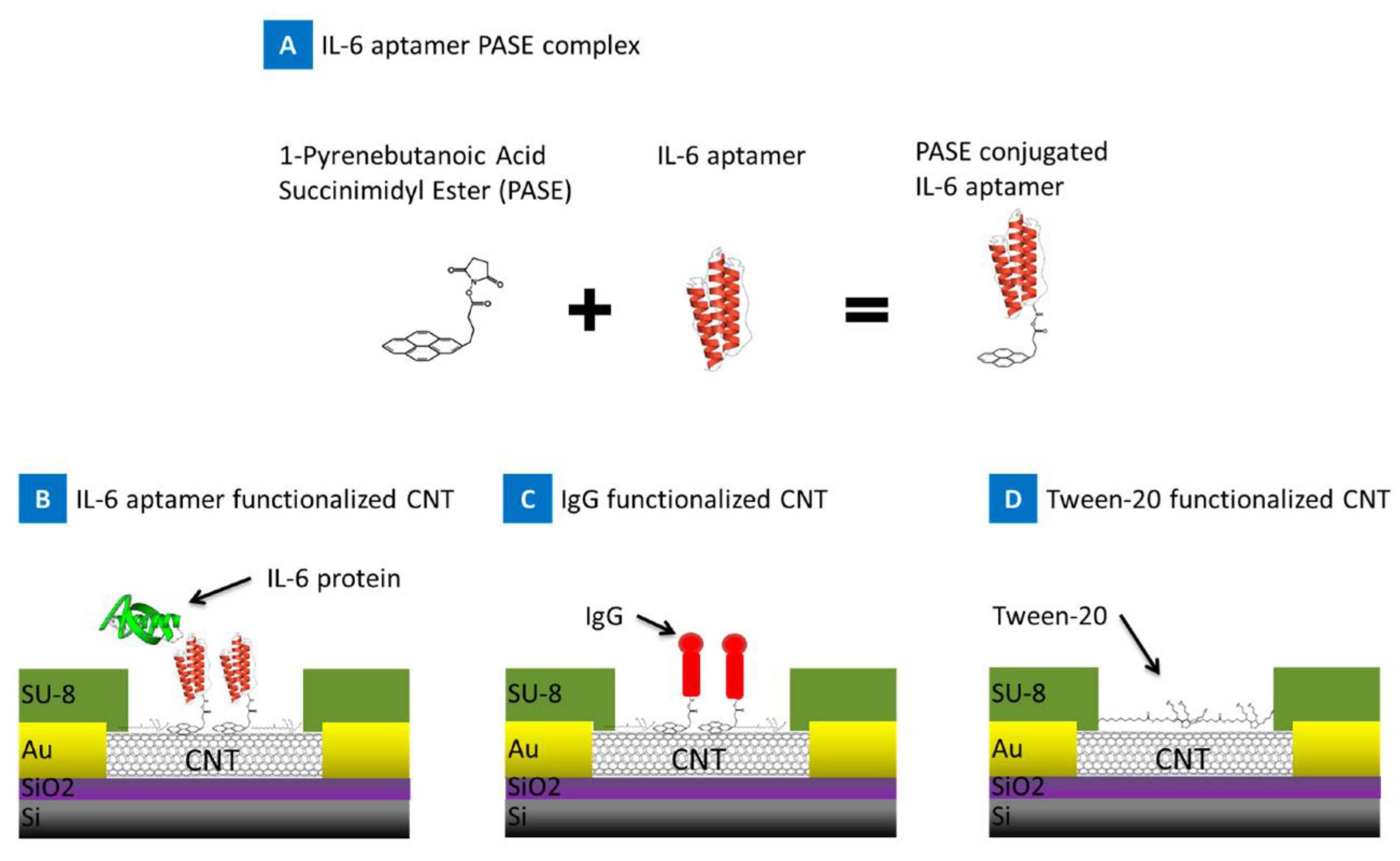
| 1. [151] | SiO2/GO-Ethanol/Ab liquid-gate | - | Anti-IL-6 Ab | BSA Ethanolamine | SiO2 + APTES + GO: Silanization, electrostatic attachment SiO2/GO + Ethanol: Carbon deposition on the edges of GO flakes by Ethanol chemical vapor deposition SiO2/GO-Ethanol + 1-pyrenebutanoic acid, succinimidyl ester + Ab: Amide bond formation, covalent attachment of Ab |
| 2. [100] | Si/SiO2/SWCNT/PASE/Apt liquid gate Ni/Au/Si/SiO2 source and drain electrodes | - | Anti-IL-6 RNA Apt | - | SWCNT + PASE: pyrene rings adsorption onto sidewalls of nanotube SWCNT/PASE + 5’-NH2 modified Apt: amide bond formation CNT + Si/SiO2: photolithography, etching |
| 3. [152] | PDMS/G/Apt gate | - | Anti-IFNγ Apt | - | Graphene grown with low pressure chemical vapor deposition Source & drain electrodes placed on Ag terminals; VG applied w.r.t. Ag/AgCl 5’ pyrene-Apt (DNA) + G: π-stacking; immobilization |
| Sl. No. [Ref.] | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [151] | FET based detection | 4.7 pg/mL | - | - | - | IL-6 in buffer | - | - | - |
| 2. [100] | FET based detection | 1 pg/mL | 1–100 pg/mL | BSA, PBS | (real-time detection) | IL-6 in MgCl2/PBS Spiked blood | - | - | - |
| 3. [152] | FET based detection | 83 pM | ~0 nM- ~10 µM | BSA, Papain | - | rIFNγ in PBS | - | - | - |
2.1.9. Biosensors for Multiplexed Cytokine Detection
| Sl. No. Ref. | Transducer Components | Redox Species | Bio-Recognition Element | Blocking Agent | Preparation and Immobilization (Brief) |
|---|---|---|---|---|---|
| 1. [153] | GCE/Graphene-Chitosan/AuNP/anti-IL-6 Ab1, anti-IL-17 Ab1 PS-Cd2+/PDDA/AuNP/anti-IL-6 Ab2 PS-Fc/PDDA/AuNP/anti-IL-17 Ab2 | Cd1+/2+ Fe2+/3 + of Ferrocene | Anti-IL-6 Ab1, Ab2 Anti-IL-17 Ab1, Ab2 | BSA | PVP/C2H5OH + AIBN + St + Cd(NO3)2: synthesis of PS-Cd2+ PVP/C2H5OH + AIBN + St + Fc: synthesis of PS-Fc PS-Cd2 + or PS-Fc + PDDA: coating PS/PDDA(+ve) + AuNP(-ve): electrostatic adsorption PS/PDDA/AuNP + Ab2 (anti-IL-6 for PS-Cd2 + & anti-IL-17 for PS-Fc): adsorption Graphene + Chitosan + AuNP: functionalization Graphene-Chitosan/AuNP + anti-IL-6 Ab1, anti-IL-17 Ab1: adsorption; drop-casted on GCE Tetrahydrofuran (THF) added to sensor for PS dissolution, followed by evaporation |
| 2. [154] | (1) SiNW/APTES-Glu-anti-IL-6 Ab gate (2) SiNW/APTES-Glu-anti-TNFα Ab gate | - | Anti-IL-6 Ab Anti-TNFα Ab | Ethanolamine | SiNW fabricated with top-down method SiNW (surface layer SiO2) + APTES: silanization SiNW/APTES(-NH2 gr.) + Glu(-CHO gr.): imine bond formation SiNW/APTES/Glu(-CHO gr.) + Ab(-NH2 gr.): imine bond formation IL-6, TNFα secreted: (i) by macrophage cell line, stimulated by bacterial endotoxin lipopolysaccharide (LPS), measured every 3 h; (ii) in rat serum stimulated by LPS |
| 3. [155] | (1) Si/SiO2/Ti-Ni-Au/CMA/anti-IL-10 Ab (2) Si/SiO2/Ti-Ni-Au/CMA/anti-IL-1β Ab | [Fe(CN)6]4−/3− | Anti-IL-10 Ab Anti-IL-1β Ab | - | Ti, Ni, Au trilayer deposited on SiO2/Si by physical vapor deposition; microelectrode designed by photolithography + etching Si/SiO2/Ti-Ni-Au + CMA: reduction, grafting with CV Si/SiO2/Ti-Ni-Au/CMA + Ab: amide bond formation with EDC/NHS catalyst |
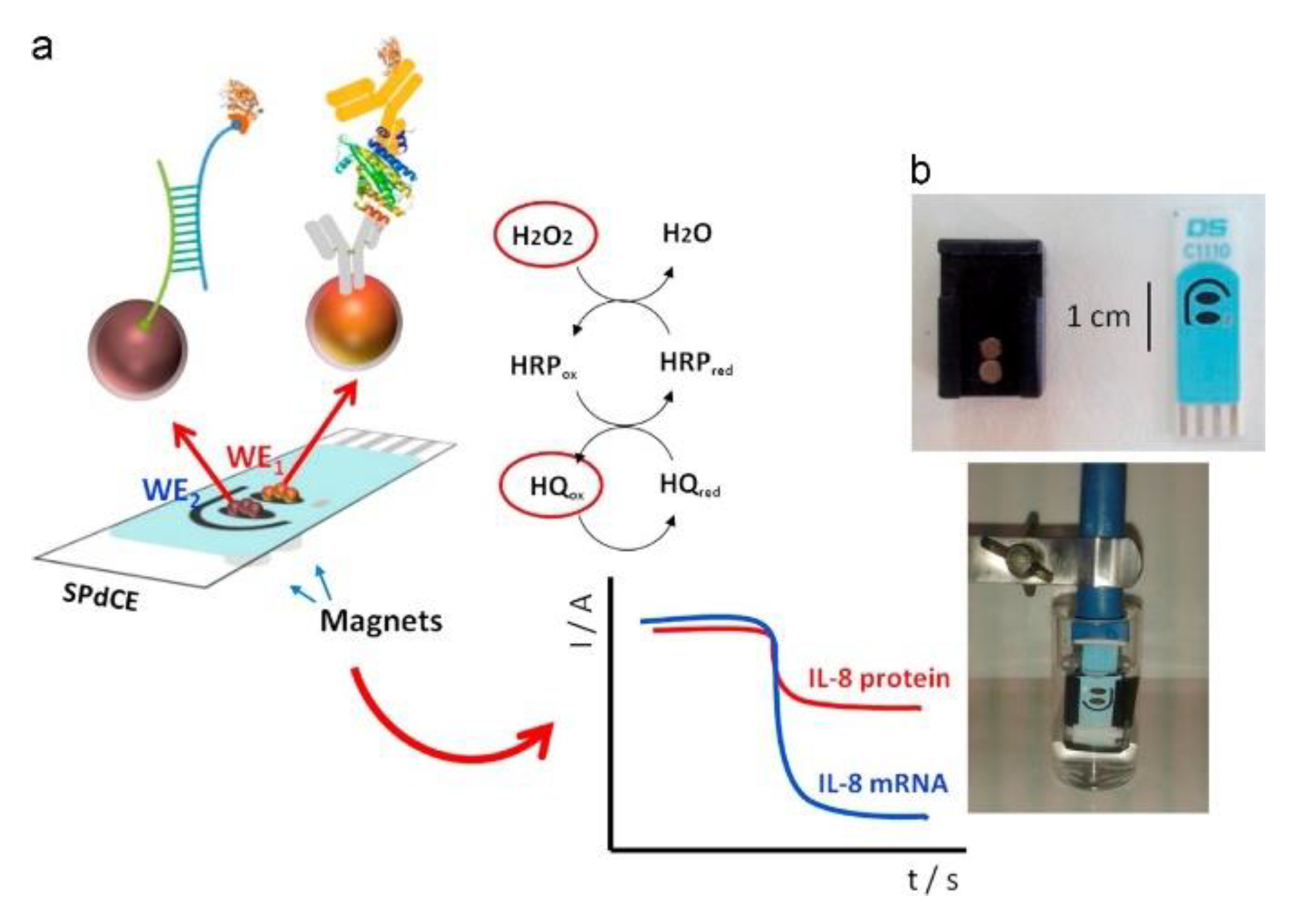
| 4. [156] | SPdCE (1) IL-8 protein WE: Mb-Ab Bt-Ab2 SAv-HRP (2) IL-8 mRNA WE: Mb-SAv Bt-hcDNA SAv-HRP Bt-tDNA | Hydroquinone/benzoquinone H2O2/H2O | Ab, hcDNA | Ethanolamine | Mb-COOH + Ab1(-NH2 gr.): amide bond formation with EDC/NHS catalyst Bt + SAv: affinity bonding mRNA associated synthetic oligonucleotide (tDNA) biotinylated separately Mbs separated from sample with magnetic separator; immobilized on SPdCE with neodymium magnet |
| Sl. No. Ref. | Detection Technique | Limit of Detection | Range of Detection | Interfering Species Tested | Incubation Time | Sample Type | Reproducibility | Stability | Repeatability |
|---|---|---|---|---|---|---|---|---|---|
| 1. [153] | SWV | IL-6: 0.5 pg/mL IL-17: 1 pg/mL | IL-6: 1–1000 pg/mL IL-17: 2–1000 pg/mL | PSA, hIgG, TNFα | +IL-6, IL-17/1 h +PS-Cd2+/Ab2, PS-Fc-Ab2/1 h | IL-6, IL-17 in buffer Human serum sample | (5 assays, at [IL-6] = 10 pg/mL, [IL-17] = 10 pg/mL) RSD ≤ 4.8% | - | - |
| 2. [154] | FET based detection | IL-6: 100 fg/mL TNFα: 100 fg/mL | - | Leptin, Resistin | (real-time) | TNFα, IL-6 in: PBS DMEM/FBS culture media Rat serum sample | - | - | - |
| 3. [155] | EIS | IL-10: 0.3 pg/mL IL-1β: 0.7 pg/mL | IL-10: 1–15 pg/mL IL-1β: 1–15 pg/mL | IL-6 | +IL-10 or IL-1β/30 min | IL-10 or IL-1β in buffer | - | - | - |
| 4. [156] | CA | IL-8 mRNA: 0.21 nM IL-8 protein: 72.4 pg/mL (undiluted saliva) | - | (1) IL-8 mRNA WE: Non-complementary DNA, single base-mismatched DNA (2) IL-8 protein WE: IL-6, lysozyme, LPO | (1) Mb-SAv+ Bt-hcDNA/60 min + IL-8, Bt-tDNA/30 min +SAv-HRP/15 min (2) Mb-Ab1+ IL-8, Bt-tDNA/30 min +Bt-Ab2/30 min +SAv-HRP/45 min | IL-8, Bt-tDNA in: PBS-Tween20 Spiked saliva Undiluted saliva samples | (5 assays, at [IL-8 mRNA] = 2.5 nM, [IL-8 protein] = 600 pg/mL, PBST buffer) RSDIL-8 mRNA = 7.7% RSDIL-8 protein = 8.3% | Signal within control limits: Within 11 days for IL-8 mRNA; Within 30 days for IL-8 protein | (disposable) |
3. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.A.; Punt, J.; Stranford, S.A. Kuby Immunology, 7th ed.; W.H. Freeman: New York, NY, USA, 2013; ISBN 9781429219198. [Google Scholar]
- Liles, W.; Van Voorhis, W. Nomenclature and Biologic Significance of Cytokines Involved in Inflammation and the Host Immune Response. J. Infect. Dis. 1995, 172, 1573–1580. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Molares-Vila, A.; Sánchez-Blanco, E.; Sánchez-Blanco, B. Study on Certain Biomarkers of Inflammation in Psoriasis Through “OMICS” Platforms. Open Biochem. J. 2014, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2016. [Google Scholar]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C. A Historical Review of Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Chalupa, P.; Beran, O.; Herwald, H.; Kaspříková, N.; Holub, M. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection 2011, 39, 411–417. [Google Scholar] [CrossRef]
- Holub, M.; Lawrence, D.A.; Andersen, N.; Davidová, A.; Beran, O.; Marešová, V.; Chalupa, P. Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Dhar, S.K.; Vishnupriyan, K.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef]
- Prendergast, G.C. Immune escape as a fundamental trait of cancer: Focus on IDO. Oncogene 2008, 27, 3889–3900. [Google Scholar] [CrossRef]
- Navegantes, K.C.; Gomes, R.S.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 1–21. [Google Scholar] [CrossRef]
- Loo, S.W.; Pui, T.S. Cytokine and cancer biomarkers detection: The dawn of electrochemical paper-based biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Okada, R.; Suzuki, Y.; Takagi, M.; Yamazaki, T.; Sumi, T.; Aoki, T.; Ohnuma, S.; Aoki, T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer 2005, 8, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Lee, S.; Kim, H.M.; Chun, S.; Engleman, E.G.; Kim, H.C.; Kang, E.S. A novel type of blood biomarker: Distinct changes of cytokine-induced stat phosphorylation in blood t cells between colorectal cancer patients and healthy individuals. Cancers 2019, 11, 1157. [Google Scholar] [CrossRef]
- Asselin-Paturel, C.; Echchakir, H.; Carayol, G.; Gay, F.; Opolon, P.; Grunenwald, D.; Chouaib, S.; Mami-Chouatb, F. Quantitative analysis of Th1, Th2 and TGF-β1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int. J. Cancer 1998, 77, 7–12. [Google Scholar] [CrossRef]
- Meyer-Siegler, K.L.; Leifheit, E.C.; Vera, P.L. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer 2004, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: Implications for survival. Cancer Immunol. Immunother. 2006, 55, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.; Isenberg, D.A. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000, 43, 1431–1442. [Google Scholar] [CrossRef]
- Morahan, G.; Huang, D.; Ymer, S.I.; Cancilla, M.R.; Stephen, K.; Dabadghao, P.; Werther, G.; Tait, B.D.; Harrison, L.C.; Colman, P.G. Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL12B allele. Nat. Genet. 2001, 27, 218–221. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. Immunotherapy of autoimmunity and cancer: The penalty for success. Nat. Rev. Immunol. 2008, 8, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.; Stahl, M.; Seehra, J.S. 1.9 A crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997, 16, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorganic Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Swardfager, W.; Lanctt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef]
- Cizza, G.; Marques, A.H.; Eskandari, F.; Christie, I.C.; Torvik, S.; Silverman, M.N.; Phillips, T.M.; Sternberg, E.M. Elevated Neuroimmune Biomarkers in Sweat Patches and Plasma of Premenopausal Women with Major Depressive Disorder in Remission: The POWER Study. Biol. Psychiatry 2008, 64, 907–911. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Heikkilä, K.; Ebrahim, S.; Lawlor, D.A. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur. J. Cancer 2008, 44, 937–945. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Yamagishi, J.-I.; Kawashima, H.; Matsuo, N.; Ohue, M.; Yamayoshi, M.; Fukui, T.; Kotani, H.; Furuta, R.; Nakano, K.; Yamada, M. Mutational analysis of structure-activity relationships in human tumor necrosis factor-alpha. Protein Eng. Des. Sel. 1990, 3, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hung, M.C.; Klostergaard, J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry 1996, 35, 8216–8225. [Google Scholar] [CrossRef]
- Van Deventer, S.J.H. Tumour necrosis factor and Crohn’s disease. Gut 1997, 40, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Gracia, J.M.; Zvaifler, N.J.; Firestein, G.S. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheu. J. Clin. Investig. 1990, 86, 1790–1798. [Google Scholar] [CrossRef]
- Bal, A.; Unlu, E.; Bahar, G.; Aydog, E.; Eksioglu, E.; Yorgancioglu, R. Comparison of serum IL-1β, sIL-2R, IL-6, and TNF-α levels with disease activity parameters in ankylosing spondylitis. Clin. Rheumatol. 2007, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Ohmoto, Y.; Mizutani, T.; Murata, M.; Shimizu, M. Role of increased production of monocytes TNF-α, IL-1β and IL-6 in psoriasis: Relation to focal infection, disease activity and responses to treatments. J. Dermatol. Sci. 1997, 14, 145–153. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Ijzermans, J.N.M.; Marquet, R.L. Interferon-gamma: A Review. Immunobiology 1989, 179, 456–473. [Google Scholar] [CrossRef]
- Walter, M.R.; Windsor, W.T.; Nagabhushan, T.L.; Lundell, D.J.; Lunn, C.A.; Zauodny, P.J.; Narula, S.K. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature 1995, 376, 230–235. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Buntinx, M.; Ameloot, M.; Steels, P.; Janssen, P.; Medaer, R.; Geusens, P.; Raus, J.; Stinissen, P. Interferon-γ-induced calcium influx in T lymphocytes of multiple sclerosis and rheumatoid arthritis patients: A complementary mechanism for T cell activation? J. Neuroimmunol. 2002, 124, 70–82. [Google Scholar] [CrossRef]
- Skurkovich, S.; Boiko, A.; Beliaeva, I.; Buglak, A.; Alekseeva, T.; Smirnova, N.; Kulakova, O.; Tchechonin, V.; Gurova, O.; Deomina, T.; et al. Randomized study of antibodies to IFN-γ and TNF-α in secondary progressive multiple sclerosis. Mult. Scler. 2001, 7, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kabeer, B.S.A.; Paramasivam, P.; Raja, A. Interferon gamma and interferon gamma inducible protein-10 in detecting tuberculosis infection. J. Infect. 2012, 64, 573–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakefield, L.M.; Smith, D.M.; Broz, S.; Jackson, M.; Levinson, A.D.; Sporn, M.B. Recombinant TGF-β1 is synthesized as a two-component latent complex that shares some structural features with the native platelet latent TGF-β1 complex. Growth Factors 1989, 1, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Bommireddy, R.; Doetschman, T. TGFβ1 and Treg cells: Alliance for tolerance. Trends Mol. Med. 2007, 13, 492–501. [Google Scholar] [CrossRef]
- Wakefield, L.M.; Letterio, J.J.; Chen, T.; Danielpour, D.; Allison, R.S.; Pai, L.H.; Denicoff, A.M.; Noone, M.H.; Cowan, K.H.; O’Shaughnessy, J.A. Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin. Cancer Res. 1995, 1, 1. [Google Scholar]
- Bauer, M.; Schuppan, D. TGFβ1 in liver fibrosis: Time to change paradigms? FEBS Lett. 2001, 502, 1–3. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, X.; Zeng, Y.; Zhu, Y.; Zhang, Z.; Liu, Y.; Wang, J.; Li, X.; Bi, J.; Kong, C. TGF-β1 promotes the migration and invasion of bladder carcinoma cells by increasing fascin1 expression. Oncol. Rep. 2016, 36, 977–983. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Boche, D.; Cunningham, C.; Docagne, F.; Scott, H.; Perry, V.H. TGFβ1 regulates the inflammatory response during chronic neurodegeneration. Neurobiol. Dis. 2006, 22, 638–650. [Google Scholar] [CrossRef]
- Monastero, R.N.; Pentyala, S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int. J. Inflam. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.A.; Masada, M.P.; Kaldahl, W.B.; DuBois, L.M.; Kornman, K.S.; Choi, J.-I.; Kalkwarf, K.L.; Allison, A.C. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J. Clin. Periodontol. 1993, 20, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, N.C.; Guo, R.F.; Hollmann, T.J.; Gao, H.; Neff, T.A.; Reuben, J.S.; Speyer, C.L.; Sarma, J.V.; Wetsel, R.A.; Zetoune, F.S.; et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004, 18, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Poiesi, C.; Merli, S.; Cassani, G. Tumor Necrosis Factor (TNF) α quantification by ELISA and bioassay: Effects of TNFα-soluble TNF receptor (p55) complex dissociation during assay incubations. J. Immunol. Methods 1994, 177, 191–198. [Google Scholar] [CrossRef]
- de Kossodo, S.; Houba, V.; Grau, G.E. Assaying tumor necrosis factor concentrations in human serum a WHO International Collaborative Study. J. Immunol. Methods 1995, 182, 107–114. [Google Scholar] [CrossRef]
- Bouyón, R.; Santana, H.; Pérez, E.M.; Hernández, N.; Furrazola, G.; Abrahantes, M.C. Development and validation of an enzyme-linked immunosorbent assay (ELISA) for recombinant human gamma interferon. J. Immunoass. Immunochem. 2003, 24, 1–10. [Google Scholar] [CrossRef]
- Pal, T.; Dutta, S.K.; Mandal, S.; Saha, B.; Tripathi, A. Differential clinical symptoms among acute phase Indian patients revealed significant association with dengue viral load and serum IFN-gamma level. J. Clin. Virol. 2014, 61, 365–370. [Google Scholar] [CrossRef]
- Adler, H.L.; McCurdy, M.A.; Kattan, M.W.; Timme, T.L.; Scardino, P.T.; Thompson, T.C. Elevated levels of circulating interleukin-6 and transforming growth factor-β1 in patients with metastatic prostatic carcinoma. J. Urol. 1999, 161, 182–187. [Google Scholar] [CrossRef]
- Andrade, P.; Hoogland, G.; Garcia, M.A.; Steinbusch, H.W.; Daemen, M.A.; Visser-Vandewalle, V. Elevated IL-1b and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur. Spine J. 2013, 22, 714–720. [Google Scholar] [CrossRef]
- Kellar, K.L.; Kalwar, R.R.; Dubois, K.A.; Crouse, D.; Chafin, W.D.; Kane, B.E. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry 2001, 45, 27–36. [Google Scholar] [CrossRef]
- Chen, R.; Lowe, L.; Wilson, J.D.; Crowther, E.; Tzeggai, K.; Bishop, J.E.; Varro, R. Simultaneous Quantification of Six Human Cytokines in a Single Sample Using Microparticle-based Flow Cytometric Technology. Clin. Chem. 1999, 45, 1693–1694. [Google Scholar] [CrossRef] [PubMed]
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical chemistry of cytokines—A review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Dongxu, X.; Fedarko, N.S.; Kuchel, G.A. Elisa and Multiplex Technologies for Cytokine. J. Gerontol. Biol. Sci. Med. Sci. 2008, 63, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.R.; Lu, D.; Bottomly, K. A polymerase chain reaction assay for the detection and quantitation of cytokine gene expression in small numbers of cells. J. Immunol. Methods 1992, 151, 277–287. [Google Scholar] [CrossRef]
- Phillips, T.M.; Dickens, B.F. Analysis of recombinant cytokines in human body fluids by immunoaffinity capillary electrophoresis. Electrophoresis 1998, 19, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Wartmann, D.; Mathie, R.A.; Dostálek, J.; Ertl, P. Microfluidic platform for multiplexed cell sampling and time-resolved spr-based cytokine sensing. In Proceedings of the IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2015; Volime 45, pp. 785–788. [Google Scholar]
- Prieto, B.; Miguel, D.; Costa, M.; Coto, D.; Alvarez, F.V. New quantitative electrochemiluminescence method (ECLIA) for interleukin-6 (IL-6) measurement. Clin. Chem. Lab. Med. 2010, 48, 835–838. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Barron, J.C.; Rusling, J.F. Carbon nanotube microwell array for sensitive electrochemiluminescent detection of cancer biomarker proteins. Anal. Chem. 2011, 83, 6698–6703. [Google Scholar] [CrossRef]
- Kurita, R.; Arai, K.; Nakamoto, K.; Kato, D.; Niwa, O. Development of electrogenerated chemiluminescence-based enzyme linked immunosorbent assay for sub-pM detection. Anal. Chem. 2010, 82, 1692–1697. [Google Scholar] [CrossRef]
- Hun, X.; Zhang, Z. Functionalized fluorescent core-shell nanoparticles used as a fluorescent labels in fluoroimmunoassay for IL-6. Biosens. Bioelectron. 2007, 22, 2743–2748. [Google Scholar] [CrossRef]
- Cesaro-Tadic, S.; Dernick, G.; Juncker, D.; Buurman, G.; Kropshofer, H.; Michel, B.; Fattinger, C.; Delamarche, E. High-sensitivity miniaturized immunoassays for tumor necrosis factor α using microfluidic systems. Lab Chip 2004, 4, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Solary, E.; Guiguet, M.; Zeller, V.; Casasnovas, R.-O.; Caillot, D.; Chavanet, P.; Guy, H.; Mack, G. Radioimmunoassay for the measurement of serum IL-6 and its correlation with tumour cell mass parameters in multiple myeloma. Am. J. Hematol. 1992, 39, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kobayashi, D.; Sasaki, M.; Araake, H.; Kida, T.; Yagihashi, A.; Yajima, T.; Kameshima, H.; Watanabe, N. Detection of human serum tumor necrosis factor-α in healthy donors, using a highly sensitive immuno-PCR assay. Clin. Chem. 1999, 45, 665–669. [Google Scholar] [CrossRef]
- Liu, G.; Qi, M.; Hutchinson, M.R.; Yang, G.; Goldys, E.M. Recent advances in cytokine detection by immunosensing. Biosens. Bioelectron. 2016, 79, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Shakouri, S.K.; Dolati, S. Biosensors: A novel approach to and recent discovery in detection of cytokines. Cytokine 2020, 136, 155272. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Revisiting electrochemical biosensing in the 21st century society for inflammatory cytokines involved in autoimmune, neurodegenerative, cardiac, viral and cancer diseases. Sensors 2021, 21, 189. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry; Wiley-VCH: Weinheim, Germany, 2006; ISBN 9780471678793. [Google Scholar]
- Estrela, P.; Stewart, A.G.; Yan, F.; Migliorato, P. Field effect detection of biomolecular interactions. Electrochim. Acta 2005, 50, 4995–5000. [Google Scholar] [CrossRef]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Dutta, G.; Lillehoj, P.B. An ultrasensitive enzyme-free electrochemical immunosensor based on redox cycling amplification using methylene blue. Analyst 2017, 142, 3492–3499. [Google Scholar] [CrossRef]
- Jolly, P.; Formisano, N.; Tkáč, J.; Kasák, P.; Frost, C.G.; Estrela, P. Label-free impedimetric aptasensor with antifouling surface chemistry: A prostate specific antigen case study. Sens. Actuators B Chem. 2015, 209, 306–312. [Google Scholar] [CrossRef]
- Dutta, G.; Regoutz, A.; Moschou, D. Enzyme-assisted glucose quantification for a painless Lab-on-PCB patch implementation. Biosens. Bioelectron. 2020, 167, 112484. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Park, S.; Singh, A.; Seo, J.; Kim, S.; Yang, H. Low-Interference Washing-Free Electrochemical Immunosensor Using Glycerol-3-phosphate Dehydrogenase as an Enzyme Label. Anal. Chem. 2015, 87, 3574–3578. [Google Scholar] [CrossRef]
- Dutta, G.; Nagarajan, S.; Lapidus, L.J.; Lillehoj, P.B. Enzyme-free electrochemical immunosensor based on methylene blue and the electro-oxidation of hydrazine on Pt nanoparticles. Biosens. Bioelectron. 2017, 92, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Kim, S.; Park, S.; Yang, H. Washing-free heterogeneous immunosensor using proximity-dependent electron mediation between an enzyme label and an electrode. Anal. Chem. 2014, 86, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Jallow, A.A.; Paul, D.; Moschou, D. Label-free electrochemical detection of S. mutans exploiting commercially fabricated printed circuit board sensing electrodes. Micromachines 2019, 10, 575. [Google Scholar] [CrossRef]
- Lillehoj, P.B.; Kaplan, C.W.; He, J.; Shi, W.; Ho, C.M. Rapid, Electrical Impedance Detection of Bacterial Pathogens Using Immobilized Antimicrobial Peptides. J. Lab. Autom. 2014, 19, 42–49. [Google Scholar] [CrossRef]
- Lillehoj, P.B.; Wei, F.; Ho, C.M. A self-pumping lab-on-a-chip for rapid detection of botulinum toxin. Lab Chip 2010, 10, 2265–2270. [Google Scholar] [CrossRef]
- Estrela, P.; Paul, D.; Song, Q.; Stadler, L.K.J.; Wang, L.; Huq, E.; Davis, J.J.; Ferrigno, P.K.; Migliorato, P. Label-free sub-picomolar protein detection with field-effect transistors. Anal. Chem. 2010, 82, 3531–3536. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Cui, D. Recent advances in nanotechnology applied to biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Munge, B.S.; Krause, C.E.; Malhotra, R.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Electrochemical immunosensors for interleukin-6. Comparison of carbon nanotube forest and gold nanoparticle platforms. Electrochem. Commun. 2009, 11, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A highly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Feng, L.N.; Ren, Z.J.; Jiang, L.P.; Zhu, J.J. Synthesis of silver nanoparticle-hollow titanium phosphate sphere hybrid as a label for ultrasensitive electrochemical detection of human interleukin-6. Small 2011, 7, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, M. Electrochemical sensor utilizing ferrocene loaded porous polyelectrolyte nanoparticles as label for the detection of protein biomarker IL-6. Sens. Actuators B Chem. 2011, 158, 361–365. [Google Scholar] [CrossRef]
- Kongsuphol, P.; Ng, H.H.; Pursey, J.P.; Arya, S.K.; Wong, C.C.; Stulz, E.; Park, M.K. EIS-based biosensor for ultra-sensitive detection of TNF-α from non-diluted human serum. Biosens. Bioelectron. 2014, 61, 274–279. [Google Scholar] [CrossRef]
- Khosravi, F.; Loeian, S.M.; Panchapakesan, B. Ultrasensitive label-free sensing of IL-6 based on PASE functionalized carbon nanotube micro-arrays with RNA-aptamers as molecular recognition elements. Biosensors 2017, 7, 17. [Google Scholar] [CrossRef]
- Liang, K.Z.; Mu, W.J. Flow-injection immuno-bioassay for interleukin-6 in humans based on gold nanoparticles modified screen-printed graphite electrodes. Anal. Chim. Acta 2006, 580, 128–135. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
- Wu, Y.; Belmonte, I.; Sykes, K.S.; Xiao, Y.; White, R.J. Perspective on the Future Role of Aptamers in Analytical Chemistry. Anal. Chem. 2019, 91, 15335–15344. [Google Scholar] [CrossRef]
- Vidotti, M.; Carvalhal, R.F.; Mendes, R.K.; Ferreira, D.C.M.; Kubota, L.T. Biosensors based on gold nanostructures. J. Braz. Chem. Soc. 2011, 22, 3–20. [Google Scholar] [CrossRef]
- Kumar, L.S.S.; Wang, X.; Hagen, J.; Naik, R.; Papautsky, I.; Heikenfeld, J. Label free nano-aptasensor for interleukin-6 in protein-dilute bio fluids such as sweat. Anal. Methods 2016, 8, 3440–3444. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tuleouva, N.; Ramanculov, E.; Revzin, A. Aptamer-based electrochemical biosensor for interferon gamma detection. Anal. Chem. 2010, 82, 8131–8136. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.; Zhang, L.; Jiang, J.; Yu, R. An electrochemical aptasensor based on hybridization chain reaction with enzyme-signal amplification for interferon-gamma detection. Biosens. Bioelectron. 2012, 36, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Cho, M.; Han, S.Y.; Shim, Y.B.; Ku, J.; Ban, C. A simple and direct electrochemical detection of interferon-γ using its RNA and DNA aptamers. Biosens. Bioelectron. 2008, 23, 1819–1824. [Google Scholar] [CrossRef]
- Matharu, Z.; Patel, D.; Gao, Y.; Haque, A.; Zhou, Q.; Revzin, A. Detecting transforming growth factor-β release from liver cells using an aptasensor integrated with microfluidics. Anal. Chem. 2014, 86, 8865–8872. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Zhang, G.; Zhang, X.; Fang, B.; Wang, L. Dual amplification strategy for the fabrication of highly sensitive interleukin-6 amperometric immunosensor based on poly-dopamine. Langmuir 2011, 27, 1224–1231. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Ye, X.; Yan, Y.; Huang, L.; Jiang, Z.; Tan, S.; Cai, X. Electrochemical immunosensor for interferon-γ based on disposable ITO detector and HRP-antibody-conjugated nano gold as signal tag. Mater. Sci. Eng. C 2016, 59, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for the determination of the cytokine interferon gamma (IFN-γ) in saliva. Talanta 2020, 211, 120761. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Martínez-García, G.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for sensitive determination of transforming growth factor (TGF)—β1 in urine. Biosens. Bioelectron. 2017, 88, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; Arellano, L.M.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Viologen-functionalized single-walled carbon nanotubes as carrier nanotags for electrochemical immunosensing. Application to TGF-β1 cytokine. Biosens. Bioelectron. 2017, 98, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon nanotubes functionalized by click chemistry as scaffolds for the preparation of electrochemical immunosensors. Application to the determination of TGF-beta 1 cytokine. Analyst 2016, 141, 5730–5737. [Google Scholar] [CrossRef]
- Moschou, D.; Greathead, L.; Pantelidis, P.; Kelleher, P.; Morgan, H.; Prodromakis, T. Amperometric IFN-γ immunosensors with commercially fabricated PCB sensing electrodes. Biosens. Bioelectron. 2016, 86, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, Y.; Jiang, L.P.; Zhu, J.J. Electrochemical immunosensor of tumor necrosis factor α based on alkaline phosphatase functionalized nanospheres. Biosens. Bioelectron. 2011, 26, 1890–1894. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Kongsuphol, P.; Park, M.K. Off surface matrix based on-chip electrochemical biosensor platform for protein biomarker detection in undiluted serum. Biosens. Bioelectron. 2017, 92, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Estrela, P. Electrochemical ELISA Protein Biosensing in Undiluted Serum Using a Polypyrrole-Based Platform. Sensors 2020, 20, 2857. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.; Frishman, W. Interleukin 1β: A proinflammatory target for preventing atherosclerotic heart disease. Cardiol. Rev. 2014, 22, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef]
- Sun, Z.; Deng, L.; Gan, H.; Shen, R.; Yang, M.; Zhang, Y. Sensitive immunosensor for tumor necrosis factor α based on dual signal amplification of ferrocene modified self-assembled peptide nanowire and glucose oxidase functionalized gold nanorod. Biosens. Bioelectron. 2013, 39, 215–219. [Google Scholar] [CrossRef]
- Yang, T.; Wang, S.; Jin, H.; Bao, W.; Huang, S.; Wang, J. An electrochemical impedance sensor for the label-free ultrasensitive detection of interleukin-6 antigen. Sens. Actuators B Chem. 2013, 178, 310–315. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Baraket, A.; Longo, A.; Vatteroni, M.; Zine, N.; Bausells, J.; Fuoco, R.; Di Francesco, F.; Salvo, P.; Karanasiou, G.S.; et al. Electrochemical biosensor platform for TNF-α cytokines detection in both artificial and human saliva: Heart failure. Sens. Actuators B Chem. 2017, 251, 1026–1033. [Google Scholar] [CrossRef]
- Dijksma, M.; Kamp, B.; Hoogvliet, J.C.; Van Bennekom, W.P. Development of an electrochemical immunosensor for direct detection of interferon-γ at the attomolar level. Anal. Chem. 2001, 73, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bao, J.; Lu, Y.; Zhang, D.; Luo, S.; Cheng, X.; Zhang, Q.; Li, S.; Liu, Q. Biomarkers of liver fibrosis detecting with electrochemical immunosensor on clinical serum. Sens. Actuators B Chem. 2016, 222, 127–132. [Google Scholar] [CrossRef]
- Caruso, R.; Trunfio, S.; Milazzo, F.; Campolo, J.; De Maria, R.; Colombo, T.; Parolini, M.; Cannata, A.; Russo, C.; Paino, R.; et al. Early expression of pro- and anti-inflammatory cytokines in left ventricular assist device recipients with multiple organ failure syndrome. ASAIO J. 2010, 56, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Yaakoubi, N.; Trivella, M.G.; Zabala, M.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. Cytokine detection using diazonium modified gold microelectrodes onto polyimide substrates with integrated AG/AGCL reference electrode. Procedia Eng. 2012, 47, 1181–1184. [Google Scholar] [CrossRef]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A novel biosensor based on hafnium oxide: Application for early stage detection of human interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Pui, T.S.; Kongsuphol, P.; Arya, S.K.; Bansal, T. Detection of tumor necrosis factor (TNF-α) in cell culture medium with label free electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2013, 181, 494–500. [Google Scholar] [CrossRef]
- Mukaida, N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, 566–577. [Google Scholar] [CrossRef]
- Sharma, R.; Deacon, S.E.; Nowak, D.; George, S.E.; Szymonik, M.P.; Tang, A.A.S.; Tomlinson, D.C.; Davies, A.G.; McPherson, M.J.; Wälti, C. Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/ml sensitivity. Biosens. Bioelectron. 2016, 80, 607–613. [Google Scholar] [CrossRef]
- Russell, C.; Ward, A.C.; Vezza, V.; Hoskisson, P.; Alcorn, D.; Steenson, D.P.; Corrigan, D.K. Development of a needle shaped microelectrode for electrochemical detection of the sepsis biomarker interleukin-6 (IL-6) in real time. Biosens. Bioelectron. 2019, 126, 806–814. [Google Scholar] [CrossRef]
- Deng, C.; Qu, F.; Sun, H.; Yang, M. Sensitive electrochemical immunosensor based on enlarged and surface charged gold nanoparticles mediated electron transfer. Sens. Actuators B Chem. 2011, 160, 471–474. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Y.; Hu, L.H.; Jiang, L.P.; Zhu, J.J. “Proof-of-principle” concept for ultrasensitive detection of cytokines based on the electrically heated carbon paste electrode. Chem. Commun. 2011, 47, 6551–6553. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; He, T.T.; Jiang, F.; Abdel-Halim, E.S.; Zhu, J.J. Ultrasensitive multi-analyte electrochemical immunoassay based on GNR-modified heated screen-printed carbon electrodes and PS@PDA-metal labels for rapid detection of MMP-9 and IL-6. Biosens. Bioelectron. 2014, 55, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hua, X.; Wu, Y.; Pan, X.; Liu, S. Polymer-functionalized silica nanosphere labels for ultrasensitive detection of tumor necrosis factor-alpha. Anal. Chem. 2011, 83, 6800–6809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mazurek, G.H.; Alocilja, E.C. Measurement of Interferon Gamma Concentration Using an Electrochemical Immunosensor. J. Electrochem. Soc. 2016, 163, B140–B145. [Google Scholar] [CrossRef]
- Li, T.; Si, Z.; Hu, L.; Qi, H.; Yang, M. Prussian Blue-functionalized ceria nanoparticles as label for ultrasensitive detection of tumor necrosis factor-α. Sens. Actuators B Chem. 2012, 171–172, 1060–1065. [Google Scholar] [CrossRef]
- Weng, S.; Chen, M.; Zhao, C.; Liu, A.; Lin, L.; Liu, Q.; Lin, J.; Lin, X. Label-free electrochemical immunosensor based on K3[Fe(CN)6] as signal for facile and sensitive determination of tumor necrosis factor-alpha. Sens. Actuators B Chem. 2013, 184, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Ma, A.; Chen, L.; Liang, H.; Litifu, A.; Xue, F. Fabrication of electrochemical immunosensor for interferon-γ determination and its application of tuberculosis diagnosis. Int. J. Electrochem. Sci. 2017, 12, 7262–7271. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Engelhard, M.H.; Lin, Y. Sensitive Immunoassay of a Biomarker Tumor Necrosis Factor-r Based on Poly(guanine)-Functionalized Silica Nanoparticle Label. Anal. Chem. 2006, 78, 6974–6979. [Google Scholar] [CrossRef] [PubMed]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Amperometric magnetoimmunoassay for the direct detection of tumor necrosis factor alpha biomarker in human serum. Anal. Chim. Acta 2014, 838, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi, F.; Enayati, L.; Sánchez, I.C.; Motaghed, R.; Mascini, M.; Palchetti, I. Electrochemical bioassay for the detection of TNF-α using magnetic beads and disposable screen-printed array of electrodes. Bioanalysis 2013, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. An immunological interleukine-6 capacitive biosensor using perturbation with a potentiostatic step. Biosens. Bioelectron. 1998, 13, 1061–1068. [Google Scholar] [CrossRef]
- Messina, G.A.; Panini, N.V.; Martinez, N.A.; Raba, J. Microfluidic immunosensor design for the quantification of interleukin-6 in human serum samples. Anal. Biochem. 2008, 380, 262–267. [Google Scholar] [CrossRef]
- Ruecha, N.; Shin, K.; Chailapakul, O.; Rodthongkum, N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens. Actuators B Chem. 2019, 279, 298–304. [Google Scholar] [CrossRef]
- Huang, J.; Harvey, J.; Derrick Fam, W.H.; Nimmo, M.A.; Alfred Tok, I.Y. Novel biosensor for interleukin-6 detection. Procedia Eng. 2013, 60, 195–200. [Google Scholar] [CrossRef]
- Farid, S.; Meshik, X.; Choi, M.; Mukherjee, S.; Lan, Y.; Parikh, D.; Poduri, S.; Baterdene, U.; Huang, C.E.; Wang, Y.Y.; et al. Detection of Interferon gamma using graphene and aptamer based FET-like electrochemical biosensor. Biosens. Bioelectron. 2015, 71, 294–299. [Google Scholar] [CrossRef]
- Li, T.; Shu, B.; Jiang, B.; Ding, L.; Qi, H.; Yang, M.; Qu, F. Ultrasensitive multiplexed protein biomarker detection based on electrochemical tag incorporated polystyrene spheres as label. Sens. Actuators B Chem. 2013, 186, 768–773. [Google Scholar] [CrossRef]
- Pui, T.S.; Agarwal, A.; Ye, F.; Huang, Y.; Chen, P. Nanoelectronic detection of triggered secretion of pro-inflammatory cytokines using CMOS compatible silicon nanowires. Biosens. Bioelectron. 2011, 26, 2746–2750. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Gamella, M.; Pingarrón, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 543–548. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, N.; Lillehoj, P.B.; Estrela, P.; Dutta, G. Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors 2021, 11, 94. https://doi.org/10.3390/bios11030094
Dutta N, Lillehoj PB, Estrela P, Dutta G. Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors. 2021; 11(3):94. https://doi.org/10.3390/bios11030094
Chicago/Turabian StyleDutta, Nirmita, Peter B. Lillehoj, Pedro Estrela, and Gorachand Dutta. 2021. "Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future" Biosensors 11, no. 3: 94. https://doi.org/10.3390/bios11030094
APA StyleDutta, N., Lillehoj, P. B., Estrela, P., & Dutta, G. (2021). Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors, 11(3), 94. https://doi.org/10.3390/bios11030094







