Dielectrophoretic Characterization of Tenogenically Differentiating Mesenchymal Stem Cells
Abstract
1. Introduction
1.1. Theory of DEP
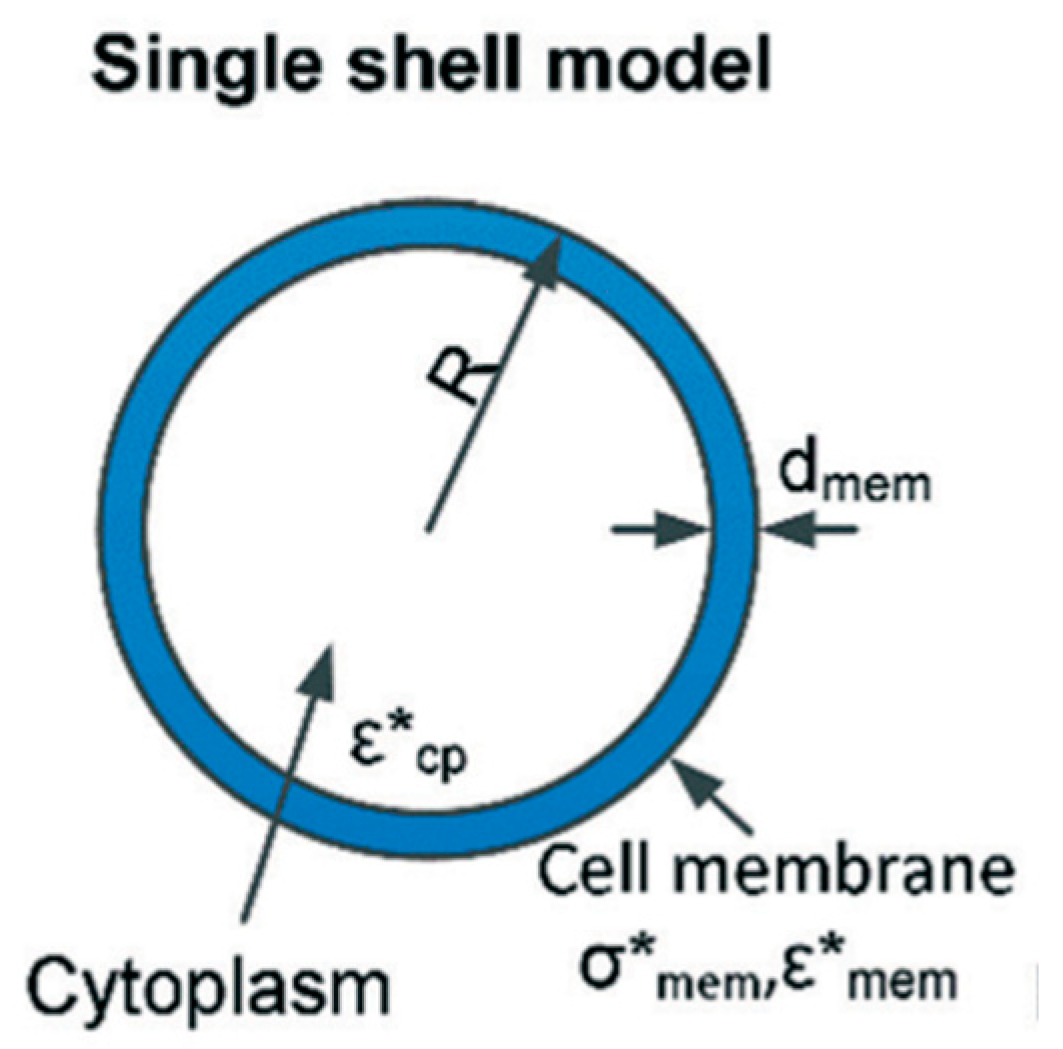
1.2. Single Shell Model
2. Materials and Methods
2.1. Overview
2.2. Cell Culture
2.3. DEP Suspending Medium
2.4. DEP Experimental Setup
2.4.1. Microwell Fabrication
2.4.2. DEP Crossover Frequency Experiments
2.5. Statistical Analysis
2.6. Image Processing
2.7. Curve-Fitting Procedure
3. Results and Discussion
3.1. DEP Crossover Frequency Response of MSCs
3.2. Variance in Cell Size
3.3. Quantification of Dielectric Properties
3.3.1. Initial Estimates for Modeling
3.3.2. Modeling of Membrane Properties
3.3.3. Modeling of Cytoplasmic Parameters
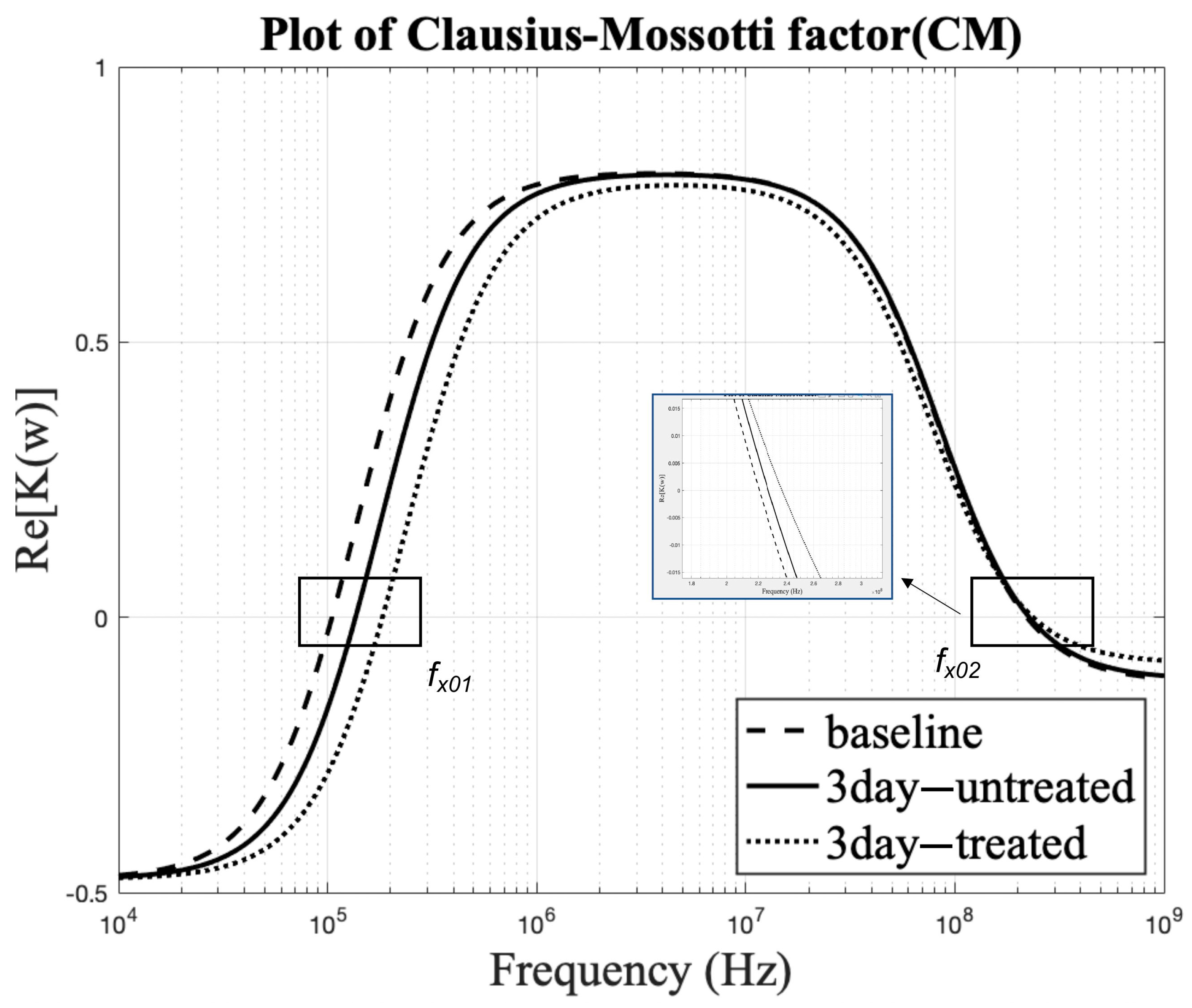
3.4. Effect on Clausius–Mossotti Factor as a Function of Frequency
3.5. Comparison of the Theoretical Model to Experimental Frequencies
3.6. Cell Viability and Throughput
3.7. Limitations
3.8. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, M.-Q.; Huang, Y.-Q.; Lu, N.-S.; Shu, G.; Zhu, X.-T.; Wang, L.-N.; Gao, P.; Xi, Q.-Y.; Zhang, Y.-L.; Wang, S.-B.; et al. Characterization and Differentiation into Adipocytes and Myocytes of Porcine Bone Marrow Mesenchymal Stem Cells. J. Integr. Agric. 2014, 13, 837–848. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Büth, H.; Cho, S.; Impidjati, N.; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Bai, Y.; Zhang, L.; Zhang, B.; Zagidullin, N.; Carvalho, K.; Du, Z.; Cai, B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications. Stem Cell Res. Ther. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Chen, Z.L.; Piao, Y.J. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J. Biosci. Bioeng. 2005, 100, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Tuan, R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 443–454. [Google Scholar] [CrossRef]
- Yoshioka, J.; Ohsugi, Y.; Yoshitomi, T.; Yasukawa, T.; Sasaki, N.; Yoshimoto, K. Label-Free Rapid Separation and Enrichment of Bone Marrow-Derived Mesenchymal Stem Cells from a Heterogeneous Cell Mixture Using a Dielectrophoresis Device. Sensors 2018, 18, 3007. [Google Scholar] [CrossRef]
- Shin, K.S.; Na, K.H.; Lee, H.J.; Kim, D.G.; Shin, S.J.; Kim, J.K.; Kim, G.J. Characterization of Fetal Tissue-derived Mesenchymal Stem Cells. Int. J. Stem Cells 2009, 2, 51–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Łos, M.J.; Skubis, A.; Ghavami, S. Chapter 2—Stem Cells. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 5–16. [Google Scholar]
- Giduthuri, A.T.; Theodossiou, S.K.; Schiele, N.R.; Srivastava, S.K. Dielectrophoresis as a tool for electrophysiological characterization of stem cells. Biophys. Rev. 2020, 1, 011304. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Martin, B.J. Mesenchymal Stem Cells and Their Potential as Cardiac Therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Zhang, Z.; Yang, J.; Hu, S.; Shen, Z. Mesenchymal Stem Cells for Cardiac Regenerative Therapy: Optimization of Cell Differentiation Strategy. Stem Cells Int. 2015, 2015, 524756. [Google Scholar] [CrossRef]
- Adams, T.N.G.; Turner, P.A.; Janorkar, A.V.; Zhao, F.; Minerick, A.R. Characterizing the dielectric properties of human mesenchymal stem cells and the effects of charged elastin-like polypeptide copolymer treatment. Biomicrofluidics 2014, 8, 054109. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Subramony, S.D.; Dargis, B.R.; Castillo, M.; Azeloglu, E.U.; Tracey, M.S.; Su, A.; Lu, H.H. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 2013, 34, 1942–1953. [Google Scholar] [CrossRef]
- Goncalves, A.I.; Rodrigues, M.T.; Lee, S.; Atala, A.; Yoo, J.J.; Reis, R.L.; Gomes, M.E. Understanding the Role of Growth Factors in Modulating Stem Cell Tenogenesis. PLoS One 2013, 8, e83734. [Google Scholar] [CrossRef]
- Schiele, N.R.; Marturano, J.E.; Kuo, C.K. Mechanical factors in embryonic tendon development: Potential cues for stem cell tenogenesis. Curr. Opin. Biotechnol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Ramdass, B.; Koka, P.S. Ligament and tendon repair through regeneration using mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 84–88. [Google Scholar] [CrossRef][Green Version]
- Brown, J.P.; Galassi, T.V.; Stoppato, M.; Schiele, N.R.; Kuo, C.K. Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res. Ther. 2015, 6, 89. [Google Scholar] [CrossRef]
- Pevsner-Fischer, M.; Levin, S.; Zipori, D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. Rep. 2011, 7, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.; Wu, Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.T.; Butler, D.L.; Boivin, G.P.; Florer, J.B.; Schantz, E.J.; Wenstrup, R.J. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J. Orthop. Res. 2004, 22, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Li, H.; Wang, F.; Cheng, K.; Zhou, G.; Zhang, W.; Ye, M.; Cao, Y.; Liu, W.; et al. A proteomic analysis of engineered tendon formation under dynamic mechanical loading in vitro. Biomaterials 2011, 32, 4085–4095. [Google Scholar] [CrossRef]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Hewitt, Z.; Forsyth, N.R.; Waterfall, M.; Wojtacha, D.; Thomson, A.J.; McWhir, J. Fluorescence-activated single cell sorting of human embryonic stem cells. Cloning Stem Cells 2006, 8, 225–234. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef]
- Zhu, B.; Murthy, S.K. Stem Cell Separation Technologies. Curr. Opin. Chem. Eng. 2014, 2, 3–7. [Google Scholar] [CrossRef]
- Castagnola, A.; Eda, S.; Jurat-Fuentes, J.L. Monitoring stem cell proliferation and differentiation in primary midgut cell cultures from Heliothis virescens larvae using flow cytometry. Differentiation 2011, 81, 192–198. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, J.; Wang, X.B.; Becker, F.F.; Gascoyne, P.R. The removal of human breast cancer cells from hematopoietic CD34+ stem cells by dielectrophoretic field-flow-fractionation. J. Hematother. Stem Cell Res. 1999, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; Talary, M.S.; Pethig, R.; Burnett, A.K.; Mills, K.I. The dielectrophoresis enrichment of CD34+ cells from peripheral blood stem cell harvests. Bone Marrow Transpl. 1996, 18, 777–782. [Google Scholar]
- Talary, M.S.; Mills, K.I.; Hoy, T.; Burnett, A.K.; Pethig, R. Dielectrophoretic separation and enrichment of CD34+cell subpopulation from bone marrow and peripheral blood stem cells. Med. Biol. Eng. Comput. 1995, 33, 235–237. [Google Scholar] [CrossRef]
- Flanagan, L.A.; Lu, J.; Wang, L.; Marchenko, S.A.; Jeon, N.L.; Lee, A.P.; Monuki, E.S. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells 2008, 26, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suen, C.-W.; Zhang, J.-F.; Li, G. Current concepts on tenogenic differentiation and clinical applications. J. Orthop. Transl. 2017, 9, 28–42. [Google Scholar] [CrossRef]
- Yafouz, B.; Kadri, N.A.; Ibrahim, F. Dielectrophoretic manipulation and separation of microparticles using microarray dot electrodes. Sensors 2014, 14, 6356–6369. [Google Scholar] [CrossRef]
- Weng, P.-Y.; Chen, I.A.; Yeh, C.-K.; Chen, P.-Y.; Juang, J.-Y. Size-dependent dielectrophoretic crossover frequency of spherical particles. Biomicrofluidics 2016, 10. [Google Scholar] [CrossRef]
- Pethig, R.; Menachery, A.; Pells, S.; DeSousa, P. Dielectrophoresis: A Review of Applications for Stem Cell Research. J. Biomed. Biotechnol. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Adekanmbi, E.O.; Srivastava, S.K. Dielectrophoretic applications for disease diagnostics using lab-on-a-chip platforms. Lab Chip 2016, 16, 2148. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.R. Dielectrophoresis: Theory, Methodology and Biological Applications; John Wiley & Sons, Ltd: West Sussex, UK, 2017. [Google Scholar]
- Adekanmbi, E.O.; Srivastava, S.K. Dielectric characterization of bioparticles via electrokinetics: The past, present, and the future. Appl. Phys. Rev. 2019, 6, 041313. [Google Scholar] [CrossRef]
- Morgan, H.G.N. Dielectrophoresis. Encyclopedia of Microfluidics and Nanofluids; Springer Science: New York, NY, USA, 2014; pp. 1–11. [Google Scholar]
- Gimsa, J.; Marszalek, P.; Loewe, U.; Tsong, T.Y. Dielectrophoresis and electrorotation of neurospora slime and murine myeloma cells. Biophys. J. 1991, 60, 749–760. [Google Scholar] [CrossRef][Green Version]
- Chung, C.; Waterfall, M.; Pells, S.; Menachery, A.; Smith, S.; Pethig, R. Dielectrophoretic Characterisation of Mammalian Cells above 100 MHz. J. Electr. Bioimpedance 2011, 2, 64. [Google Scholar] [CrossRef]
- Theodossiou, S.K.; Tokle, J.; Schiele, N.R. TGFbeta2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2019, 508, 889–893. [Google Scholar] [CrossRef]
- Havis, E.; Bonnin, M.A.; de Lima, J.E.; Charvet, B.; Milet, C.; Duprez, D. TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development 2016, 143, 3839–3851. [Google Scholar] [CrossRef] [PubMed]
- Pryce, B.A.; Watson, S.S.; Murchison, N.D.; Staverosky, J.A.; Dünker, N.; Schweitzer, R. Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development 2009, 136, 1351–1361. [Google Scholar] [CrossRef]
- Scott, A.; Danielson, P.; Abraham, T.; Fong, G.; Sampaio, A.V.; Underhill, T.M. Mechanical force modulates scleraxis expression in bioartificial tendons. J. Musculoskelet Neuronal Interact. 2011, 11, 124–132. [Google Scholar]
- Chiok, K.L.; Paul, N.C.; Adekanmbi, E.O.; Srivastava, S.K.; Shah, D.H. Dimethyl adenosine transferase (KsgA) contributes to cell-envelope fitness in Salmonella Enteritidis. Microbiol. Res. 2018, 216, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Adekanmbi, E.O.; Ueti, M.W.; Rinaldi, B.; Suarez, C.E.; Srivastava, S.K. Insulator-based dielectrophoretic diagnostic tool for babesiosis. Biomicrofluidics 2016, 10, 033108. [Google Scholar] [CrossRef]
- Adekanmbi, E.O.; Dustin, J.; Srivastava, S.K. Electro-osmotic surface effects generation in an electrokinetic-based transport device: A comparison of RF and MW plasma generating sources. Electrophoresis 2019, 40, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Vykoukal, D.M.; Gascoyne, P.R.; Vykoukal, J. Dielectric characterization of complete mononuclear and polymorphonuclear blood cell subpopulations for label-free discrimination. Integr. Biol. (Camb) 2009, 1, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Noshari, J.; Becker, F.F.; Pethig, R. Use of dielectrophoretic collection spectra for characterizing differences between normal and cancerous cells. IEEE Trans. Ind. Appl. 1994, 30, 829. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Liu, L.; Tseng, L.; Ye, Q.; Wu, Y.L.; Bain, D.J.; Ho, C. A New Method for Preparing Mesenchymal Stem Cells and Labeling with Ferumoxytol for Cell Tracking by MRI. Sci. Rep. 2016, 6, 26271. [Google Scholar] [CrossRef] [PubMed]
- Velugotla, S.; Pells, S.; Mjoseng, H.K.; Duffy, C.R.E.; Smith, S. Dielectrophoresis based discrimination of human embryonic stem cells from differentiating derivatives. Biomicrofluidics 2012, 6, 44113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Q.; Sun, H.; Chen, D.; Li, Z.; Fan, B.; George, J.; Xue, C.; Cui, Z.; Wang, J.; et al. Electrical Property Characterization of Neural Stem Cells in Differentiation. PLoS ONE 2016, 11, e0158044. [Google Scholar] [CrossRef]
- Adams, T.N.G.; Jiang, A.Y.L.; Vyas, P.D.; Flanagan, L.A. Separation of neural stem cells by whole cell membrane capacitance using dielectrophoresis. Methods 2018, 133, 91–103. [Google Scholar] [CrossRef]
- Lo, Y.J.; Lei, U.; Chen, K.Y.; Lin, Y.Y.; Huang, C.C.; Wu, M.S.; Yang, P.C. Derivation of the cell dielectric properties based on Clausius-Mossotti factor. Appl. Phys. Lett. 2014, 104, 113702. [Google Scholar] [CrossRef]
- Lu, J.; Barrios, C.A.; Dickson, A.R.; Nourse, J.L.; Lee, A.P.; Flanagan, L.A. Advancing practical usage of microtechnology: A study of the functional consequences of dielectrophoresis on neural stem cells. Integr. Biol. (Camb) 2012, 4, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.G.; Li, Y.; Arulmoli, J.; McDonnell, L.P.; Akil, A. Increasing label-free stem cell sorting capacity to reach transplantation-scale throughput. Biomicrofluidics 2014, 8, 064106. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bessette, P.H.; Qian, J.; Meinhart, C.D.; Daugherty, P.S.; Soh, H.T. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl. Acad. Sci. USA 2005, 102, 15757. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, B.; Kim, B. The potential of a dielectrophoresis activated cell sorter (DACS) as a next generation cell sorter. Micro Nano Syst. Lett. 2016, 4, 2. [Google Scholar] [CrossRef]
- Theodossiou, S.K.; Murray, J.B.; Hold, L.A.; Courtright, J.M.; Carper, A.M.; Schiele, N.R. Akt signaling is activated by TGFβ2 and impacts tenogenic induction of mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 1–12. [Google Scholar] [CrossRef]


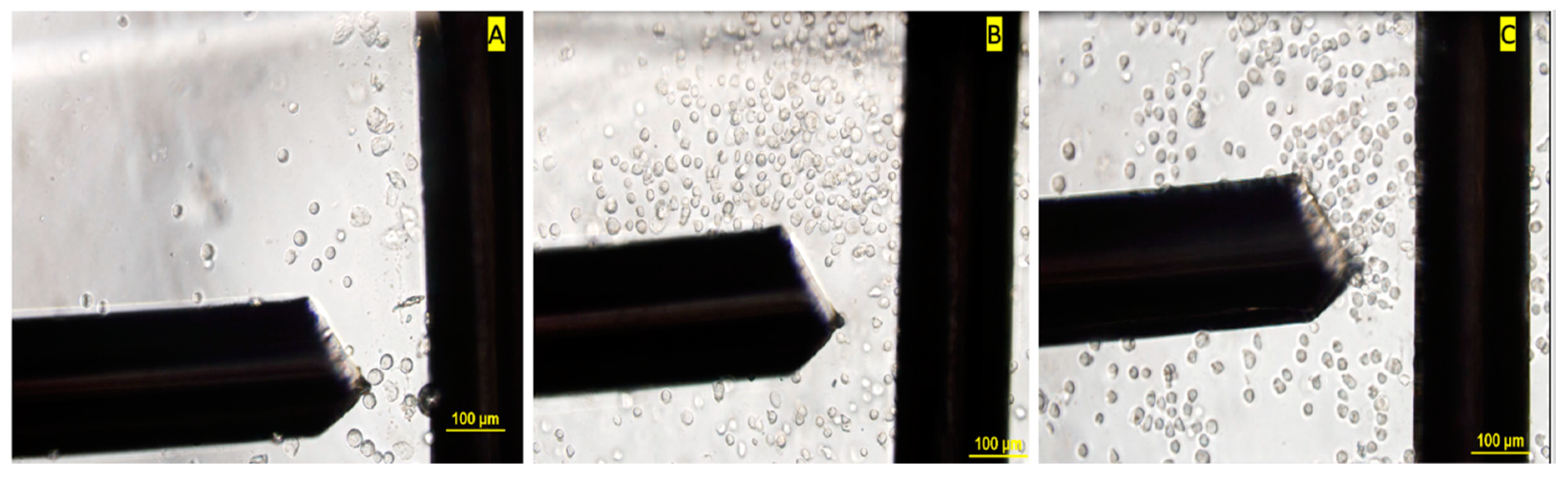
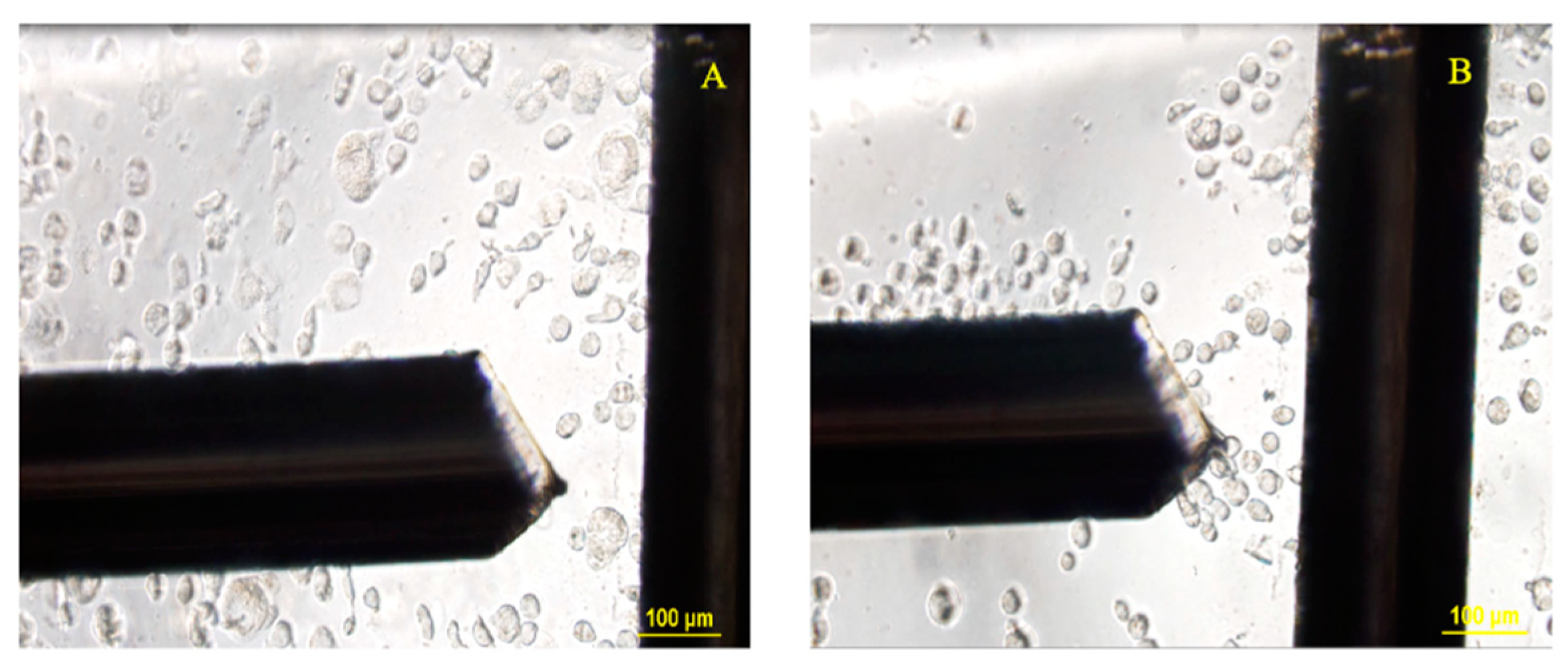

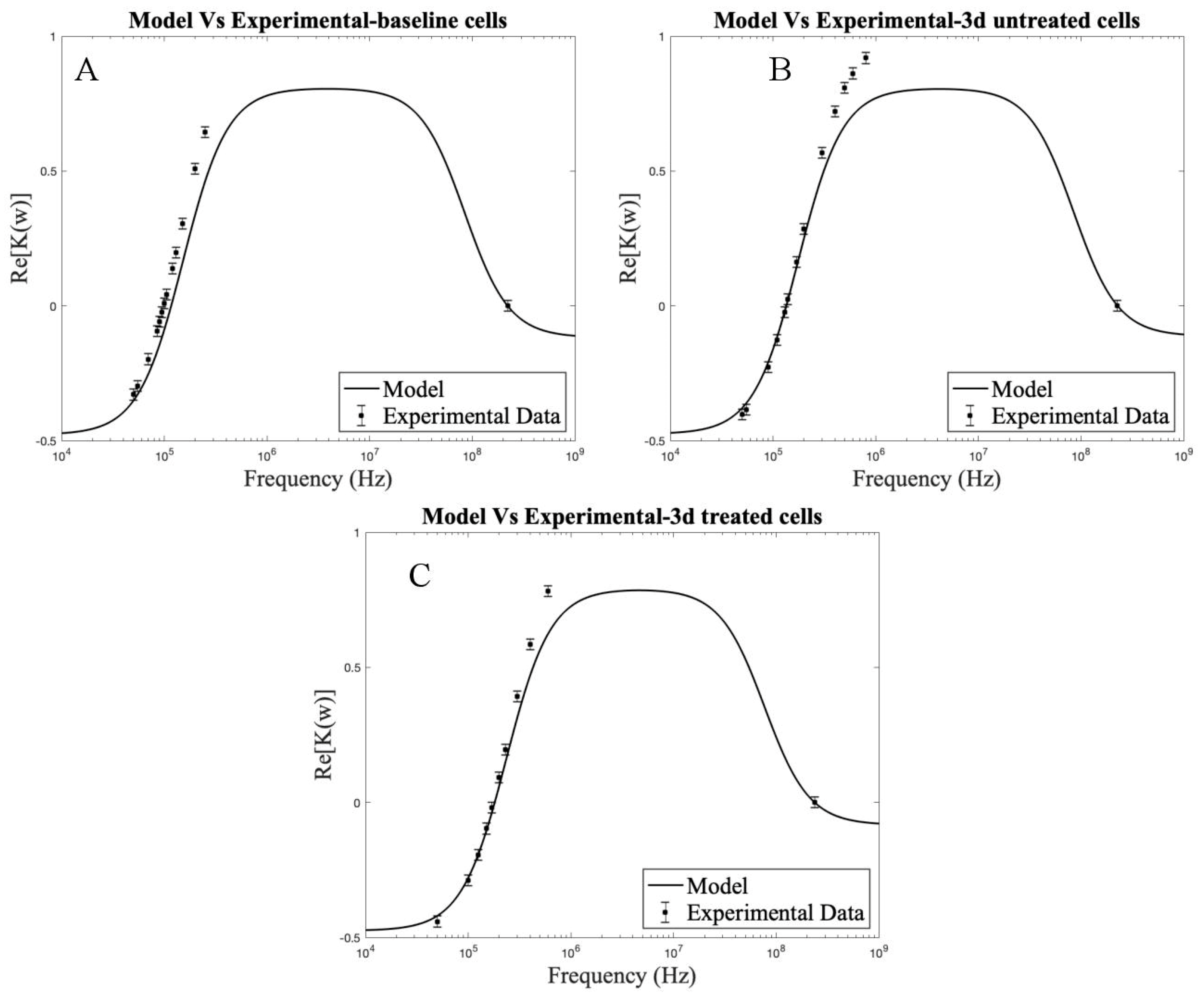
| Timepoint | Treatment | No Treatment |
|---|---|---|
| First Crossover Frequency (fx1) | ||
| Baseline | 95–100 kHz | |
| Day 0 (8 Vpp) | 180–220 kHz | 200–210 kHz |
| Day 3 (8 Vpp) | 165–190 kHz | 128–140 kHz |
| Day 7 (8 Vpp) | 110–130 kHz (small); 50–70 kHz (elongated); 150–175 kHz (big) | 135–150 kHz (small); 250–300 kHz (big) |
| Second Crossover Frequency (fx2) | ||
| Baseline | 225–230 MHz | |
| Day 0 (13 dBm) | 215–230 MHz | 200–205 MHz |
| Day 3 (13 dBm) | 235–240 MHz | 220–230 MHz |
| Day 7 (13 dBm) | 240 MHz | 215 MHz |
| Radius (In μm) of Mesenchymal Stem Cells | |||||
|---|---|---|---|---|---|
| Baseline (Control) | 3 d–No Treatment | 3 d–Treatment | Reference | ||
| Current study (murine) | 8.91 ± 0.091 | 10.10 ± 0.19 | 10.11 ± 0.21 | ||
| Adams et al. (human) | 13.20 | [18,45]* | |||
| Velugotla et al. (human) | H1-MSCs | 7.53 ± 1.55 | [61] | ||
| H9-MSCs | 6.25 ± 1.14 | ||||
| Liu et al. (murine) | 8.67 ± 0.95 | [60] | |||
| Cell Component | Permittivity | Conductivity (S/m) |
|---|---|---|
| Membrane | 6.5–11 | 10−3–10−8 |
| Cytoplasm | 50–100 | 0.3–1.5 |
| Property | Control | 3 d–No Treatment | 3 d–Treatment |
|---|---|---|---|
| Whole-cell capacitance (pF) | 3.83 ± 0.012 | 3.19 ± 0.08 | 2.46 ± 0.1 |
| Relative permittivity | 3.03 ± 0.01 | 1.97 ± 0.05 | 1.51 ± 0.06 |
| Cell Group | Conductivity (S/m) | |
|---|---|---|
| Baseline (control group) | 0.88 ± 0.01 | 55 ± 2 |
| 3 d–no treatment | 0.88 ± 0.02 | 55 ± 1 |
| 3 d–treatment | 0.82 ± 0.02 | 62 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giduthuri, A.T.; Theodossiou, S.K.; Schiele, N.R.; Srivastava, S.K. Dielectrophoretic Characterization of Tenogenically Differentiating Mesenchymal Stem Cells. Biosensors 2021, 11, 50. https://doi.org/10.3390/bios11020050
Giduthuri AT, Theodossiou SK, Schiele NR, Srivastava SK. Dielectrophoretic Characterization of Tenogenically Differentiating Mesenchymal Stem Cells. Biosensors. 2021; 11(2):50. https://doi.org/10.3390/bios11020050
Chicago/Turabian StyleGiduthuri, Anthony T., Sophia K. Theodossiou, Nathan R. Schiele, and Soumya K. Srivastava. 2021. "Dielectrophoretic Characterization of Tenogenically Differentiating Mesenchymal Stem Cells" Biosensors 11, no. 2: 50. https://doi.org/10.3390/bios11020050
APA StyleGiduthuri, A. T., Theodossiou, S. K., Schiele, N. R., & Srivastava, S. K. (2021). Dielectrophoretic Characterization of Tenogenically Differentiating Mesenchymal Stem Cells. Biosensors, 11(2), 50. https://doi.org/10.3390/bios11020050





