cAMP Biosensors Based on Genetically Encoded Fluorescent/Luminescent Proteins
Abstract
1. Introduction
2. Fluorescence-Based cAMP Sensors
2.1. Single Fluorescent Protein (FP)-Based cAMP Sensors
2.1.1. cAMP Difference Detector in Situ (cADDis)
2.1.2. GAkdYmut
2.1.3. Fluorescent cAMP Indicator (Flamindo)
2.1.4. Flamindo2
2.1.5. Pink Flamindo
2.1.6. Red Fluorescent Indicator for cAMP (R-FlincA)
2.1.7. cAMPr
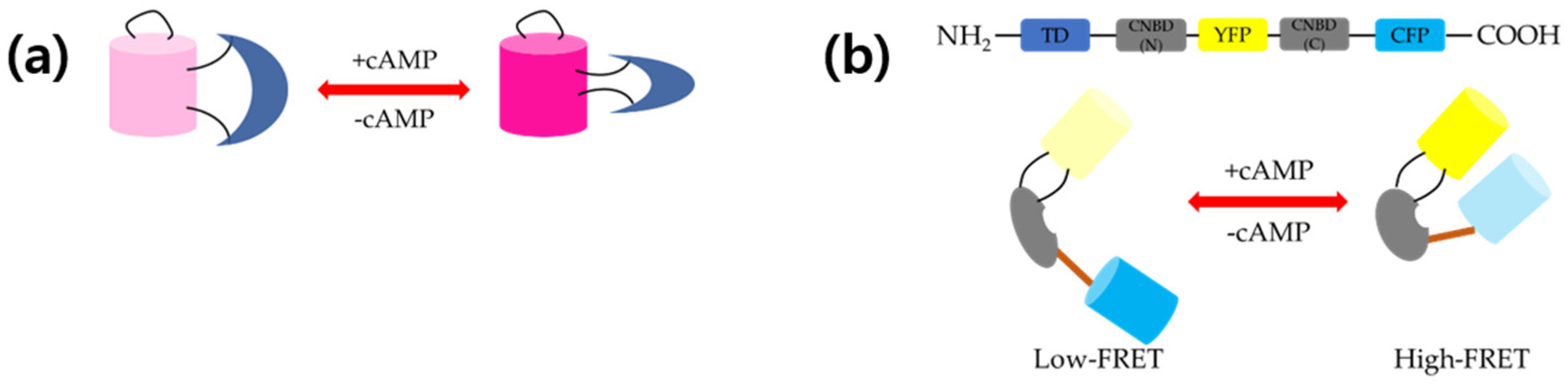
2.2. FRET-Based cAMP Sensors
2.2.1. PKA-Based Sensors
2.2.2. Epac-Based Sensors
2.2.3. Other Sensors
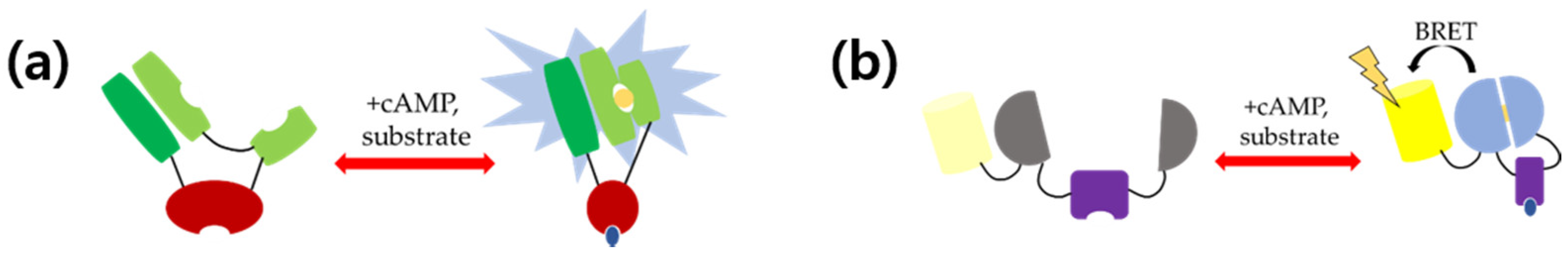
3. Single Luciferase-Based cAMP Sensors
4. BRET-Based cAMP Sensors
4.1. Epac1-Based cAMP Sensor
4.2. Nano Lantern-Based cAMP Sensor
4.3. PKA-Based cAMP Sensor
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santillán, M.; Mackey, M.C. Influence of Catabolite Repression and Inducer Exclusion on the Bistable Behavior of the lac Operon. Biophys. J. 2004, 86, 1282–1292. [Google Scholar] [CrossRef][Green Version]
- Taylor, S.S.; Kim, C.; Cheng, C.Y.; Brown, S.H.; Wu, J.; Kannan, N. Signaling through cAMP and cAMP-dependent protein kinase: Diverse strategies for drug design. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zufall, F.; Shepherd, G.M.; Barnstable, C.J. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr. Opin. Neurobiol. 1997, 7, 404–412. [Google Scholar] [CrossRef]
- De Rooij, J.; Zwartkruis, F.J.T.; Verheijen, M.H.G.; Cool, R.H.; Nijman, S.M.B.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef]
- Schindler, R.F.; Brand, T. The Popeye domain containing protein family—A novel class of cAMP effectors with important functions in multiple tissues. Prog. Biophys. Mol. Biol. 2016, 120, 28–36. [Google Scholar] [CrossRef]
- Krähling, A.M.; Alvarez, L.; Debowski, K.; Van, Q.; Gunkel, M.; Irsen, S.; Al-Amoudi, A.; Strünker, T.; Kremmer, E.; Krause, E. CRIS—A novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet. 2013, 9, e1003960. [Google Scholar]
- Overington, J.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Williams, C. cAMP detection methods in HTS: Selecting the best from the rest. Nat. Rev. Drug Discov. 2004, 3, 125–135. [Google Scholar] [CrossRef]
- Adams, S.R.; Harootunian, A.T.; Buechler, Y.J.; Taylor, S.S.; Tsien, R.Y. Fluorescence ratio imaging of cyclic AMP in single cells. Nat. Cell Biol. 1991, 349, 694–697. [Google Scholar] [CrossRef]
- Bacskai, B.J.; Hochner, B.; Mahaut-Smith, M.; Adams, S.R.; Kaang, B.K.; Kandel, E.R.; Tsien, R.Y. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 1993, 260, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, H.; Arias-Palomo, E.; Hadders, M.A.; Schwede, F.; Llorca, O.; Bos, J.L. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nat. Cell Biol. 2008, 455, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Tewson, P.H.; Martinka, S.; Shaner, N.C.; Hughes, T.E.; Quinn, A.M. New DAG and cAMP Sensors Optimized for Live-Cell Assays in Automated Laboratories. J. Biomol. Screen. 2015, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Tewson, P.; Martinka, S.; Shaner, N.; Berlot, C.; Quinn, A.M.; Hughes, T.E. Assay for Detecting Gαi-Mediated Decreases in cAMP in Living Cells. SLAS Discov. Adv. Life Sci. R&D 2018, 23, 898–906. [Google Scholar] [CrossRef]
- Dackor, R.T.; Cheng, J.; Voltz, J.W.; Card, J.W.; Ferguson, C.D.; Garrett, R.C.; Bradbury, J.A.; DeGraff, L.M.; Lih, F.B.; Tomer, K.B.; et al. Prostaglandin E2 protects murine lungs from bleomycin-induced pulmonary fibrosis and lung dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L645–L655. [Google Scholar] [CrossRef]
- Nunez, F.J.; Schulte, N.A.; Fogel, D.M.; Michalski, J.; Rennard, S.I.; Penn, R.B.; Toews, M.L.; Ostrom, R.S. Agonist-specific desensitization of PGE2-stimulated cAMP signaling due to upregulated phosphodiesterase expression in human lung fibroblasts. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 843–856. [Google Scholar] [CrossRef]
- Thomas, M.; Martinka, S.; Hughes, T. Design for fast optogenetic screen in mammalian cells For Next Gen Ca2+ Sensors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bonnot, A.; Guiot, E.; Hepp, R.; Cavellini, L.; Tricoire, L.; Lambolez, B. Single-fluorophore biosensors based on conformation-sensitive GFP variants. FASEB J. 2014, 28, 1375–1385. [Google Scholar] [CrossRef]
- Heikal, A.A.; Hess, S.T.; Baird, G.S.; Tsien, R.Y.; Webb, W.W. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: Coral red (dsRed) and yellow (Citrine). Proc. Natl. Acad. Sci. USA 2000, 97, 11996–12001. [Google Scholar] [CrossRef]
- Kitaguchi, T.; Oya, M.; Wada, Y.; Tsuboi, T.; Miyawaki, A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem. J. 2013, 450, 365–373. [Google Scholar] [CrossRef]
- Odaka, H.; Arai, S.; Inoue, T.; Kitaguchi, T. Genetically-Encoded Yellow Fluorescent cAMP Indicator with an Expanded Dynamic Range for Dual-Color Imaging. PLoS ONE 2014, 9, e100252. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Fu, B.; Zhao, Y.L.; Li, W.H. Effects of berbamine on intracellular calcium concentration in cultured HeLa cells. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1999, 20, 1011–1014. [Google Scholar]
- Matsuda, S.; Eharada, K.; Ito, M.; Takizawa, M.; Wongso, D.; Tsuboi, T.; Kitaguchi, T. Generation of a cGMP Indicator with an Expanded Dynamic Range by Optimization of Amino Acid Linkers between a Fluorescent Protein and PDE5α. ACS Sens. 2017, 2, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ito, M.; Wang, X.; Tanaka, M.; Wongso, D.; Konno, A.; Hirai, H.; Hirase, H.; Tsuboi, T.; Kitaguchi, T. Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging. Sci. Rep. 2017, 7, 7351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, L.; Matsuda, T.; Zhao, Y.; Rebane, A.; Drobizhev, M.; Chang, Y.-F.; Araki, S.; Arai, Y.; March, K.; et al. Improved Orange and Red Ca2+ Indicators and Photophysical Considerations for Optogenetic Applications. ACS Chem. Neurosci. 2013, 4, 963–972. [Google Scholar] [CrossRef]
- Ohta, Y.; Furuta, T.; Nagai, T.; Horikawa, K. Red fluorescent cAMP indicator with increased affinity and expanded dynamic range. Sci. Rep. 2018, 8, 1866. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderon, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolo, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef]
- Hackley, C.R.; Mazzoni, E.O.; Blau, J. cAMPr: A single-wavelength fluorescent sensor for cyclic AMP. Sci. Signal. 2018, 11, eaah3738. [Google Scholar] [CrossRef]
- Simonds, W.F. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 1999, 20, 66–73. [Google Scholar] [CrossRef]
- Brandon, E.P.; Idzerda, R.L.; McKnight, G.S. PKA isoforms, neural pathways, and behaviour: Making the connection. Curr. Opin. Neurobiol. 1997, 7, 397–403. [Google Scholar] [CrossRef]
- Zaccolo, M.; De Giorgi, F.; Cho, C.Y.; Feng, L.; Knapp, T.; Negulescu, P.A.; Taylor, S.S.; Tsien, R.Y.; Pozzan, T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat. Cell Biol. 2000, 2, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Mongillo, M.; McSorley, T.; Evellin, S.; Sood, A.; Lissandron, V.; Terrin, A.; Huston, E.; Hannawacker, A.; Lohse, M.J.; Pozzan, T.; et al. Fluorescence Resonance Energy Transfer–Based Analysis of cAMP Dynamics in Live Neonatal Rat Cardiac Myocytes Reveals Distinct Functions of Compartmentalized Phosphodiesterases. Circ. Res. 2004, 95, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bagorda, A.; Das, S.; Rericha, E.C.; Chen, D.; Davidson, J.M.; Parent, C.A. Real-time measurements of cAMP production in live Dictyostelium cells. J. Cell Sci. 2009, 122, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Pozzan, T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 2002, 295, 1711–1715. [Google Scholar] [CrossRef]
- Herget, S.; Lohse, M.J.; Nikolaev, V.O. Real-time monitoring of phosphodiesterase inhibition in intact cells. Cell. Signal. 2008, 20, 1423–1431. [Google Scholar] [CrossRef]
- Surdo, N.C.; Berrera, M.; Koschinski, A.; Brescia, M.; Machado, M.R.; Carr, C.; Wright, P.; Gorelik, J.; Morotti, S.; Grandi, E.; et al. FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 2017, 8, 15031. [Google Scholar] [CrossRef]
- Depry, C.; Allen, M.D.; Zhang, J. Visualization of PKA activity in plasma membrane microdomains. Mol. BioSyst. 2011, 7, 52–58. [Google Scholar] [CrossRef]
- Musheshe, N.; Lobo, M.J.; Schmidt, M.; Zaccolo, M. Targeting FRET-Based Reporters for cAMP and PKA Activity Using AKAP79. Sensors 2018, 18, 2164. [Google Scholar] [CrossRef]
- Ohta, Y.; Kamagata, T.; Mukai, A.; Takada, S.; Nagai, T.; Horikawa, K. Nontrivial Effect of the Color-Exchange of a Donor/Acceptor Pair in the Engineering of Förster Resonance Energy Transfer (FRET)-Based Indicators. ACS Chem. Biol. 2016, 11, 1816–1822. [Google Scholar] [CrossRef]
- Ponsioen, B.; Zhao, J.; Riedl, J.; Zwartkruis, F.; Van Der Krogt, G.; Zaccolo, M.; Moolenaar, W.H.; Bos, J.L.; Jalink, K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004, 5, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Schwiening, C.J. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J. Physiol. 2002, 544, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.; Lin, S.; Long, R.; Paul, S. N-methyl-D-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J. Neurosci. 1994, 14, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Everett, K.L.; Cooper, D.M. cAMP measurements with FRET-based sensors in excitable cells. Biochem. Soc. Trans. 2012, 40, 179–183. [Google Scholar] [CrossRef][Green Version]
- Koschinski, A.; Zaccolo, M. Activation of PKA in cell requires higher concentration of cAMP than in vitro: Implications for compartmentalization of cAMP signalling. Sci. Rep. 2017, 7, 14090. [Google Scholar] [CrossRef]
- Griesbeck, O.; Baird, G.S.; Campbell, R.E.; Zacharias, D.A.; Tsien, R.Y. Reducing the Environmental Sensitivity of Yellow Fluorescent Protein. J. Biol. Chem. 2001, 276, 29188–29194. [Google Scholar] [CrossRef]
- Everett, K.L.; Cooper, D.M.F. An Improved Targeted cAMP Sensor to Study the Regulation of Adenylyl Cyclase 8 by Ca2+ Entry through Voltage-Gated Channels. PLoS ONE 2013, 8, e75942. [Google Scholar] [CrossRef]
- Salonikidis, P.S.; Niebert, M.; Ullrich, T.; Bao, G.; Zeug, A.; Richter, D.W. An Ion-insensitive cAMP Biosensor for Long Term Quantitative Ratiometric Fluorescence Resonance Energy Transfer (FRET) Measurements under Variable Physiological Conditions. J. Biol. Chem. 2011, 286, 23419–23431. [Google Scholar] [CrossRef]
- Goedhart, J.; Von Stetten, D.; Noirclerc-Savoye, M.; Lelimousin, M.; Joosen, L.; Hink, M.A.; Van Weeren, L.; Gadella, T.W.; Royant, A. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat. Commun. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Markwardt, M.L.; Kremers, G.-J.; Kraft, C.A.; Ray, K.; Cranfill, P.J.C.; Wilson, K.A.; Day, R.N.; Wachter, R.M.; Davidson, M.W.; Rizzo, M.A. An Improved Cerulean Fluorescent Protein with Enhanced Brightness and Reduced Reversible Photoswitching. PLoS ONE 2011, 6, e17896. [Google Scholar] [CrossRef]
- Klarenbeek, J.; Goedhart, J.; Van Batenburg, A.; Groenewald, D.; Jalink, K. Fourth-Generation Epac-Based FRET Sensors for cAMP Feature Exceptional Brightness, Photostability and Dynamic Range: Characterization of Dedicated Sensors for FLIM, for Ratiometry and with High Affinity. PLoS ONE 2015, 10, e0122513. [Google Scholar] [CrossRef]
- Klarenbeek, J.; Jalink, K. Detecting cAMP with an Epac-Based FRET Sensor in Single Living Cells. Methods Mol. Biol 2013, 1071, 49–58. [Google Scholar] [CrossRef]
- Muntean, B.S.; Zucca, S.; MacMullen, C.M.; Dao, M.T.; Johnston, C.; Iwamoto, H.; Blakely, R.D.; Davis, R.L.; Martemyanov, K.A. Interrogating the Spatiotemporal Landscape of Neuromodulatory GPCR Signaling by Real-Time Imaging of cAMP in Intact Neurons and Circuits. Cell Rep. 2018, 22, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Bünemann, M.; Hein, L.; Hannawacker, A.; Lohse, M.J. Novel Single Chain cAMP Sensors for Receptor-induced Signal Propagation. J. Biol. Chem. 2004, 279, 37215–37218. [Google Scholar] [CrossRef]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar] [CrossRef]
- Violin, J.D.; DiPilato, L.M.; Yildirim, N.; Elston, T.C.; Zhang, J.; Lefkowitz, R.J. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 2008, 283, 2949–2961. [Google Scholar] [CrossRef]
- DiPilato, L.M.; Zhang, J. The role of membrane microdomains in shaping β2-adrenergic receptor-mediated cAMP dynamics. Mol. BioSyst. 2009, 5, 832–837. [Google Scholar] [CrossRef]
- Smith, F.D.; Esseltine, J.L.; Nygren, P.J.; Veesler, D.; Byrne, D.P.; Vonderach, M.; Strashnov, I.; E Eyers, C.; A Eyers, P.; Langeberg, L.K.; et al. Local protein kinase A action proceeds through intact holoenzymes. Science 2017, 356, 1288–1293. [Google Scholar] [CrossRef]
- Iancu, R.V.; Ramamurthy, G.; Warrier, S.; Nikolaev, V.O.; Lohse, M.J.; Jones, S.W.; Harvey, R.D. Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am. J. Physiol. Physiol. 2008, 295, C414–C422. [Google Scholar] [CrossRef]
- Hong, K.P.; Spitzer, N.C.; Nicol, X. Improved molecular toolkit for cAMP studies in live cells. BMC Res. Notes 2011, 4, 241–245. [Google Scholar] [CrossRef]
- Berisha, F.; Götz, K.R.; Wegener, J.W.; Jungen, C.; Pape, U.; Kraft, A.E.; Warnke, S.; Lindner, D.; Westermann, D.; Blankenberg, S.; et al. Direct monitoring of cAMP at the cardiac ryanodine receptor using a novel targeted fluorescence biosensor mouse. bioRxiv 2019. [Google Scholar] [CrossRef]
- Romero, F.; Santana-Calvo, C.; Sánchez-Guevara, Y.; Nishigaki, T. FRET-based binding assay between a fluorescent cAMP analogue and a cyclic nucleotide-binding domain tagged with a CFP. FEBS Lett. 2017, 591, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Börner, S.; Schwede, F.; Schlipp, A.; Berisha, F.; Calebiro, D.; Lohse, M.J.; Nikolaev, V.O. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat. Protoc. 2011, 6, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, J.B.; Goedhart, J.; Hink, M.A.; Gadella, T.W.; Jalink, K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PLoS ONE 2011, 6, e19170. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D.; Gao, L.; Ustione, A.; Bedard, N.; Kester, R.; Piston, D.W.; Tkaczyk, T.S. Real-time hyperspectral fluorescence imaging of pancreatic β-cell dynamics with the image mapping spectrometer. J. Cell Sci. 2012, 125, 4833–4840. [Google Scholar] [CrossRef]
- Vilardaga, J.-P.; Bünemann, M.; Krasel, C.; Castro, M.; Lohse, M.J. Measurement of the millisecond activation switch of G protein–coupled receptors in living cells. Nat. Biotechnol. 2003, 21, 807–812. [Google Scholar] [CrossRef]
- Nimigean, C.M.; Shane, T.; A Miller, C. A Cyclic Nucleotide Modulated Prokaryotic K+ Channel. J. Gen. Physiol. 2004, 124, 203–210. [Google Scholar] [CrossRef]
- Peuker, S.; Cukkemane, A.; Held, M.; Noé, F.; Kaupp, U.B.; Seifert, R. Kinetics of Ligand-Receptor Interaction Reveals an Induced-Fit Mode of Binding in a Cyclic Nucleotide-Activated Protein. Biophys. J. 2013, 104, 63–74. [Google Scholar] [CrossRef]
- Cukkemane, A.; Grüter, B.; Novak, K.; Gensch, T.; Bönigk, W.; Gerharz, T.; Kaupp, U.B.; Seifert, R. Subunits act independently in a cyclic nucleotide-activated K + channel. EMBO Rep. 2007, 8, 749–755. [Google Scholar] [CrossRef]
- Mukherjee, S.; Jansen, V.; Jikeli, J.F.; Hamzeh, H.; Alvarez, L.; Dombrowski, M.; Balbach, M.; Strünker, T.; Seifert, R.; Kaupp, U.B.; et al. A novel biosensor to study cAMP dynamics in cilia and flagella. ELife 2016, 5, e14052. [Google Scholar] [CrossRef]
- Aye-Han, N.-N.; Allen, M.D.; Ni, Q.; Zhangy, J. Parallel tracking of cAMP and PKA signaling dynamics in living cells with FRET-based fluorescent biosensors. Mol. BioSyst. 2012, 8, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Bunemann, M.; Schmitteckert, E.; Lohse, M.J.; Engelhardt, S. Cyclic AMP Imaging in Adult Cardiac Myocytes Reveals Far-Reaching β1-Adrenergic but Locally Confined β2-Adrenergic Receptor–Mediated Signaling. Circ. Res. 2006, 99, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Binkowski, B.F.; Butler, B.L.; Stecha, P.F.; Lewis, M.K.; Wood, K. Novel Genetically Encoded Biosensors Using Firefly Luciferase. ACS Chem. Biol. 2008, 3, 346–351. [Google Scholar] [CrossRef]
- Binkowski, B.; Fan, F.; Wood, K. Engineered luciferases for molecular sensing in living cells. Curr. Opin. Biotechnol. 2009, 20, 14–18. [Google Scholar] [CrossRef]
- Binkowski, B.F.; Butler, B.L.; Stecha, P.F.; Eggers, C.T.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Wood, M.G.; Encell, L.P.; Fan, F.; et al. A Luminescent Biosensor with Increased Dynamic Range for Intracellular cAMP. ACS Chem. Biol. 2011, 6, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- DiRaddo, J.O.; Miller, E.J.; Hathaway, H.A.; Grajkowska, E.; Wroblewska, B.; Wolfe, B.B.; Liotta, D.C.; Wroblewski, J.T. A real-time method for measuring cAMP production modulated by Galphai/o-coupled metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2014, 349, 373–382. [Google Scholar] [CrossRef]
- Kumar, M.; Hsiao, K.; Vidugiriene, J.; Goueli, S. A Bioluminescent-Based, HTS-Compatible Assay to Monitor G-Protein-Coupled Receptor Modulation of Cellular Cyclic AMP. Assay Drug Dev. Technol. 2007, 5, 237–246. [Google Scholar] [CrossRef]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.P.; Feng, B.; et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef]
- Mondal, S.; Hsiao, K.; Goueli, S. Utility of Adenosine Monophosphate Detection System for Monitoring the Activities of Diverse Enzyme Reactions. Assay Drug Dev. Technol. 2017, 15, 330–340. [Google Scholar] [CrossRef]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-Level Laser Therapy for Wound Healing: Mechanism and Efficacy. Dermatol. Surg. 2005, 32, 239–244. [Google Scholar] [CrossRef]
- Medrado, A.R.; Pugliese, L.S.; Reis, S.R.A.; Andrade, Z.A. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg. Med. 2003, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Johnson, M.I.; Lopes-Martins, R.A.B.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Couppé, C.; Chow, R.T.; Tunér, J.; Ljunggren, E.A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003, 49, 107–116. [Google Scholar] [CrossRef]
- De Morais, N.C.R.; Barbosa, A.M.; Vale, M.L.; Villaverde, A.B.; De Lima, C.J.; Cogo, J.C.; Zamuner, S.R. Anti-Inflammatory Effect of Low-Level Laser and Light-Emitting Diode in Zymosan-Induced Arthritis. Photomed. Laser Surg. 2010, 28, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Moretti, A.I.S.; Abrahão, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Ms, N.A.P. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, L.; Yang, H.; Chen, J.; Wang, Y.; Li, H.; Xie, S.; Zeng, H. Measuring the dynamics of cyclic adenosine monophosphate level in living cells induced by low-level laser irradiation using bioluminescence resonance energy transfer. J. Biomed. Opt. 2015, 20, 51029. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Murtiashaw, M.H.; Boije, H.; Fleet, S.E. A Mutagenesis Study of the Putative Luciferin Binding Site Residues of Firefly Luciferase. Biochemistry 2003, 42, 10429–10436. [Google Scholar] [CrossRef]
- Loening, A.M.; Fenn, T.D.; Wu, A.M.; Gambhir, S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. 2006, 19, 391–400. [Google Scholar] [CrossRef]
- Ward, W.W.; Cormier, M.J. In vitro energy transfer in Renilla bioluminescence. J. Phys. Chem. 1976, 80, 2289–2291. [Google Scholar] [CrossRef]
- Baubet, V.; Le Mouellic, H.; Campbell, A.K.; Lucas-Meunier, E.; Fossier, P.; Brûlet, P. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc. Natl. Acad. Sci. USA 2000, 97, 7260–7265. [Google Scholar] [CrossRef]
- Hoshino, H.; Nakajima, Y.; Ohmiya, Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat. Chem. Biol. 2007, 4, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Barak, L.S.; Salahpour, A.; Zhang, X.; Masri, B.; Sotnikova, T.D.; Ramsey, A.J.; Violin, J.D.; Lefkowitz, R.J.; Caron, M.G.; Gainetdinov, R.R. Pharmacological Characterization of Membrane-Expressed Human Trace Amine-Associated Receptor 1 (TAAR1) by a Bioluminescence Resonance Energy Transfer cAMP Biosensor. Mol. Pharmacol. 2008, 74, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/ β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Salahpour, A.; Masri, B.; Sotnikova, T.D.; Messa, M.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. Functional Interaction between Trace Amine-Associated Receptor 1 and Dopamine D2 Receptor. Mol. Pharmacol. 2011, 80, 416–425. [Google Scholar] [CrossRef]
- Jiang, L.I.; Collins, J.; Davis, R.; Lin, K.-M.; DeCamp, D.; Roach, T.; Hsueh, R.; Rebres, R.A.; Ross, E.M.; Taussig, R.; et al. Use of a cAMP BRET Sensor to Characterize a Novel Regulation of cAMP by the Sphingosine 1-Phosphate/G13 Pathway. J. Biol. Chem. 2007, 282, 10576–10584. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Landomiel, F.; Gallay, N.; Jegot, G.; Poupon, A.; Crepieux, P.; Reiter, E. Assessing gonadotropin receptor function by resonance rnergy transfer-based assays. Front. Endocrinol. (Lausanne) 2015, 6, 130. [Google Scholar] [CrossRef]
- Saito, K.; Chang, Y.-F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef]
- Prinz, A.; Diskar, M.; Erlbruch, A.; Herberg, F.W. Novel, isotype-specific sensors for protein kinase A subunit interaction based on bioluminescence resonance energy transfer (BRET). Cell. Signal. 2006, 18, 1616–1625. [Google Scholar] [CrossRef]
| Method | Structure | Properties | Kd | Application | References |
|---|---|---|---|---|---|
| Single FP | cpGFP-Epac2 | Upward/downward | 10~100 μM | Baculovirus, mammalian cell | [12,13] |
| Single FP | cpmNeonGreen-Epac2, cpmApple-Epac2 | Green/Red | NA | Mammalian cell | [14] |
| Single FP | mApple-mEpac1 | Fast response time, red fluorescence | 7.2 μM | Mammalian cell, mice | [24] |
| Single FP | cpmApple-PKA | High affinity | 0.3 μM | Mammalian cell, amoeba | [27] |
| FRET | CNBD(N)-YFP-CNBD(C)-CFP | Consistent dynamic range, | NA | Mammalian cell, cardiac myocyte, rat | [37,38,39] |
| FRET | cpVenus-PKA-ECFP | Large dynamic range | 37.2 nM | Mammalian cell, amoeba | [40] |
| FRET | mTurquois2-Epac1-cpVenus-cpVenus | Bright, good affinity | 9.5~4.0 μM | Mammalian cell, mice, FLIM | [51,52,53] |
| FRET | cpVenus-Epac1-Cerulean | Good dynamic range | NA | Mammalian cell | [58] |
| Single Luciferase | FLuc(N)-PKA-FLuc(C) | Large dynamic range, broad range of linear response | NA | Mammalian cell | [73,74,75] |
| BRET | RLuc-Epac1-Citrine | Real-time dynamic assessment | NA | Mammalian cell | [92] |
| BRET | cpCitrine-hEpac1-RLuc | Good dynamic range | 8.8 μM | Mammalian cell | [95,96] |
| BRET | Venus-RLuc(N)-Epac1-RLuc(C) | Bright, small | 1.6 μM | Amoeba | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Shin, S.; Bae, S.W. cAMP Biosensors Based on Genetically Encoded Fluorescent/Luminescent Proteins. Biosensors 2021, 11, 39. https://doi.org/10.3390/bios11020039
Kim N, Shin S, Bae SW. cAMP Biosensors Based on Genetically Encoded Fluorescent/Luminescent Proteins. Biosensors. 2021; 11(2):39. https://doi.org/10.3390/bios11020039
Chicago/Turabian StyleKim, Namdoo, Seunghan Shin, and Se Won Bae. 2021. "cAMP Biosensors Based on Genetically Encoded Fluorescent/Luminescent Proteins" Biosensors 11, no. 2: 39. https://doi.org/10.3390/bios11020039
APA StyleKim, N., Shin, S., & Bae, S. W. (2021). cAMP Biosensors Based on Genetically Encoded Fluorescent/Luminescent Proteins. Biosensors, 11(2), 39. https://doi.org/10.3390/bios11020039





