Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration
Abstract
:1. Introduction
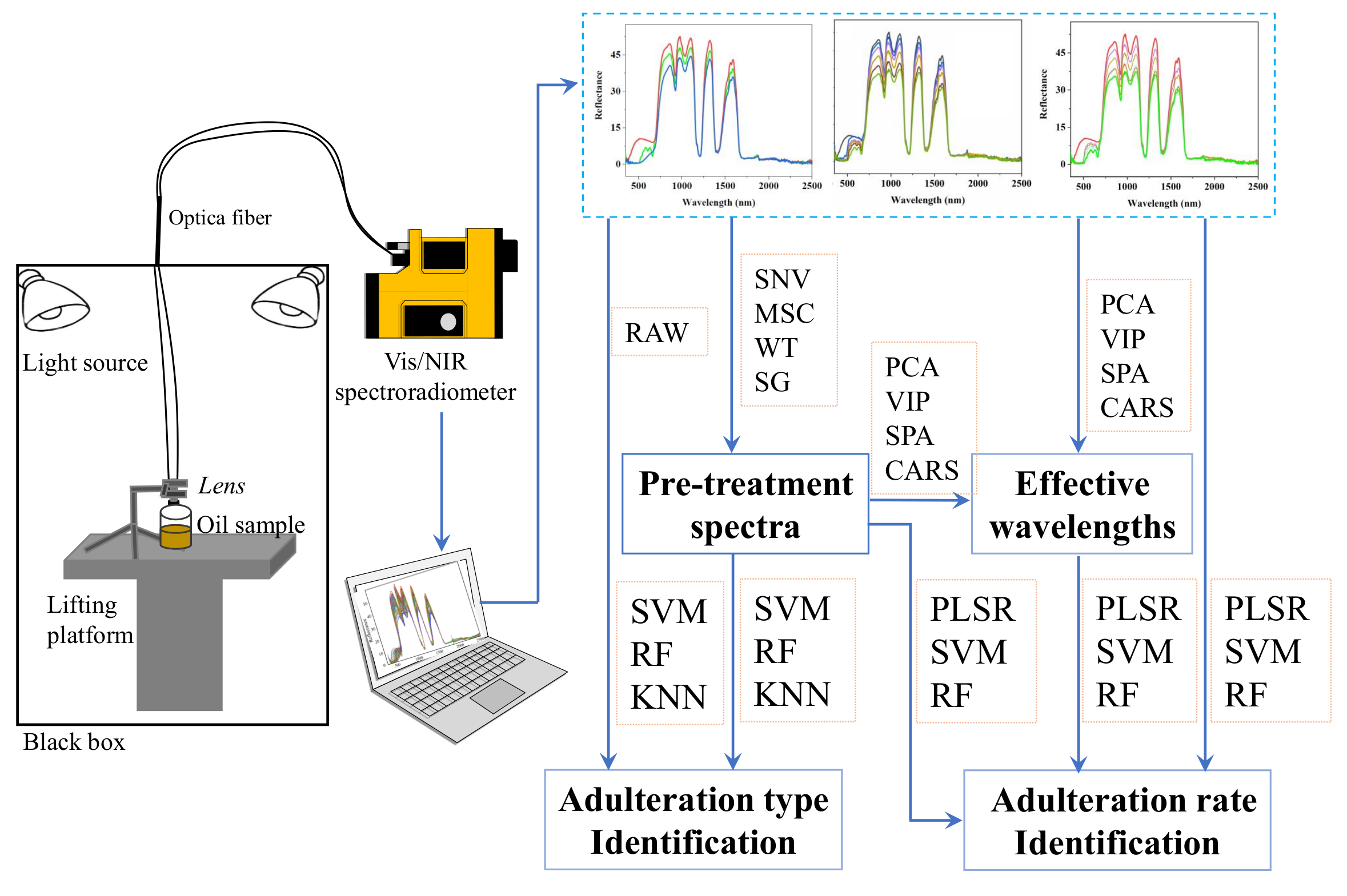
2. Materials and Methods
2.1. Preparation of Experimental Samples
2.2. The Measurement of Reflectance Spectra
2.3. Preprocessing of Reflectance Spectra
2.4. Selection of Effective Wavelengths of Reflectance Spectra
2.5. Adulteration Types and Adulteration Rate Prediction Models
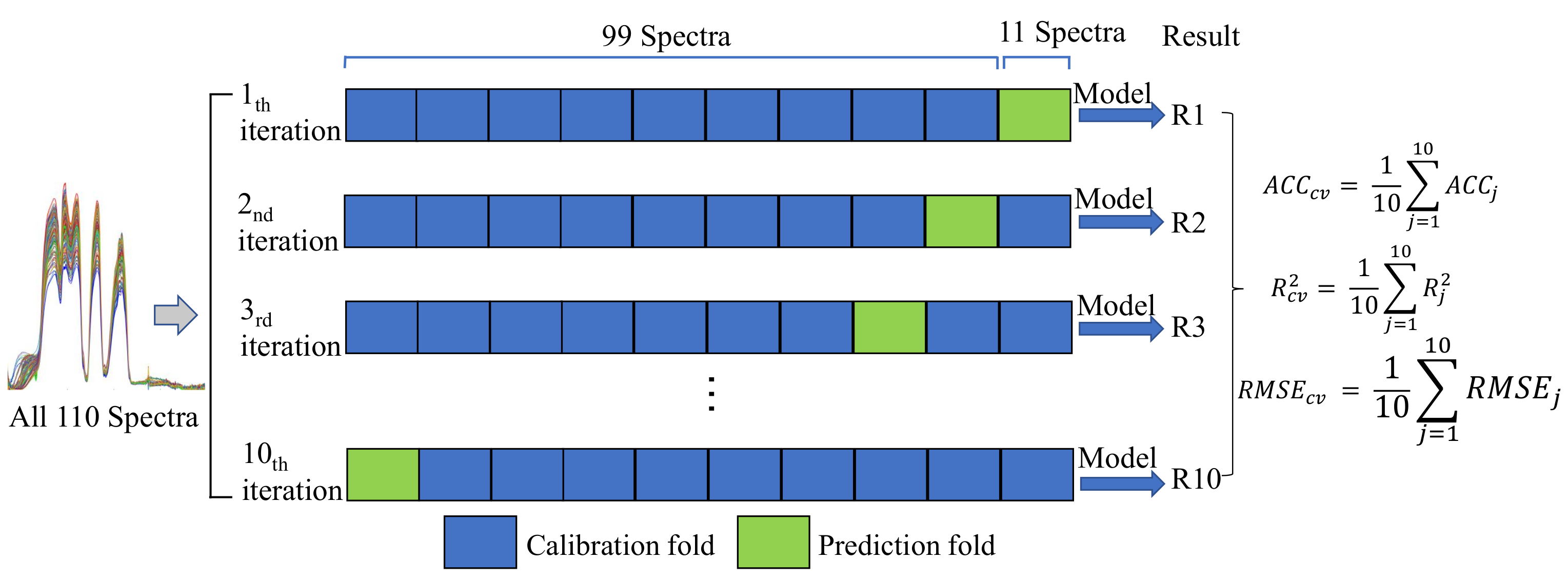
2.6. Performance Evaluation
3. Results and Discussion
3.1. The Vis-NIR Reflectance Spectra of the Adulteration Oil Samples
3.2. Qualitative Analysis of Vis-NIR Spectra to Identify Oil Adulteration Type
3.3. Quantitative Analysis with Full Wavelengths of Vis-NIR Spectra
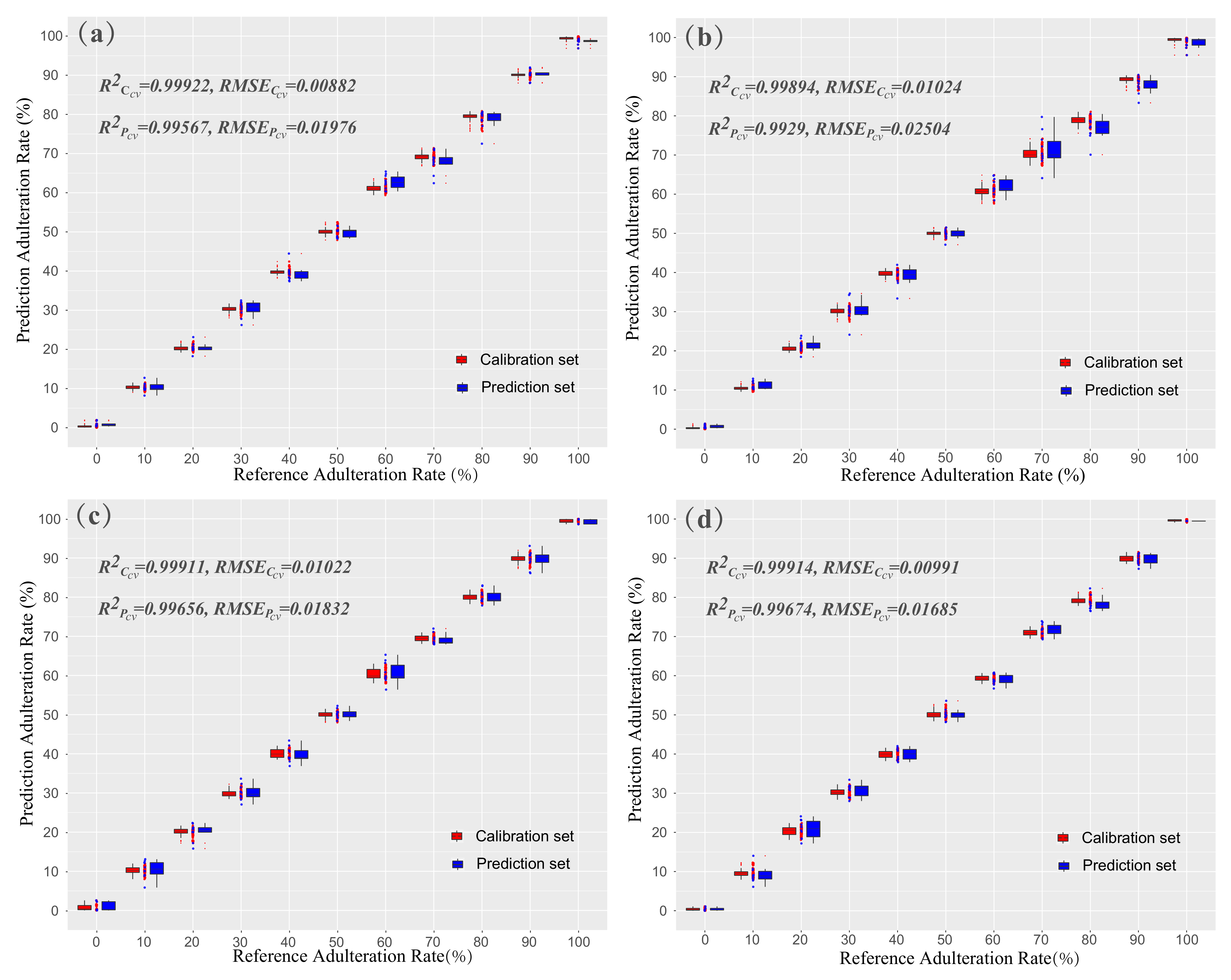
3.4. Quantitative Analysis with Effective Wavelengths of Vis-NIR Spectra
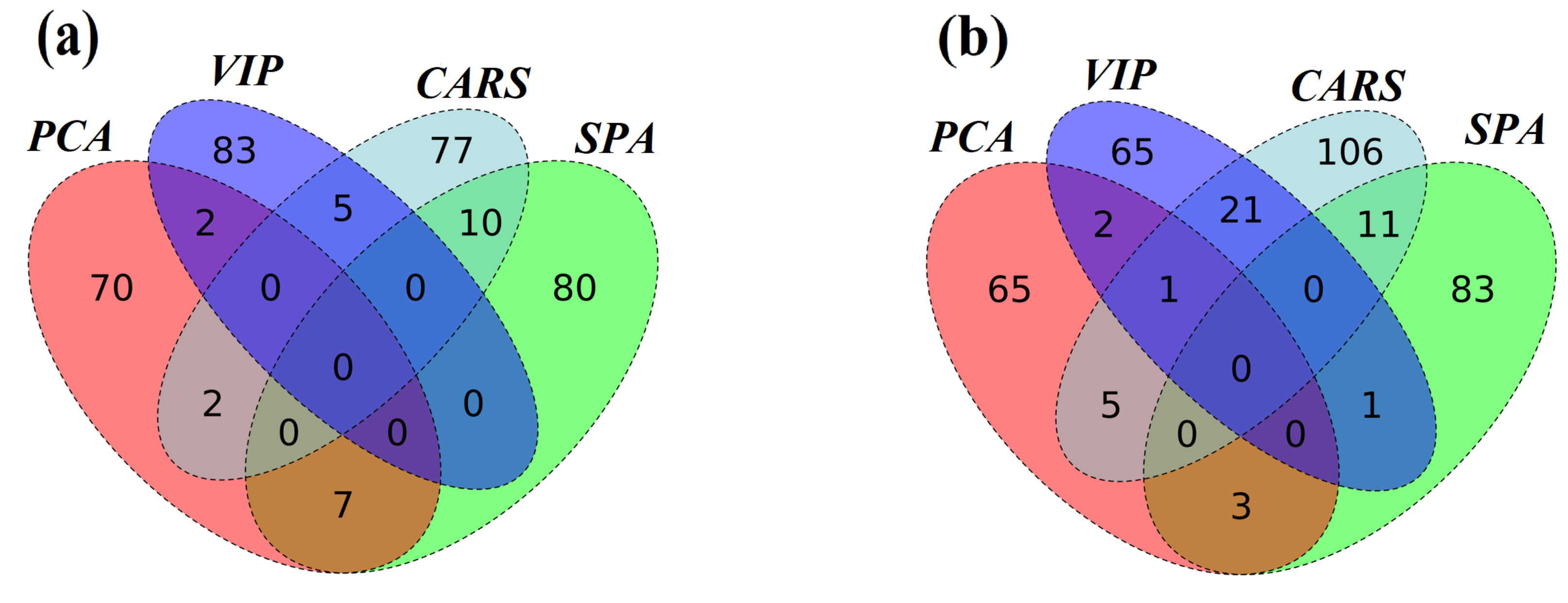
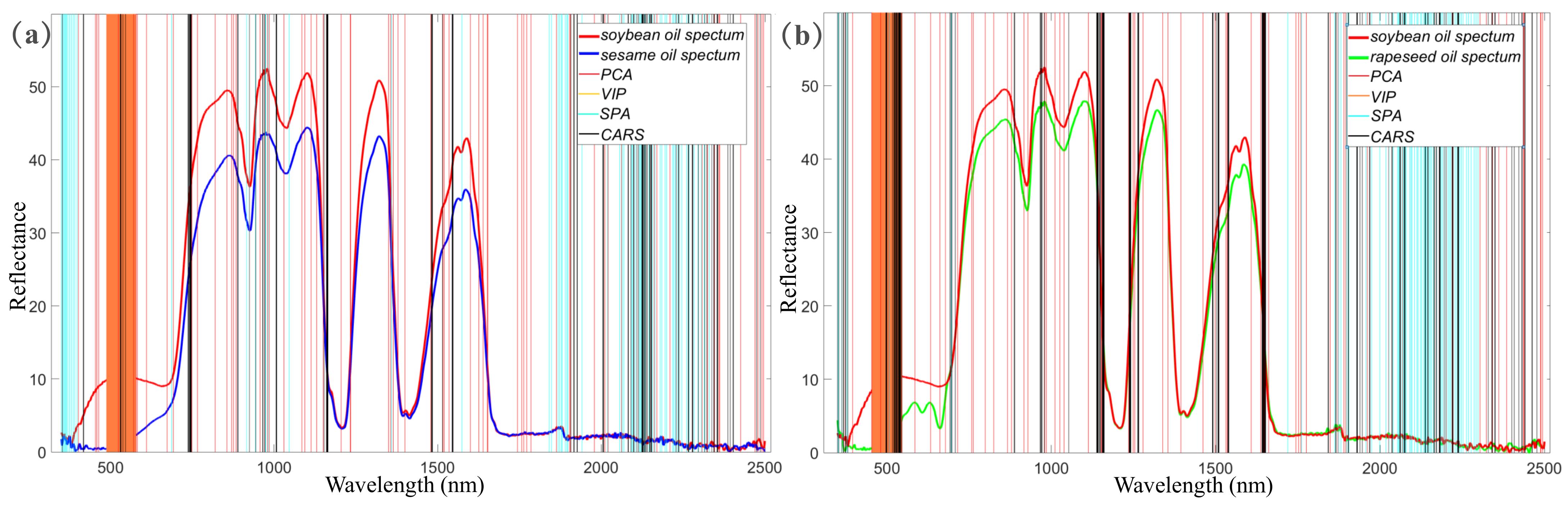
3.5. Analysis of the Distribution and Characteristics of the Screened Effective Wavelengths
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.R.; Shafique, A.; Azeem, F.; Nadeem, H.U.; Siddique, M.H.; Zubair, M.; Rasool, D.; Rasul, I. Edible oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–126. [Google Scholar]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar]
- Zhang, L.; Shuai, Q.; Li, P.; Zhang, Q.; Ma, F.; Zhang, W.; Ding, X. Ion mobility spectrometry fingerprints: A rapid detection technology for adulteration of sesame oil. Food Chem. 2016, 192, 60–66. [Google Scholar]
- Nasopoulou, C.; Zabetakis, I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds. A review. LWT 2012, 47, 217–224. [Google Scholar]
- Zhang, Y.; Dong, W.; Zhang, B.; Wang, X. Research on detection method of adulterated olive oil by Raman spectroscopy and least squares support vector machine. Spectrosc. Spectr. Anal. 2012, 32, 1554–1558. [Google Scholar]
- Zhao, H.; Wang, Y.; Xu, X.; Ren, H.; Li, L.; Xiang, L.; Zhong, W. Detection of adulterated vegetable oils containing waste cooking oils based on the contents and ratios of cholesterol, β-sitosterol, and campesterol by gas chromatography/mass spectrometry. J. AOAC Int. 2015, 98, 1645–1654. [Google Scholar]
- Zhang, X.F.; Zou, M.Q.; Qi, X.H.; Liu, F.; Zhang, C.; Yin, F. Quantitative detection of adulterated olive oil by Raman spectroscopy and chemometrics. J. Raman Spectrosc. 2011, 42, 1784–1788. [Google Scholar]
- Torrecilla, J.S.; García, J.; García, S.; Rodríguez, F. Application of lag-k autocorrelation coefficient and the TGA signals approach to detecting and quantifying adulterations of extra virgin olive oil with inferior edible oils. Anal. Chim. Acta 2011, 688, 140–145. [Google Scholar]
- Lim, K.; Pan, K.; Yu, Z.; Xiao, R.H. Pattern recognition based on machine learning identifies oil adulteration and edible oil mixtures. Nat. Commun. 2020, 11, 5353. [Google Scholar]
- Apetrei, I.; Apetrei, C. Detection of virgin olive oil adulteration using a voltammetric e-tongue. Comput. Electron. Agric. 2014, 108, 148–154. [Google Scholar]
- Harzalli, U.; Rodrigues, N.; Veloso, A.C.; Dias, L.G.; Pereira, J.A.; Oueslati, S.; Peres, A.M. A taste sensor device for unmasking admixing of rancid or winey-vinegary olive oil to extra virgin olive oil. Comput. Electron. Agric. 2018, 144, 222–231. [Google Scholar]
- Kakouri, E.; Revelou, P.K.; Kanakis, C.; Daferera, D.; Pappas, C.S.; Tarantilis, P.A. Authentication of the Botanical and Geographical Origin and Detection of Adulteration of Olive Oil Using Gas Chromatography, Infrared and Raman Spectroscopy Techniques: A Review. Foods 2021, 10, 1565. [Google Scholar]
- Luka, M.F.; Akun, E. Investigation of trace metals in different varieties of olive oils from northern Cyprus and their variation in accumulation using ICP-MS and multivariate techniques. Environ. Earth Sci. 2019, 78, 1–10. [Google Scholar]
- Zhou, R.Z.; Jiang, J.; Mao, T.; Zhao, Y.S.; Lu, Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography–tandem mass spectrometry. Food Chem. 2016, 207, 43–50. [Google Scholar]
- Savio, M.; Ortiz, M.S.; Almeida, C.A.; Olsina, R.A.; Martinez, L.D.; Gil, R.A. Multielemental analysis in vegetable edible oils by inductively coupled plasma mass spectrometry after solubilisation with tetramethylammonium hydroxide. Food Chem. 2014, 159, 433–438. [Google Scholar]
- Yuan, J.J.; Wang, C.Z.; Chen, H.X.; Ye, J.Z.; Zhou, H. Identification and detection of adulterated Camellia Oleifera Abel. Oils by near infrared transmittance spectroscopy. Int. J. Food Prop. 2016, 19, 300–313. [Google Scholar]
- Luna, A.S.; da Silva, A.P.; Pinho, J.S.; Ferré, J.; Boqué, R. Rapid characterization of transgenic and non-transgenic soybean oils by chemometric methods using NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 115–119. [Google Scholar]
- Salguero-Chaparro, L.; Baeten, V.; Abbas, O.; Peña-Rodríguez, F. On-line analysis of intact olive fruits by vis–NIR spectroscopy: Optimisation of the acquisition parameters. J. Food Eng. 2012, 112, 152–157. [Google Scholar]
- Chakraborty, S.; Weindorf, D.C.; Li, B.; Aldabaa, A.A.A.; Ghosh, R.K.; Paul, S.; Ali, M.N. Development of a hybrid proximal sensing method for rapid identification of petroleum contaminated soils. Sci. Total Environ. 2015, 514, 399–408. [Google Scholar]
- Jiang, H.; Zhang, L.; Yang, H.; Chen, X.; Wang, S.; Li, X.; Liu, K. An evaluation of prediction accuracy and stability of a new 314 vegetation index for estimating vegetation leaf area index. In Multispectral, Hyperspectral, and Ultraspectral Remote Sensing Technology, Techniques and Applications V; SPIE Asia-Pacific Remote Sensing: Beijing, China, 2014; Volume 9263, p. 92631B. [Google Scholar]
- Jamwal, R.; Kumari, S.; Balan, B.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid and non-destructive approach for the detection of fried mustard oil adulteration in pure mustard oil via ATR-FTIR spectroscopy-chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118822. [Google Scholar]
- Hou, X.; Wang, G.; Su, G.; Wang, X.; Nie, S. Rapid identification of edible oil species using supervised support vector machine based on low-field nuclear magnetic resonance relaxation features. Food Chem. 2019, 280, 139–145. [Google Scholar]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar]
- Zhang, Y.; Li, T.; Chen, H.; Chen, S.; Guo, P.; Li, Y. Excitation wavelength analysis of a laser-induced fluorescence technique for quantification of extra virgin olive oil adulteration. Appl. Opt. 2019, 58, 4484–4491. [Google Scholar]
- Zhang, W.; Li, N.; Feng, Y.; Su, S.; Li, T.; Liang, B. A unique quantitative method of acid value of edible oils and studying the impact of heating on edible oils by UV–Vis spectrometry. Food Chem. 2015, 185, 326–332. [Google Scholar]
- Su, N.; Pan, F.; Wang, L.; Weng, S. Rapid Detection of Fatty Acids in Edible Oils Using Vis-NIR Reflectance Spectroscopy with Multivariate Methods. Biosensors 2021, 11, 261. [Google Scholar]
- Lin, P.; Chen, Y.; He, Y. Identification of geographical origin of olive oil using visible and near-infrared spectroscopy technique combined with chemometrics. Food Bioprocess Technol. 2012, 5, 235–242. [Google Scholar]
- Maleki, M.; Mouazen, A.; Ramon, H.; De Baerdemaeker, J. Multiplicative scatter correction during on-line measurement with near infrared spectroscopy. Biosyst. Eng. 2007, 96, 427–433. [Google Scholar]
- Wang, W.; Peng, Y.; Sun, H.; Zheng, X.; Wei, W. Real-time inspection of pork quality attributes using dual-band spectroscopy. J. Food Eng. 2018, 237, 103–109. [Google Scholar]
- Chen, H.; Pan, T.; Chen, J.; Lu, Q. Waveband selection for NIR spectroscopy analysis of soil organic matter based on SG smoothing and MWPLS methods. Chemom. Intell. Lab. Syst. 2011, 107, 139–146. [Google Scholar]
- Ebadi, L.; Shafri, H.Z. A stable and accurate wavelet-based method for noise reduction from hyperspectral vegetation spectrum. Earth Sci. Inform. 2015, 8, 411–425. [Google Scholar]
- Xie, C.; Chu, B.; He, Y. Prediction of banana color and firmness using a novel wavelengths selection method of hyperspectral imaging. Food Chem. 2018, 245, 132–140. [Google Scholar]
- Gosselin, R.; Rodrigue, D.; Duchesne, C. A Bootstrap-VIP approach for selecting wavelength intervals in spectral imaging applications. Chemom. Intell. Lab. Syst. 2010, 100, 12–21. [Google Scholar]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in partial least squares regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar]
- Tan, C.H.; Kong, I.; Irfan, U.; Solihin, M.I.; Pui, L.P. Edible Oils Adulteration: A Review on Regulatory Compliance and Its Detection Technologies. J. Oleo Sci. 2021, 70, 1343–1356. [Google Scholar]


| Model | Pretreatment | ||
|---|---|---|---|
| SVM | RAW | 1.00000 | 0.97895 |
| SNV | 1.00000 | 1.00000 | |
| MSC | 1.00000 | 1.00000 | |
| SG | 1.00000 | 0.98947 | |
| WT | 1.00000 | 0.99474 | |
| RF | RAW | 1.00000 | 0.97368 |
| SNV | 1.00000 | 0.99474 | |
| MSC | 1.00000 | 0.99474 | |
| SG | 1.00000 | 0.99474 | |
| WT | 1.00000 | 0.99474 | |
| KNN | RAW | 0.95556 | 0.81579 |
| SNV | 0.99766 | 0.98947 | |
| MSC | 0.99766 | 0.98947 | |
| SG | 0.84971 | 0.66316 | |
| WT | 0.96491 | 0.84737 |
| Model | Pretreatment | ||||
|---|---|---|---|---|---|
| PLSR | RAW | 0.99945 | 0.01058 | 0.96727 | 0.05285 |
| SNV | 0.99923 | 0.01201 | 0.97667 | 0.04597 | |
| MSC | 0.99919 | 0.01212 | 0.97721 | 0.04560 | |
| SG | 0.99999 | 0.00144 | 0.97126 | 0.05197 | |
| WT | 1.00000 | 0.00067 | 0.97282 | 0.05116 | |
| RF | RAW | 0.99879 | 0.01099 | 0.99201 | 0.02673 |
| SNV | 0.99921 | 0.00885 | 0.99531 | 0.02030 | |
| MSC | 0.99922 | 0.00882 | 0.99567 | 0.01976 | |
| SG | 0.99540 | 0.02142 | 0.97058 | 0.05306 | |
| WT | 0.99807 | 0.01388 | 0.98824 | 0.03380 | |
| SVR | RAW | 0.99274 | 0.02694 | 0.96790 | 0.05265 |
| SNV | 0.99330 | 0.02588 | 0.98178 | 0.04114 | |
| MSC | 0.99331 | 0.02585 | 0.98209 | 0.04083 | |
| SG | 0.99268 | 0.02706 | 0.96829 | 0.05473 | |
| WT | 0.99193 | 0.02840 | 0.96695 | 0.05647 |
| Model | Pretreatment | ||||
|---|---|---|---|---|---|
| PLSR | RAW | 0.99965 | 0.00873 | 0.98420 | 0.03775 |
| SNV | 0.99955 | 0.00948 | 0.99019 | 0.02902 | |
| MSC | 0.99953 | 0.00958 | 0.99008 | 0.02919 | |
| SG | 1.00000 | 0.00076 | 0.98258 | 0.04026 | |
| WT | 1.00000 | 0.00036 | 0.98210 | 0.04097 | |
| RF | RAW | 0.99896 | 0.01018 | 0.99353 | 0.02454 |
| SNV | 0.99891 | 0.01038 | 0.99277 | 0.02533 | |
| MSC | 0.99894 | 0.01024 | 0.9929 | 0.02504 | |
| SG | 0.99871 | 0.01133 | 0.99124 | 0.02862 | |
| WT | 0.99890 | 0.01044 | 0.99390 | 0.02372 | |
| SVR | RAW | 0.99235 | 0.02766 | 0.97932 | 0.04443 |
| SNV | 0.99415 | 0.02418 | 0.98820 | 0.03281 | |
| MSC | 0.99410 | 0.02429 | 0.98814 | 0.03293 | |
| SG | 0.99289 | 0.02667 | 0.97625 | 0.04716 | |
| WT | 0.99237 | 0.02763 | 0.97201 | 0.05162 |
| Adulteration Type | Method | Pretreatment | Spectral Selection | Number | ||||
|---|---|---|---|---|---|---|---|---|
| Sesame oil adulterated with soybean oil | RF | SNV | PCA | 83 | 0.99881 | 0.01091 | 0.99275 | 0.02470 |
| PLSR | SNV | SPA | 97 | 0.99621 | 0.02107 | 0.94951 | 0.06516 | |
| RF | SNV | VIP | 90 | 0.99896 | 0.01014 | 0.99377 | 0.02364 | |
| PLSR | MSC | CARS | 94 | 0.99911 | 0.01022 | 0.99656 | 0.01832 | |
| Rapeseed oil adulterated with soybean oil | RF | RAW | PCA | 98 | 0.99836 | 0.01280 | 0.99047 | 0.03050 |
| RF | RAW | SPA | 81 | 0.98602 | 0.03730 | 0.92778 | 0.08287 | |
| RF | RAW | VIP | 90 | 0.99938 | 0.00789 | 0.99587 | 0.01913 | |
| PLSR | MSC | CARS | 144 | 0.99914 | 0.00991 | 0.99675 | 0.01685 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N.; Weng, S.; Wang, L.; Xu, T. Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration. Biosensors 2021, 11, 492. https://doi.org/10.3390/bios11120492
Su N, Weng S, Wang L, Xu T. Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration. Biosensors. 2021; 11(12):492. https://doi.org/10.3390/bios11120492
Chicago/Turabian StyleSu, Ning, Shizhuang Weng, Liusan Wang, and Taosheng Xu. 2021. "Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration" Biosensors 11, no. 12: 492. https://doi.org/10.3390/bios11120492
APA StyleSu, N., Weng, S., Wang, L., & Xu, T. (2021). Reflectance Spectroscopy with Multivariate Methods for Non-Destructive Discrimination of Edible Oil Adulteration. Biosensors, 11(12), 492. https://doi.org/10.3390/bios11120492





