Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis
Abstract
:1. Introduction
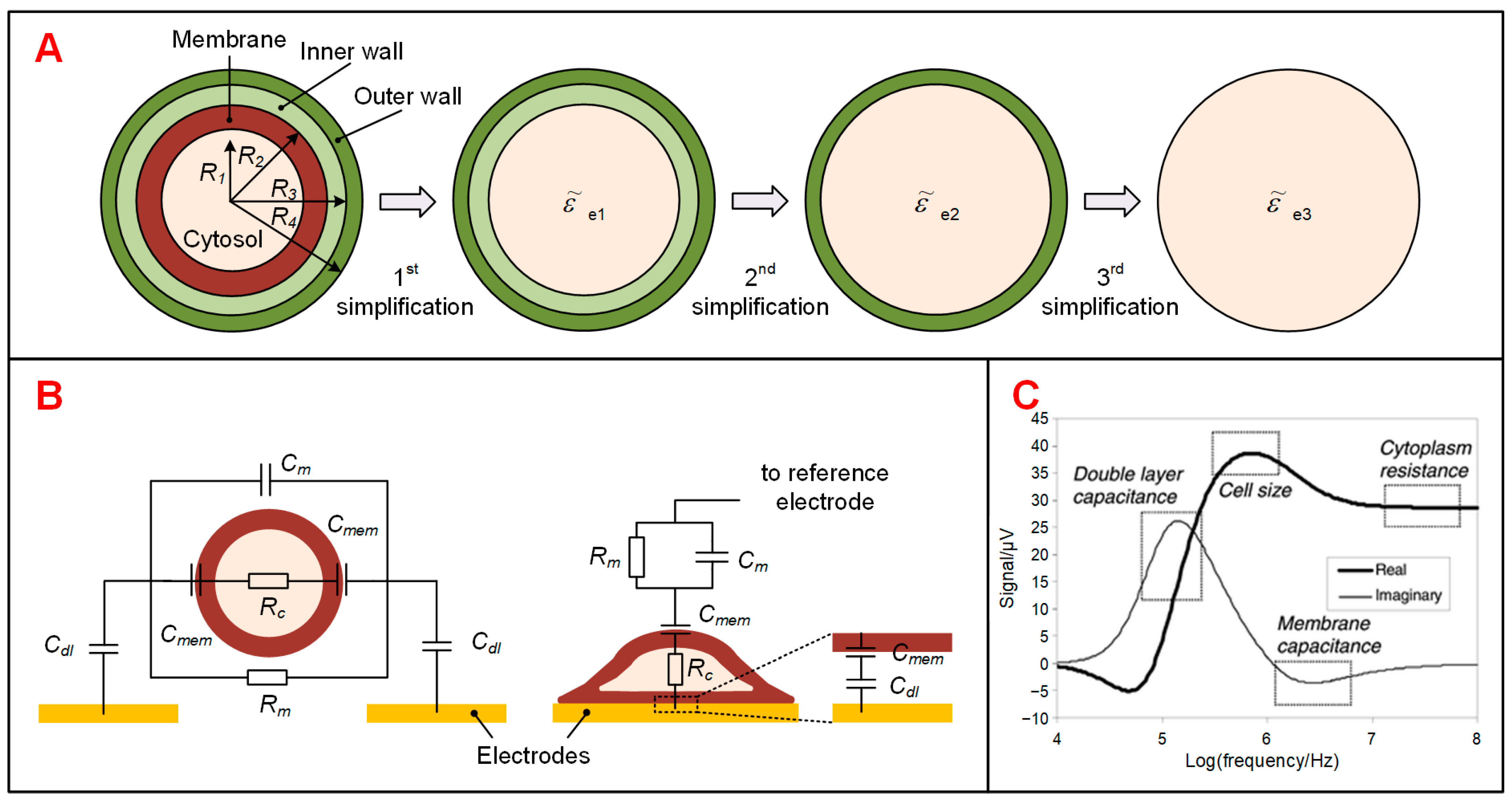
2. Theory and Modeling
3. Device Designs for Sensing Single-Cell Impedance
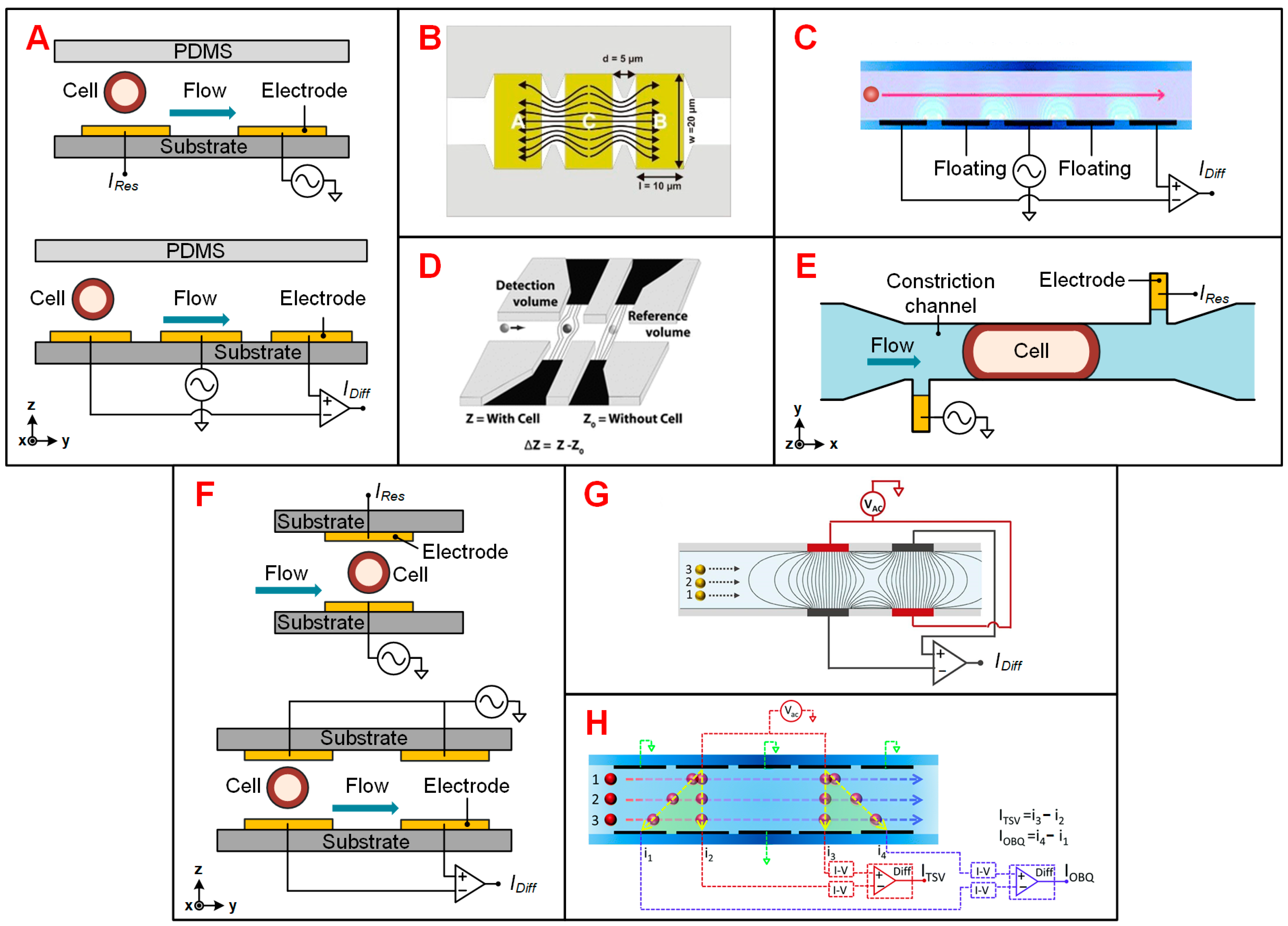
3.1. Impedance Flow Cytometry (IFC)
3.1.1. Electrode Configurations
3.1.2. Particle Positioning
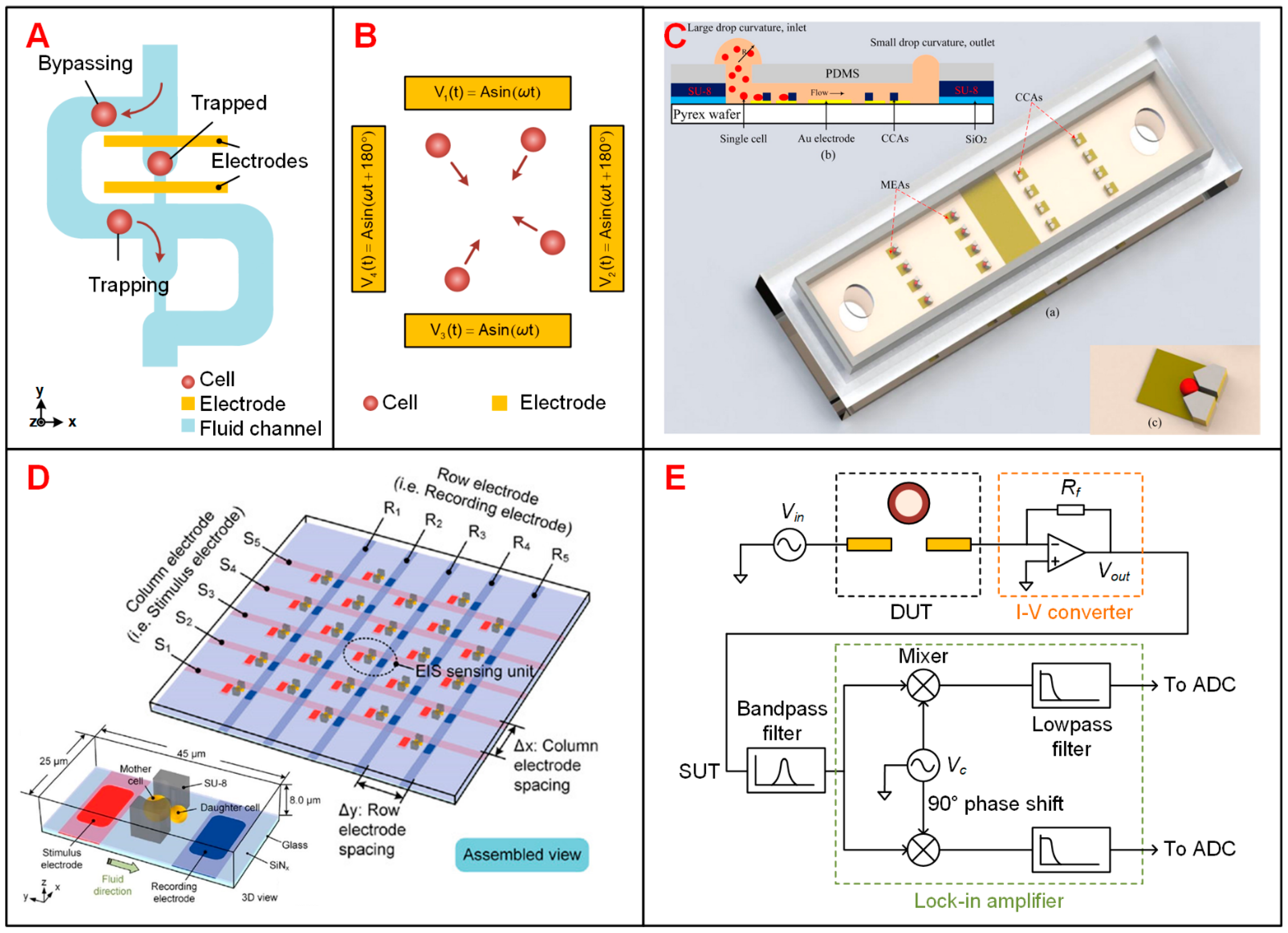
3.2. Electrical Impedance Spectroscopy (EIS) Sensing Devices
3.2.1. Trapping of Suspended Single Cells
3.2.2. Electrical Cell-Substrate Impedance Sensing (ECIS)
3.2.3. Advanced Design to Increase the Throughput of EIS Devices
3.3. Instruments and Portable Platforms for Electrical Impedance Sensing Technology
3.4. CMOS-Based Impedance Sensing Devices
4. Applications of Single-Cell Impedance Sensing Technology
4.1. IFC to Detect Flowing Single Cells
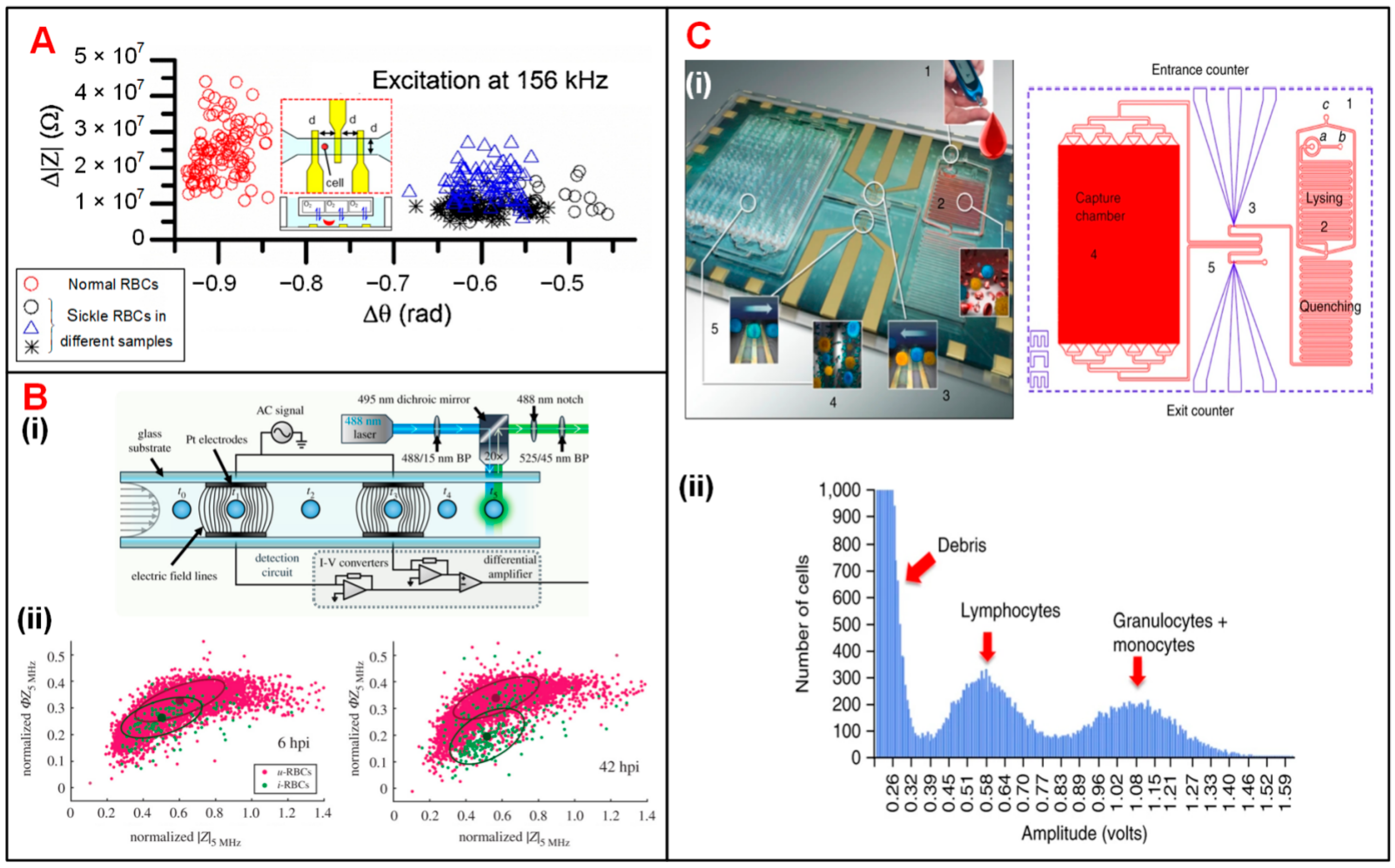
4.1.1. Blood Cells
4.1.2. Tumor Cells
4.1.3. Stem Cells
4.1.4. Plant Cells
4.1.5. Microbes
4.2. EIS to Detect Suspended or Adherent Single Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef]
- Graf, T.; Stadtfeld, M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 2008, 3, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Hunter, K. Host genetics influence tumour metastasis. Nat. Rev. Cancer. 2006, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bodovitz, S. Single cell analysis: The new frontier in ‘omics’. Trends Biotechnol. 2010, 28, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbrecht, L.; Dittrich, P.S. Recent advances in the analysis of single cells. Anal. Chem. 2017, 89, 2–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, R.N.; Kim, S. Microfluidic platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Song, H.; Ahn, H.; Kim, T.; Jung, J.; Cho, S.K.; Shin, D.-M.; Choi, J.-R.; Hwang, Y.-H.; Kim, K. A Review of Advanced Impedance Biosensors with Microfluidic Chips for Single-Cell Analysis. Biosensors 2021, 11, 412. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Marcus, J.S.; Anderson, W.F.; Quake, S.R. Microfluidic single-cell mRNA isolation and analysis. Anal. Chem. 2006, 78, 3084–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, H.; van den Berg, A. Microfluidic devices for cellomics: A review. Sens. Actuators B Chem. 2003, 92, 315–325. [Google Scholar] [CrossRef]
- Gao, D.; Jin, F.; Zhou, M.; Jiang, Y. Recent advances in single cell manipulation and biochemical analysis on microfluidics. Analyst 2019, 144, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.P.; Westervelt, R.M. Dielectrophoresis tweezers for single cell manipulation. Biomed. Microdevices 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Lin, S.C.; Mao, X.; Huang, T.J. Surface acoustic wave (SAW) acoustophoresis: Now and beyond. Lab Chip 2012, 12, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Ramser, K.; Hanstorp, D. Optical manipulation for single-cell studies. J. Biophotonics 2010, 3, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Rettig, J.R.; Folch, A. Large-scale single-cell trapping and imaging using microwell arrays. Anal. Chem. 2005, 77, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Ryley, J.; Pereira-Smith, O.M. Microfluidics device for single cell gene expression analysis in Saccharomyces cerevisiae. Yeast 2006, 23, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.I.; Morgan, B.; Trautwein, J.; et al. Inertial focusing for tumor antigen–dependent and –independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013, 5, 179ra147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Volponi, J.V.; Oliver, A.E.; Parikh, A.N.; Simmons, B.A.; Singh, S. In vivo lipidomics using single-cell Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 3809–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R.; Fuchs, B.M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 2008, 6, 339–348. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, Q.; Zhang, Y.; Lin, J.M. Recent advances in microchip-mass spectrometry for biological analysis. Trends Analyt. Chem. 2014, 53, 84–97. [Google Scholar] [CrossRef]
- Actis, P.; Tokar, S.; Clausmeyer, J.; Babakinejad, B. Electrochemical nanoprobes for single-cell analysis. ACS Nano 2014, 8, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Morgan, H.; Sun, T.; Holmes, D.; Gawad, S.; Green, N.G. Single cell dielectric spectroscopy. J. Phys. D Appl. Phys. 2007, 40, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Hatamie, A.; Ewing, A.G. Nanoelectrochemical analysis inside a single living cell. Curr. Opin. Electrochem. 2020, 22, 94–101. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Nicolas, G.G.; Hywel, M. Analysis and numerical modeling methods for impedance analysis of single cells on-chip. Nano 2008, 3, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Gawad, S.; Cheung, K.; Seger, U.; Bertsch, A.; Renaud, P. Dielectric spectroscopy in a micromachined flow cytometer: Theoretical and practical considerations. Lab Chip 2004, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Asami, K. Characterization of heterogeneous systems by dielectric spectroscopy. Prog. Polym. Sci. 2002, 27, 1617–1659. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Gawad, S.; Morgan, H. Analytical electric field and sensitivity analysis for two microfluidic impedance cytometer designs. IET Nanobiotechnol. 2007, 1, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Morgan, H.; Green, N.G. Analytical solutions of ac electrokinetics in interdigitated electrode arrays: Electric field, dielectrophoretic and traveling-wave dielectrophoretic forces. Phys. Rev. E 2007, 76, 046610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, J.L.S.; Otero, A.S.; Madronero, J.R.; Martin, S.M.S. Dielectric characterization of the yeast cell budding cycle. Prog. Electromagn. Res. 2013, 134, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Gawad, S.; Schild, L.; Renaud, P.H. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.C.; Di Berardino, M.; Schade-Kampmann, G.; Hebeisen, M.; Pierzchalski, A.; Bocsi, J.; Mittag, A.; Tarnok, A. Microfluidic impedance-based flow cytometry. Cytometry A 2010, 77, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yuan, D. Continuous microfluidic 3D focusing enabling microflow cytometry for single-cell analysis. Talanta 2021, 221, 121401. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, H.; Solsona, M.; Cottet, J.; Gauthier, M.; Renaud, P.; Bolopion, A. Positional dependence of particles and cells in microfluidic electrical impedance flow cytometry origin, challenges and opportunities. Lab Chip 2020, 20, 3665–3689. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Trujillo, R.; Castillo-Fernandez, O.; Garrido, M.; Arundell, M.; Valencia, A.; Gomila, G. High-speed particle detection in a micro-Coulter counter with two-dimensional adjustable aperture. Biosens. Bioelectron. 2008, 24, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, J.; Clausen, C.H.; Rodriguez-Trujillo, R.; Svendsen, W.E. Study of paclitaxel-treated HeLa cells by differential electrical impedance flow cytometry. Biosensors 2014, 4, 257–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, C.; Skands, G.; Bertelsen, C.; Svendsen, W. Coplanar electrode layout optimized for increased sensitivity for electrical impedance spectroscopy. Micromachines 2014, 6, 110–120. [Google Scholar] [CrossRef]
- Carminati, M.; Ferrari, G.; Vahey, M.D.; Voldman, J.; Sampietro, M. Miniaturized impedance flow cytometer: Design rules and integrated readout. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- De Ninno, A.; Errico, V.; Bertani, F.R.; Businaro, L.; Bisegna, P.; Caselli, F. Coplanar electrode microfluidic chip enabling accurate sheathless impedance cytometry. Lab Chip 2017, 17, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, A.; Braschler, T.; Renaud, P. A unified approach to dielectric single cell analysis: Impedance and dielectrophoretic force spectroscopy. Lab Chip 2010, 10, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Tan, H.; Chen, D.; Wang, Y.; Xu, Y.; Wang, J.; Chen, J. Development of microfluidic platform to high-throughput quantify single-cell intrinsic bioelectrical markers of tumor cell lines, subtypes and patient tumor cells. Sens. Actuators B Chem. 2020, 317, 128231. [Google Scholar] [CrossRef]
- Caselli, F.; De Ninno, A.; Reale, R.; Businaro, L.; Bisegna, P. A novel wiring scheme for standard chips enabling high-accuracy impedance cytometry. Sens. Actuators B Chem. 2018, 256, 580–589. [Google Scholar] [CrossRef]
- Spencer, D.; Caselli, F.; Bisegna, P.; Morgan, H. High accuracy particle analysis using sheathless microfluidic impedance cytometry. Lab Chip 2016, 16, 2467–2473. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Cheng, Z.; Xu, Y.; Liu, R.; Li, Q.; Cheng, J. A sheath-less electric impedance micro-flow cytometry device for rapid label-free cell classification and viability testing. Anal. Methods 2017, 9, 1201–1212. [Google Scholar] [CrossRef]
- Demierre, N.; Braschler, T.; Muller, R.; Renaud, P. Focusing and continuous separation of cells in a microfluidic device using lateral dielectrophoresis. Sens. Actuators B Chem. 2008, 132, 388–396. [Google Scholar] [CrossRef]
- Demierre, N.; Braschler, T.; Linderholm, P.; Seger, U.; van Lintel, H.; Renaud, P. Characterization and optimization of liquid electrodes for lateral dielectrophoresis. Lab Chip 2007, 7, 355–365. [Google Scholar] [CrossRef]
- Shaker, M.; Colella, L.; Caselli, F.; Bisegna, P.; Renaud, P. An impedance-based flow microcytometer for single cell morphology discrimination. Lab Chip 2014, 14, 2548–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottet, J.; Kehren, A.; van Lintel, H.; Buret, F.; Frénéa-Robin, M.; Renaud, P. How to improve the sensitivity of coplanar electrodes and micro channel design in electrical impedance flow cytometry: A study. Microfluid. Nanofluid. 2019, 23, 11. [Google Scholar] [CrossRef]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation. Cytometry A 2005, 65, 124–132. [Google Scholar] [CrossRef]
- Spencer, D.; Hollis, V.; Morgan, H. Microfluidic impedance cytometry of tumour cells in blood. Biomicrofluidics 2014, 8, 064124. [Google Scholar] [CrossRef] [Green Version]
- Clausen, C.H.; Dimaki, M.; Bertelsen, C.V.; Skands, G.E.; Rodriguez-Trujillo, R.; Thomsen, J.D.; Svendsen, W.E. Bacteria detection and differentiation using impedance flow cytometry. Sensors 2018, 18, 3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürgel, S.C.; Escobedo, C.; Haandbæk, N.; Hierlemann, A. On-chip electroporation and impedance spectroscopy of single-cells. Sens. Actuators B Chem. 2015, 210, 82–90. [Google Scholar] [CrossRef]
- Spencer, D.; Morgan, H. Positional dependence of particles in microfludic impedance cytometry. Lab Chip 2011, 11, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yang, L.; Hu, S.; Liu, K.; Jia, J.; Yao, J. Position Calibration of a Single Cell Measurement with Electrochemical Impedance Spectroscopy. IEEE Sens. J. 2021, 1. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Tan, Q.; Shojaei-Baghini, E.; Zhang, Y.L.; Li, J.; Prasad, P.; You, L.; Wu, X.Y.; Sun, Y. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab Chip 2011, 11, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, K.; Chen, D.; Fan, B.; Xu, Y.; Ye, Y.; Wang, J.; Chen, J.; Huang, C. Development of microfluidic impedance cytometry enabling the quantification of specific membrane capacitance and cytoplasm conductivity from 100,000 single cells. Biosens. Bioelectron. 2018, 111, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shojaei-Baghini, E.; Azad, A.; Wang, C.; Sun, Y. High-throughput biophysical measurement of human red blood cells. Lab Chip 2012, 12, 2560–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Chen, L.; Zhang, S.; Wang, J.; Duan, X. Label-free and simultaneous mechanical and electrical characterization of single plant cells using microfluidic impedance flow cytometry. Anal. Chem. 2020, 92, 14568–14575. [Google Scholar] [CrossRef]
- Watkins, N.; Venkatesan, B.M.; Toner, M.; Rodriguez, W.; Bashir, R. A robust electrical microcytometer with 3-dimensional hydrofocusing. Lab Chip 2009, 9, 3177–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Jeon, C.S.; Hwang, I.; Ko, J.; Lee, S.; Choo, J.; Boo, J.H.; Kim, H.C.; Chung, T.D. A flow cytometry-based submicron-sized bacterial detection system using a movable virtual wall. Lab Chip 2014, 14, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Grenvall, C.; Antfolk, C.; Bisgaard, C.Z.; Laurell, T. Two-dimensional acoustic particle focusing enables sheathless chip Coulter counter with planar electrode configuration. Lab Chip 2014, 14, 4629–4637. [Google Scholar] [CrossRef]
- Ng, J.W.; Collins, D.J.; Devendran, C.; Ai, Y.; Neild, A. Flow-rate-insensitive deterministic particle sorting using a combination of travelling and standing surface acoustic waves. Microfluid. Nanofluid. 2016, 20, 151. [Google Scholar] [CrossRef]
- Sriphutkiat, Y.; Zhou, Y. Particle accumulation in a microchannel and its reduction by a standing surface acoustic wave (SSAW). Sensors 2017, 17, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mernier, G.; Duqi, E.; Renaud, P. Characterization of a novel impedance cytometer design and its integration with lateral focusing by dielectrophoresis. Lab Chip 2012, 12, 4344–4349. [Google Scholar] [CrossRef] [PubMed]
- Evander, M.; Ricco, A.J.; Morser, J.; Kovacs, G.T.; Leung, L.L.; Giovangrandi, L. Microfluidic impedance cytometer for platelet analysis. Lab Chip 2013, 13, 722–729. [Google Scholar] [CrossRef]
- Carlo, D.D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, D.; Wu, L.Y.; Lee, L.P. Dynamic single cell culture array. Lab Chip 2006, 6, 1445–1449. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2006, 104, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Jang, L.S.; Wang, M.H. Microfluidic device for cell capture and impedance measurement. Biomed. Microdevices 2007, 9, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Dynamic microarray system with gentle retrieval mechanism for cell-encapsulating hydrogel beads. Lab Chip 2008, 8, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Malleo, D.; Nevill, J.T.; Lee, L.P.; Morgan, H. Continuous differential impedance spectroscopy of single cells. Microfluid. Nanofluid. 2010, 9, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.H.; Yamamoto, T.; Sakai, Y.; Fujii, T.; Kim, B. Development of microfluidic device for electrical/physical characterization of single cell. J. Microelectromech. Syst. 2006, 15, 287–295. [Google Scholar] [CrossRef]
- Han, K.H.; Han, A.; Frazier, A.B. Microsystems for isolation and electrophysiological analysis of breast cancer cells from blood. Biosens. Bioelectron. 2006, 21, 1907–1914. [Google Scholar] [CrossRef]
- Younghak, C.; Hyun Soo, K.; Frazier, A.B.; Chen, Z.G.; Dong Moon, S.; Han, A. Whole-cell impedance analysis for highly and poorly metastatic cancer cells. J. Microelectromech. Syst. 2009, 18, 808–817. [Google Scholar] [CrossRef]
- Lan, K.C.; Jang, L.S. Integration of single-cell trapping and impedance measurement utilizing microwell electrodes. Biosens. Bioelectron. 2011, 26, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Heida, T.; Rutten, W.L.C.; Marani, E. Understanding dielectrophoretic trapping of neuronal cells: Modelling electric field, electrodeliquid interface and fluid flow. J. Phys. D Appl. Phys. 2002, 35, 1592–1602. [Google Scholar] [CrossRef]
- Rosenthal, A.; Voldman, J. Dielectrophoretic traps for single-particle patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef] [Green Version]
- Jang, L.S.; Huang, P.H.; Lan, K.C. Single-cell trapping utilizing negative dielectrophoretic quadrupole and microwell electrodes. Biosens. Bioelectron. 2009, 24, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Shi, Y.; Lao, Z.; Ni, J.; Li, G.; Hu, Y.; Li, J.; Chu, J.; Wu, D.; Sugioka, K. Real-time two-photon lithography in controlled flow to create a single-microparticle array and particle-cluster array for optofluidic imaging. Lab Chip 2018, 18, 442–450. [Google Scholar] [CrossRef]
- Tang, W.; Tang, D.; Ni, Z.; Xiang, N.; Yi, H. A portable single-cell analysis system integrating hydrodynamic trapping with broadband impedance spectroscopy. Sci. China Technol. Sci. 2017, 60, 1707–1715. [Google Scholar] [CrossRef]
- Zhu, Z.; Frey, O.; Franke, F.; Haandbaek, N.; Hierlemann, A. Real-time monitoring of immobilized single yeast cells through multifrequency electrical impedance spectroscopy. Anal. Bioanal. Chem. 2014, 406, 7015–7025. [Google Scholar] [CrossRef] [Green Version]
- Taff, B.M.; Voldman, J. A scalable addressable positive-dielectrophoretic cell-sorting array. Anal. Chem. 2005, 77, 7976–7983. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Yin, T.-I.; Reyes, D.; Urban, G.A. Microfluidic chip with integrated electrical cell-Impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal. Chem. 2013, 85, 11068–11076. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhu, Z.; Zhang, Z.; Xu, F.; Marchisio, M.A.; Wang, Z.; Pan, D.; Zhao, X.; Huang, Q.A. Design and 3D modeling investigation of a microfluidic electrode array for electrical impedance measurement of single yeast cells. Electrophoresis 2021, 42, 1996–2009. [Google Scholar] [CrossRef]
- Asphahani, F.; Zhang, M. Cellular impedance biosensors for drug screening and toxin detection. Analyst 2007, 132, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.T.A. Electronic sensors with living cellular components. Proc. IEEE 2003, 91, 915–929. [Google Scholar] [CrossRef]
- Seriburi, P.; McGuire, S.; Shastry, A.; Böhringer, K.F.; Meldrum, D.R. Measurement of the cell-substrate separation and the projected area of an individual adherent cell using electric cell-substrate impedance sensing. Anal. Chem. 2008, 80, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Thein, M.; Asphahani, F.; Cheng, A.; Buckmaster, R.; Zhang, M.; Xu, J. Response characteristics of single-cell impedance sensors employed with surface-modified microelectrodes. Biosens. Bioelectron. 2010, 25, 1963–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.L.; Wang, M.H. 24 h observation of a single HeLa cell by impedance measurement and numerical modeling. Sens. Actuators B Chem. 2016, 229, 225–231. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Zhu, R. Microchip with single-cell impedance measurements for monitoring osteogenic differentiation of mesenchymal stem cells under electrical stimulation. Anal. Chem. 2020, 92, 12579–12587. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Zhu, X.; Zhang, X.; He, J.; Li, C.Z. Microelectromechanical system-based sensing arrays for comparative in vitro nanotoxicity assessment at single cell and small cell-population using electrochemical impedance spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 5804–5812. [Google Scholar] [CrossRef]
- Zhu, Z.; Frey, O.; Haandbaek, N.; Franke, F.; Rudolf, F.; Hierlemann, A. Time-lapse electrical impedance spectroscopy for monitoring the cell cycle of single immobilized S. pombe cells. Sci. Rep. 2015, 5, 17180. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Basu, S.; Laue, E.; Seshia, A.A. Single cell studies of mouse embryonic stem cell (mESC) differentiation by electrical impedance measurements in a microfluidic device. Biosens. Bioelectron. 2016, 81, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Zhu, R. Controllable in-situ cell electroporation with cell positioning and impedance monitoring using micro electrode array. Sci. Rep. 2016, 6, 31392. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, L.; Cao, Z.; Zhou, H.; Yang, W. A high-speed electrical impedance measurement circuit based on information-filtering demodulation. Meas. Sci. Technol. 2014, 25, 075010. [Google Scholar] [CrossRef]
- Kishore, K.; Akbar, S.A. Evolution of lock-in amplifier as portable sensor interface platform: A review. IEEE Sens. J. 2020, 20, 10345–10354. [Google Scholar] [CrossRef]
- Zurich Instruments. Available online: https://www.zhinst.com/others/en (accessed on 14 September 2021).
- Liquid Instruments. Available online: https://www.liquidinstruments.com/company/ (accessed on 14 September 2021).
- NF Corporation. Available online: https://www.nfcorp.co.jp/english/index.html (accessed on 14 September 2021).
- SRS Home Page. Available online: https://thinksrs.com/index.html (accessed on 14 September 2021).
- SBT Instruments. Available online: https://sbtinstruments.com/ (accessed on 12 November 2021).
- Sine Scientific Instruments. Available online: https://www.ssi-instrument.com/ (accessed on 21 November 2021).
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Geng, Y.; Zhang, X.; Chen, D.; Cai, Z.; Wang, M.; Zhu, Z.; Wang, Z. A wide-band digital lock-in amplifier and its application in microfluidic impedance measurement. Sensors 2019, 19, 3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wong, C.C.; Pui, T.S.; Nadipalli, R.; Weerasekera, R.; Chandran, J.; Yu, H.; Rahman, A.R.A. CMOS high density electrical impedance biosensor array for tumor cell detection. Sens. Actuators B Chem. 2012, 173, 903–907. [Google Scholar] [CrossRef]
- Gamo, K.; Nakazato, K.; Niitsu, K. Design, theoretical analysis, and experimental verification of a CMOS current integrator with 1.2 × 2.05 µm2 microelectrode array for high-sensitivity bacterial counting. Jpn. J. Appl. Phys. 2017, 56, 01AH01. [Google Scholar] [CrossRef]
- Viswam, V.; Bounik, R.; Shadmani, A.; Dragas, J.; Urwyler, C.; Boos, J.A.; Obien, M.E.J.; Muller, J.; Chen, Y.; Hierlemann, A. Impedance spectroscopy and electrophysiological imaging of cells with a high-density CMOS microelectrode array system. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiang, Y.; Alvarez, O.; Du, E. Electrical impedance microflow cytometry with oxygen control for detection of sickle cells. Sens. Actuators B Chem. 2018, 255, 2392–2398. [Google Scholar] [CrossRef]
- Hassan, U.; Watkins, N.N.; Reddy, B., Jr.; Damhorst, G.; Bashir, R. Microfluidic differential immunocapture biochip for specific leukocyte counting. Nat. Protoc. 2016, 11, 714–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.; Morgan, H. Single cell impedance cytometry for identification and counting of CD4 T-cells in human blood using impedance labels. Anal. Chem. 2010, 82, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Ha, S.; Diez-Silva, M.; Dao, M.; Suresh, S.; Chandrakasan, A.P. Electric impedance microflow cytometry for characterization of cell disease states. Lab Chip 2013, 13, 3903–3909. [Google Scholar] [CrossRef] [Green Version]
- Honrado, C.; Ciuffreda, L.; Spencer, D.; Ranford-Cartwright, L.; Morgan, H. Dielectric characterization of Plasmodium falciparum-infected red blood cells using microfluidic impedance cytometry. J. R. Soc. Interface 2018, 15, 20180416. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Kim, K.B.; Jeon, C.S.; Hwang, I.; Lee, S.; Kim, H.K.; Kim, H.C.; Chung, T.D. A label-free DC impedance-based microcytometer for circulating rare cancer cell counting. Lab Chip 2013, 13, 970–977. [Google Scholar] [CrossRef]
- Han, S.I.; Han, K.H. Electrical detection method for circulating tumor cells using graphene nanoplates. Anal. Chem. 2015, 87, 10585–10592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, M.; Chen, D.; Zhao, X.; Xue, C.; Hao, R.; Yue, W.; Wang, J.; Chen, J. Single-cell electrical phenotyping enabling the classification of mouse tumor samples. Sci. Rep. 2016, 6, 19487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, S.P.; Coston, A.; Berlin, A. Micro-electrical impedance spectroscopy and identification of patient-derived, dissociated tumor cells. IEEE Trans. Nanobiosci. 2019, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ghassemi, P.; Strobl, J.S.; Agah, M. Biophysical phenotyping of cells via impedance spectroscopy in parallel cyclic deformability channels. Biomicrofluidics 2019, 13, 044103. [Google Scholar] [CrossRef]
- McGrath, J.S.; Honrado, C.; Moore, J.H.; Adair, S.J.; Varhue, W.B.; Salahi, A.; Farmehini, V.; Goudreau, B.J.; Nagdas, S.; Blais, E.M.; et al. Electrophysiology-based stratification of pancreatic tumorigenicity by label-free single-cell impedance cytometry. Anal. Chim. Acta 2020, 1101, 90–98. [Google Scholar] [CrossRef]
- Ostermann, M.; Sauter, A.; Xue, Y.; Birkeland, E.; Schoelermann, J.; Holst, B.; Cimpan, M.R. Label-free impedance flow cytometry for nanotoxicity screening. Sci. Rep. 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Hou, J.M.; Ward, T.H.; Blackhall, F.H.; Dive, C. Circulating tumour cells: Their utility in cancer management and predicting outcomes. Ther. Adv. Med. Oncol. 2010, 2, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, C.; Buth, H.; Cho, S.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Y.; Rosano, J.M.; Prabhakarpandian, B.; Garson, C.; Pant, K.; Lai, E. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells. Lab Chip 2013, 13, 2300–2310. [Google Scholar] [CrossRef]
- Song, H.; Rosano, J.M.; Wang, Y.; Garson, C.J.; Prabhakarpandian, B.; Pant, K.; Klarmann, G.J.; Perantoni, A.; Alvarez, L.M.; Lai, E. Identification of mesenchymal stem cell differentiation state using dual-micropore microfluidic impedance flow cytometry. Anal. Methods 2016, 8, 7437–7444. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Sun, H.; Chen, D.; Li, Z.; Fan, B.; George, J.; Xue, C.; Cui, Z.; Wang, J.; et al. Electrical property characterization of neural stem cells in differentiation. PLoS ONE 2016, 11, e0158044. [Google Scholar] [CrossRef] [Green Version]
- Xavier, M.; de Andres, M.C.; Spencer, D.; Oreffo, R.O.C.; Morgan, H. Size and dielectric properties of skeletal stem cells change critically after enrichment and expansion from human bone marrow: Consequences for microfluidic cell sorting. J. R. Soc. Interface 2017, 14, 20170233. [Google Scholar] [CrossRef] [Green Version]
- Heidmann, I.; Schade-Kampmann, G.; Lambalk, J.; Ottiger, M.; Di Berardino, M. Impedance flow cytometry: A novel technique in pollen analysis. PLoS ONE 2016, 11, e0165531. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, I.; Di Berardino, M. Impedance flow cytometry as a tool to analyze microspore and pollen quality. Methods Mol. Biol. 2017, 1669, 339–354. [Google Scholar]
- Impe, D.; Reitz, J.; Kopnick, C.; Rolletschek, H.; Borner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for wheat. Front. Plant Sci. 2019, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Ascari, L.; Cristofori, V.; Macri, F.; Botta, R.; Silvestri, C.; De Gregorio, T.; Huerta, E.S.; Di Berardino, M.; Kaufmann, S.; Siniscalco, C. Hazelnut pollen phenotyping using label-free impedance flow cytometry. Front. Plant Sci. 2020, 11, 615922. [Google Scholar] [CrossRef] [PubMed]
- Canonge, J.; Philippot, M.; Leblanc, C.; Potin, P.; Bodin, M. Impedance flow cytometry allows the early prediction of embryo yields in wheat (Triticum aestivum L.) microspore cultures. Plant Sci. 2020, 300, 110586. [Google Scholar] [CrossRef] [PubMed]
- Bernabini, C.; Holmes, D.; Morgan, H. Micro-impedance cytometry for detection and analysis of micron-sized particles and bacteria. Lab Chip 2011, 11, 407–412. [Google Scholar] [CrossRef] [PubMed]
- David, F.; Hebeisen, M.; Schade, G.; Franco-Lara, E.; Di Berardino, M. Viability and membrane potential analysis of Bacillus megaterium cells by impedance flow cytometry. Biotechnol. Bioeng. 2012, 109, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.T.; Bilican, I. Capacitive detection of single bacterium from drinking water with a detailed investigation of electrical flow cytometry. Sens. Actuator A Phys. 2018, 269, 454–463. [Google Scholar] [CrossRef]
- McGrath, J.S.; Honrado, C.; Spencer, D.; Horton, B.; Bridle, H.L.; Morgan, H. Analysis of parasitic protozoa at the single-cell level using microfluidic impedance cytometry. Sci. Rep. 2017, 7, 2601. [Google Scholar] [CrossRef] [Green Version]
- Chawla, K.; Burgel, S.C.; Schmidt, G.W.; Kaltenbach, H.M.; Rudolf, F.; Frey, O.; Hierlemann, A. Integrating impedance-based growth-rate monitoring into a microfluidic cell culture platform for live-cell microscopy. Microsyst. Nanoeng. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.; Schade, G.; Kaufmann, S.; Berardino, M.D. Rapid determination of general cell status, cell viability, and optimal harvest time in eukaryotic cell cultures by impedance flow cytometry. Appl. Microbiol. Biotechnol. 2019, 103, 8619–8629. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, Z.; Ge, X.; Zhao, X.; Hao, L.; Cheng, Z.; Zhou, W.; Du, Y.; Wang, L.; Tian, F.; et al. Particle self-aligning, focusing, and electric impedance microcytometer device for label-free single cell morphology discrimination and yeast budding analysis. Anal. Chem. 2019, 91, 13398–13406. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, C.V.; Franco, J.C.; Skands, G.E.; Dimaki, M.; Svendsen, W.E. Investigating the use of impedance flow cytometry for classifying the viability state of E. coli. Sensors 2020, 20, 6339. [Google Scholar] [CrossRef]
- Spencer, D.C.; Paton, T.F.; Mulroney, K.T.; Inglis, T.J.J.; Sutton, J.M.; Morgan, H. A fast impedance-based antimicrobial susceptibility test. Nat. Commun. 2020, 11, 5328. [Google Scholar] [CrossRef] [PubMed]
- van Beers, E.J.; Samsel, L.; Mendelsohn, L.; Saiyed, R.; Fertrin, K.Y.; Brantner, C.A.; Daniels, M.P.; Nichols, J.; McCoy, J.P.; Kato, G.J. Imaging flow cytometry for automated detection of hypoxia-induced erythrocyte shape change in sickle cell disease. Am. J. Hematol. 2014, 89, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küttel, C.; Nascimento, E.; Demierre, N.; Silva, T.; Braschler, T.; Renaud, P.; Oliva, A.G. Label-free detection of Babesia bovis infected red blood cells using impedance spectroscopy on a microfabricated flow cytometer. Acta Trop. 2007, 102, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.; Pettigrew, D.; Reccius, C.H.; Gwyer, J.D.; van Berkel, C.; Holloway, J.; Davies, D.E.; Morgan, H. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 2009, 9, 2881–2889. [Google Scholar] [CrossRef]
- Hassan, U.; Reddy, B., Jr.; Damhorst, G.; Sonoiki, O.; Ghonge, T.; Yang, C.; Bashir, R. A microfluidic biochip for complete blood cell counts at the point-of-care. Technology 2015, 3, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, N.N.; Hassan, U.; Damhorst, G.; Ni, H.; Vaid, A.; Rodriguez, W.; Bashir, R. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci. Transl. Med. 2013, 5, 214ra170. [Google Scholar] [CrossRef]
- Durand-Smet, P.; Chastrette, N.; Guiroy, A.; Richert, A.; Berne-Dedieu, A.; Szecsi, J.; Boudaoud, A.; Frachisse, J.-M.; Bendahmane, M.; Hamant, O.; et al. A comparative mechanical analysis of plant and animal cells reveals convergence across kingdoms. Biophys. J. 2014, 107, 2237–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Han, Z.; Fan, X.; Zhang, S.; Wang, J.; Duan, X. An impedance-coupled microfluidic device for single-cell analysis of primary cell wall regeneration. Biosens. Bioelectron. 2020, 165, 112374. [Google Scholar] [CrossRef] [PubMed]
- Houssin, T.; Follet, J.; Follet, A.; Dei-Cas, E.; Senez, V. Label-free analysis of water-polluting parasite by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2010, 25, 1122–1129. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.W.; Yun, J.; Seo, S.; Park, C.J.; Lee, J.Z.; Lee, J.H. Microelectrical impedance spectroscopy for the differentiation between normal and cancerous human urothelial cell lines: Real-time electrical impedance measurement at an optimal frequency. BioMed Res. Int. 2016, 2016, 8748023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.-L.; Lan, K.-C.; Jang, L.-S. Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sens. Actuators B Chem. 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Primiceri, E.; Chiriaco, M.S.; Dioguardi, F.; Monteduro, A.G.; D’Amone, E.; Rinaldi, R.; Giannelli, G.; Maruccio, G. Automatic transwell assay by an EIS cell chip to monitor cell migration. Lab Chip 2011, 11, 4081–4086. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Zhu, R. Single-cell individualized electroporation with real-time impedance monitoring using a microelectrode array chip. Microsyst. Nanoeng. 2020, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, T.; Zhu, R. Long-term and label-free monitoring for osteogenic differentiation of mesenchymal stem cells using force sensor and impedance measurement. J. Mater. Chem. B 2020, 8, 9913–9920. [Google Scholar] [CrossRef]
- Ghenim, L.; Kaji, H.; Hoshino, Y.; Ishibashi, T.; Haguet, V.; Gidrol, X.; Nishizawa, M. Monitoring impedance changes associated with motility and mitosis of a single cell. Lab Chip 2010, 10, 2546–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Frey, O.; Ottoz, D.S.; Rudolf, F.; Hierlemann, A. Microfluidic single-cell cultivation chip with controllable immobilization and selective release of yeast cells. Lab Chip 2012, 12, 906–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowell, L.L.; Yakisich, J.S.; Aufderheide, B.; Adams, T.N.G. Electrical impedance spectroscopy for monitoring chemoresistance of cancer cells. Micromachines 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
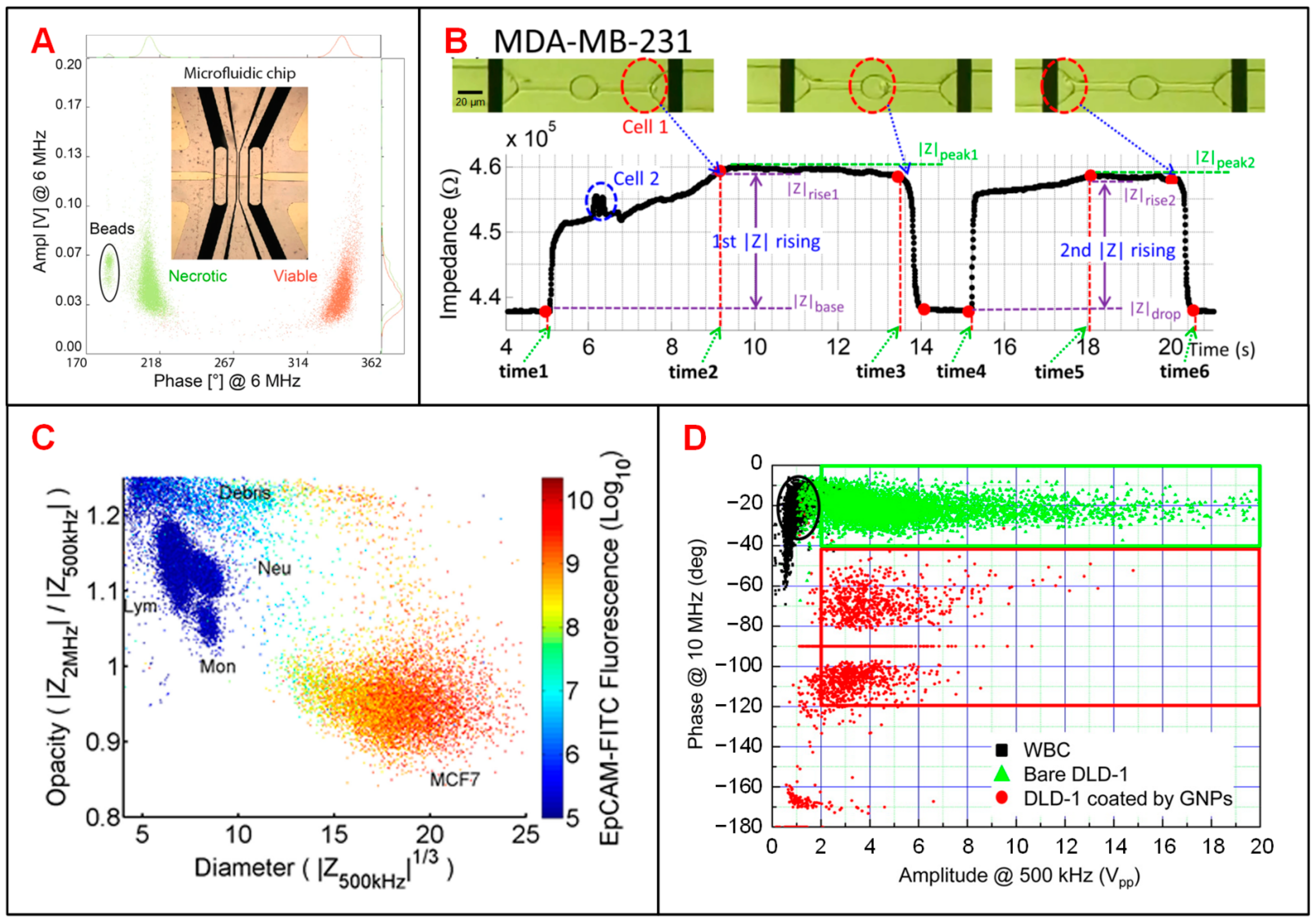
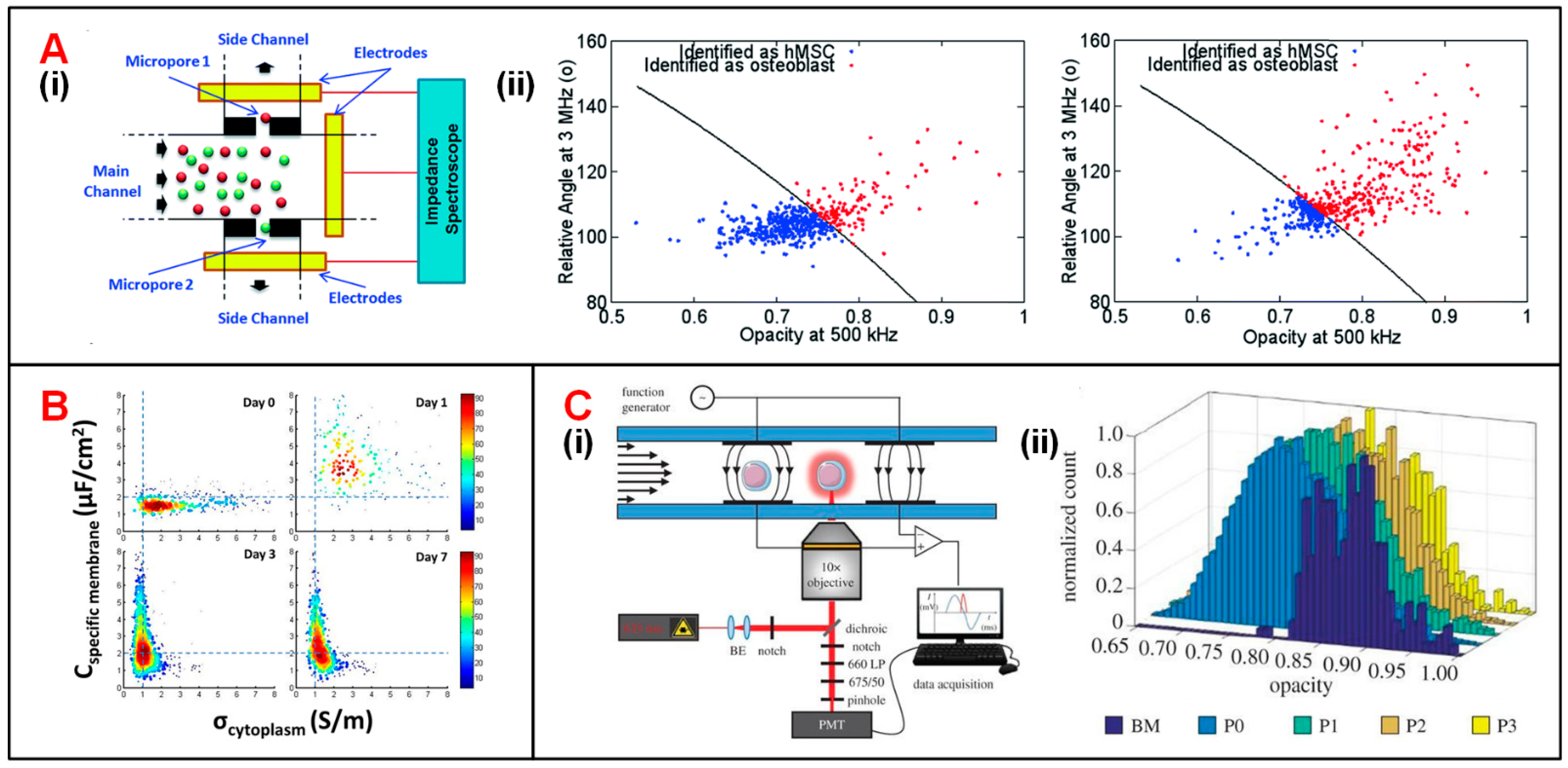
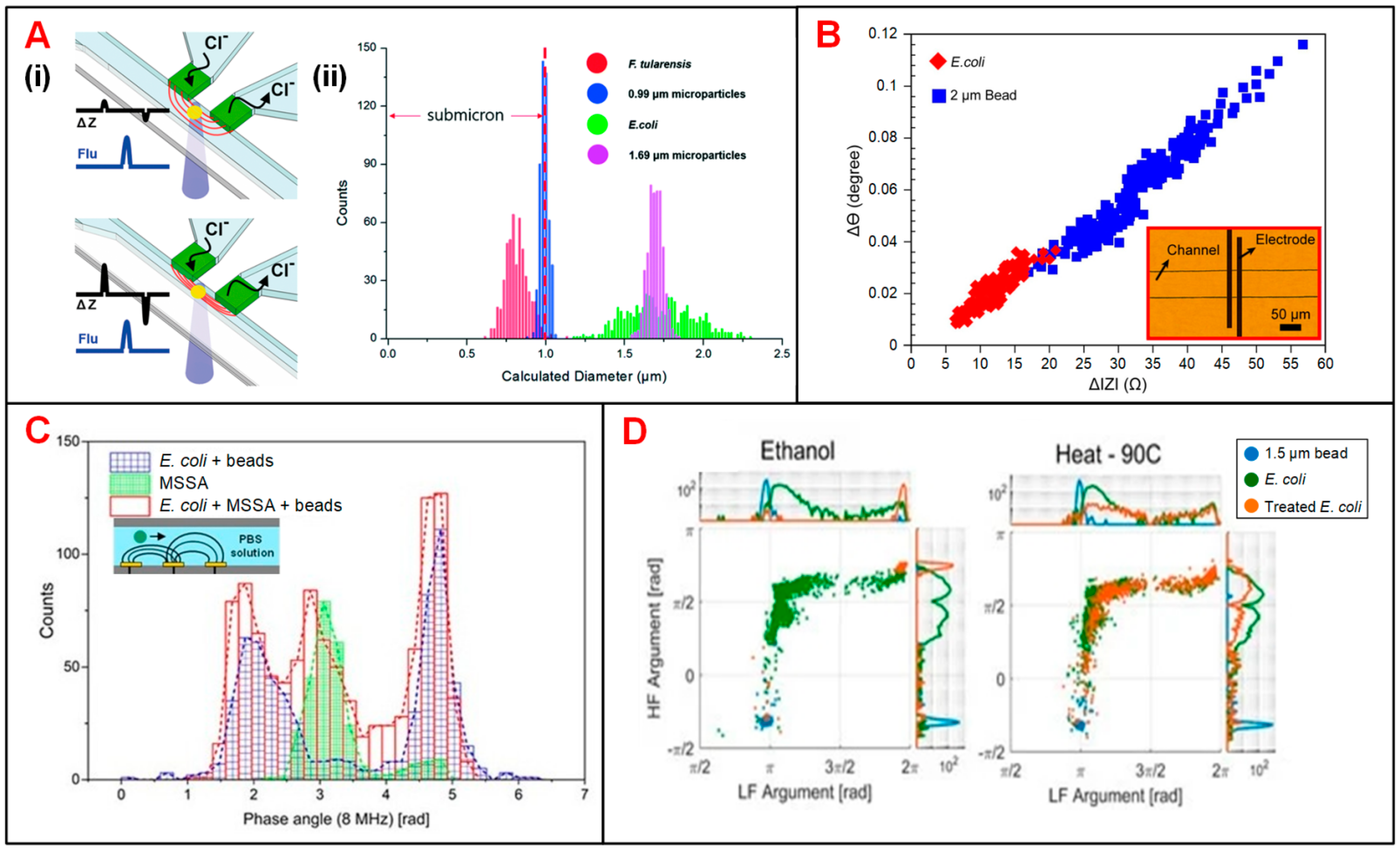
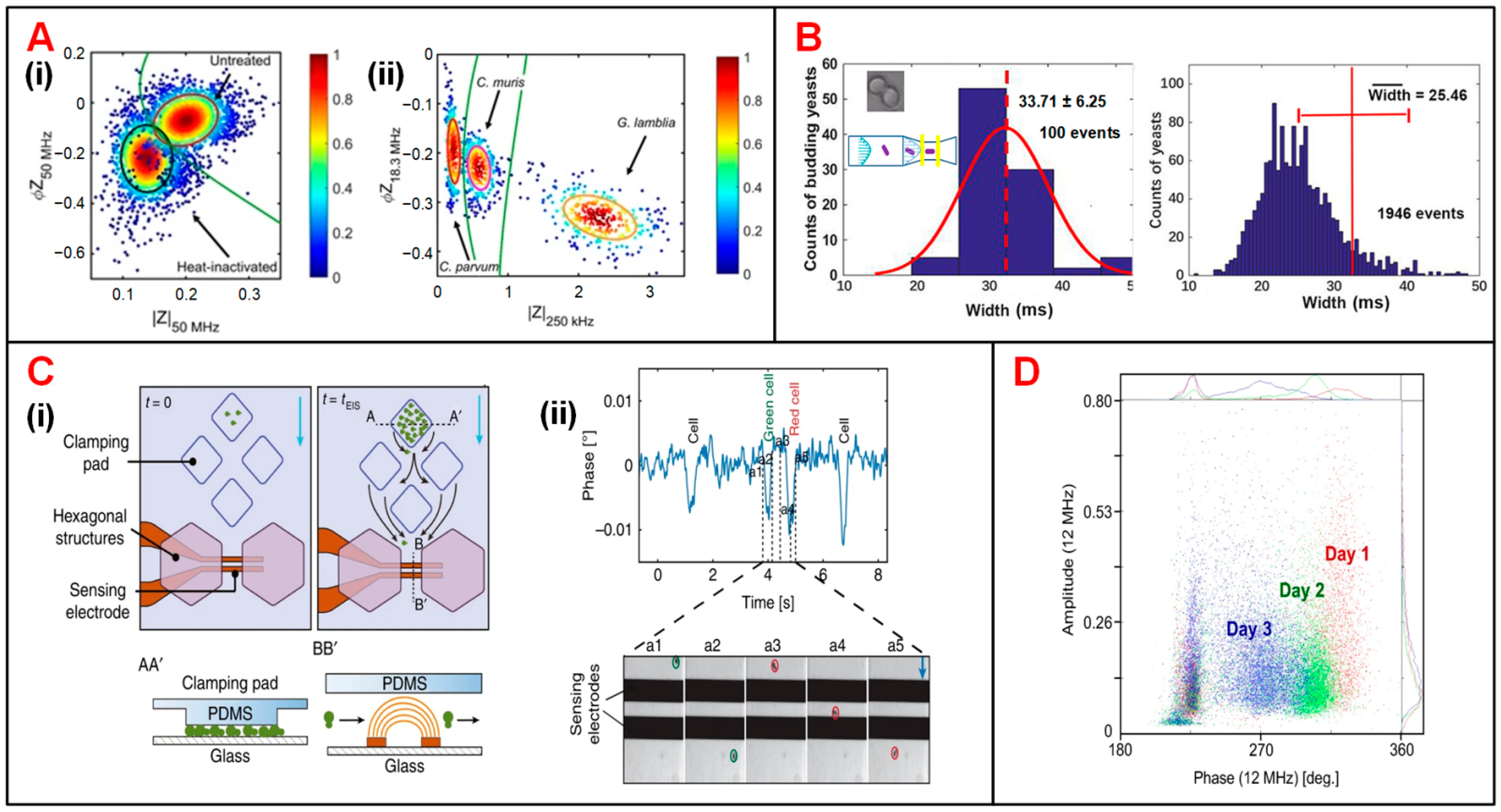
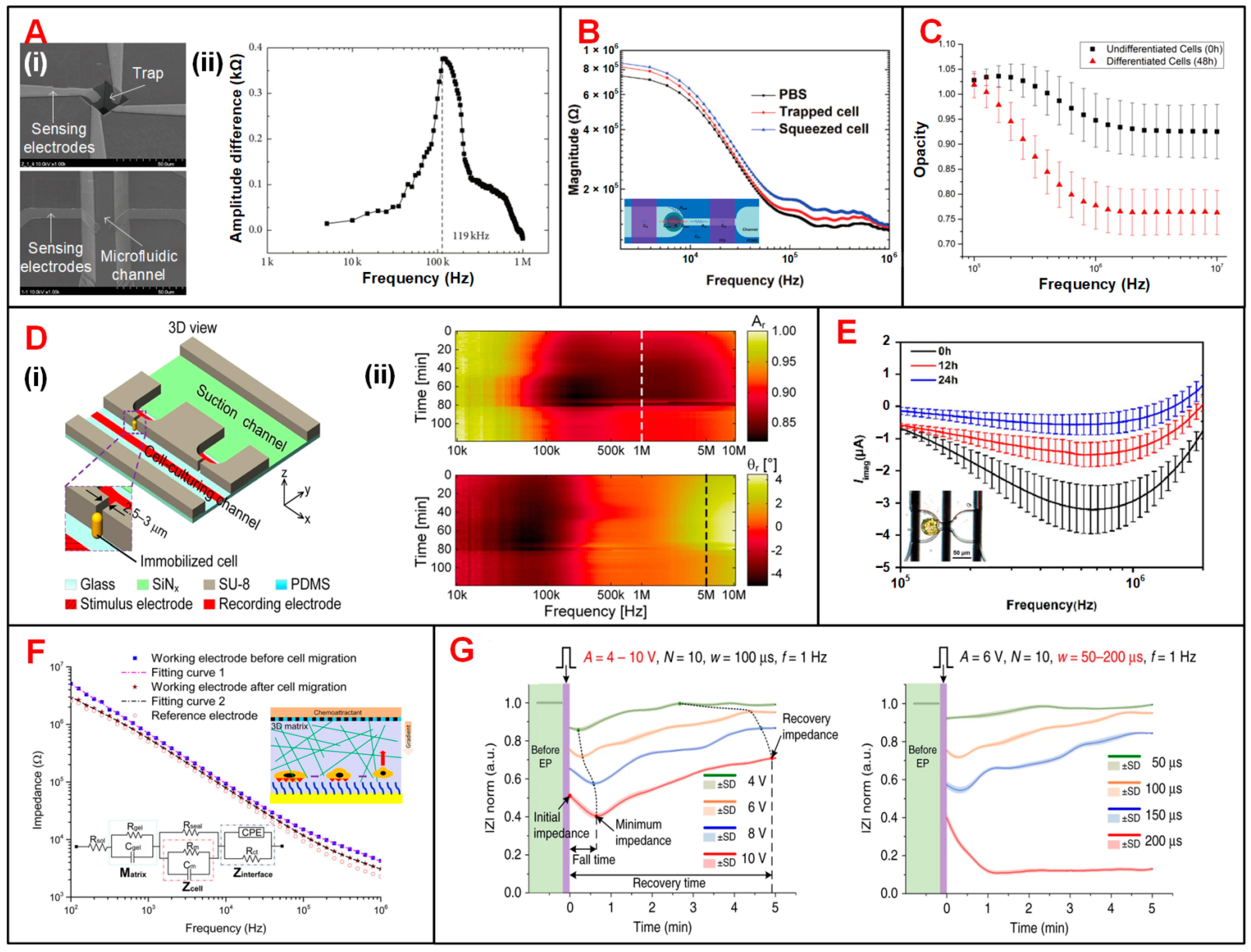
| Category | First Author (Year) | Electrode and Fluidic Layouts | Frequency | Target Cells | Application | Ref. |
|---|---|---|---|---|---|---|
| Blood cells | Holmes (2010) | 2 coplanar electrode pairs | 503 kHz and 10 MHz | CD4 T-cells | Cell counting | [112] |
| Du (2013) | 1 coplanar electrode pair | 2 MHz | Red blood cells | Detection of malaria-infected cells | [113] | |
| Hassan (2016) | 2 coplanar electrode pairs | 303 kHz and 1.7 MHz | CD4 and CD8 T-cells | Cell counting | [111] | |
| Liu (2018) | 2 coplanar electrode pairs | 156 kHz, 500 kHz and 3 MHz | Red blood cells | Detection of sickle cells | [110] | |
| Honrdo (2018) | 2 facing electrode pairs, fluorescence detection | 2–8 MHz | Red blood cells | Detection of malaria-infected cells | [114] | |
| Tumor cells | Choi (2013) | Two polyelectrolyte gel electrodes | DC | OVCAR-3 cells | Cell recognition | [115] |
| Spencer (2014) | 2 facing electrode pairs | 0.5 MHz and 2 MHz | MCF-7 cells | Cell recognition | [52] | |
| Han (2015) | 2 facing electrode pairs | 500 kHz and 10 MHz | DLD-1 cells | Cell recognition | [116] | |
| Zhao (2016) | μCPC with constriction channel | 1 kHz and 100 kHz | A549 and H1299 cells | Cell screening | [117] | |
| Desai (2019) | 2 coplanar electrode pairs, sheath flow focusing | 250 kHz | Thyroid, breast, lung, and ovarian cancer cells | Cell recognition | [118] | |
| Ren (2019) | 1 coplanar electrode pair, 2 constriction channels | 1 kHz, 10 kHz, 100 kHz, and 1 MHz | MDA-MB-231 cells | Cell recognition | [119] | |
| McGrath (2020) | 5 facing electrode pairs | 500 kHz–50 MHz | Six types of pancreatic ductal adenocarcinoma cell | Cell screening | [120] | |
| Ostermann (2020) | 2 facing electrode pairs | 6 MHz | U937 cells | Viability assay | [121] | |
| Zhang (2020) | 1 coplanar electrode pair, asymmetrical constriction channel | 100 kHz and 250 kHz | A549 and Hep G2 cells | Cell screening | [43] | |
| Stem cells | Song (2016) | C-shaped arranged coplanar electrodes | 500 kHz and 3 MHz | Mesenchymal stem cells | Monitoring differentiation process | [125] |
| Xavier (2017) | 2 facing electrode pairs, fluorescence detection | 500 Hz and 2MHz | Skeletal stem cells | Monitoring differentiation process | [127] | |
| Plant cells | Heidmann (2016) | 2 facing electrode pairs | 500 Hz and 12 MHz | Tobacco pollen | Viability assay | [128] |
| Heidmann (2017) | 2 facing electrode pairs | 500 kHz, 3 MHz and 12 MHz | Tomato, pepper, potato and wind pollinators pollen | Viability assay | [129] | |
| Impe (2019) | 2 facing electrode pairs | 1 MHz | Wheat pollen | Viability assay | [130] | |
| Ascari (2020) | 2 facing electrode pairs | 2 MHz and 8 MHz | Hazelnut pollen | Viability assay | [131] | |
| Canonge (2020) | 2 facing electrode pairs | 500 kHz and 12 MHz | Wheat microspore | Monitoring androgenesis process | [132] | |
| Han (2020) | 2 coplanar electrode pairs, constriction channel | 500 kHz and 5 MHz | Herbaceous Arabidopsis thaliana and woody Populus trichocarpa | Cell screening | [60] | |
| Microbes | Choi (2014) | 2 polyelectrolytic gel electrodes, sheath focusing | DC | F. tularensis and E. coli | Cell recognition | [62] |
| Mcgrath (2017) | 2 facing electrode pairs | 250 kHz, 18.3 MHz and 50 MHz | C. parvum | Viability assay | [136] | |
| Guler (2018) | 1 coplanar electrode pairs | 2 MHz | E. coli | Cell recognition | [135] | |
| Clausen (2018) | 2 coplanar electrode pairs 2 facing electrode pairs | 200 kHz and 7 MHz | E. coli | Cell recognition | [53] | |
| Chawla (2018) | 1 coplanar electrode pairs | 1.12 MHz and 1.5 MHz | S. cerevisiae cells | Monitoring cell growth rate | [137] | |
| Xie (2019) | 1 coplanar electrode pairs | 1 MHz | S. cerevisiae cells | Reproductive performance assessment | [139] | |
| Opitz (2019) | 2 facing electrode pairs | 0.5 MHz, 10 MHz and 12 MHz | S. cerevisiae cells | Viability assay | [138] | |
| Bertelsen (2020) | 2 facing electrode pairs | 366 kHz and 6.9 MHz | E. coli | Determination of the viability of E. coli | [140] | |
| Spencer (2020) | 4 facing electrode pairs | 5 MHz and 40 MHz | K. pneumoniae | Antimicrobial susceptibility tests | [141] |
| First Author (Year) | Techniques | Frequency Range | Throughput | OT | Target Cells | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Primiceri (2011) | ECIS | 1 Hz to 1 MHz | / | 4 h | Hepatocellular carcinoma cells | Monitoring cell migration | [152] |
| Hong (2012) | DEP traps | 20 kHz to 101 kHz | / | / | A549, MDA-MB-231, MCF-7, and HeLa cells | Electrical characteristics analysis of cancer cells | [151] |
| Nguyen (2013) | Hydrodynamic traps and ECIS | 100 Hz to 1 MHz | 16 | / | MDA-MB-231 and MCF-7 cells | Monitoring cell capture, adhesion, and spreading process | [85] |
| Zhu (2014) | Negative pressure traps | 10 kHz to 10 MHz | 10 | 42 min | S. cerevisiae cells | Monitoring bud growth and cell motion | [83] |
| Zhu (2015) | Negative pressure traps | 10 kHz to 10 MHz | 10 | 120 min | S. pombe cells | Cell cycle determination | [94] |
| Zhou (2016) | Hydrodynamic traps | 100 Hz to 20 MHz | 10 | 48 h | Mouse embryonic stem cells | Monitoring the differentiation process | [95] |
| Park (2016) | Negative pressure traps | 5 kHz to 1 MHz | 5 | / | Cancerous human urothelial cells (TCCSUP) | Cell recognition | [150] |
| Tsai (2016) | Hydrodynamic traps | 10 kHz to 100 kHz | 3 | 24 h | HeLa cells | Monitoring electrical characteristics | [91] |
| Tang (2017) | Hydrodynamic traps | 1.953 kHz to 1 MHz | 10 | / | MCF-7 cells | Monitoring the capture process and cell screening | [82] |
| Chen (2020) | Hydrodynamic traps | 100 kHz to 2 MHz | / | 24 h | Arabidopsis mesophyll cells | Monitoring the regeneration process of primary cell wall | [148] |
| Zhang (2020) | DEP traps and ECIS | 100 kHz | 32 | 5 min | HeLa, MCF-7, and 293T cells | Monitoring the recovery process after electroporation | [153] |
| Zhang (2020) | DEP traps and ECIS | 100 kHz | 32 | 21 days | Mesenchymal stem cells | Monitoring differentiation process | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Huang, X.; Liu, K.; Lan, T.; Wang, Z.; Zhu, Z. Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors 2021, 11, 470. https://doi.org/10.3390/bios11110470
Zhang Z, Huang X, Liu K, Lan T, Wang Z, Zhu Z. Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors. 2021; 11(11):470. https://doi.org/10.3390/bios11110470
Chicago/Turabian StyleZhang, Zhao, Xiaowen Huang, Ke Liu, Tiancong Lan, Zixin Wang, and Zhen Zhu. 2021. "Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis" Biosensors 11, no. 11: 470. https://doi.org/10.3390/bios11110470
APA StyleZhang, Z., Huang, X., Liu, K., Lan, T., Wang, Z., & Zhu, Z. (2021). Recent Advances in Electrical Impedance Sensing Technology for Single-Cell Analysis. Biosensors, 11(11), 470. https://doi.org/10.3390/bios11110470






