Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis
Abstract
:1. Introduction
2. Preparation Methods and Sensing Mechanism of Cu NCs
2.1. Preparation Methods
2.1.1. Blue Emission
2.1.2. Green Emission
2.1.3. Orange/Red Emission
2.1.4. Near Infrared Emission
2.2. Sensing Mechanisms
2.2.1. Turn Off
2.2.2. Turn On
2.2.3. Ratiometric Analysis
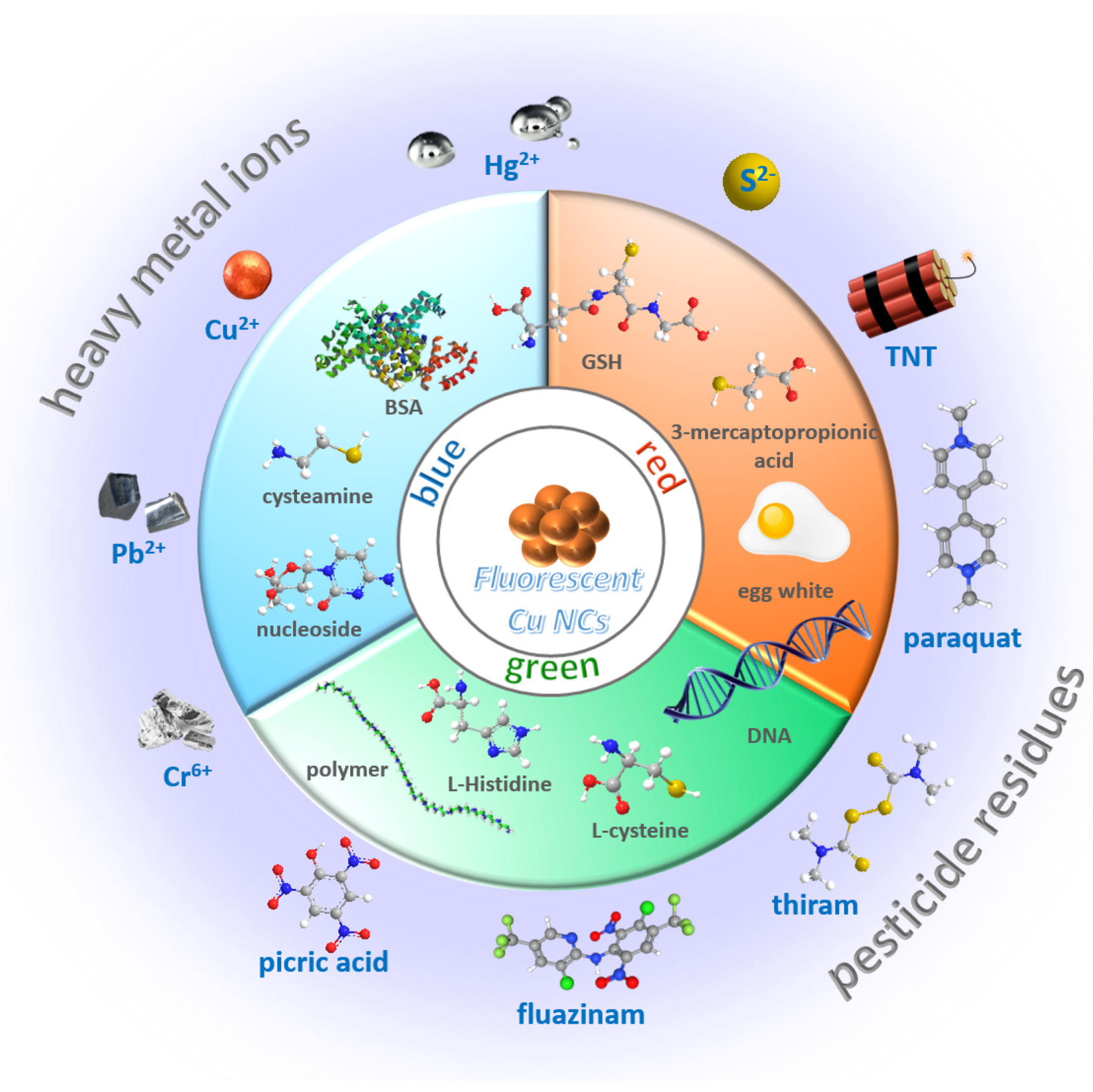
3. Sensing Applications Based on Cu NCs
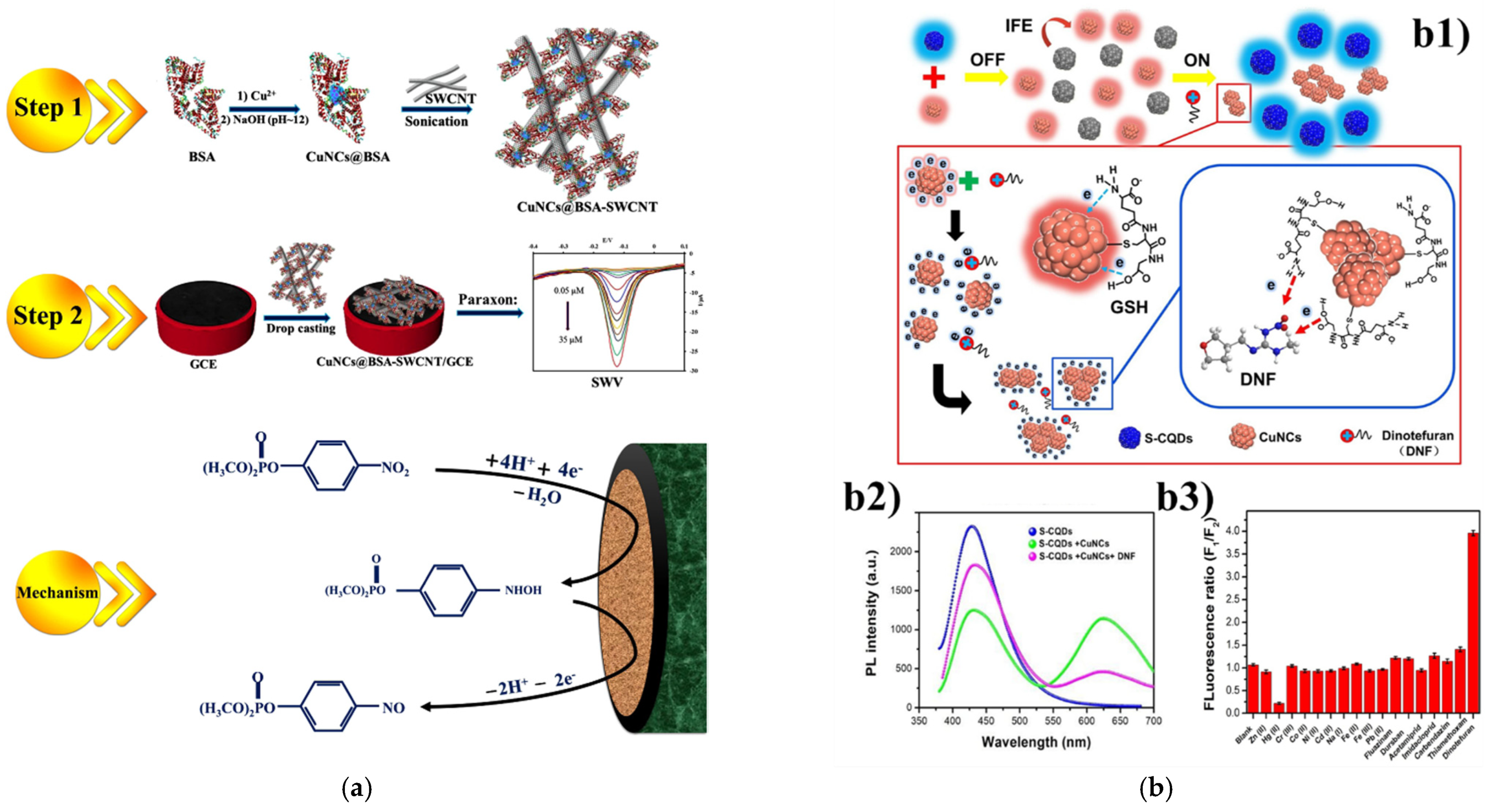
3.1. Pesticides as Target Analytes
3.2. Heavy Metals as Target Analytes
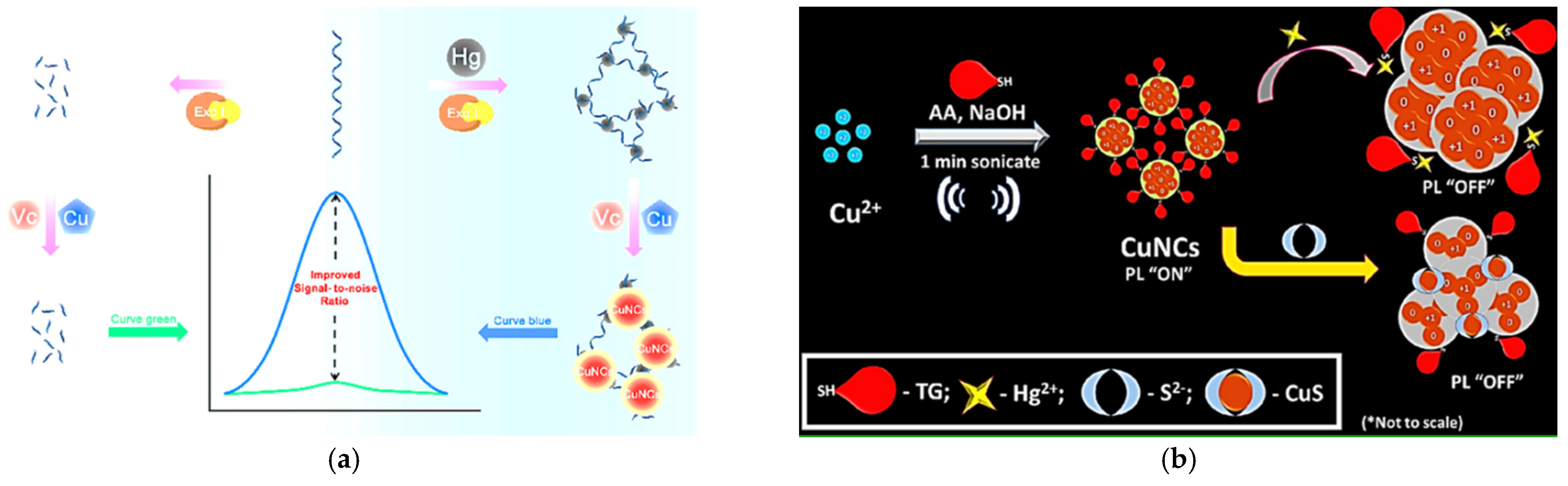
3.2.1. Mercury Ions
3.2.2. Lead Ions
3.2.3. Chromate Anions
3.2.4. Copper Ions
3.3. Sulfide as Target Analytes
3.4. Others
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2019, 120, 526–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Astruc, D. Atomically precise copper nanoclusters and their applications. Coord. Chem. Rev. 2018, 359, 112–126. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Rogach, A.L. Synthesis, optical properties and applications of light-emitting copper nanoclusters. Nanoscale Horiz. 2017, 2, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Baghdasaryan, A.; Bürgi, T. Copper nanoclusters: Designed synthesis, structural diversity, and multiplatform applications. Nanoscale 2021, 13, 6283–6340. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Development of Copper Nanoclusters for in vitro and in vivo Theranostic Applications. Adv. Mater. 2020, 32, 1906872. [Google Scholar] [CrossRef]
- Shahsavari, S.; Hadian-Ghazvini, S.; Hooriabad Saboor, F.; Menbari Oskouie, I.; Hasany, M.; Simchi, A.; Rogach, A.L. Ligand functionalized copper nanoclusters for versatile applications in catalysis, sensing, bioimaging, and optoelectronics. Mater. Chem. Front. 2019, 3, 2326–2356. [Google Scholar] [CrossRef]
- Hu, X.; Liu, T.; Zhuang, Y.; Wang, W.; Li, Y.; Fan, W.; Huang, Y. Recent advances in the analytical applications of copper nanoclusters. TrAC Trends Anal. Chem. 2016, 77, 66–75. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Waworuntu, E.H.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, R.; Shi, L.; Zhang, C.; Zhang, Y.; Zhou, Y.; Dong, C.; Li, G.; Shuang, S. Aggregation/assembly induced emission based on silk fibroin-templated fluorescent copper nanoclusters for “turn-on” detection of S2−. Sens. Actuators B Chem. 2019, 279, 361–368. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, D.; Zhang, H. Self-Assembly Driven Aggregation-Induced Emission of Copper Nanoclusters: A Novel Technology for Lighting. ACS Appl. Mater. Interfaces 2017, 10, 12071–12080. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Hajian, A.; Shahriyari, A. Protein capped Cu nanoclusters-swcnt nanocomposite as a novel candidate of high performance platform for organophosphates enzymeless biosensor. Biosens. Bioelectron. 2017, 89, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, W.; Huang, Y. Copper nanocluster-based fluorescent probe for sensitive and selective detection of Hg(2+) in water and food stuff. Talanta 2016, 154, 409–415. [Google Scholar] [CrossRef] [PubMed]
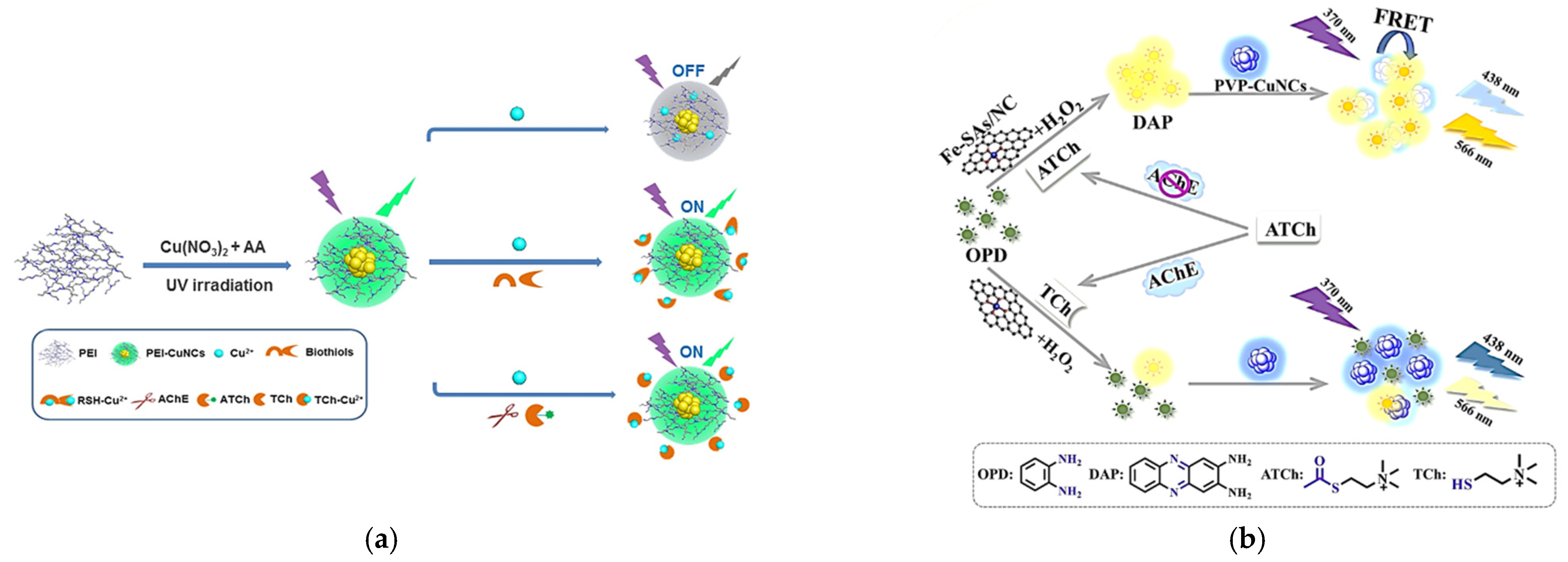
- Wang, M.; Liu, L.; Xie, X.; Zhou, X.; Lin, Z.; Su, X. Single-atom iron containing nanozyme with peroxidase-like activity and copper nanoclusters based ratio fluorescent strategy for acetylcholinesterase activity sensing. Sens. Actuators B Chem. 2020, 313, 128023. [Google Scholar] [CrossRef]
- Xia, J.; Wei, X.; Chen, X.; Shu, Y.; Wang, J. Folic acid modified copper nanoclusters for fluorescent imaging of cancer cells with over-expressed folate receptor. Microchim. Acta 2018, 185, 205. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Zhuang, Q.; Ni, Y. Label-free photoluminescence assay for nitrofurantoin detection in lake water samples using adenosine-stabilized copper nanoclusters as nanoprobes. Talanta 2018, 179, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; Zhu, S.; Luo, Y.; Zhuo, Y.; Dou, Y. One-step synthesis and applications of fluorescent Cu nanoclusters stabilized by l-cysteine in aqueous solution. Anal. Chim. Acta 2014, 847, 49–54. [Google Scholar] [CrossRef]
- Goswami, N.; Giri, A.; Bootharaju, M.S.; Xavier, P.L.; Pradeep, T.; Pal, S.K. Copper Quantum Clusters in Protein Matrix: Potential Sensor of Pb2+ Ion. Anal. Chem. 2011, 83, 9676–9680. [Google Scholar] [CrossRef]
- Xiaoqing, L.; Ruiyi, L.; Zaijun, L.; Xiulan, S.; Zhouping, W.; Junkang, L. Fast synthesis of copper nanoclusters through the use of hydrogen peroxide additive and their application for the fluorescence detection of Hg2+ in water samples. New J. Chem. 2015, 39, 5240–5248. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, J.; Wang, Q.; He, Y.; Ge, Y.; Song, C. Copper nanoclusters coated with bovine serum albumin as a regenerable fluorescent probe for copper(II) ion. Microchim. Acta 2014, 182, 909–915. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chiu, T.-C.; Hu, C.-C. Fluorescence-tunable copper nanoclusters and their application in hexavalent chromium sensing. RSC Adv. 2019, 9, 9228–9234. [Google Scholar] [CrossRef] [Green Version]
- Bao, Z.; Zhang, K.; Jian, J.; Hu, Z.; Yuan, K.; Shao, H.; Peng, K.; Jiang, Z.; Zapien, J.A.; Yan, Y.; et al. Strongly fluorescent cysteamine-coated copper nanoclusters as a fluorescent probe for determination of picric acid. Microchim. Acta 2018, 185, 507. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.H.; Wu, S.P. Cysteamine-capped gold-copper nanoclusters for fluorometric determination and imaging of chromium(VI) and dopamine. Mikrochim. Acta 2019, 186, 788. [Google Scholar] [CrossRef]
- Khonkayan, K.; Sansuk, S.; Srijaranai, S.; Tuntulani, T.; Saiyasombat, C.; Busayaporn, W.; Ngeontae, W. New approach for detection of chromate ion by preconcentration with mixed metal hydroxide coupled with fluorescence sensing of copper nanoclusters. Microchim. Acta 2017, 184, 2965–2974. [Google Scholar] [CrossRef]
- Huang, H.; Li, H.; Feng, J.-J.; Feng, H.; Wang, A.-J.; Qian, Z. One-pot green synthesis of highly fluorescent glutathione-stabilized copper nanoclusters for Fe3+ sensing. Sens. Actuators B Chem. 2017, 241, 292–297. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, S.; Wang, Y.; Wang, M.; Liao, M.; Kou, X. Glutathione-stabilized Cu nanocluster-based fluorescent probe for sensitive and selective detection of Hg2+ in water. Luminescence 2017, 32, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Yu, Y.; Lin, B.; Zhang, L.; Cao, Y.; Guo, M. A novel signal amplification strategy based on the use of copper nanoclusters for ratiometric fluorimetric determination of o-phenylenediamine. Mikrochim. Acta 2019, 186, 206. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, Y.; Han, Y.; Liu, J.; Ma, S.; Zhang, H.; Chen, X. pH-Regulated Synthesis of Trypsin-Templated Copper Nanoclusters with Blue and Yellow Fluorescent Emission. ACS Omega 2017, 2, 9109–9117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, S.; Kundu, S. pH-Dependent reversible emission behaviour of lysozyme coated fluorescent copper nanoclusters. J. Lumin. 2020, 228, 117607. [Google Scholar] [CrossRef]
- Ghosh, R.; Sahoo, A.K.; Ghosh, S.S.; Paul, A.; Chattopadhyay, A. Blue-Emitting Copper Nanoclusters Synthesized in the Presence of Lysozyme as Candidates for Cell Labeling. ACS Appl. Mater. Interfaces 2014, 6, 3822–3828. [Google Scholar] [CrossRef]
- Mao, A.; Wei, C. Cytosine-rich ssDNA-templated fluorescent silver and copper/silver nanoclusters: Optical properties and sensitive detection for mercury(II). Mikrochim. Acta 2019, 186, 541. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, N.; Lv, X.; Jia, Q. UV-light-induced synthesis of PEI-CuNCs based on Cu2+-quenched fluorescence turn-on assay for sensitive detection of biothiols, acetylcholinesterase activity and inhibitor. Sens. Actuators B Chem. 2018, 259, 226–232. [Google Scholar] [CrossRef]
- Li, L.; Hou, C.; Li, J.; Yang, Y.; Hou, J.; Ma, Y.; He, Q.; Luo, H.; Huo, D. Fluazinam direct detection based on the inner filter effect using a copper nanocluster fluorescent probe. Anal. Methods 2019, 11, 4637–4643. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; He, Y.; Ge, Y.; Song, G.; Zhou, J. Sensitive Naked-eye and Fluorescence Determination of Acetylcholinesterase Activity using Cu/Ag Nanoclusters Based on Inner Filter Effect. ChemistrySelect 2019, 4, 7639–7644. [Google Scholar] [CrossRef]
- Dutta, A.; Goswami, U.; Chattopadhyay, A. Probing Cancer Cells through Intracellular Aggregation-Induced Emission Kinetic Rate of Copper Nanoclusters. ACS Appl. Mater. Interfaces 2018, 10, 19459–19472. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Xiong, Y.; Cepe, K.; Schneider, J.; Zboril, R.; Lee, C.-S.; Rogach, A.L. Incorporating Copper Nanoclusters into Metal-Organic Frameworks: Confinement-Assisted Emission Enhancement and Application for Trinitrotoluene Detection. Part. Part. Syst. Charact. 2017, 34, 1700029. [Google Scholar] [CrossRef]
- Patel, R.; Bothra, S.; Kumar, R.; Crisponi, G.; Sahoo, S.K. Pyridoxamine driven selective turn-off detection of picric acid using glutathione stabilized fluorescent copper nanoclusters and its applications with chemically modified cellulose strips. Biosens. Bioelectron. 2018, 102, 196–203. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Yu, X.L.; Li, J.; Liu, B.H.; Zhang, Z.P. A facile stage for Cu2+ ions detection by formation and aggregation of Cu nanoclusters. Microchem. J. 2019, 145, 517–522. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Huang, Y.; Xu, Z.; Lin, H.; Zhang, C. Facile sonochemical synthesis of pH-responsive copper nanoclusters for selective and sensitive detection of Pb2+ in living cells. Analyst 2015, 140, 5634–5639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, W.; Bao, J.; Wang, Z.; Dai, Z. Fluorescence Regulation of Copper Nanoclusters via DNA Template Manipulation toward Design of a High Signal-to-Noise Ratio Biosensor. ACS Appl. Mater. Interfaces 2018, 10, 6965–6971. [Google Scholar] [CrossRef]
- He, J.-L.; Wang, X.-X.; Mei, T.-T.; Wu, L.; Zeng, J.-L.; Wang, J.-H.; Wang, J.; Yu, D.; Cao, Z. DNA-templated copper nanoclusters obtained via TdT isothermal nucleic acid amplification for mercury(ii) assay. Anal. Methods 2019, 11, 4165–4172. [Google Scholar] [CrossRef]
- Su, Y.-T.; Lan, G.-Y.; Chen, W.-Y.; Chang, H.-T. Detection of Copper Ions Through Recovery of the Fluorescence of DNA-Templated Copper/Silver Nanoclusters in the Presence of Mercaptopropionic Acid. Anal. Chem. 2010, 82, 8566–8572. [Google Scholar] [CrossRef]
- Ou, L.J.; Huang, J.K.; Lv, X.L.; Huang, N. DsDNA-templated fluorescent copper nanoclusters for ultrasensitive label-free detection of Pb2+ ion. Chin. J. Anal. Lab. 2016, 35, 899–902. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Hou, T.; Ge, L.; Li, F. A facile, sensitive, and highly specific trinitrophenol assay based on target-induced synergetic effects of acid induction and electron transfer towards DNA-templated copper nanoclusters. Talanta 2016, 160, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bhamore, J.R.; Jha, S.; Mungara, A.K.; Singhal, R.K.; Sonkeshariya, D.; Kailasa, S.K. One-step green synthetic approach for the preparation of multicolor emitting copper nanoclusters and their applications in chemical species sensing and bioimaging. Biosens. Bioelectron. 2016, 80, 243–248. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, F.; Li, Y. Facile synthesis of near-infrared emitting dBSA-templated Cu nanoclusters for sensitive detection of heparin. J. Mater. Chem. B 2018, 6, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Nematulloev, S.; Huang, R.W.; Yin, J.; Shkurenko, A.; Dong, C.; Ghosh, A.; Alamer, B.; Naphade, R.; Hedhili, M.N.; Maity, P.; et al. [Cu15(PPh3)6(PET)13]2+: A Copper Nanocluster with Crystallization Enhanced Photoluminescence. Small 2021, 17, 2006839. [Google Scholar] [CrossRef]
- Dong, W.; Sun, C.; Sun, M.; Ge, H.; Asiri, A.M.; Marwani, H.M.; Ni, R.; Wang, S. Fluorescent Copper Nanoclusters for the Iodide-Enhanced Detection of Hypochlorous Acid. ACS Appl. Nano Mater. 2019, 3, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Shojaeifard, Z.; Heidari, N.; Hemmateenejad, B. Bimetallic AuCu nanoclusters-based florescent chemosensor for sensitive detection of Fe(3+) in environmental and biological systems. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2019, 209, 202–208. [Google Scholar] [CrossRef]
- Luo, M.; Wei, J.; Zhao, Y.; Sun, Y.; Liang, H.; Wang, S.; Li, P. Fluorescent and visual detection of methyl-paraoxon by using boron-and nitrogen-doped carbon dots. Microchem. J. 2020, 154, 104547. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Dong, J.; Wang, S.; Li, P. Fluorometric determination of pesticides and organophosphates using nanoceria as a phosphatase mimic and an inner filter effect on carbon nanodots. Microchim. Acta 2019, 186, 66. [Google Scholar] [CrossRef]
- Cao, H.; Chen, Z.; Zheng, H.; Huang, Y. Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens. Bioelectron. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, S.; Priya, A.; Datta, S.; Mukherjee, S. Luminescent Copper Nanoclusters as a Specific Cell-Imaging Probe and a Selective Metal Ion Sensor. J. Phys. Chem. C 2015, 119, 24657–24664. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Deshmukh, B.; Haran, V.; Jha, S.; Singhal, R.K.; Lenka, N.; Kailasa, S.K.; Murthy, Z.V.P. One-step eco-friendly approach for the fabrication of synergistically engineered fluorescent copper nanoclusters: Sensing of Hg2+ ion and cellular uptake and bioimaging properties. New J. Chem. 2018, 42, 1510–1520. [Google Scholar] [CrossRef]
- Sahu, D.K.; Singha, D.; Sahu, K. Sensing of iron(III)-biomolecules by surfactant-free fluorescent copper nanoclusters. Sens. Bio-Sens. Res. 2019, 22, 100250. [Google Scholar] [CrossRef]
- Boonmee, C.; Promarak, V.; Tuntulani, T.; Ngeontae, W. Cysteamine-capped copper nanoclusters as a highly selective turn-on fluorescent assay for the detection of aluminum ions. Talanta 2018, 178, 796–804. [Google Scholar] [CrossRef]
- Han, B.-Y.; Hou, X.-F.; Xiang, R.-C.; Yu, M.-B.; Li, Y.; Peng, T.-T.; He, G.-H. Detection of Lead Ion Based on Aggregation-induced Emission of Copper Nanoclusters. Chin. J. Anal. Chem. 2017, 45, 23–27. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Zhang, Z.; Ni, Y. Cytidine-stabilized copper nanoclusters as a fluorescent probe for sensing of copper ions and hemin. RSC Adv. 2018, 8, 9057–9062. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, Z.; Jia, Q. Anchoring copper nanoclusters to Zn-containing hydroxy double salt: Construction of 2D surface confinement induced enhanced emission toward bio-enzyme sensing and light-emitting diode fabrication. Chem. Commun. 2020, 56, 3081–3084. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Zhang, X.; Chai, H.; Huang, Y. One-pot synthesis of the CuNCs/ZIF-8 nanocomposites for sensitively detecting H2O2 and screening of oxidase activity. Biosens. Bioelectron. 2018, 105, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.E.S.; Wei, X.; Chen, X.; Wang, J. Confinement of AuAg NCs in a Pomegranate-Type Silica Architecture for Improved Copper Ion Sensing and Imaging. ACS Appl. Mater. Interfaces 2019, 11, 21150–21158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, N.; Jia, Q. Investigation of the surface confinement effect of copper nanoclusters: Construction of an ultrasensitive fluorescence turn-on bio-enzyme sensing platform. Nanoscale 2019, 11, 21927–21933. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, W.; Feng, X.; Lin, L.; Nie, P.; Shi, J.; Zou, X.; He, Y. Sensing of mercury ions in Porphyra by Copper @ Gold nanoclusters based ratiometric fluorescent aptasensor. Food Chem. 2021, 344, 128694. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, J.; Zou, J.; Cheng, Z.; Huang, Z.; Gu, L.; Zhong, Z.; Li, S.; Wang, Y.; Li, P. Electrochemical detection of methyl-paraoxon based on bifunctional nanozyme with catalytic activity and signal amplification effect. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Wang, W.; Gunasekaran, S. Nanozymes-based biosensors for food quality and safety. TrAC Trends Anal. Chem. 2020, 126, 115841. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Cheng, Z.; Wei, J.; Yang, L.; Zhong, Z.; Hu, H.; Wang, Y.; Zhou, B.; Li, P. Emerging core–shell nanostructures for surface-enhanced Raman scattering (SERS) detection of pesticide residues. Chem. Eng. J. 2021, 424, 130323. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Q.; Zou, T.; Kong, Y.; Su, L.; Ma, D.; Wang, Y. Dual-emission ratiometric fluorescent detection of dinotefuran based on sulfur-doped carbon quantum dots and copper nanocluster hybrid. Sens. Actuators B Chem. 2020, 321, 128534. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Feng, L. Specific detection and discrimination of dithiocarbamates using CTAB-encapsulated fluorescent copper nanoclusters. Talanta 2020, 210, 120627. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Hu, Y.; Xia, Y.; Shen, Q.; Nie, Z.; Huang, Y.; Yao, S. A fluorometric assay for acetylcholinesterase activity and inhibitor detection based on DNA-templated copper/silver nanoclusters. Biosens. Bioelectron. 2013, 47, 345–349. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, Y.; Li, X.; Lian, L.; Wang, X.; Gao, W.; Zhu, B.; Liu, X.; Lou, D. Ultrasensitive Biosensor for Detection of Mercury(II) Ions Based on DNA-Cu Nanoclusters and Exonuclease III-assisted Signal Amplification. Anal. Sci. 2018, 34, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gao, X.; Zhuang, X.; Tian, C.; Wang, Z.; Li, Y.; Rogach, A.L. A specific electrochemiluminescence sensor for selective and ultra-sensitive mercury(ii) detection based on dithiothreitol functionalized copper nanocluster/carbon nitride nanocomposites. Analyst 2019, 144, 4425–4431. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhu, R.; Pang, S.; Tian, F.; Zhang, C. One-step Green Synthetic Approach for the Preparation of Orange Light Emitting Copper Nanoclusters for Sensitive Detection of Mercury(II) Ions. ChemistrySelect 2020, 5, 165–170. [Google Scholar] [CrossRef]
- Maruthupandi, M.; Thiruppathi, D.; Vasimalai, N. One minute synthesis of green fluorescent copper nanocluster: The preparation of smartphone aided paper-based kit for on-site monitoring of nanomolar level mercury and sulfide ions in environmental samples. J. Hazard. Mater. 2020, 392, 122294. [Google Scholar] [CrossRef]
- Benavides, J.; Quijada-Garrido, I.; Garcia, O. The synthesis of switch-off fluorescent water-stable copper nanocluster Hg(2+) sensors via a simple one-pot approach by an in situ metal reduction strategy in the presence of a thiolated polymer ligand template. Nanoscale 2020, 12, 944–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, D.N.; Crofts, E.J.; Akemann, C.; Gurdziel, K.; Farr, R.; Baker, B.B.; Weber, D.; Baker, T.R. Developmental exposure to Pb2+ induces transgenerational changes to zebrafish brain transcriptome. Chemosphere 2020, 244, 125527. [Google Scholar] [CrossRef]
- Vineeth Daniel, P.; Kamthan, M.; Gera, R.; Dogra, S.; Gautam, K.; Ghosh, D.; Mondal, P. Chronic exposure to Pb2+ perturbs ChREBP transactivation and coerces hepatic dyslipidemia. FEBS Lett. 2019, 593, 3084–3097. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; et al. Copper nanoclusters @ nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef]
- Bai, H.; Tu, Z.; Liu, Y.; Tai, Q.; Guo, Z.; Liu, S. Dual-emission carbon dots-stabilized copper nanoclusters for ratiometric and visual detection of Cr2O7(2−) ions and Cd(2+) ions. J. Hazard. Mater. 2020, 386, 121654. [Google Scholar] [CrossRef]
- Maayan, G.; Behar, A.E.; Sabater, L.; Baskin, M.; Hureau, C. A Water-Soluble Peptoid Chelator that Can Remove Cu2+ from Amyloid-β and Stop the Formation of Reactive Oxygen Species Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2021, 60, 2–12. [Google Scholar] [CrossRef]
- Migliorini, C.; Porciatti, E.; Luczkowski, M.; Valensin, D. Structural characterization of Cu2+, Ni2+ and Zn2+ binding sites of model peptides associated with neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 352–368. [Google Scholar] [CrossRef]
- Liu, Z.C.; Qi, J.W.; Hu, C.; Zhang, L.; Song, W.; Liang, R.P.; Qiu, J.D. Cu nanoclusters-based ratiometric fluorescence probe for ratiometric and visualization detection of copper ions. Anal. Chim. Acta 2015, 895, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zuo, L.; Huang, X.; Liu, S.; Yang, G.; Li, S.; Lv, C. Colorimetric determination of lead(II) or mercury(II) based on target induced switching of the enzyme-like activity of metallothionein-stabilized copper nanoclusters. Mikrochim. Acta 2019, 186, 250. [Google Scholar] [CrossRef]
- Wang, H.-B.; Bai, H.-Y.; Wang, Y.-S.; Gan, T.; Liu, Y.-M. Highly selective fluorimetric and colorimetric sensing of mercury(II) by exploiting the self-assembly-induced emission of 4-chlorothiophenol capped copper nanoclusters. Microchim. Acta 2020, 187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, B. Synthesis of Protein-Directed Orange/Red-Emitting Copper Nanoclusters via Hydroxylamine Hydrochloride Reduction Approach and Their Applications on Hg2+ Sensing. Nano 2016, 11, 1650108. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Wang, J.; Niu, L.; Zhang, Y.; Liu, X.; Liu, C.; Yang, S.; Qi, H.; Liu, A. Selective colorimetric sensing of sub-nanomolar Hg2+ based on its significantly enhancing peroxidase mimics of silver/copper nanoclusters. Analyst 2021, 146, 4630–4635. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pang, S.; Wang, M.; Wu, M.; Li, P.; Bai, J.; Yang, X. Dual-emission carbon dots-copper nanoclusters ratiometric photoluminescent nano-composites for highly sensitive and selective detection of Hg2+. Ceram. Int. 2021, 47, 18238–18245. [Google Scholar] [CrossRef]
- Zhong, K.; Hao, C.; Liu, H.; Yang, H.; Sun, R. Synthesis of dual-emissive ratiometric probe of BSA-Au NCs and BSA-Cu NCs and their sensitive and selective detection of copper and mercury ions. J. Photochem. Photobiol. A Chem. 2021, 408. [Google Scholar] [CrossRef]
- Feng, D.-Q.; Zhu, W.; Liu, G.; Wang, W. Dual-modal light scattering and fluorometric detection of lead ion by stimuli-responsive aggregation of BSA-stabilized copper nanoclusters. RSC Adv. 2016, 6, 96729–96734. [Google Scholar] [CrossRef]
- Li, M.; Cai, Y.; Peng, C.; Wei, X.; Wang, Z. DNA dendrimer–templated copper nanoparticles: Self-assembly, aggregation-induced emission enhancement and sensing of lead ions. Microchim. Acta 2021, 188. [Google Scholar] [CrossRef]
- Cao, X.; Bai, Y.; Liu, F.; Li, F.; Luo, Y. ‘Turn-off’ fluorescence strategy for determination of hexavalent chromium ions based on copper nanoclusters. Luminescence 2020, 36, 229–236. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Yang, S.I. A selective fluorescence turn-on sensing system for evaluation of Cu2+ polluted water based on ultra-fast formation of fluorescent copper nanoclusters. Anal. Methods 2015, 7, 2278–2282. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Wang, X.; Zhuang, X.; Tian, C.; Luan, F.; Fu, X. Functionalized Copper Nanoclusters-Based Fluorescent Probe with Aggregation-Induced Emission Property for Selective Detection of Sulfide Ions in Food Additives. J. Agric. Food Chem. 2020, 68, 11301–11308. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.; Peng, Y.; Chen, Z.; Gao, X.; Cheng, L.; Mei, X. Development of ratiometric sensing and white light-harvesting materials based on all-copper nanoclusters. Nanoscale Adv. 2019, 1, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.C.; Li, Y.C.; Ma, J.Y.; Huang, J.Y.; Chen, C.F.; Chang, H.T. Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci. Rep. 2016, 6, 24882. [Google Scholar] [CrossRef] [Green Version]
- Shojaeifard, Z.; Hemmateenejad, B.; Shamsipur, M.; Ahmadi, R. Dual fluorometric and colorimetric sensor based on quenching effect of copper (II) sulfate on the copper nanocluster for determination of sulfide ion in water samples. J. Photochem. Photobiol. A Chem. 2019, 384, 112030. [Google Scholar] [CrossRef]
- Wen, Z.; Song, S.; Hu, T.; Wang, C.; Qu, F.; Wang, P.; Yang, M. A dual emission nanocomposite prepared from copper nanoclusters and carbon dots as a ratiometric fluorescent probe for sulfide and gaseous H2S. Mikrochim. Acta 2019, 186, 258. [Google Scholar] [CrossRef] [PubMed]
- Sivasankaran, U.; Radecki, J.; Radecka, H.; Girish Kumar, K. Copper nanoclusters: An efficient fluorescence sensing platform for quinoline yellow. Luminescence 2019, 34, 243–248. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, L.; Qi, K.; Deng, C.; Zhang, C. Blue-emitting glutathione-capped copper nanoclusters as fluorescent probes for the highly specific biosensing of furazolidone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Y.; Du, P.; Li, J.; Zhang, Z.; Lu, X. Portable smartphone platform integrated with fluorescent test strip based on Eu3+-functionalized copper nanoclusters for on-site visual recognition of a pathogenic biomarker. Sens. Actuators B Chem. 2021, 332, 129495. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; John, S.A. Inner filter effect based selective detection of picric acid in aqueous solution using green luminescent copper nanoclusters. New J. Chem. 2018, 42, 7223–7229. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.G.; Han, L.; Ling, Y.; Liao, L.L.; Mo, S.; Luo, H.Q.; Li, N.B. Copper nanoclusters with strong fluorescence emission as a sensing platform for sensitive and selective detection of picric acid. Anal. Methods 2018, 10, 4251–4256. [Google Scholar] [CrossRef]
- Lian, N.; Zhang, Y.; Liu, D.; Tang, J.; Wu, H. Copper nanoclusters as a turn-on fluorescent probe for sensitive and selective detection of quinolones. Microchem. J. 2021, 164, 105989. [Google Scholar] [CrossRef]
- Ling, Y.; Li, J.X.; Qu, F.; Li, N.B.; Luo, H.Q. Rapid fluorescence assay for Sudan dyes using polyethyleneimine-coated copper nanoclusters. Microchim. Acta 2014, 181, 1069–1075. [Google Scholar] [CrossRef]
- Xu, S.; Deng, M.; Sui, Y.; Zhang, Y.; Chen, F. Ultrasensitive determination of bisphenol A in water by inhibition of copper nanoclusters-enhanced chemiluminescence from the luminol–KMnO4 system. RSC Adv. 2014, 4, 44644–44649. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, W.; Yu, X.; Xu, J.; Chen, H. Poly thymine stabilized copper nanoclusters as a fluorescence probe for melamine sensing. Talanta 2015, 144, 642–647. [Google Scholar] [CrossRef]


| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Paraoxon | Cu NCs @ BSA-SWCNT/GCE | 325/420 | electrochemical method | 0.05–0.5 μM 0.5–35 μM | 12.8 nM | water | [12] |
| 2 | Thiram Paraquat | Egg white- Cu NCs | 344/600 | turn off | 0.5–1000 μM 0.2–1000 μM | 70 nM 49 nM | water | [45] |
| 3 | Metham sodium | CTAB-Cu NCs | 254/620 | fluorescent based colorimetric method | 1–100 mg kg−1 | 0.63 mg kg−1 | apple, pear and cherry tomato | [68] |
| 4 | Fluazina | L-Cys-Cu NCs | 365/497 | turn off | 0.05–25 µM | 1.4 nM | pears and cabbage | [33] |
| 5 | o-Phenylenediamine | GSH-Cu NCs | 334/432 | ratiometric | 0.15–110 μg L−1 | 93 ng L−1 | industry water | [27] |
| 6 | Nitrofurantoin | Adenosine-Cu NCs | 285/417 | turn off | 0.05–4.0 μM | 30 nM | lake water | [16] |
| 7 | Dinotefuran | S-CQDs/Cu NCs | 330/430 | ratiometric | 10–500 μM | 7.04 μM | honey | [67] |
| 8 | AChE Methamidophos | DNA-Cu/Ag NCs | 480/565 | turn off turn on | 0.05–2.0 U L−1 — | 0.05 UL−1 0.075 mg L−1 (IC50) | water and vegetable | [69] |
| 10 | AChE | L-His-Cu/Ag NCs | 390/485 | turn off | 0.1–1.0 UL−1 and 1.0–7.0 UL−1 | 0.03 UL−1 | — | [34] |
| 9 | AChE | PVP-Cu NCs | 370/438 | ratiometric | 2.0–70 UL−1 | 0.56 UL−1 | human serum sample | [14] |
| 11 | AChE | PEI-Cu NCs | 365/495 | turn on | 3–200 UL−1 | 1.38 UL−1 | human serum sample | [32] |
| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Hg2+ | Cu NCs@P-8B | 400/535 | turn off | 10–100 μM | 10 μM | aqueous solution | [74] |
| 2 | Hg2+ | Curcuminoids-Cu NCs | 350/440 | turn off | 0.5 nM–25 µM | 0.12 nM | water | [54] |
| 3 | Hg2+ | Cu NCs | 340/560 | turn off | 2–40 μM | 23 nM | water | [72] |
| 4 | Hg2++ | Trypsin-Cu NCs | 360/567 | turn off | 0.1−100 μM | 30 nM | human urine and serum samples | [28] |
| 5 | Hg2+ | TdT-INAA-DNA-Cu NCs | 343/600 | turn off | 0.2–500 nM | 76 pM | environmental water | [41] |
| 6 | Hg2+ | GSH–Cu NCs | 360/445 | turn off | 10 nM–10 μM | 3.3 nM | water and rice | [13] |
| 7 | Hg2+ | poly(30T) DNA-Cu NCs | 340/650 | turn on | 50 pM–2.5 μM and 2.5–500 μM | 16 pM | lake water | [40] |
| 8 | Hg2+ | DTT-Cu NCs/CNNS nanocomposite | 395/615 | electrochemiluminescence | 0.05–10 nM | 0.01 nM | lake and tap water | [71] |
| 9 | Hg2+ | Metallothionein–Cu NCs | — | UV-VIS | 97 nm–2.3 μM and 3.1–15.6 μM | 43.8 nM | environmental water | [82] |
| 10 | Hg2+ | GSH-Cu NCs | 375/440 | turn off | 0.04−60 μM | 22 nM | water | [26] |
| 11 | Hg2+ | Cytosine rich- ssDNA-Cu/Ag NCs | 470/550 | turn off | 40–550 nM | 2.4 nM | lake and tap water | [31] |
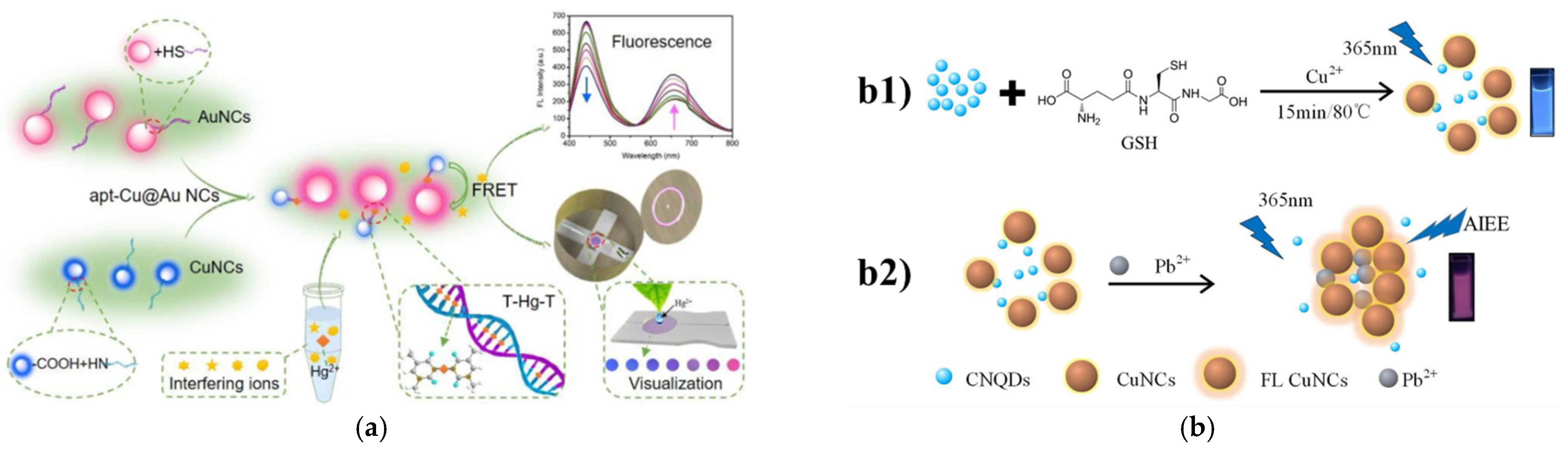
| 12 | Hg2+ | apt-Cu@Au NCs | 470/656 | ratiometric | 0.1–9.0 μM | 4.92 nM | porphyra | [63] |
| 13 | Hg2+ | 4-chlorothiophenol-Cu NCs | 330/605 | turn off | 1–500 nM | 0.3 nM | environmental water | [83] |
| 14 | Hg2+ | BSA-Cu NCs | 320/420 | turn off | 0.01 nM–10 μM | 4.7 pM | water | [19] |
| 15 | Hg2+ | BSA-Cu NCs | 395/645 | turn off | 20–1000 nM | 0.2 nM | — | [84] |
| 16 | Hg2+ | L-Cys-Cu NCs | 375/480 | turn off | 0.1–1000 μM | 24 nM | human urine sample | [17] |
| 17 | Hg2+ | dsDNA-Cu NCs | 570/595 | turn off | 0.04−8 nM | 4 pM | water | [70] |
| 18 | Hg2+ | Ag/Cu NCs | colorimetric | turn on | 0.1–700 nM | 0.05 nM | aqueous sample | [85] |
| 19 | Hg2+ | CDs-CuNCs | 345/430,647 | ratiometric | 0–4000 nM | 0.31 nM | Tap, lake water | [86] |
| 20 | Hg2+ | BSA-Cu NCs/ BSA-Au NCs | 365/398,616 | ratiometric | 0.06–1 µM and 1–4 µM | 19.4 nM | Tap, mineral, lake water | [87] |
| 21 | Pb2+ | BSA-Cu NCs | 324/401 (fluorescent); 324/396 (light scattering) | turn off; turn on | 30–180 nM; 3–21 nM | 10 nM; 1 nM | environmental water | [88] |
| 22 | Pb2+ | BSA-Cu NCs | 325/410 | turn off | 0–200 ppm | — | — | [18] |
| 23 | Pb2+ | GSH-Cu NCs | 360/607 | turn on | 200–700 μM | 106 μM | water | [57] |
| 24 | Pb2+ | Cu NCs-CNQDs | 365/468, 632 | ratiometric | 0.01–2.5 mg L−1 | 0.0031 mg L−1 | porphyra | [77] |
| 25 | Pb2+ | Metallothionein–Cu NCs | — | UV-VIS | 0.7–96 μM | 142 nM | environmental water | [82] |
| 26 | Pb2+ | dsDNA-Cu NCs | 340/605 | turn off | 0–150 pM | 5.2 pM | tap water | [43] |
| 27 | Pb2+ | GSH-Cu NCs | 420/606 | turn off | 1–160 nM | 1 nM | — | [39] |
| 28 | Pb2+ | Cu NASs | 340/590 | turn off | 2–100 nM | 0.75 nM | aqueous sample | [89] |
| 29 | Cr2O72− | GSH@CDs-Cu NCs | 360/450,750 | ratiometric | 0–20 μM | 0.9 μM | tap water, spring water samples and human urine | [78] |
| 30 | Cr(VI) | DAMP-Cu NCs | 357/428 | turn off | 0–150 μM | 8.5 μM | water | [24] |
| 31 | Cr(VI) | Thiosalicylic acid/Cysteamine-Cu NCs | 355/411 | turn off | 0.1–1000 μM | 30 nM | water | [21] |
| 32 | Cr(VI) | Cysteamine-Au/Cu NCs | 350/436 | turn off | 0.2–100 μM | 80 nM | water and human urine sample | [23] |
| 33 | Cr(Ⅵ) | Cu NCs@TA | 360/430 | turn off | 0.03–60 µM | 5 nM | water sample | [90] |
| 34 | Cu2+ | D-Penicillamine -Cu NCs | 391/673 | turn on | 0.95–6.35 ppm | 0.3 ppm | tap water | [91] |
| 35 | Cu2+ | CdSe QDs @ hPEI-Cu NCs | 380/495,625 | ratiometric | 0.022–8.8 μM | 8.9 nM | river water | [81] |
| 36 | Cu2+ | GSH- Cu NCs | 330/615 | turn on | 0.25–10 μM | 170 nM | chalcocite | [38] |
| 37 | Cu2+ | DNA-Cu/Ag NCs | 480/576 | turn on | 5–200 nM | 2.7 nM | soil and pond water | [42] |
| 38 | Cu2+ | Cytidine-Cu NCs | 300/380 | turn on | 0.05–2.0 µM | 32 nM | lake water | [58] |
| 39 | Cu2+ | BSA-Cu NCs | 340/420 | turn off | 0.02–34 μM | 1 nM | tap water | [20] |
| 40 | Cu2+ | BSA-Cu NCs/ BSA-Au NCs | 365/398,616 | ratiometric | 0.1–1 µM and 1–5 µM | 23.4 nM | Tap, mineral, lake water | [87] |
| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | H2S | PSS-PA-Cu NCs | 325/655 | turn-off | 2–10 μM | 650 nM | spring water | [94] |
| 2 | S2− | G-R-Cu NCs | 400/490, 610 | ratiometric | 0.1–10 μM and 0.1–10 mM; | 100 nM | chicken blood | [93] |
| 3 | S2− | TA-Cu NCs | 360/441 | turn-off colorimetric | 0.7–80 μM 6–130 μM | 0.1 μM 2.0 μM | natural water | [95] |
| 4 | S2− | Cu2+@MPA-Cu NCs | 350/610 | turn-off | 0−600 μM | 26.3 nM | food additives | [92] |
| 5 | S2− and H2S | Cu NCs-CQD | 365/469,622 | ratiometric | 26–128 nM | 4.3 nM | — | [96] |
| 6 | S2− | Cu NCs | 326/422 | turn-on | 5–110 μM | 0.286 μM | tap water and river water | [10] |
| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | picric acid | Cu NCs-CA | 393/480 | turn-off | 1–80 μM | 0.14 μM | tap water, lake water and river water | [22] |
| 2 | trinitrophenol | DNA-Cu NCs | 340/627 | turn-off | 0.1–100 μM | 0.03 μM | water samples | [44] |
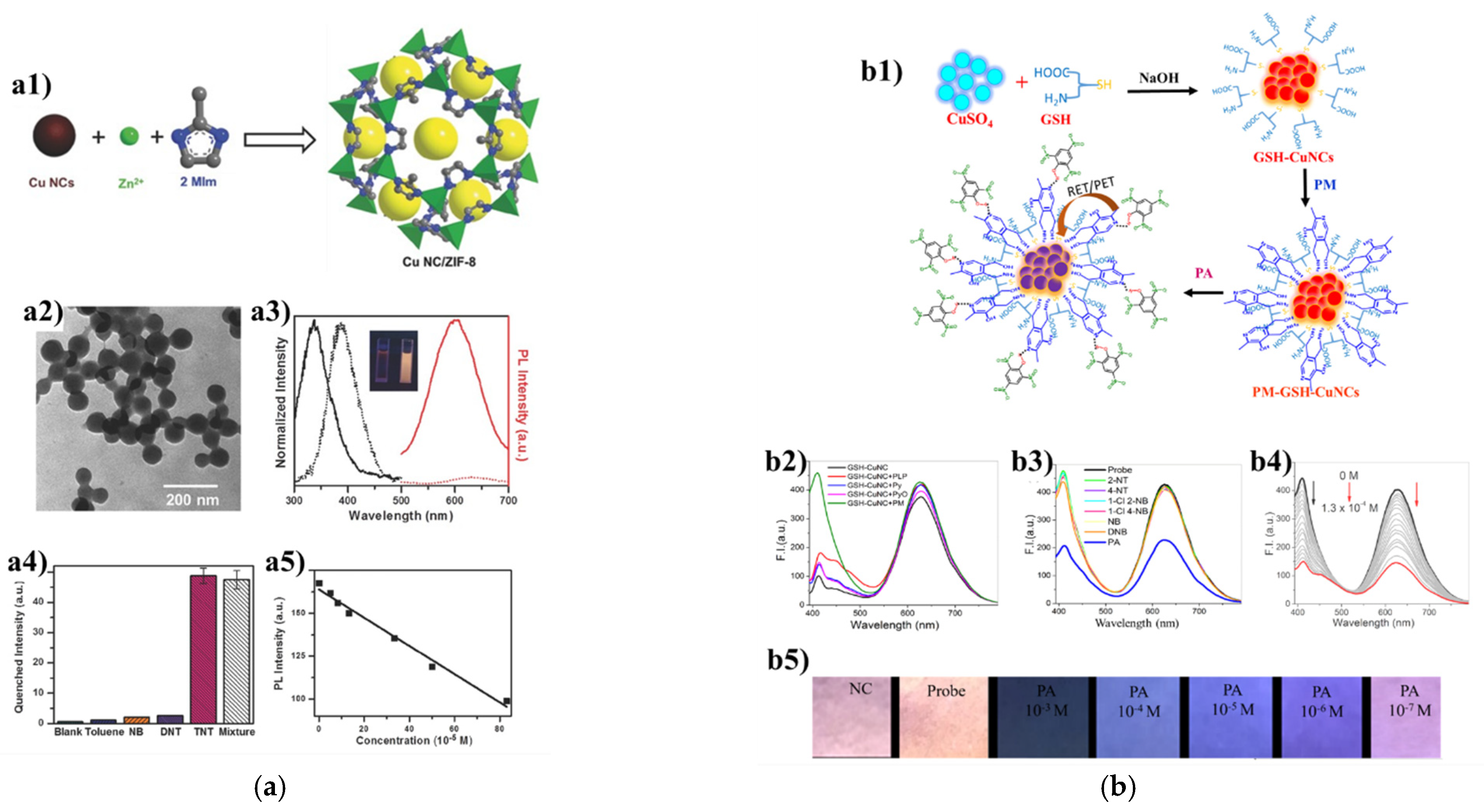
| 3 | picric acid | PM-GSH-Cu NCs | 360/625 | turn-off | 9.9–43 μM | 2.74 μM | water and matchstick | [37] |
| 4 | picric acid | Cys–Cu NCs | 370/494 | turn-off | 2.5–25 mM | 0.19 mM | tap and lake water | [100] |
| 5 | trinitrotoluene | CuNC/ZIF-8 | 365/600 | turn-off | 5–80 μM | 8.5 μM | tap water | [36] |
| 6 | picric acid | Cu NCs | 350/430 | turn-off | 2–40 mM | 0.98 mM | water samples | [101] |
| 7 | quinoline yellow | L-Cys-Cu NCs | 380/422,617 | ratiometric | 0.2–5.5 µM | 110 nM | candies and soft drink | [97] |
| 8 | furazolidone | GSH-Cu NCs | 366/426 | turn off | 0.05–60 µM | 12 nM | aqueous sample | [98] |
| 9 | DPA | GSH-Cu NCs | 380/422,617 | ratiometric | 0–20 µM | 8 nM | aqueous sample | [99] |
| 10 | quinolones | Cys-Cu NCs | 368/475 | turn on | 0.5–40 µM | 8 nM | tablets | [102] |
| 11 | Sudan dyes Ⅰ Sudan dyes Ⅱ Sudan dyes Ⅲ Sudan dyes Ⅳ | PEI-Cu NCs | 355/480 | turn off | 0.1–30 µM 0.1–30 µM 0.1–25 µM 0.1–25 µM | 65 nM 70 nM 45 nM 50 nM | chilli powder sample | [103] |
| 12 | Bisphenol A | BSA-Cu NCs | chemiluminescence | turn off | 0.001–10 µM | 0.12 nM | water sample | [104] |
| 13 | Melamine | T30-Cu NCs | 345/598 | turn on | 0.1–6 µM | 95 nM | Milk | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Cheng, Z.; Luo, M.; Hu, H.; Xia, C. Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis. Biosensors 2021, 11, 424. https://doi.org/10.3390/bios11110424
Xue Y, Cheng Z, Luo M, Hu H, Xia C. Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis. Biosensors. 2021; 11(11):424. https://doi.org/10.3390/bios11110424
Chicago/Turabian StyleXue, Yan, Zehua Cheng, Mai Luo, Hao Hu, and Chenglai Xia. 2021. "Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis" Biosensors 11, no. 11: 424. https://doi.org/10.3390/bios11110424
APA StyleXue, Y., Cheng, Z., Luo, M., Hu, H., & Xia, C. (2021). Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis. Biosensors, 11(11), 424. https://doi.org/10.3390/bios11110424






