Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors
Abstract
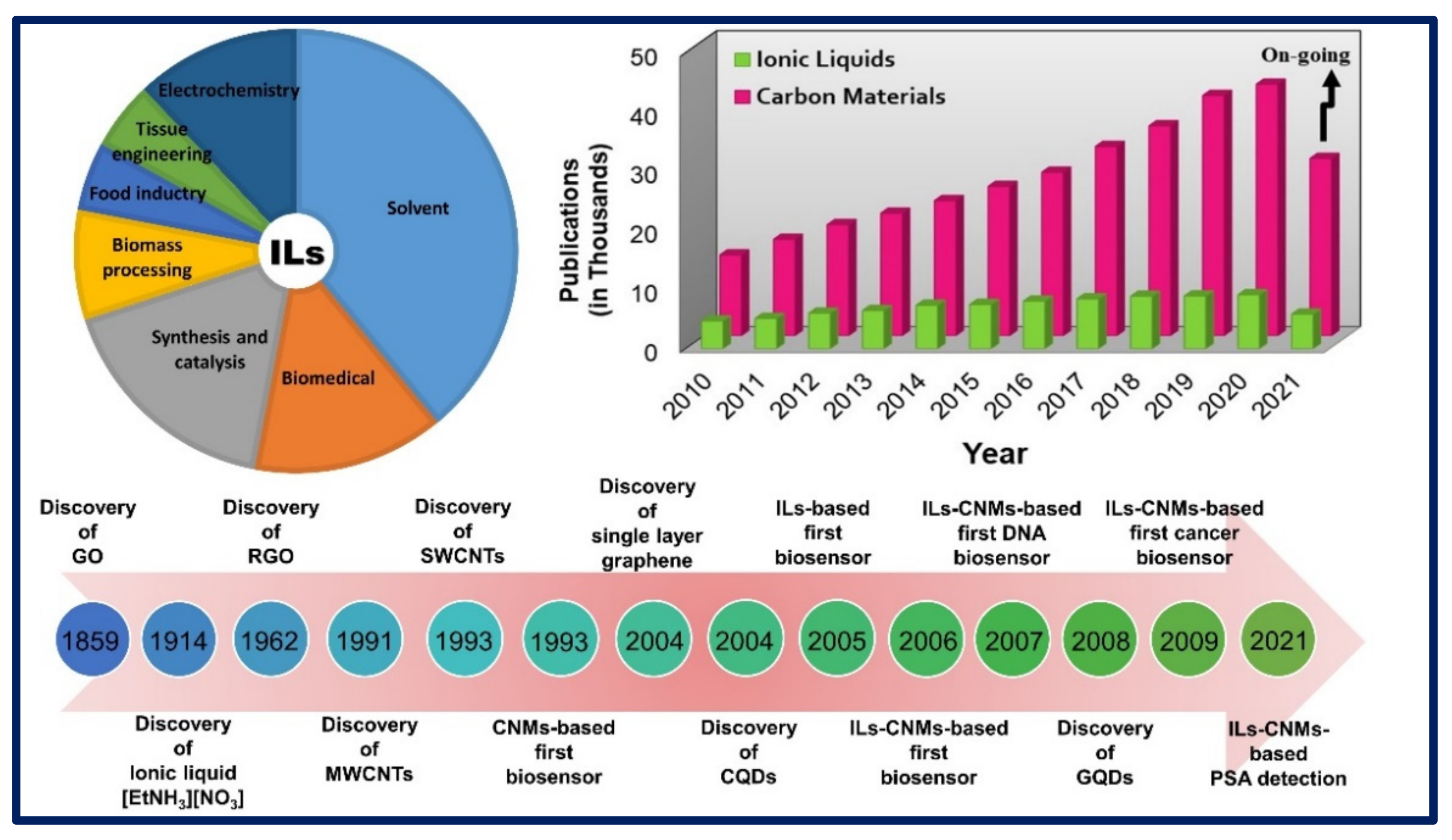
:1. Introduction
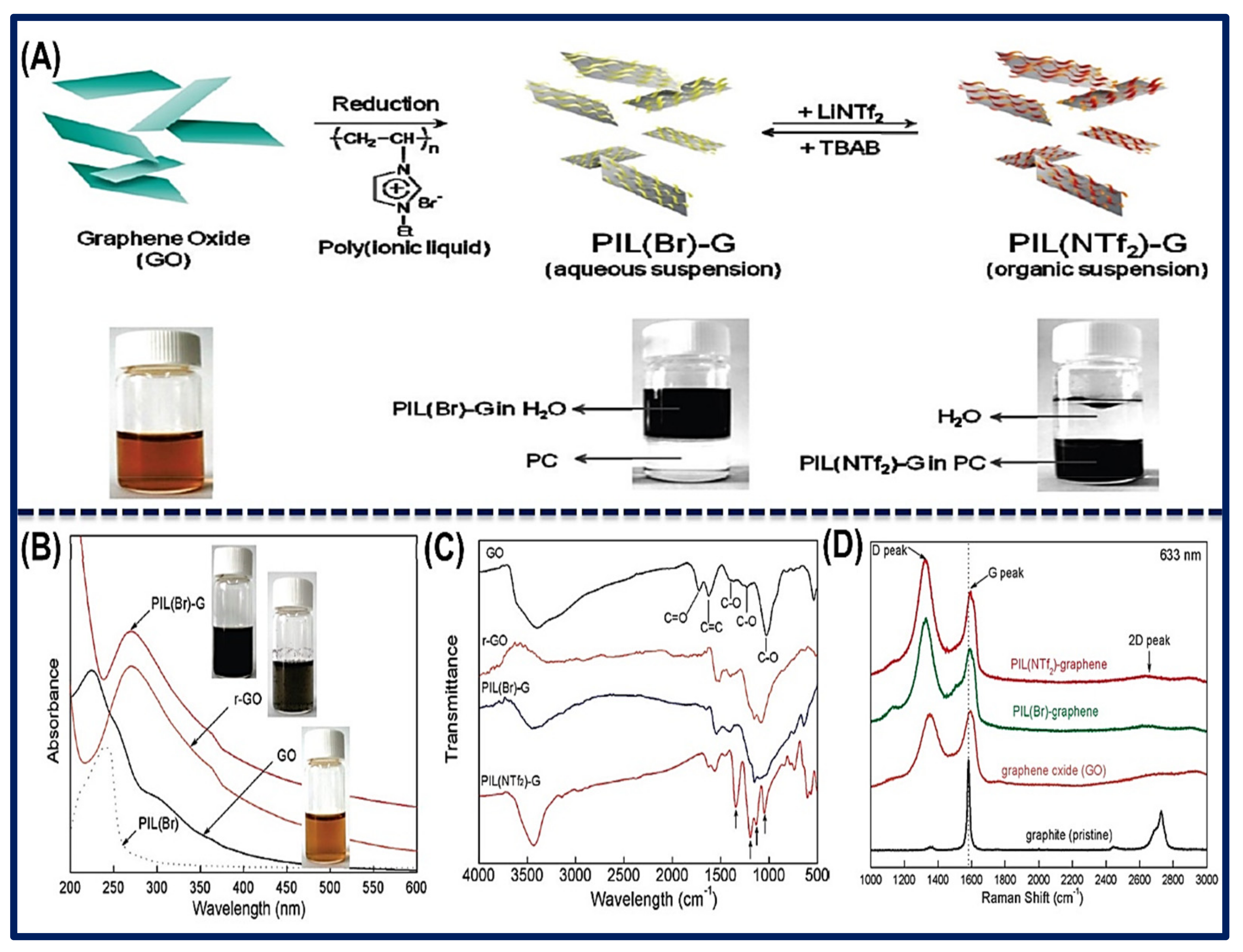
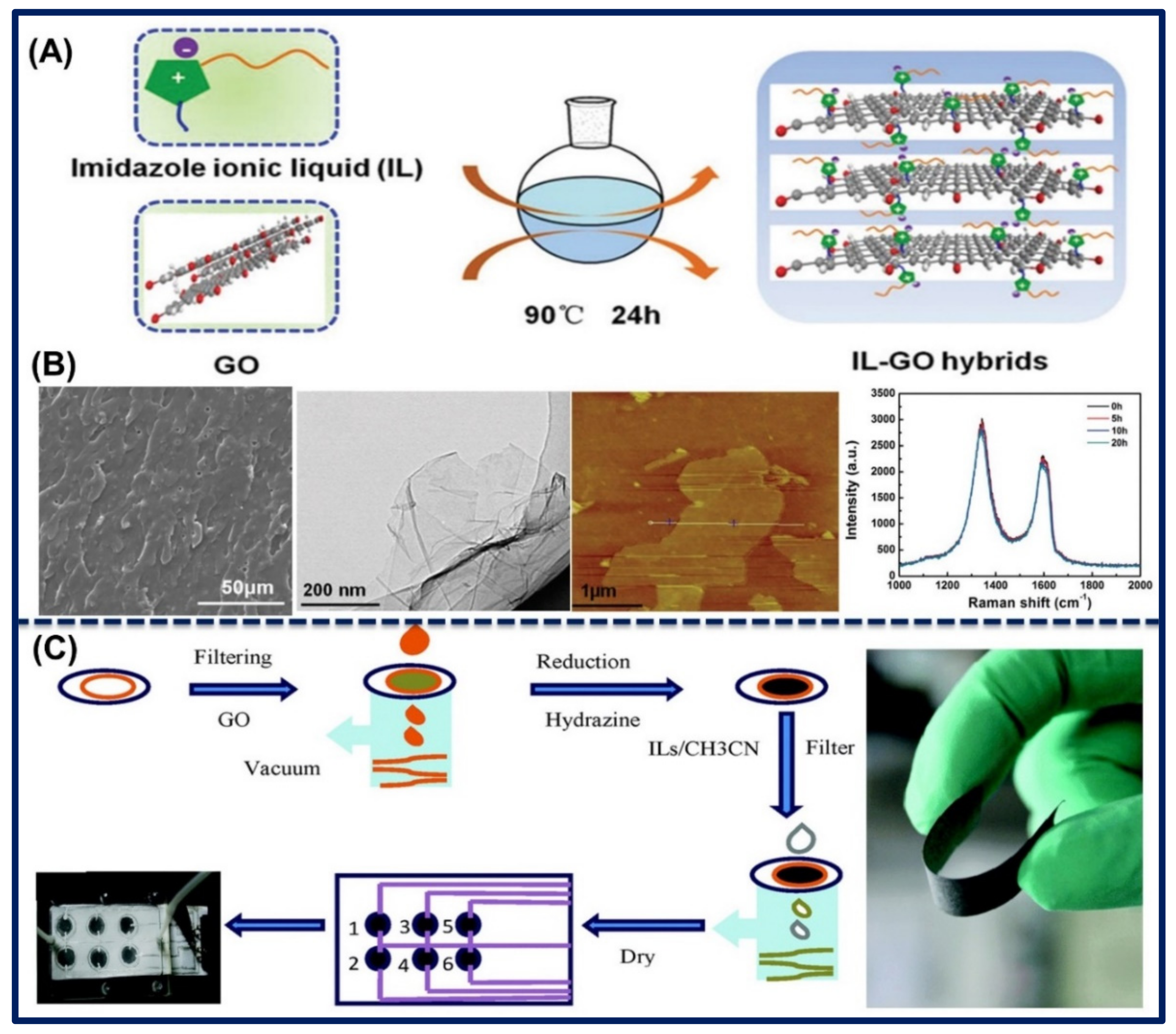
2. Synthesis of Functional Ionic Liquid-Carbon Hybrid Nanomaterials.
3. Properties of Ionic Liquids
3.1. Electrochemical Properties of Ionic Liquids
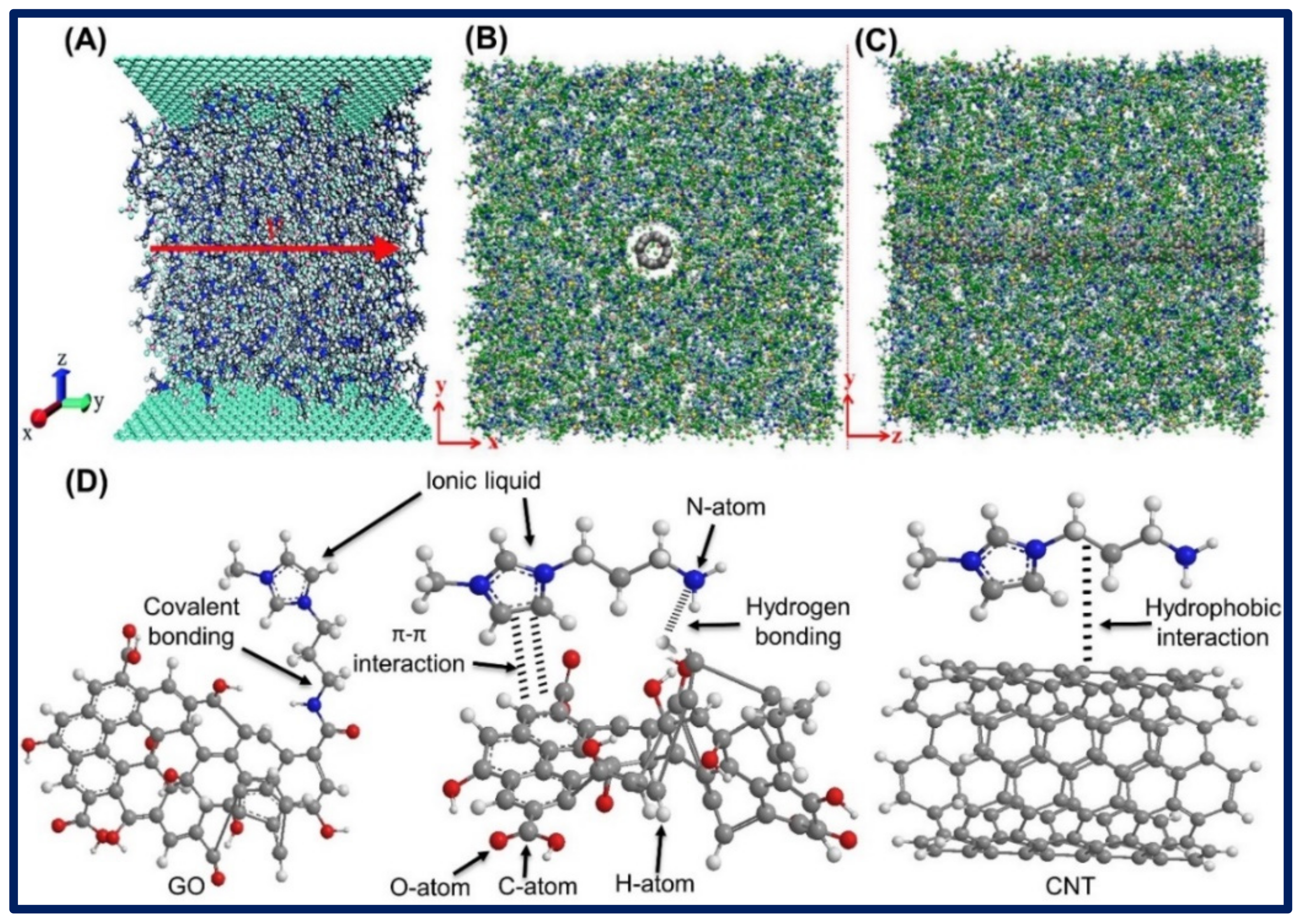
3.2. Bonding of Ionic Liquid-Carbon Hybrid Nanomaterials
4. Ionic Liquids–Carbon-Based Functional Nanomaterials in Biosensing Applications
4.1. Electrochemical Analytic for Cancer Biomarkers
4.2. Electrochemical Analysis of Cardiac Biomarkers
4.3. Electrochemical Investigation of Immunoglobulins
4.4. Detection of Neurotransmitters
4.5. Detection of Glucose
4.6. Detection of Other Markers
5. Conclusions and Future Viewpoints
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Name |
| [BMIM][BF4] | 1-Butyl-3-Methylimidazolium Tetrafluoroborate |
| [EMIM][BF4] | 1-Ethyl-3-Methylimidazolium Tetrafluoroborate |
| [AMIM][TFSI] | 1-Allyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide |
| [BMIM][TFSI] | 1-Butyl-3-Methyllimidazolium Bis(tri-fluoromethylsulfonyl) imide |
| [APMIM]Cl | 1-Aminopropyl-3-Methylimidazolium Chloride |
| [BMIM]Cl | 1-Butyl-3-Methylimidazolium Chloride |
| [BMIM]Br | 1-Butyl-3-Methylimidazolium Bromide |
| [DMIM]Br | 1-Decyl-3-Methylimidazolium Bromide |
| [BMIM][PF6] | 1-Butyl-3-Methyliidazolium Hexafluorophosphate |
| [BMIM][TFSI] | 1-butyl-3-methyllimidazoliumbis (tri-fluoromethylsulfonyl) imide |
| [BP][BF4] | 1-Butyl-Pyridine Tetrafluoroborate |
| [OMIM][BF4] | 1-Methyl-3-Octylimidazolium Tetrafluoroborate |
| [EMIM][BTI] | 1-Ethyl-3-Methylimidazolium Bis(tri-fluoromethylsulfonyl)imide |
| [BMIM][BTI] | 1-Butyl-3-Methyllimidazolium Bis(tri-fluoromethylsulfonyl)imide |
| [BDMIM][BF4] | 1-Butyl-2,3-Dimethylimidazolium Tetrafluoroborate |
| [BMIM][Ac] | 1-Butyl-3-Methylimidazolium Acetate |
| [BMIM][NTf2] | 1-Butyl-3-Methyl Imidazolium Bis(trifluoro-methylsulfonyl)amide |
| [VEIM][BF4] | 1-Vinyl-3-Ethylimidazolium Tetrafluoroborate |
| [NMIM][Cl] | 1-(nonyl)-3-methylimidazolium chloride |
| [CMMIM]Cl | Carboxymethyl-3-Methylimidazolium Chloride |
| [EMIM][F3MeS] | 1-Ethyl-3-Methylimidazolium Trifluoromethyl Sulfonate |
| [EMIM][SCN] | 1-Ethyl-3-Methylimidazolium Thiocyanate |
| [EMIM][DCA] | 1-Ethyl-3-Methylimidazolium Dicyanamide |
| [Pyr14][Tf2N] | N-Butyl-N-Methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide |
| [N8,8,8,1][NTf2] | N-Methyl-N-Trioctylammonium Bis(trifluoromethylsulfonyl)imide |
| [Et3S][NTf2] | Triethylsulphonium Bis(trifluoromethylsulfonyl)imide |
References
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of ionic liquids in analytical chemistry. Anal. Chem. 2018, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- Tiago, G.A.; Matias, I.A.; Ribeiro, A.P.; Martins, L.M. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta. 2008, 607, 126–135. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Niranjan, T.; Redhi, G.G. Ionic liquid based electrochemical sensors and their applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 367–387. [Google Scholar]
- Wang, X.; Hao, J. Recent advances in ionic Liquid-Based electrochemical biosensors. Sci. Bull. 2016, 61, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.R.; Etzold, B.J. Ionic liquids in electrocatalysis. J. Enery Chem. 2016, 25, 199–207. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bostrom, T. Application of ionic liquids in solar cells and batteries: A review. Curr. Org. Chem. 2015, 19, 556–566. [Google Scholar] [CrossRef]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Meira, R.M.; Correia, D.M.; Ribeiro, S.; Costa, P.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S.; Ribeiro, C. Ionic-liquid-based electroactive polymer composites for muscle tissue engineering. ACS Appl. Polym. Mater. 2019, 1, 2649–2658. [Google Scholar] [CrossRef] [Green Version]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Pedro, S.N.; R Freire, C.S.; Silvestre, A.J.; Freire, M.G. The role of ionic liquids in the pharmaceutical field: An overview of relevant applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Liu, P.; Jin, K.; Wong, W.; Wang, Y.; Liang, T.; He, M.; Li, H.; Lu, C.; Tang, X.; Zong, Y.; et al. Ionic liquid functionalized non-releasing antibacterial hydrogel dressing coupled with electrical stimulation for the promotion of diabetic wound healing. Chem. Eng. J. 2021, 415, 129025. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial ionic liquid-based materials for biomedical applications. Adv. Funct. Mater. 2021, 2104148. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous nanomaterials employed in the development of electrochemical sensors based on screen-printing technique—A review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Huang, J. Two dimensional soft material: New faces of graphene oxide. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Du, X. Electrochemical sensors based on carbon nanomaterial used in diagnosing metabolic disease. Front. Chem. 2020, 8, 651. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Facure, M.H.; Schneider, R.; Lima, J.; Mercante, L.A.; Correa, D.S. Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey. Electrochem 2021, 2, 32. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F.; Nazari, M.; Parnianchi, F.; Santoro, C. Carbon Nanodots in Electrochemical Sensors and Biosensors: A Review. ChemElectroChem 2021, 8, 15–35. [Google Scholar] [CrossRef]
- Wang, F.T.; Wang, L.; Xu, J.; Huang, K.J.; Wu, X. Synthesis and modification of carbon dots for advanced biosensing application. Analyst 2021, 146, 4418–4435. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid. State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.J.; Gupta, V.; Bandhoria, P.; Khosla, A. Recent advances in carbon nanomaterials as electrochemical biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. Trends. Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.J.; Min, D.H. Emerging approaches for graphene oxide biosensor. Anal. Chem. 2017, 89, 232–248. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene-Based Sensors for the Detection of Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef]
- Jiménez-Marín, E.; Moreno-Valenzuela, J.; Trejo-Valdez, M.; Martinez-Rivas, A.; Vargas-García, J.R.; Torres-Torres, C. Laser-induced electrical signal filtering by multilayer reduced graphene oxide decorated with Au nanoparticles. Opt. Express 2019, 27, 7330–7343. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Prospects of ionic liquids application in electronic and bioelectronic nose instruments. Trends. Anal. Chem. 2017, 93, 23–36. [Google Scholar]
- Dideikin, A.T.; Vul, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Madurani, K.A.; Suprapto, S.; Machrita, N.I.; Bahar, S.L.; Illiya, W.; Kurniawan, F. Progress in graphene synthesis and its application: History, challenge and the future outlook for research and industry. ECS J. Solid State Sci. Technol. 2020, 9, 093013. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Dong, S. Electrochemical characteristics of facile prepared carbon nanotubes–ionic liquid gel modified microelectrode and application in bioelectrochemistry. Electrochem. Commun. 2006, 8, 1429–1434. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, F.; Ren, R.; Lin, J.M. An electrochemical biosensor for α-fetoprotein based on carbon paste electrode constructed of room temperature ionic liquid and gold nanoparticles. Talanta 2009, 78, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, P.; Wilkins, E. Biosensor for continuos glucose monitoring. Biotechnol. Bioeng. 1994, 43, 262–266. [Google Scholar] [CrossRef]
- Ferrag, C.; Kerman, K. Grand Challenges in Nanomaterial-Based Electrochemical Sensors. Front. Sens. 2020, 1, 5. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 1–36. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Carbon nanomaterial-based biosensors: A review of design and applications. IEEE Nanotechnol. Mag. 2019, 13, 4–14. [Google Scholar] [CrossRef]
- Shiddiky, M.J.; Torriero, A.A. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; AlSaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic liquid-carbon nanomaterial hybrids for electrochemical sensor applications: A review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E. Ionic liquid-mediated functionalization of graphene-based materials for versatile applications: A review. Graphene Technol. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Khare, V.; Pham, M.Q.; Kumari, N.; Yoon, H.S.; Kim, C.S.; Park, J.I.; Ahn, S.H. Graphene–ionic liquid based hybrid nanomaterials as novel lubricant for low friction and wear. ACS Appl. Mater. Interfaces 2013, 5, 4063–4075. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Wang, B.; Tang, W.; Lu, H.; Huang, Z. Hydrothermal synthesis of ionic liquid-capped carbon quantum dots with high thermal stability and anion responsiveness. J. Mater. Sci. 2015, 50, 5411–5418. [Google Scholar] [CrossRef]
- Xi, F.; Liu, L.; Wu, Q.; Lin, X. One-step construction of biosensor based on chitosan–ionic liquid–horseradish peroxidase biocomposite formed by electrodeposition. Biosens. Bioelectron. 2008, 24, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Q.; Ju, M.; Li, W. New developments in material preparation using a combination of ionic liquids and microwave irradiation. Nanomaterials 2019, 9, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Chen, Y.; Ma, Q.; Liu, R.; Liu, X. Layer-by-layer assembled ionic-liquid functionalized graphene–polyaniline nanocomposite with enhanced electrochemical sensing properties. J. Mater. Chem. C 2014, 2, 4818–4827. [Google Scholar] [CrossRef]
- Rizzo, C.; Marullo, S.; D’Anna, F. Carbon-based ionic liquid gels: Alternative adsorbents for pharmaceutically active compounds in wastewater. Environ. Sci. Nano 2021, 8, 131–145. [Google Scholar] [CrossRef]
- Kang, X.; Sun, X.; Han, B. Synthesis of functional nanomaterials in ionic liquids. Adv. Mater. 2016, 28, 1011–1030. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Timur, S. Ionic Liquids from Biocompatibility and Electrochemical Aspects toward Applying in Biosensing Devices. Anal. Chem. 2018, 90, 640–648. [Google Scholar] [CrossRef]
- Kim, T.; Lee, H.; Kim, J.; Suh, K.S. Synthesis of phase transferable graphene sheets using ionic liquid polymers. ACS Nano 2010, 4, 1612–1618. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Gu, Y.; Xue, B.; Li, Y. A new and efficient method of graphene oxide immobilized with ionic liquids: Promoted catalytic activity for CO2 cycloaddition. Mater. Chem. Phys. 2018, 208, 68–76. [Google Scholar] [CrossRef]
- Ramesh, K.; Siboro, S.A.; Kim, D.W.; Lim, K.T. Ultrasound-accelerated covalent-functionalization of reduced graphene oxide with imidazolium-based poly (ionic liquid) s by Diels-Alder click reaction for supercapacitors. React. Funct. Polym. 2020, 152, 104605. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Zhu, M. Amine-terminated ionic liquid modified graphene oxide/copper nanocomposite toward efficient lubrication. Appl. Surf. Sci. 2019, 491, 105–115. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Wen, Y.; Liu, J.; Li, X.; Zeng, H.; Xue, Z.; Zhou, X.; Xie, X. Noncovalent engineering of carbon nanotube surface by imidazolium ionic liquids: A promising strategy for enhancing thermal conductivity of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105517. [Google Scholar] [CrossRef]
- Park, C.H.; Schroeder, V.; Kim, B.J.; Swager, T.M. Ionic liquid-carbon nanotube sensor arrays for human breath related volatile organic compounds. ACS Sens. 2018, 3, 2432–2437. [Google Scholar] [CrossRef]
- Shu, Y.; Lu, J.; Mao, Q.X.; Song, R.S.; Wang, X.Y.; Chen, X.W.; Wang, J.H. Ionic liquid mediated organophilic carbon dots for drug delivery and bioimaging. Carbon 2017, 114, 324–333. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Huang, Z.; Lu, D.; Zhu, A.; Shi, G. Ionic liquids modified graphene oxide composites: A high efficient adsorbent for phthalates from aqueous solution. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Qiu, S.; Du, P.; Zhao, H.; Wang, L. An ionic liquid–graphene oxide hybrid nanomaterial: Synthesis and anticorrosive applications. Nanoscale 2018, 10, 8115–8124. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Chen, Q.; Lin, L.; Jiang, S.; Zhou, H.; Zhao, J.; Wu, J. A paper-supported graphene-ionic liquid array for e-nose application. Chem. Comm. 2016, 52, 3042–3045. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Poly (ionic liquid)-stabilized graphene nanoinks for scalable 3D printing of graphene aerogels. ACS Appl. Nano Mater. 2020, 3, 11608–11619. [Google Scholar] [CrossRef]
- Vatani, M.; Vatani, M.; Choi, J.W. Multi-layer stretchable pressure sensors using ionic liquids and carbon nanotubes. Appl. Phys. Lett. 2016, 108, 061908. [Google Scholar] [CrossRef]
- Singh, V.V.; Nigam, A.K.; Batra, A.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Applications of ionic liquids in electrochemical sensors and biosensors. Int. J. Electrochem. 2012, 19, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wang, X.; Jin, J.; Zhang, Q.; Chen, J. Electrochemical biosensing platform based on amino acid ionic liquid functionalized graphene for ultrasensitive biosensing applications. Biosens. Bioelectron. 2014, 62, 134–139. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Aldroubi, S.; Brun, N.; Malham, I.B.; Mehdi, A. When graphene meets ionic liquids: A good match for the design of functional materials. Nanoscale 2021, 13, 2750–2779. [Google Scholar] [CrossRef]
- Wang, C.H.; Wu, C.H.; Wu, J.W.; Lee, M.T.; Chang, J.K.; Ger, M.D.; Sun, C.L. The effects of ionic liquid on the electrochemical sensing performance of graphene-and carbon nanotube-based electrodes. Analyst 2013, 138, 576–582. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, J.M. Applications of ionic liquids. Chem. Rec. 2012, 12, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E. Recent Advances in Ionic Liquids in Biomedicine. Adv. Sci. 2021, 8(17), 2004819. [Google Scholar] [CrossRef] [PubMed]
- Silvester, D.S. Recent advances in the use of ionic liquids for electrochemical sensing. Analyst 2011, 136, 4871–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Muthukumar, S.; Prasad, S. Room-temperature ionic liquids for electrochemical application with special focus on gas sensors. J. Electrochem. Soc. 2019, 167, 037511. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Guan, Y.; Shao, Q.; Chen, W.; Zhang, J.; Zhang, X.; Deng, Y. Flow-induced voltage generation by driving imidazolium-based ionic liquids over a graphene nano-channel. J. Mater. Chem. A Mater. 2018, 6, 11941–11950. [Google Scholar] [CrossRef]
- Wang, X.; Fu, F.; Peng, K.; Huang, Q.; Li, W.; Chen, X.; Yang, Z. Understanding of competitive hydrogen bond behavior of imidazolium-based ionic liquid mixture around single-walled carbon nanotubes. J. Phys. Chem. C 2020, 124, 6634–6645. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef]
- Wang, J.; Chu, H.; Li, Y. Why single-walled carbon nanotubes can be dispersed in imidazolium-based ionic liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2-based advanced biosensors: A review. ACS Appl. Nano Mater. 2017, 1, 2–25. [Google Scholar] [CrossRef]
- Pal, M.; Khan, R. Graphene oxide layer decorated gold nanoparticles based immunosensor for the detection of prostate cancer risk factor. Anal. Biochem. 2017, 536, 51–58. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-care biosensor-based diagnosis of COVID-19 holds promise to combat current and future pandemics. ACS Appl. Bio Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef]
- Xie, Z.L.; Su, D.S. Ionic liquid based approaches to carbon materials synthesis. Eur. J. Inorg. Chem. 2015, 2015, 1137–1147. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Musameh, M.M.; Abu-Yousef, I.; Yousef, J.M.; Kanan, S.M.; Xiao, L.; Davies, S.G.; Russell, A.; Compton, R.G. Carbon nanotube-ionic liquid composite sensors and biosensors. Anal. Chem. 2009, 81, 435–442. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Galpothdeniya, W.I.S.; McCarter, K.S.; De Rooy, S.L.; Regmi, B.P.; Das, S.; Hasan, F.; Tagge, A.; Warner, I.M. Ionic liquid-based optoelectronic sensor arrays for chemical detection. RSC Adv. 2014, 4, 7225–7234. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. Trends. Analyt. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Xu, B.; Zhao, F.; Zeng, B. Ionic liquid functionalized 3D graphene-carbon nanotubes—AuPd nanoparticles-molecularly imprinted copolymer based paracetamol electrochemical sensor: Preparation, characterization and application. Talanta 2021, 224, 121845. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. A novel ratiometric electrochemical sensor for the selective detection of citrinin based on molecularly imprinted poly (thionine) on ionic liquid decorated boron and nitrogen co-doped hierarchical porous carbon. Food Chem. 2021, 363, 130385. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Zhang, M.; Zhao, F.; Zeng, B. Ionic liquid assisted molecular self-assemble and molecular imprinting on gold nanoparticles decorated boron-doped ordered mesoporous carbon for the detection of zearalenone. Talanta 2020, 217, 121032. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Taher, M.A.; Karimi-Maleh, H. A sensitive and fast approach for voltammetric analysis of bisphenol a as a toxic compound in food products using a Pt-SWCNTs/ionic liquid modified sensor. Food Chem. Toxicol. 2021, 152, 112166. [Google Scholar] [CrossRef]
- Liu, N.; Liu, J.; Niu, X.; Wang, J.; Guo, R.; Mo, Z. An electrochemical chiral sensor based on the synergy of chiral ionic liquid and 3D-NGMWCNT for tryptophan enantioselective recognition. Microchim. Acta 2021, 188, 1–13. [Google Scholar] [CrossRef]
- Silva, T.A.; Wong, A.; Fatibello-Filho, O. Electrochemical sensor based on ionic liquid and carbon black for voltammetric determination of Allura red colorant at nanomolar levels in soft drink powders. Talanta 2020, 209, 120588. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, X.; Liu, P.; He, M.; Li, C.; Wang, Y. Near-infrared photoactive Yb-MOF functionalized with a large conjugate ionic liquid: Synthesis and application for photoelectrochemical immunosensing of carcinoma embryonic antigen. Nanoscale 2021, 13, 9757–9765. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, Y.J.; Bao, T.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Y.; Nie, G. Electrochemical immunosensor for the carcinoembryonic antigen based on a nanocomposite consisting of reduced graphene oxide, gold nanoparticles and poly (indole-6-carboxylic acid). Microchim. Acta 2016, 183, 2925–2932. [Google Scholar] [CrossRef]
- Liu, N.; Ma, Z. Au–ionic liquid functionalized reduced graphene oxide immunosensing platform for simultaneous electrochemical detection of multiple analytes. Biosens. Bioelectron. 2014, 51, 84–190. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, X.; Zhang, W.; Jia, L.P.; Ma, R.N.; Jia, W.L.; Wang, H.S. A dual-potential electrochemiluminescence sensor for ratiometric detection of carcinoembryonic antigen based on single luminophor. Sens. Actuators B Chem. 2020, 325, 128776. [Google Scholar] [CrossRef]
- Wang, X.; Shang, L.; Zhang, W.; Jia, L.P.; Ma, R.N.; Jia, W.L.; Wang, H.S. An ultrasensitive luminol cathodic electrochemiluminescence probe with highly porous Pt on ionic liquid functionalized graphene film as platform for carcinoembryonic antigen sensing. Biosens. Bioelectron. 2019, 141, 111436. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Sun, X.; Ma, H.; Zhang, Y.; Wei, Q. Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on Au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens. Bioelectron. 2017, 94, 141–147. [Google Scholar] [CrossRef]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan-graphene-ionic liquid-ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef]
- Kesici, E.; Eksin, E.; Erdem, A. An impedimetric biosensor based on ionic liquid-modified graphite electrodes developed for microRNA-34a detection. Sensors 2018, 18, 2868. [Google Scholar] [CrossRef] [Green Version]
- Yaralı, E.; Kanat, E.; Erac, Y.; Erdem, A. Ionic Liquid Modified Single-use Electrode Developed for Voltammetric Detection of miRNA-34a and its Application to Real Samples. Electroanalysis 2020, 32, 384–393. [Google Scholar] [CrossRef]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S. Electrochemical genosensor based on carbon nanotube/amine-ionic liquid functionalized reduced graphene oxide nanoplatform for detection of human papillomavirus (HPV16)-related head and neck cancer. J. Pharm. Biomed. Anal. 2020, 179, 112989. [Google Scholar] [CrossRef]
- Yan, H.; Tang, X.; Zhu, X.; Zeng, Y.; Lu, X.; Yin, Z.; Lu, Y.; Yang, Y.; Li, L. Sandwich-type electrochemical immunosensor for highly sensitive determination of cardiac troponin I using carboxyl-terminated ionic liquid and helical carbon nanotube composite as platform and ferrocenecarboxylic acid as signal label. Sens. Actuators B Chem. 2018, 277, 234–240. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, M.; Lü, Y.; Zhang, D.; Cheng, Z.; Liu, Y.; Gao, H.; Jin, Z. Label-Free Electrochemical Immunosensor Based on a Functionalized Ionic Liquid and Helical Carbon Nanotubes for the Determination of Cardiac Troponin I. ACS Omega 2019, 4, 11888–11892. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Shen, Y. Covalent Functionalized Carbon Nanotube with Ionic Liquid and Its Application for Human Immunoglobulin G Immunosensor. Int. J. Electrochem. Sci. 2019, 14, 7560–7569. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, G.; Zhang, Y. High Sensitive Sandwich-type Electrochemical IgG Immunosensor Based on a Nanocomposite of Carbon Nanotube and Multifunctional Ionic Liquid Containing Aldehyde and Ferrocene Groups as Labels. Int. J. Electrochem. Sci. 2018, 13, 8905–8914. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, G.; Zhang, Y. A versatile matrix of an ionic liquid functionalized with aldehyde and ferrocene groups for label-free electrochemical immunosensors. Anal. Methods 2018, 10, 1612–1617. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, D.; Cui, H.; Huang, T. ZnO/porous carbon composite from a mixed-ligand MOF for ultrasensitive electrochemical immunosensing of C-reactive protein. Sens. Actuators B Chem. 2019, 284, 354–361. [Google Scholar] [CrossRef]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly imprinted electrochemical biosensor based on chitosan/ionic liquid–graphene composites modified electrode for determination of bovine serum albumin. Sens. Actuators B Chem. 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Sun, Y.; Ding, C.; Lin, Y.; Sun, W.; Luo, C. A novel ionic liquid functionalized graphene oxide supported gold nanoparticle composite film for sensitive electrochemical detection of dopamine. RSC Adv. 2017, 7, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Wang, H.; He, T.; Chen, L. Enhanced voltammetric determination of dopamine using a glassy carbon electrode modified with ionic liquid-functionalized graphene and carbon dots. Microchim. Acta 2016, 183, 3177–3182. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Nagles, E.; García-Beltrán, O.; Calderón, J.A. Evaluation of the usefulness of a novel electrochemical sensor in detecting uric acid and dopamine in the presence of ascorbic acid using a screen-printed carbon electrode modified with single walled carbon nanotubes and ionic liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Albishri, H.M.; Abd El-Hady, D. Hyphenation of enzyme/graphene oxide-ionic liquid/glassy carbon biosensors with anodic differential pulse stripping voltammetry for reliable determination of choline and acetylcholine in human serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef]
- Naeim, H.; Kheiri, F.; Sirousazar, M.; Afghan, A. Ionic liquid/reduced graphene oxide/nickel-palladium nanoparticle hybrid synthesized for non-enzymatic electrochemical glucose sensing. Electrochim. Acta 2018, 282, 137–146. [Google Scholar] [CrossRef]
- Benjamin, M.; Manoj, D.; Thenmozhi, K.; Bhagat, P.R.; Saravanakumar, D.; Senthilkumar, S. A bioinspired ionic liquid tagged cobalt-salophen complex for nonenzymatic detection of glucose. Biosens. Bioelectron. 2017, 91, 380–387. [Google Scholar] [CrossRef]
- Manoj, D.; Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Aldehyde functionalized ionic liquid on electrochemically reduced graphene oxide as a versatile platform for covalent immobilization of biomolecules and biosensing. Biosens. Bioelectron. 2018, 103, 104–112. [Google Scholar] [CrossRef]
- Janmee, N.; Preechakasedkit, P.; Rodthongkum, N.; Chailapakul, O.; Potiyaraj, P.; Ruecha, N. A Non-Enzymatic Disposable Electrochemical Sensor Based on Surface-Modified Screen-Printed Electrodes CuO-IL/rGO Nanocomposite for Single-Step Determination of Glucose in Human Urine and Electrolyte Drink. Anal. Methods 2021, 13, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, S.S.; Qiu, J. Preparation and properties of a glucose biosensor based on an ionic liquid-functionalized graphene/carbon nanotube composite. New Carbon Mater. 2020, 35, 12–19. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Arotiba, O.A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface. Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, A.R.; Zangeneh, M.M.; Jalili, F.; Soleimani, S.; Díaz-Cruz, J.M. An elegant technology for ultrasensitive impedimetric and voltammetric determination of cholestanol based on a novel molecularly imprinted electrochemical sensor. Chem. Phys. Lipids. 2020, 229, 104895. [Google Scholar] [CrossRef]
- Boobphahom, S.; Ruecha, N.; Rodthongkum, N.; Chailapakul, O.; Remcho, V.T. A copper oxide-ionic liquid/reduced graphene oxide composite sensor enabled by digital dispensing: Non-enzymatic paper-based microfluidic determination of creatinine in human blood serum. Anal. Chim. Acta 2019, 1083, 110–118. [Google Scholar] [CrossRef]
- Wang, M.; Cui, M.; Liu, W.; Liu, X.; Xu, B. Facile Synthesis of Cyclodextrin Functionalized Reduced Graphite Oxide with the Aid of Ionic Liquid for Simultaneous Determination of Guanine and Adenine. Electroanalysis 2018, 30, 842–851. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuang, X.; Chen, D.; Luan, F.; He, T.; Tian, C.; Chen, L. Simultaneous voltammetric determination of guanine and adenine using MnO 2 nanosheets and ionic liquid-functionalized graphene combined with a permeation-selective polydopamine membrane. Microchim. Acta 2019, 186, 1–10. [Google Scholar]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. An ultrasensitive electrochemical anti-lysozyme aptasensor with biorecognition surface based on aptamer/amino-rGO/ionic liquid/amino-mesosilica nanoparticles. Colloids Surf. B Biointerfaces 2019, 181, 16–24. [Google Scholar] [CrossRef]
- Anusha, T.; Bhavani, K.S.; Kumar, J.S.; Bonanni, A.; Brahman, P.K. Fabrication of handmade paper sensor based on silver-cobalt doped copolymer-ionic liquid composite for monitoring of vitamin D3 level in real samples. Microchem. J. 2021, 161, 105789. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, H. Detection of insulin-like growth factor 1 based on an electrochemical impedance spectroscopy sensor. Int. J. Electrochem. Sci. 2017, 12, 11163–11170. [Google Scholar] [CrossRef]
- Inaba, A.; Yoo, G.; Takei, Y.; Matsumoto, K.; Shimoyama, I. A graphene FET gas sensor gated by ionic liquid. In Proceedings of the 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013; pp. 969–972. [Google Scholar]
- Ye, J.; Craciun, M.F.; Koshino, M.; Russo, S.; Inoue, S.; Yuan, H.; Shimotani, H.; Morpurgo, A.F.; Iwasa, Y. Accessing the transport properties of graphene and its multilayers at high carrier density. Proc. Natl. Acad. Sci. USA 2011, 108, 13002–13006. [Google Scholar] [CrossRef] [Green Version]
- Famili, M.; Jia, C.; Liu, X.; Wang, P.; Grace, I.M.; Guo, J.; Liu, Y.; Feng, Z.; Wang, Y.; Zhao, Z.; et al. Self-assembled molecular-electronic films controlled by room temperature quantum interference. Chem 2019, 5, 474–484. [Google Scholar] [CrossRef] [Green Version]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 8 August 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Crosby, D.; Lyons, N.; Greenwood, E.; Harrison, S.; Hiom, S.; Moffat, J.; Quallo, T.; Samuel, E.; Walker, I. A roadmap for the early detection and diagnosis of cancer. Lancet Oncol. 2020, 21, 1397–1399. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, P.; Parihar, A.; Jain, S.; Kumar, N.; Dhand, C.; Murali, S.; Mishra, D.; Sanghi, S.K.; Chaurasia, J.P.; Srivastava, A.K.; et al. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: A comprehensive review. Anal. Biochem. 2020, 610, 113996. [Google Scholar] [CrossRef]
- Kumar, N.; Ranjan, P.; Sadique, M.A.; Yadav, S.; Singhal, A.; Mishra, A.; Murali, S.; Gowri1, V.S.; Khan, R. Efficiency of Nanomaterials for Electrochemical Diagnostics based Point-of-Care Detection of Non-Invasive Oral Cancer Biomarkers. Adv. Mater. Lett. 2021, 12, 21081651. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Vashistha, R.; Dangi, A.K.; Kumar, A.; Chhabra, D.; Shukla, P. Futuristic biosensors for cardiac health care: An artificial intelligence approach. 3 Biotech 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Pal, M.; Kuzikov, A.V.; Bulko, T.; Suprun, E.V.; Shumyantseva, V.V. Impedimetric immunosensor for detection of cardiovascular disorder risk biomarker. Mater. Sci. Eng. C 2016, 68, 52–58. [Google Scholar] [CrossRef]
- Pal, M.; Khan, R. Biosensor platforms for detection of cardiovascular disease risk biomarkers. In Functional Polysaccharides for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 397–431. [Google Scholar]
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef]
- Cho, I.H.; Lee, J.; Kim, J.; Kang, M.S.; Paik, J.K.; Ku, S.; Cho, H.M.; Irudayaraj, J.; Kim, D.H. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [Green Version]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The role of electrochemical immunosensors in clinical analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Si, B.; Song, E. Recent advances in the detection of neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; McCracken, S.; Hossain, M.F.; Slaughter, G. Electrochemical detection of neurotransmitters. Biosensors 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Madhurantakam, S.; Karnam, J.B.; Brabazon, D.; Takai, M.; Ahad, I.U.; Balaguru Rayappan, J.B.; Krishnan, U.M. “Nano”: An Emerging Avenue in Electrochemical Detection of Neurotransmitters. ACS Chem. Neurosci. 2020, 11, 4024–4047. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Kaushik, A.; Khan, R.; Solanki, P.R.; Pandey, P.; Alam, J.; Ahmad, S.; Malhotra, B.D. Iron oxide nanoparticles-chitosan composite based glucose biosensor. Biosens. Bioelectron. 2008, 24, 676–683. [Google Scholar] [CrossRef]






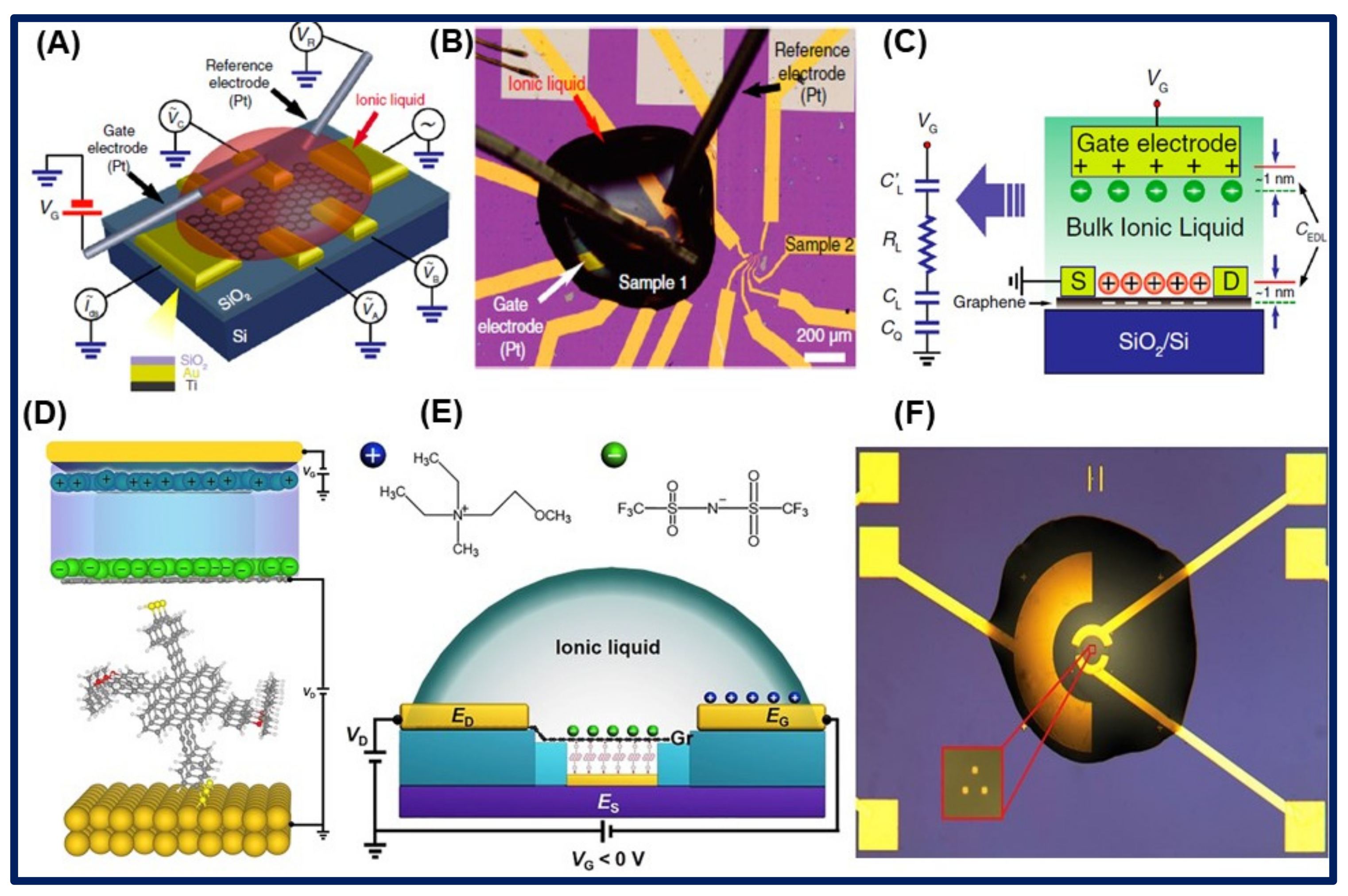
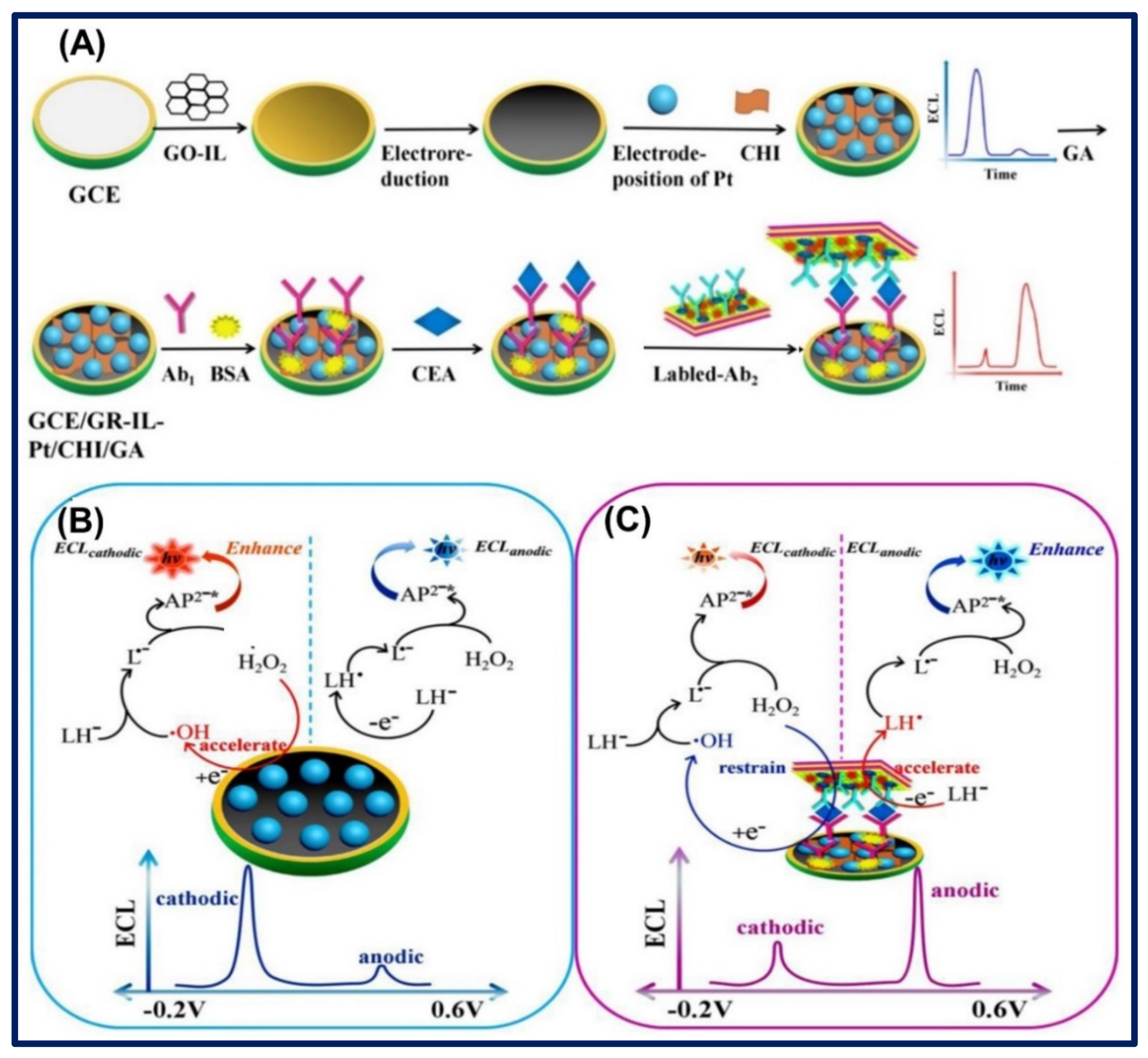
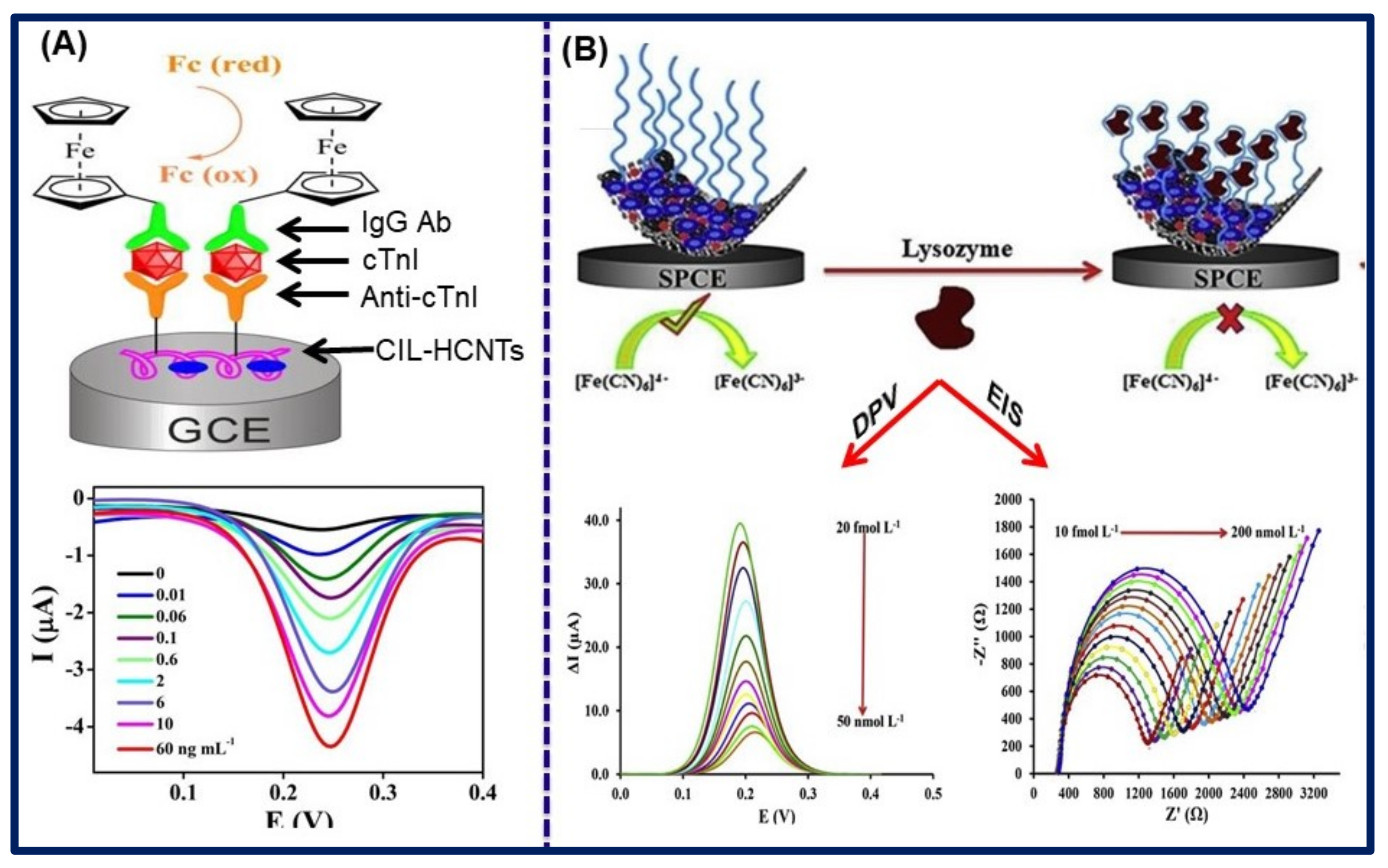

| Ionic Liquids | Conductivity (in mS/cm) | Electrochemical Window (in V) | Viscosity (in cP) | Ref. |
|---|---|---|---|---|
| [BMIM][BF4] | 3.5 | - | 154 | [88] |
| [BMIM][NTf2] | 4.0 | - | 52 | |
| [BMIM][PF6] | 1.46–1.0 | - | 308 | |
| [BMIM] [OTf] | 2.9 | - | 90 | |
| [EMIM] [NTf2] | 9.1 | - | 28–34 | |
| [EMIM][PF6] | 5.2 | - | - | |
| [EMIM][OTf] | 8.6 | 45 | ||
| [EMIM][BF4] | 12.0 | 4.3 | 33.8 | [89] |
| [EMIM][F3MeS] | 8.6 | 4.3 | 39.8 | |
| [EMIM][SCN] | 27.0 | 2.9 | 24.7 | |
| [EMIM][DCA] | 21.0 | 2.3 | 14.6 | |
| [Pyr14][Tf2N] | 2.1 | 6.6 | 0.002 | |
| [N8,8,8,1][NTf2] | 2.2 | 5.7 | 530 | |
| [Et3S][NTf2] | 8.2 | 5.5 | 5.2 |
| Nanomaterial | Ionic Liquid | Target Analyte | Sample | Technique | Linearity | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Cancer biomarker | |||||||
| GQDs-ILs-nafion | [BDMIM][BF4] | CEA | Serum | DPV | 0.5 fg/mL–0.5 ng/mL | 0.34 fg/mL | [112] |
| AuNPs/IL/PICA/RGO/GCE | [BMIM][PF6] | CEA | Spiked serum samples | DPV | 0.02–90.0 ng/mL | 0.02 ng/mL | [113] |
| IL/RGO/AuNPs | [APMIM]Cl | CEA, AFP | Serum | DPV | 0.01–100 ng/mL | CEA 0.01 ng/mL, AFP 0.006 ng/mL | [114] |
| GR-IL-Pt and Ti3C2 MXenes-AuNPs hybrids | [BMIM][PF6] | CEA | Serum | ECL | 0.1 pg/mL–10.0 ng/mL | 34.58 fg/mL | [115] |
| GR-IL-pPT | [BMIM][PF6] | CEA | Serum | ECL | 0.001 fg/mL–1.0 ng/mL | 0.0003 fg/mL | [116] |
| Au-CoS/GR and CeO2/IL doped with carboxymethyl chitosan | [BP][BF4] | PSA | Serum | DPV | 0.5 pg/mL–50.0 ng/mL | 0.16 pg/mL | [117] |
| 3D porous cryogel (CS-GR-IL-Fc cry) | [BMIM][TFSI] | PSA | Serum | DPV | 1.0 × 10−7–1.0 × 10−1 ng/mL | 4.8 × 10−8 Ng/mL | [118] |
| IL-CA-PGEs | [BMIM][PF6] | miRNA-34a | FBS | EIS | 2.0–10.0 µg/mL | 0.826 µg/mL | [119] |
| IL-CA-PGEs | [BMIM][PF6] | miRNA-34a | FBS | DPV | 1.0–7.0 µg/mL | 0.40 µg/mL | [120] |
| NH2-IL-RGO | 3-(2-aminoethyl)-1-propyl-1H-imidazol-3-ium chloride | HPV16 DNA | - | DPV | 8.5 nM–10.7 µM | 1.3 nM | [121] |
| Cardiovascular biomarker | |||||||
| CIL-HCNTs and IgG Ab-Fc-COOH | [CMMIM]Cl | cTnI | Serum | DPV | 0.01–60.0 ng/mL | 0.006 ng/mL | [122] |
| Dialdehyde-functionalized Ionic Liquid and Helical Carbon Nanotubes (DIL−HCNT) | 4-(Bromomethyl) benzaldehyde, 4,4′-Bipyridine | cTnI | - | DPV | 0.05–30.0 ng/mL | 0.03 ng/mL | [123] |
| Immunoglobulins | |||||||
| CNT-IL | Aldehyde terminated IL | IgG | Serum | DPV | 0.1–15.0 ng/mL | 0.02 ng/mL | [124] |
| CNT/Fc-IL-CHO | - | IgG | - | DPV | 0.05–30.0 ng/mL | 0.01 ng/mL | [125] |
| Nf/Fc-IL-CHO | - | IgG | - | DPV | 0.05–35.0 ng/mL | 0.03 ng/mL | [126] |
| ZnO/MPC/IL | - | CRP | - | DPV | 0.01–1000 ng/mL | 5.0 pg/mL | [127] |
| MIPs/CS/IL-GR | [BMIM][PF6] | BSA | Plasma | DPV | 1.0 × 10−10–1.0 × 10−4 g/L | 2 × 10−11 g/L | [128] |
| Neurotransmitter | |||||||
| GO/IL/AuNPs | 1-butyl-3-methyl imidazole hydrobromide | DA | Urine | DPV | 7.0 nM–5.0 mM | 2.3 nM | [129] |
| C-dots/IL-GR | 1-methylimidazole and 2-bromoethylamine hydrobromide | DA | Spiked fetal bovine serum | DPV | 0.1–600 μM | 30.0 nM | [130] |
| GQDs/IL-SPCE | [BMIM][PF6] | AA, DA, UA | - | DPV | 25.0–400 μM, 0.2–10.0 μM, and 0.5–20.0 μM, | 6.64, 0.06 and 0.03 μM, | [131] |
| Cs-SWCNT/IL | [BMIM][BF4] | DA, UA | Urine | LSV | DA 0.50–30.0 μmol/L, UA 0.50–1000 μmol/L | DA 0.16 μmol/L, UA 0.17 μmol/L | [132] |
| AChE-ChO/GO/IL | [AMIM][TFSI] | Ac, ACh | Serum | ADPSV | 5.0–1000 nmol/L | 0.885 nmol/L, 1.352 nmol/L | [133] |
| Glucose | |||||||
| IL/RGO/Ni-Pd | 1,2-Dimethylimidazole, 1-bromobutan | Glucose | Serum | Amperometric | 0.2–10.0 mM | 0.03 μM | [134] |
| Co-salophen-IL/ERGO/SPE | - | Glucose | Serum and urine | Amperometric | 0.2 μM–1.8 mM | 0.79 μM | [135] |
| CHO/IL/ERGO Azu-A/CHO-IL/SPE | (3-(3-formyl-4-hydroxy benzyl)-3- methylimidazolium hexafluorophosphate | H2O2, Glucose | H2O2 in milk, Juice, glucose in human serum | Amperometric, CV | 0.03–1.0 mM 0.05–2.4 mM | 11.5 and 17.0 μM | [136] |
| CuO-IL/RGO | - | Glucose | Urine | Chronoamperometry | 0.03–7.0 mM | 0.14 μM | [137] |
| CoPc/G/IL/SPCE | [BDMIM][BF4] | Glucose | Food, Serum | Chronoamperograms | 0.01–1.3 mM and 1.3–5.0 mM | 0.67 μM | [138] |
| IL-GR-CNTs | 1-Methylimidazole | Glucose | - | DPV | 0.004–5.0 mmol/L | 3.99 × 10−7 mol/L | [139] |
| Other markers | |||||||
| CuO-rGR/1M3OIDTFB | [OMIM][BF4] | CA, AA, UA | Real samples | SWV | 0.04–300 μM, 0.04–240 μM, 0.4–400 μM | 9.0, 9.0, and 0.08 μM | [140] |
| MIP/AuPd NPs/PDA/MWCNTs-CS-IL/GCE | [EMIM][BTI] | Cholestanol | - | EIS, DPV | EIS: 0.1–60.0 pM and DPV: 1.0–50 pM | EIS: 0.05 pM, DPV: 0.2 pM | [141] |
| CuO/IL/ERGO | [BDMIM][BF4] | Creatinine | Serum | Paper-based microfluidic | 0.01–2.0 mM | 0.22 mM | [142] |
| βCD/RGO/IL | [BMIM][PF6] | Guanine, Adenine | - | SWV | Guanine: 0.03–10.0 mM and adenine: 0.02–7.0 mM | 0.01 mM | [143] |
| PDA/MnO2/IL-GR | 2-bromoethylamine hydrobromide, 1-methyl imidazole, | Guanine, Adenine | Blood | DPV | 10.0–300 μM | Guanine: 0.25 μM and Adenine: 0.15 μM | [144] |
| Amino-rGO/IL/Amino-MSNs | [BMIM]Br | Lysozyme | - | EIS, DPV | EIS: 10.0 fmol/L–200.0 nmol/L and DPV: 20.0 fmol/L–50.0 nmol/L | 2.1 and 4.2 fmol/L | [145] |
| Co-Ag/PANI-PPY/IL@GCE | [BMIM][PF6] | Vitamin D3 | Serum and urine | SWV | 0.0125–22.5 μM and 0.025–0.125 μM | 0.0073 and 0.015 μM | [146] |
| MWCNT/IL/GCE | [BMIM][PF6] | IGF-1 | EIS | 0.4–15.0 ng/mL | 22.0 pg/mL | [147] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Chaurasia, J.P.; Srivastava, A.K. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414. https://doi.org/10.3390/bios11110414
Ranjan P, Yadav S, Sadique MA, Khan R, Chaurasia JP, Srivastava AK. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors. 2021; 11(11):414. https://doi.org/10.3390/bios11110414
Chicago/Turabian StyleRanjan, Pushpesh, Shalu Yadav, Mohd Abubakar Sadique, Raju Khan, Jamana Prasad Chaurasia, and Avanish Kumar Srivastava. 2021. "Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors" Biosensors 11, no. 11: 414. https://doi.org/10.3390/bios11110414
APA StyleRanjan, P., Yadav, S., Sadique, M. A., Khan, R., Chaurasia, J. P., & Srivastava, A. K. (2021). Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors, 11(11), 414. https://doi.org/10.3390/bios11110414





