1. Introduction
Detection of biomarkers specific to a particular disease is a standard method for diagnosis and can be used for successful detection of various diseases [
1]. A number of proteins considered as biomarkers are secreted at an early onset of a disease when the symptoms are yet to develop. A typical source to detect such biomarkers can be a patient’s blood sample. C-reactive protein (CRP) has long been used as a marker of systemic inflammation, where the level of CRP increases several hundred fold within hours of an inflammatory occurrence [
2] and it is found to be over-expressed in heart disease [
3]. Previous research has shown that CRP is dominant in the instigation of several pathogenic pathways that may cause atherosclerosis, a precursor to cardiovascular disease [
4]. According to the Center for Disease Control and Prevention, heart attack is the leading cause of death in the US with an estimated 785,000 Americans having a new coronary attack and approximately 470,000 with a recurrent attack [
5]. Thus a rapid and ultra-sensitive detection of CRP from a patient’s serum sample may be clinically significant in diagnosing cardiac events such as acute myocardial infarction. In recent years, the trend in healthcare has been towards developing devices that support personalized medicine with benefits such as faster diagnosis and therapeutic turnaround time.
Nanotechnology offers a wide range of materials that can be used both as biological recognition elements and transducers for a typical biosensor with exceptional levels of sensitivity that can be used to monitor biomarkers from different diseases. Novel properties of nanoscale materials, especially in the form of nanofibers, such as larger surface to volume ratios and size-based confinement of biomolecules, give them the potential to increase the sensitivity of the diagnostic devices by several orders of magnitude [
6,
7,
8,
9,
10,
11]. This property can be used to detect protein biomarkers such as CRP at very low concentrations and also give an early indication of a heart condition [
12,
13].
Some of the previous efforts in developing biosensors based on nanofibers involved citric acid-decorated nylon nanofibers for the detection of 3-phenoxybenzoic acid (3-PBA), a common human urinary metabolite [
14], and polyvinyl alcohol-co-ethylene (PVA-co-PE)-based nanofibrous membranes for the detection of chloramphenicol (CAP) residues in milk [
15]. One of the aspects of polyvinyl alcohol (PVA) and carbon nanotube (CNT)-based nanofibrous biological recognition elements is that they offer spatial confinement similar to the intracellular environment through porosity. The binding affinities and the rate at which a typical self-assembly process takes place can benefit from several physical constraints during the biosensing of macromolecules in vitro [
16,
17,
18]. The nanotexturing of the sensor platform enables minimization of excluded volume of the biomolecules not participating in the interaction with the biomarker, which is also evident from the increase in the concentration that is analyzed through finite element analysis of diluted species [
19]. Furthermore, even though individual fibers may have been laid on the electrode, there are still multiple fibers exhibiting a certain thickness similar to a membrane-like matrix but taking advantage of a higher specific surface area.
One of the major challenges faced by nanomaterial-based biosensors is the sensitivity and reproducibility of the results, which arises mainly because of the extreme difficulty in detecting a small quantity of protein biomarkers in serum samples [
20,
21,
22]. Hence, determining the concentration of trace biomarkers in a complex mixture is a challenge in patient diagnosis.
Amongst several diagnostic biosensors, most of the conventional ones rely on fluorescent labeling or dyes for gathering the data [
23]. Various sensing techniques that are applied to detect biomolecules at low concentrations are electrochemical analysis, surface plasmon resonance, electromagnetic measurements or mechanical actuation. Electrochemical sensors allow a label free detection of biomolecules by detecting and measuring the electrical signal [
24,
25,
26]. A variety of electrochemical techniques based on integrated devices have been employed for label free and ultra-sensitive detection of different biomarkers. These methods are based on the principles of charge transfer [
27,
28,
29], radiofrequency [
30], complementary metal oxide semiconductors (CMOS) [
31], capacitance [
32], or impedance [
33]. CMOS-based sensors are limited by the complexity of the fabrication technique, which leads to an extremely high cost of the overall biosensor device. In contrast, impedance and capacitance-based measurements are potential techniques for sensing a variety of biomolecules, mainly due to the low power consumption, ease of miniaturization and relatively low cost [
34]. It is known that the performance of any biosensor depends on the immobilization of biomolecules on a biocompatible electrode surface. Therefore, the specificity of a biosensor can be increased by increasing the overall specific binding sites on the surface of the electrode. Hence, a biocompatible electrode, with an extremely high surface area that can engender spatial confinement for favorable binding events, would be a preferred platform for antigen–antibody interactions to achieve high sensitivity and specificity.
Here, we report, an extremely sensitive diagnosing platform with an ability to detect CRP concentrations up to fg mL−1 in both phosphate buffer saline (PBS) and human serum (hs). A microchip with seven interdigitated micro-comb capacitors is integrated with a randomly aligned electrospun PVA nanofibrous mesh. Owing to the random alignment of the nanofibers, the mesh showed high porosity for macromolecule confinement and an extremely small individual fiber diameter (~250 nm), along with a larger surface area to volume ratio, providing efficient antigen–antibody interactions. Furthermore, the signal transduction is found to be chemo-electro ionic, with the protein binding resulting in a modulation of the electrical double layer at the nanofibrous surface. A 45% change in the measured impedance from the antibody saturation baseline was observed while detecting the lowest detectable dose of 1 fg mL−1. Finally, a change in the oxidation current for a gold substrate with and without PVA/CNT/anti-CRP functionalization provides crucial insights on the charge transfer resistance at the double layer capacitance interface between the metal electrode and the bulk electrolyte. The results obtained through a finite element model are in close agreement with the experimental data.
2. Materials and Methods
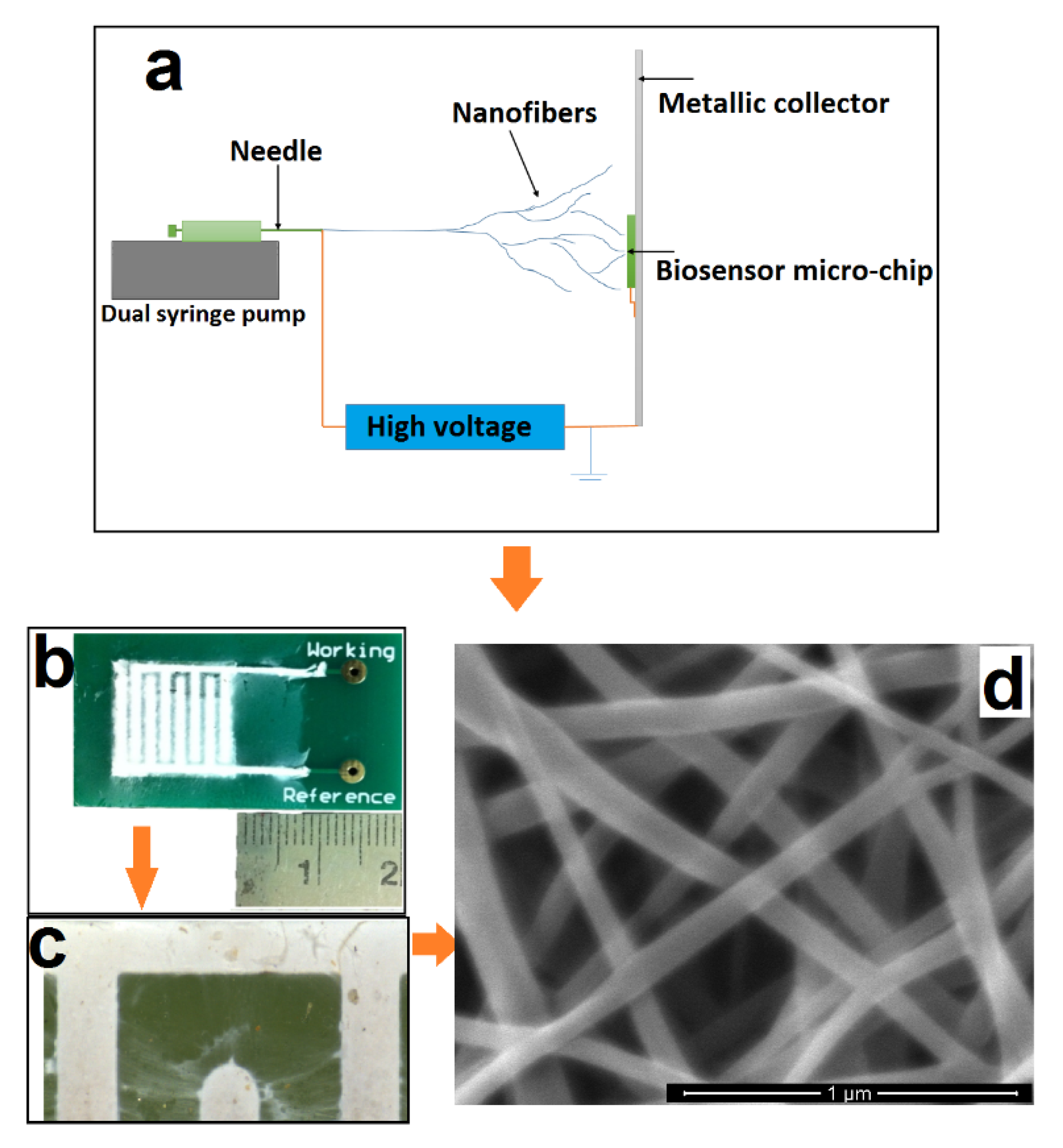
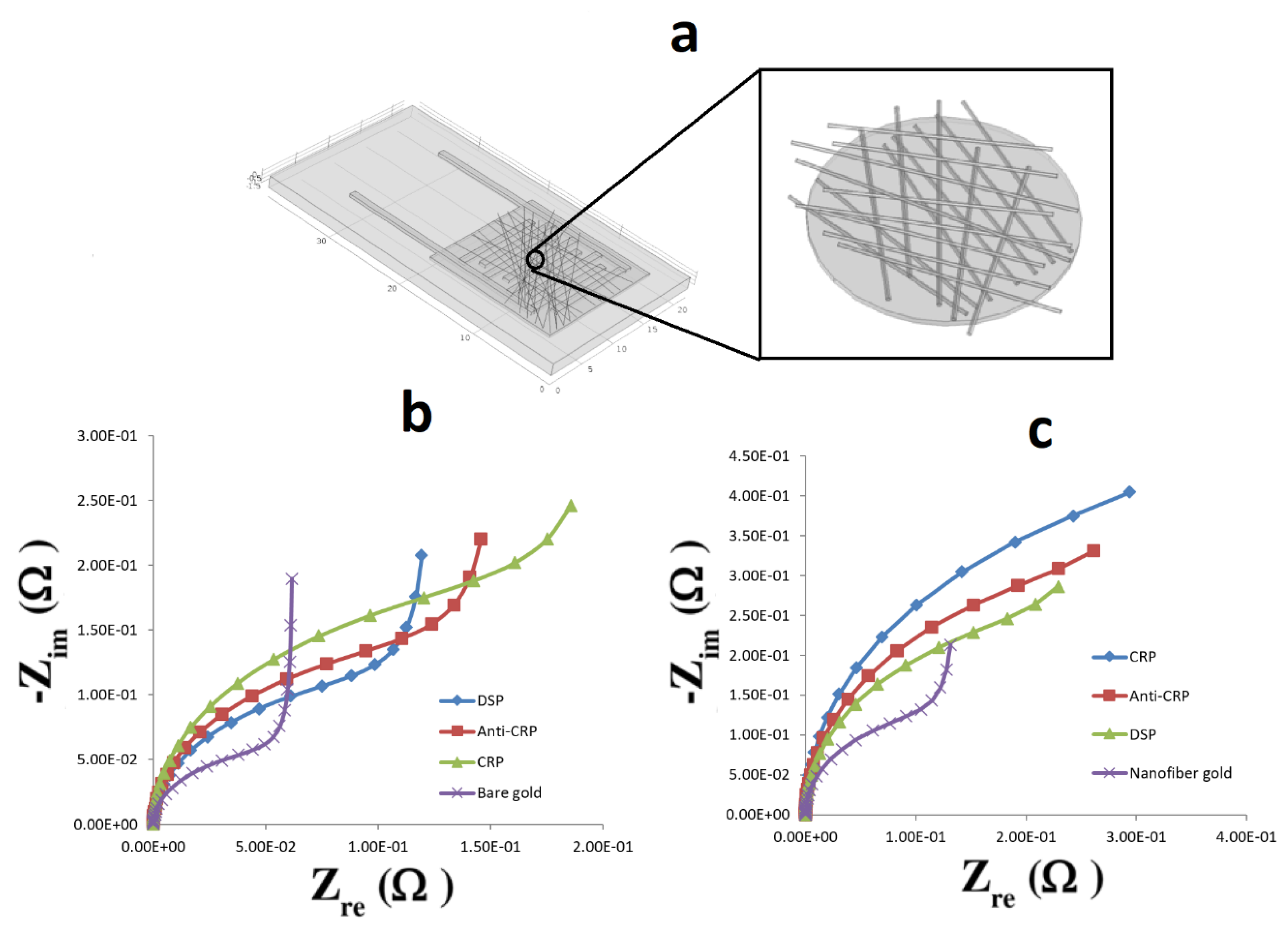
The nanoweb was fabricated using an electrospinning process (
Figure 1a). The electrospinning set up comprises a high voltage power supply and a dual syringe pump unit. A positive electrode from the high voltage power supply is connected to the needle. The nanoweb is collected on a grounded metal collector covered with insulating polyethylene. To produce the PVA-CNT nanocomposite fibrous nanoweb, we applied 15 kV of voltage across 10 cm distance between the collector and the needle tip with a flow rate of the CNT dispersed polymer solution of 1.5 mL/h.
The nanoweb biosensor chip was comprised of a base printed circuit platform. The platform is overlaid with the nanoporous nanoweb layer, which is then encapsulated by microfluidic manifolds manufactured using polydimethylsiloxane for fluid encapsulation and confinement. Based on the SEM images, the area of the pores is estimated to be in the range of 100 to 500 square nanometers. CRP is used in phosphate buffered saline as well as 50% human serum for evaluating the sensor performance.
Printed circuit board (PCB) chips comprising gold comb shaped designs of dimensions 10 mm × 10 mm in length; a finger width of 1 mm with spacings of 1 mm were manufactured with a FR4 passivation layer. After cleaning the surface of these chips with 10 mL of isopropyl alcohol (IPA) and air drying the samples for 10 min, nanowebs constituting 10 mg of CNT in 10% PVA were electrospun (
Figure 1b).
Figure 1c shows a blown-up image of the individual fingers and the presence of the nanoweb in between.
To ensure that the nanofibers are deposited on the gold microelectrodes and not on the FR4 (glass reinforced epoxy laminate printed circuit board) surface of the microchip itself, both the ‘Working’ and the ‘Reference’ electrodes were also grounded. Interestingly, the above mentioned procedure results in selective deposition of nanocomposite fibers only on the conductive electrodes allowing the FR4 surface to be clean without any deposition. The average diameter of the nanocomposite fibers is ~250 nm, as seen from the SEM image in
Figure 1d. Based on the flow rate of 1.5 mL/h and the electrospinning time of ~90 min, the thickness of the electrospun nanofibrous substrate was found to be ~0.5 mm. The presence of CNTs in the polymer matrix helps to stretch the nanofibers under the electric field, enabling continuous stretching and thereby forming a uniform fiber diameter without beaded morphology owing to the conductive nature of the CNTs. This ensures uniform fiber distribution, creating a spatially confined environment for better antigen–antibody interactions.
The dimensions of the nanowebs used for the experiments were 13.2 mm × 13.2 mm. The nanowebs were overlaid onto the metallic sensor surface using tweezers. The nanoweb was encapsulated with a polydimethylsiloxane (PDMS) manifold with dimensions of 13 mm × 13 mm. The manifold has a groove of 5 mm depth to enable the localization of the nanoweb onto the sensor surface. All the steps pertaining to the detection assay were performed on the assembled sensor chip.
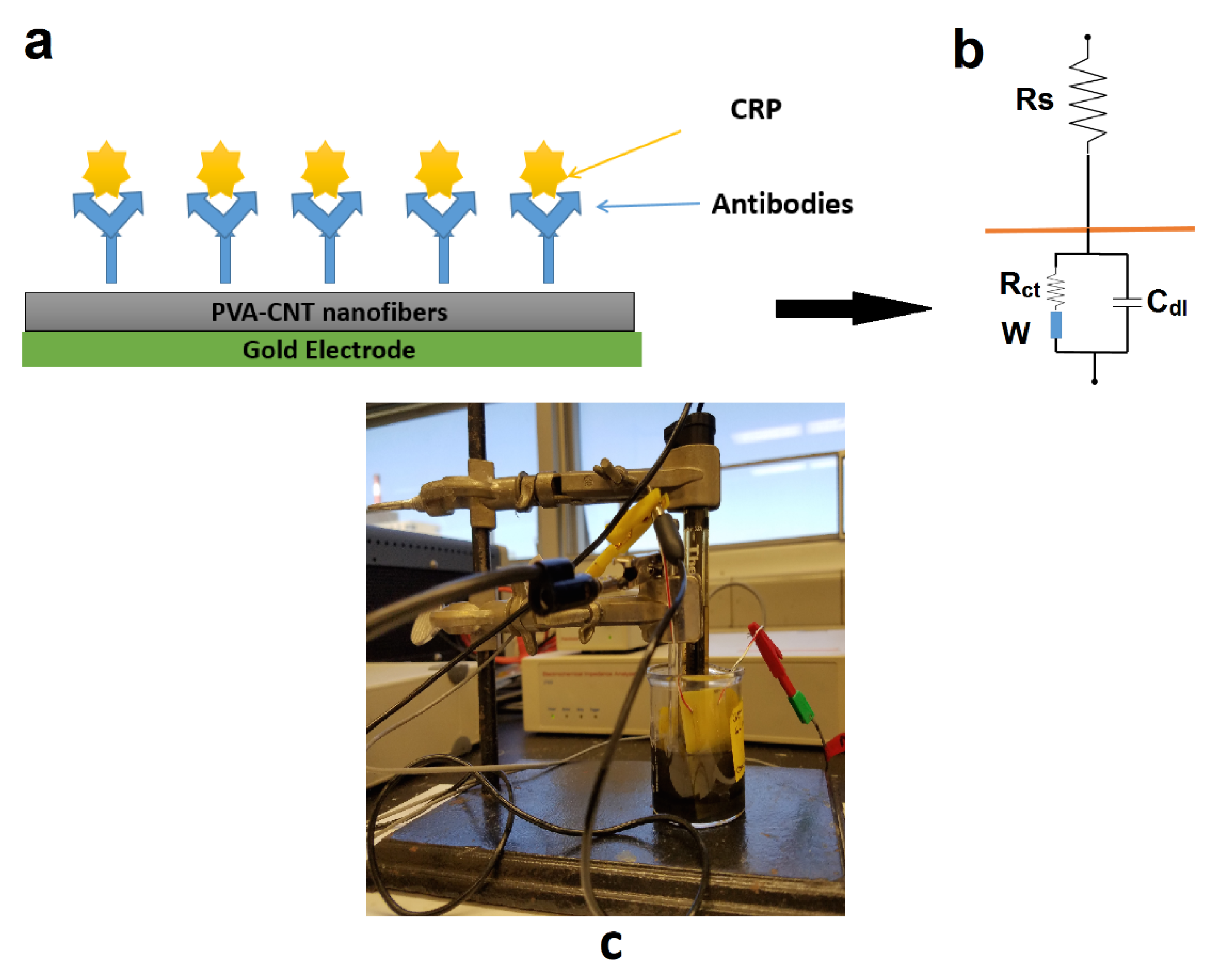
Detection of the protein binding event was achieved using electrochemical impedance spectroscopy (EIS), and electrical double layer (EDL) capacitance measurement [
9,
10,
12]. Briefly, the EDL comprises two components: the solution resistance (R
s) and the double layer capacitance (C
dl). The binding of the protein biomolecules to the covalently anchored antibodies in the nanotextured surface resulted in a modulation to the charge at the electrical double layer formed at the solid/liquid interface [
12]. This change in charge produced a change in C
dl. The capacitance change was measured as an impedance change using an impedance analyzer (Impedance/Gain Phase Analyzer, Autolabs, Avon, IN, USA).
Oscillating AC fields of 0.05 V were applied, and the frequency was scanned over a range of 40 Hz–10 kHz. Frequencies only of the lower orders (up to 10 kHz) were considered for this study. At these low frequencies C
dl undergoes major variations during protein binding at the electrical interface. The sample volume was maintained throughout the experimental trials at 150 µL, sufficient to completely wet the sensor surface. This kept the solution resistance R
s constant within this frequency regime [
10]. This EIS technique produced measurements that were purely the result of the changes in C
dl, and indicated protein binding.
Two sets of dose response experiments were performed to test the capabilities of the nanoweb sensor platform using electrochemical impedance measurements. The first set was focused on detecting CRP when aliquoted in isotonic buffer solution, 0.15 M phosphate buffer saline (PBS). Aliquots of hs-CRP ranging from 1 fg/mL to 100 ng/mL were prepared on a logarithmic scale. These concentrations were selected because they represent clinically relevant concentrations of CRP in physiological conditions. It is known that dithiobis succinimidyl propionate (DSP), which is a covalent linker, has a thiol (sulphur) group at one end that has high affinity for the gold surface while the other end, which is the NHS group, binds with the biological macromolecule (anti-CRP in this case) [
35,
36]. Based on the highly porous structure of the nanofibrous substrate, the DSP linker would permeate through these pores and anchor on the underlying gold substrate. This unique substrate now allows the interaction of the analyte (CRP) with the anti-CRP within these pores, increasing the overall sensitivity of the biosensor. Thus, after integrating the nanoweb mat and PDMS manifold onto the chip, 150 μL of 10 mM DSP crosslinker was injected into the manifold and incubated for 30 min at room temperature. The sensor surface was subsequently washed three times with 0.15 M PBS and baseline PBS measurements were taken. Following the crosslinker deposition, 150 μL of 50 μg/mL anti-CRP was incubated onto the sensor surface at 4 °C for 2 h to immobilize the receptors. After immobilizing anti-CRP on the sensing surface, 0.15 M PBS wash was performed three times followed by incubation of super block, a blocking protein that reduces the non-specific binding, on the sensor surface for 30 min at room temperature to minimize non-specific binding, which was again followed by a 0.15 M PBS wash that was performed three times. A zero dose, corresponding to 0.15 M PBS, was injected into the manifold and the measured impedance was considered as the baseline. All the impedance measurements for different dose concentrations of CRP were normalized to this baseline measurement. Starting from the lowest dose within the range, 150 μL of CRP spiked buffer was injected into the manifold, incubated for 15 min and impedance measurement was taken. The change in impedance from baseline measurement was calculated and converted into percentage change of impedance from baseline readings.
The second set of experiments focused on detecting CRP when aliquoted in CRP free human serum (hs). Aliquots of hs-CRP ranging from 1 fg/mL to 100 ng/mL were prepared on a logarithmic scale. After immobilization of anti-CRP onto the sensor surface, followed by blocking and washing, the zero dose, corresponding to CRP-free human serum, was injected into the manifold. EIS measurements were taken after 15 min incubation and considered as the baseline measurement. CRP-spiked human sera of different concentrations were subsequently injected onto the sensor surface and measured impedance was converted to percentage change from baseline impedance.
For finite element analysis, a portion of the microelectrode sensor was taken and electrochemical impedance analysis was carried out to obtain the Nyquist plots. First the area and the volume of the gold surface and the number of gold atoms at the surface of the electrode were calculated by using the concentration of redox couple as 10−3 mol/m3. At the initial stage, only oxidizing agents are present at the electrode surface and, since the electrode is bare, these agents easily get electrons from the gold surface and get reduced. The initial reaction rate, K0, at this stage was found to be ~2.5 × 10−4 cm/s. Utilizing the transport of diluted species module, DSP was diffused on the gold electrode and the surface concentration of DSP was calculated after 1600 s. From the concentration value obtained, it was found that the DSP monolayer occupied ~30% of the electrode surface with each molecule shielding around 8 gold atoms. According to this insight, ~50% of the gold atoms at the electrode surface are shielded, which affects the forward reaction rate coefficient, Kf, of the redox reaction and in turn reduces the K0 by another 50%, making it ~1.25 × 10−4 cm/s. In the same manner, the concentrations of CRP and anti-CRP were also calculated. Considering the size of anti-CRP and CRP, it was found that they could shield around 22 and 62 gold atoms respectively. Thus, 60% of the gold atoms would be shielded by anti-CRP and 70% by CRP reducing the K0 by 40% (1 × 10−4 cm/s of the initial value) after anti-CRP deposition and by 30% (7.5 × 10−5 cm/s) after CRP deposition. In the case of the nanofiber meshed electrode, we can see from the design that some part of the gold surface is occupied by nanofibers and it was estimated to be covering ~55% of the gold atoms. Thus, the initial value for K0 was found to be ~1.125 × 10−4 cm/s. Taking the same assumption for the number of gold atoms shielded by DSP, anti-CRP and CRP, we get the following values of K0: 0.5625 × 10−4 cm/s after DSP deposition, 0.45 × 10−4 cm/s and 0.3375 × 10−4 cm/s after anti-CRP and CRP deposition, respectively.
4. Discussion
The PVA/CNT nanofiber-embedded biosensor device exhibits an amplification of the measured electrochemical impedance signal associated with the protein binding. CRP immobilization is achieved using a standard immunoassay protocol. The transduction is chemo-electro ionic with the protein binding resulting in a modulation of the electrical double layer at the interface of the nanofibers. Charged groups are present both on the hydrophilic surface as well as on the hydrophobic residues that are interior to the protein molecule [
37]. Upon the application of voltage, the charged surface facilitates either repulsive or attractive forces on the ions at the electrode interface leading to a change in the dielectric thickness across the electrode [
34]. These changes in the dielectric thickness are directly proportional to the thickness of the electrical double layer. When the electrode surface becomes nanotextured as in this particular study, the effect of the modulation on the dielectric thickness is amplified primarily due to the segmentation of the electrical double layer.
The changes in the impedance were comparable in both the ionic buffer as well as in human serum. The minimization of high background in the human serum buffer may be attributed to the sieve-like behavior of the nanofibers that provide size matched confinement of the target proteins. Furthermore, given the nature of the substrate, being nanofibrous and porous, it turns out to be a better microenvironment for the analyte to interact. In addition, the pores within the nanofibrous substrate would provide an environment that may promote the phenomenon of macromolecular crowding that typically exists in confined spaces, thereby altering the behavior of human serum [
16,
17]. Interestingly, it was observed that the protein association is significantly enhanced at lower concentrations, which in turn enhanced the sensitivity of the biosensor device.
The dimensions of the composite nanofibers are ~100 µm in length and ~250 nm in diameter. The nanofibers make a good biochemical transducer due to the presence of CNT and are highly suitable for achieving electrical signal amplification associated with the detection of the biomolecules. The resulting nanostructure comprises a non-periodic array of nanoscale confined spaces, which are electrically connected through the metallic micro electrode. The nanofibers have been surface functionalized with a protocol similar to the enzyme linked immunosorbent assay.
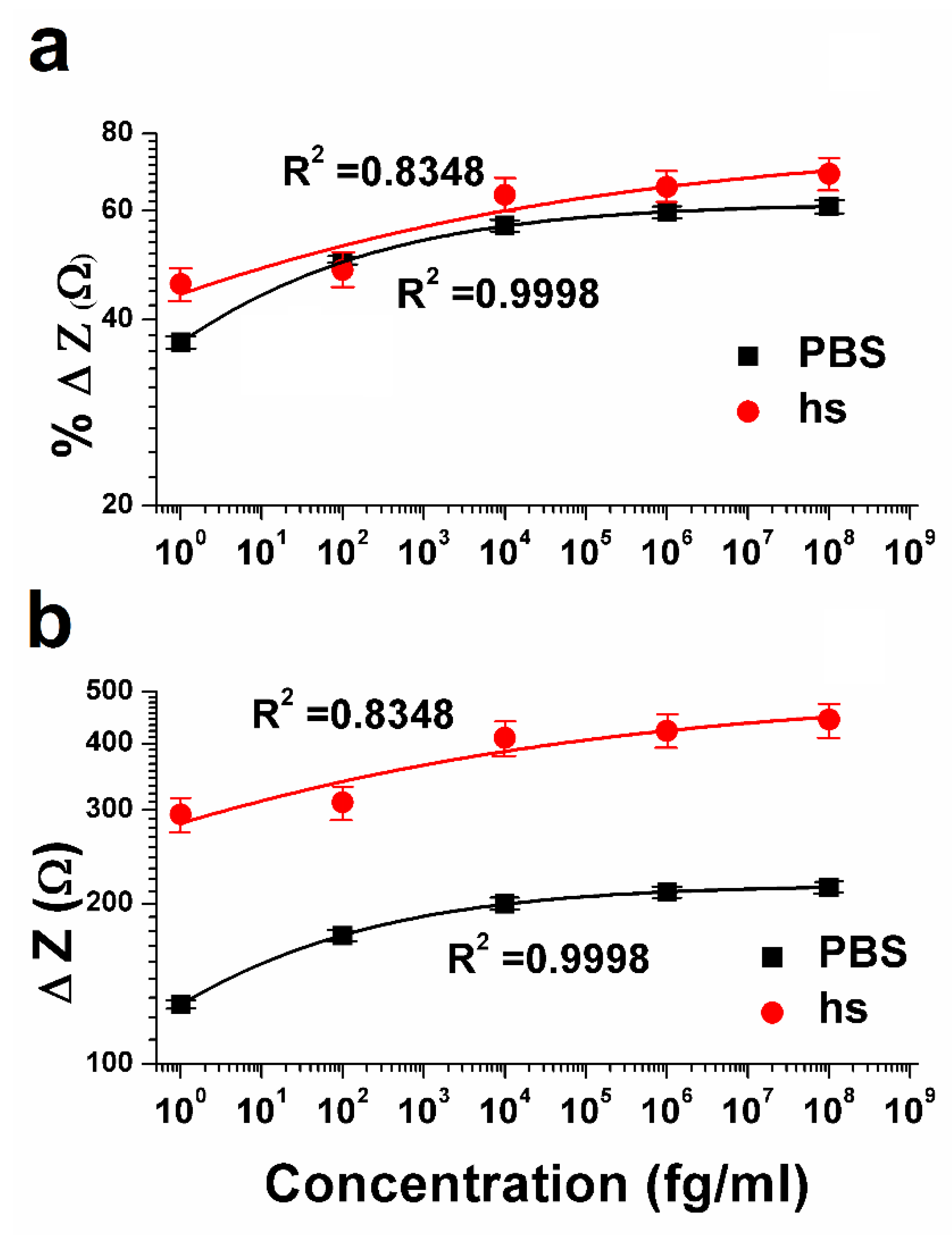
The most important characteristic of a biosensor is the calibration dependence of the impedance signal (Z) on the concentration of the analyte (C) [
38]. The behavior of the calibration curve can be understood using a suitable mathematical equation such as the Hill isotherm model [
39], which is used to describe the binding of different species onto homogenous substrates to fit the experimental data. According to this model, the antigen binding ability at one site on the macromolecule may influence different binding sites on the same macromolecule and the adsorption process is a cooperative phenomenon. As seen in
Figure 4, the biosensor response in the form of percent change in impedance (%Δ Z) and difference impedance (ΔZ), are closely fitted with the following Hill formula:
where
is the biosensor response (signal Z),
is the maximum state of the reaction reached at concentration
,
is the antigen concentration that binds the receptor sites at half concentration and
is the Hill coefficient.
For
Figure 4, the values of
(0.2221 ± 0.00374 (PBS) and 0.1050 ± 0.0862 (hs)) are calculated based on Equation (1). This low value of Hill’s coefficient at all the concentrations is a measure of the variable free energy of the interactions between biomolecules and surfaces [
40,
41,
42]. In both PBS and hs, Hill’s slope is less than 1, which is an indication of negative cooperative binding, which means that although many binding sites may be present, only specific binding occurs at a given point of time [
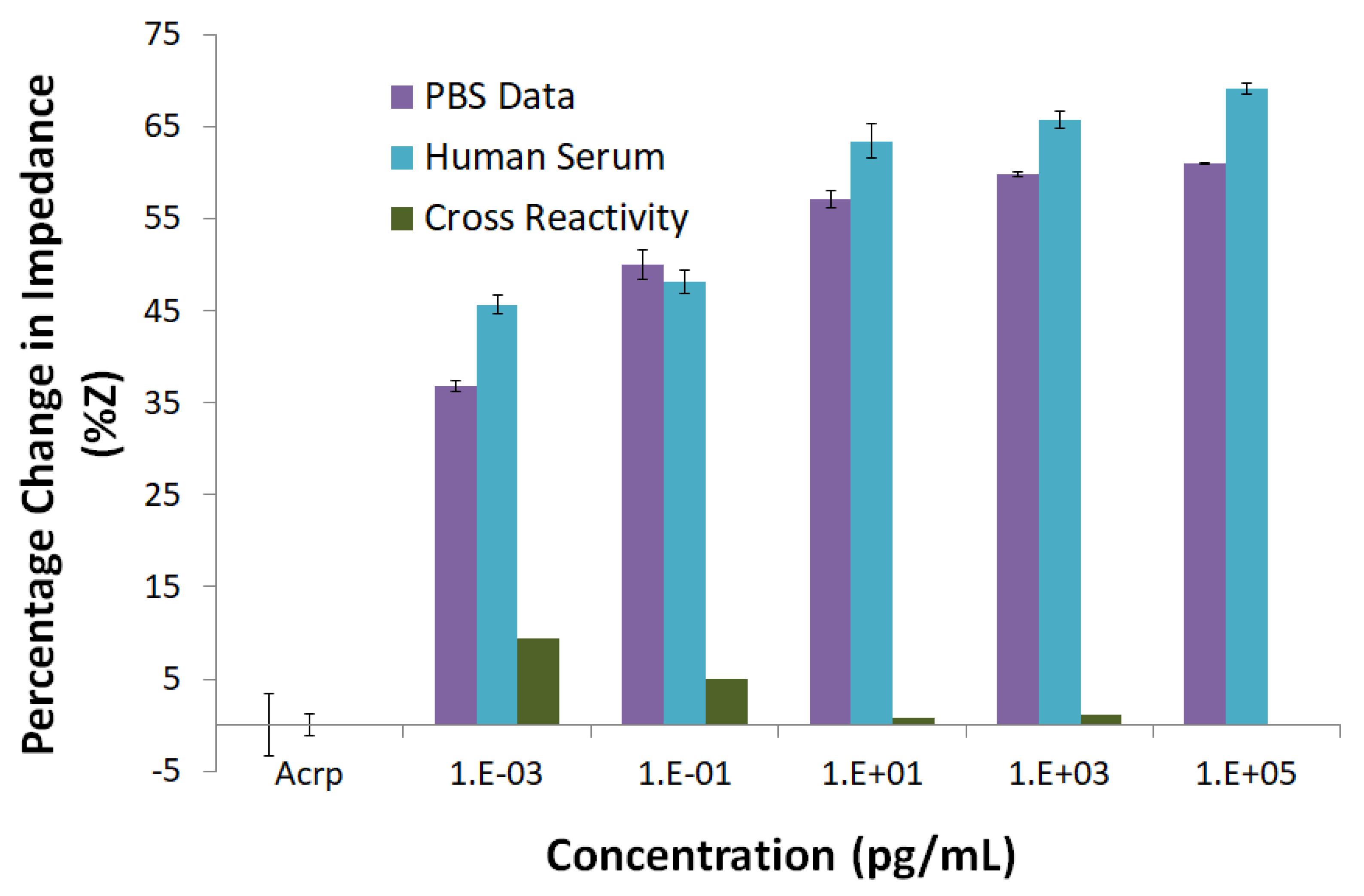
43]. This observation is also supported by the cross-reactivity study, where at 1 fg mL
−1 concentration of CRP, the percentage change in the impedance is less than 10%. The values of
are 0.1744 ± 0.0089 fg mL
−1 (PBS) and 0.0708 ± 0.2357 fg mL
−1 (hs), respectively.