Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato
Abstract
1. Introduction
1.1. Emerging Needs in Phytodiagnostics
1.2. New Technological Solutions in Phytodiagnostics and POC Testing
2. Materials and Methods
2.1. Bacterial Samples
2.2. ELISA and PCR Analysis
2.3. Lab-On-Chip Platform
- (1)
- a mixed self-assembled monolayer (SAM) of beta-mercaptoethanol and mercaptoundecanoic acid obtained by overnight incubation in ethanol solution;
- (2)
- the activation of the COOH groups of 11-mercaptoundecanoic acid (MUA) by incubation for 30 min in a solution of N-hydroxysuccinimide and N-ethyl-N-3-dimethylaminopropyl carbodiimide hydrochloride at a ratio of 1:4 in milliQ water;
- (3)
- a protein A monolayer assembled by covalent binding to the mixed SAM by a 2 h incubation in a (50 μg/mL) phosphate buffered saline (PBS) solution;
- (4)
- the disactivation of ester reactive groups by incubation in ethanolamine (1 M);
- (5)
- the passivation of the unbound surface by incubation in bovine serum albumin (BSA) (1 mg/mL);
- (6)
- the oriented immobilization of the antibodies specific for P. atrosepticum (5 ng/mL) by binding to protein A through their Fc region (fragment crystallizable region).
3. Results and Discussion
3.1. ELISA and PCR Assays
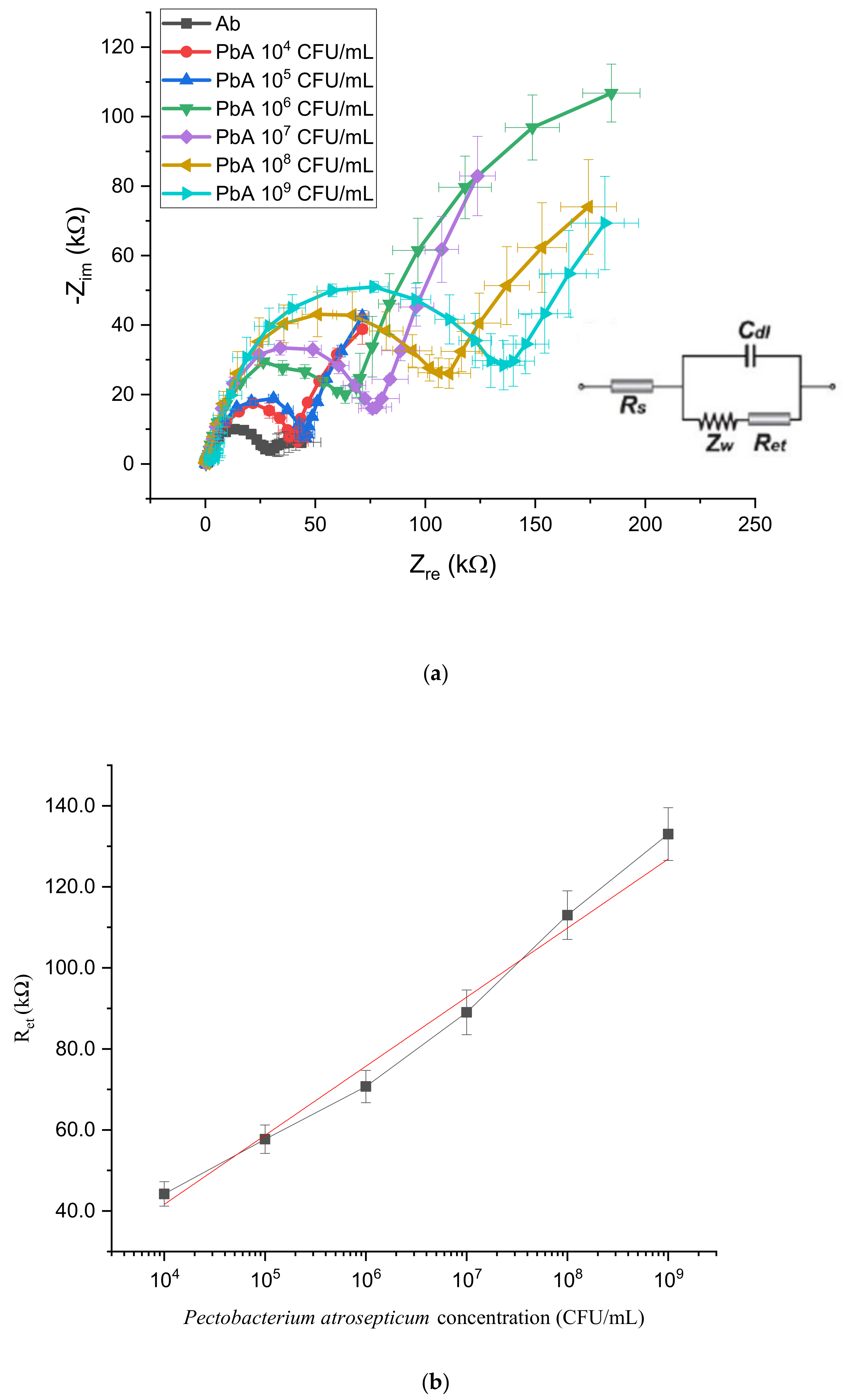
3.2. LOC Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassedy, A.; Mullins, E.; O’Kennedy, R. Sowing seeds for the future: The need for on-site plant diagnostics. Biotechnol. Adv. 2020, 39, 107358. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hibbing, M.E.; Kim, H.-S.; Reedy, R.M.; Yedidia, I.; Breuer, J.; Breuer, J.; Glasner, J.D.; Perna, N.T.; Kelman, A.; et al. Host Range and Molecular Phylogenies of the Soft Rot Enterobacterial Genera Pectobacterium and Dickeya. Phytopathology 2007, 97, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Pérombelon, M.C.M. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Hauben, L.; Moore, E.R.B.; Vauterin, L.; Steenackers, M.; Mergaert, J.; Verdonck, L.; Swings, J. Phylogenetic Position of Phytopathogens within the Enterobacteriaceae. Syst. Appl. Microbiol. 1998, 21, 384–397. [Google Scholar] [CrossRef]
- Czajkowski, R.L.; Grabe, G.J.; Wolf, J.M.v.d. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp carotovorum in naturally infected seed potatoes. Eur. J. Plant Pathol. 2009, 125, 263–275. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.; Jafra, S.; Lojkowska, E.; Potrykus, M.; van der Wolf, J.; Sledz, W. Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. Ann. Appl. Biol. 2015, 166, 18–38. [Google Scholar] [CrossRef]
- Humphris, S.N.; Cahill, G.; Elphinstone, J.G.; Kelly, R.; Parkinson, N.M.; Pritchard, L.; Toth, I.K.; Saddler, G.S. Detection of the Bacterial Potato Pathogens Pectobacterium and Dickeya spp. Using Conventional and Real-Time PCR. In Plant Pathology: Techniques and Protocols; Lacomme, C., Ed.; Springer: New York, NY, USA, 2015; pp. 1–16. [Google Scholar] [CrossRef]
- Alvarez, A.M. Integrated Approaches for Detection of Plant Pathogenic Bacteria and Diagnosis of Bacterial Diseases. Annu. Rev. Phytopathol. 2004, 42, 339–366. [Google Scholar] [CrossRef]
- Charlermroj, R.; Himananto, O.; Seepiban, C.; Kumpoosiri, M.; Warin, N.; Gajanandana, O.; Elliott, C.T.; Karoonuthaisiri, N. Antibody Array in a Multiwell Plate Format for the Sensitive and Multiplexed Detection of Important Plant Pathogens. Anal. Chem. 2014, 86, 7049–7056. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, M.S.; Primiceri, E.; De Feo, F.; Montanaro, A.; Monteduro, A.G.; Tinelli, A.; Megha, M.; Carati, D.; Maruccio, G. Simultaneous detection of multiple lower genital tract pathogens by an impedimetric immunochip. Biosens. Bioelectron. 2016, 79, 9–14. [Google Scholar] [CrossRef]
- Templier, V.; Livache, T.; Boisset, S.; Maurin, M.; Slimani, S.; Mathey, R.; Roupioz, Y. Biochips for Direct Detection and Identification of Bacteria in Blood Culture-Like Conditions. Sci. Rep. 2017, 7, 9457. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Chiriacò, M.S.; Primiceri, E.; Srivastava, D.N.; Maruccio, G. Picomolar detection of retinol binding protein 4 for early management of type II diabetes. Biosens. Bioelectron. 2019, 128, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, A.S. Future of portable devices for plant pathogen diagnosis. Lab Chip 2014, 14, 2887–2904. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, M.S.; Luvisi, A.; Primiceri, E.; Sabella, E.; De Bellis, L.; Maruccio, G. Development of a lab-on-a-chip method for rapid assay of Xylella fastidiosa subsp pauca strain CoDiRO. Sci. Rep. 2018, 8, 7376. [Google Scholar] [CrossRef] [PubMed]
- Julich, S.; Riedel, M.; Kielpinski, M.; Urban, M.; Kretschmer, R.; Wagner, S.; Fritzsche, W.; Henkel, T.; Möller, R.; Werres, S. Development of a lab-on-a-chip device for diagnosis of plant pathogens. Biosens. Bioelectron. 2011, 26, 4070–4075. [Google Scholar] [CrossRef] [PubMed]
- Boer, S.H.D.; López, M.M. New Grower-Friendly Methods for Plant Pathogen Monitoring. Annu. Rev. Phytopathol. 2012, 50, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.; Lombardo, A.; Raspagliesi, D.; Russo, M.; Catara, A.; Licciardello, G. Development and Evaluation of a Novel Probe Microarray for Genotyping Citrus tristeza virus Using an Integrated Lab-on-Chip Device. J. Plant Pathol. 2016, 98, 25–34. [Google Scholar]
- Li, X.A.; Deboer, S.H. Selection of Polymerase Chain-Reaction Primers from an Rna Intergenic Spacer Region for Specific Detection of Clavibacter-michiganensis subsp sepedonicus. Phytopathology 1995, 85, 837–842. [Google Scholar] [CrossRef]
- Deboer, S.H.; Ward, L.J. PCR detection Of Erwinia-carotovora subsp atroseptica Associated with Potato Tissue. Phytopathology 1995, 85, 854–858. [Google Scholar] [CrossRef]
- Frechon, D.; Exbrayat, P.; Helias, V.; Hyman, L.J.; Jouan, B.; Llop, P.; Lopez, M.M.; Payet, N.; Perombelon, M.C.M.; Toth, I.K.; et al. Evaluation of a PCR kit for the detection of Erwinia carotovora subsp. atroseptica on potato tubers. Potato Res. 1998, 41, 163–173. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Primiceri, E.; Monteduro, A.G.; Bove, A.; Leporatti, S.; Capello, M.; Ferri-Borgogno, S.; Rinaldi, R.; Novelli, F.; Maruccio, G. Towards pancreatic cancer diagnosis using EIS biochips. Lab Chip 2013, 13, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Primiceri, E.; Chiriacò, M.S.; D’Amone, E.; Urso, E.; Ionescu, R.E.; Rizzello, A.; Maffia, M.; Cingolani, R.; Rinaldi, R.; Maruccio, G. Real-time monitoring of copper ions-induced cytotoxicity by EIS cell chips. Biosens. Bioelectron. 2010, 25, 2711–2716. [Google Scholar] [CrossRef]
- Safenkova, I.V.; Pankratova, G.K.; Zaitsev, I.A.; Varitsev, Y.A.; Vengerov, Y.Y.; Zherdev, A.V.; Dzantiev, B.B. Multiarray on a test strip (MATS): Rapid multiplex immunodetection of priority potato pathogens. Anal. Bioanal. Chem. 2016, 408, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Safenkova, I.V.; Zaitsev, I.A.; Varitsev, Y.A.; Byzova, N.A.; Drenova, N.V.; Zherdev, A.V.; Dzantiev, B.B. Development of a lateral flow immunoassay for rapid diagnosis of potato blackleg caused by Dickeya species. Anal. Bioanal. Chem. 2017, 409, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Skandalis, N.; Dimopoulou, A.; Glynos, P.; Gizeli, E. Bacteria Murmur: Application of an Acoustic Biosensor for Plant Pathogen Detection. PLoS ONE 2015, 10, e0132773. [Google Scholar] [CrossRef]
- Yazgan, I.; Noah, N.; Toure, O.; Zhang, S.; Sadik, O. Biosensor for selective detection of E-coli in spinach using the strong affinity of derivatized mannose with fimbrial lectin. Biosens. Bioelectron. 2014, 61C, 266–273. [Google Scholar] [CrossRef]
- Zikria, Y.B.; Afzal, M.K.; Kim, S.W. Internet of Multimedia Things (IoMT): Opportunities, Challenges and Solutions. Sensors 2020, 20, 2334. [Google Scholar] [CrossRef]
- Farooq, M.S.; Riaz, S.; Abid, A.; Umer, T.; Zikria, Y.B. Role of IoT Technology in Agriculture: A Systematic Literature Review. Electronics 2020, 9, 319. [Google Scholar] [CrossRef]



| CFU/mL | ELISA | ||
|---|---|---|---|
| OD | R | Result | |
| 109 | 2 | 33 | + |
| 108 | 1.8 | 30 | + |
| 107 | 1.12 | 18.67 | + |
| 106 | 0.21 | 3.5 | + |
| 105 | 0.16 | 2.6 | + |
| 104 | 0.088 | 1.47 | - |
| 103 | 0.063 | 1.05 | - |
| 102 | 0.034 | 0.56 | - |
| 101 | 0.015 | 0.25 | - |
| Control | 0.06 | 1 | - |
| Technique | Limit of Detection |
|---|---|
| ELISA | 105 CFU/mL |
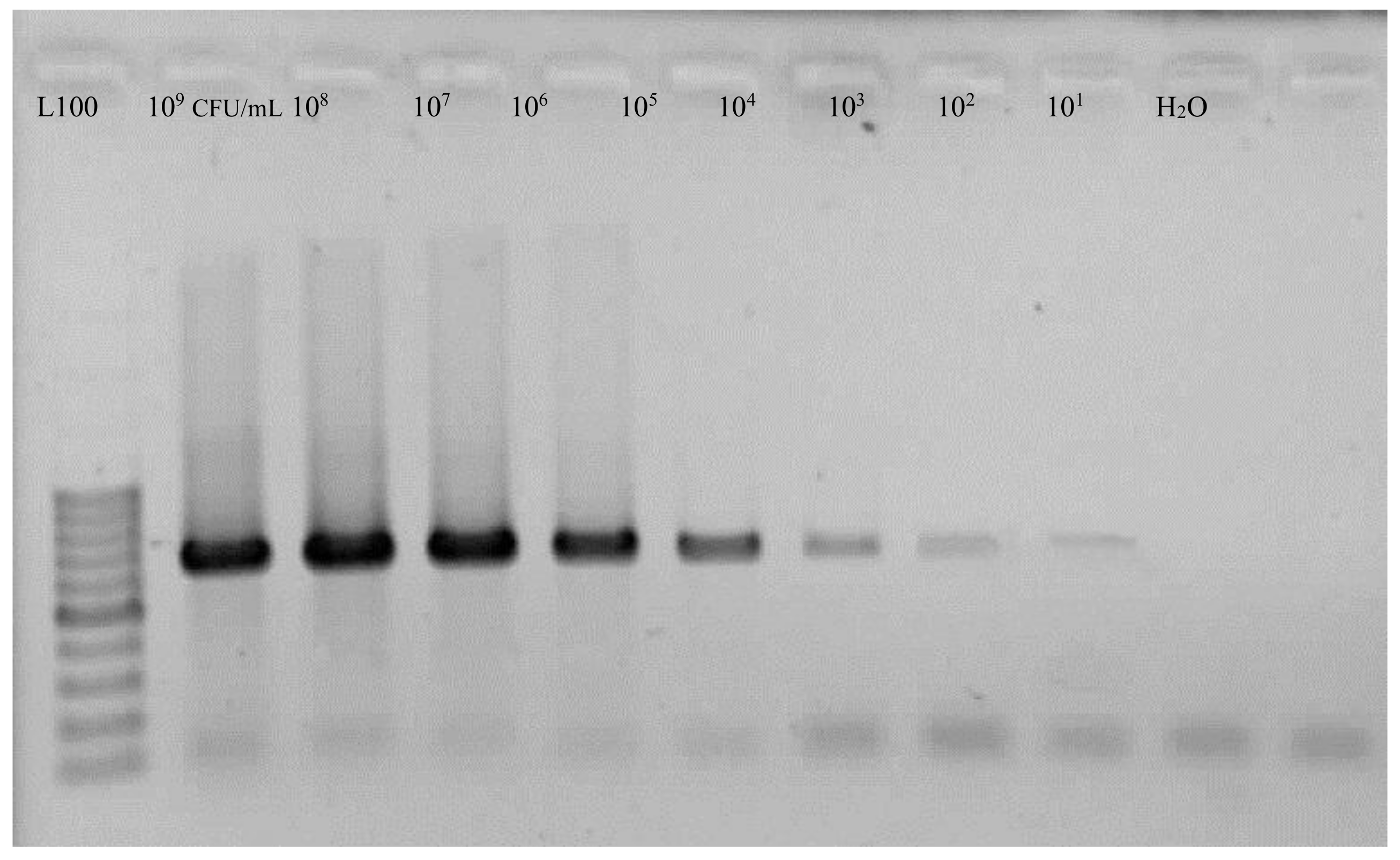
| PCR | 102 CFU/mL |
| LOC | 104 CFU/mL |
| Detection Technique | Bioreceptor | Plant | Bacteria | LOD | Ref. |
|---|---|---|---|---|---|
| Lateral Flow Immunoassay | Ab | Potato | Dickeya dianthicola and Dickeya solani | 4 × 105 CFU/mL | Safenkova et al. [25] |
| Quartz Crystal Microbance | DNA | tomato | Ralstonia solanacearum, Pseudomonas syringae pv. tomato, Xanthomonas campestris pv. vesicatoria | 103–104 CFU/mL | Papadakis et al. [26] |
| Cyclic Voltammetry | Modified carbohydrate ligand | Spinach | E. coli | 6.25 × 102 CFU/mL | Yazgan et al. [27] |
| Surface Plasmon resonance | Modified carbohydrate ligand | Spinach | E. coli | 1–104 CFU/mL | Yazgan et al. [27] |
| Lateral Flow immunoassay in multiarray on a test strip (MATS) format | Ab | Potato | Clavibacter michiganensis | 104 CFU/mL | Safenkova et al. [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi Tameh, M.; Primiceri, E.; Chiriacò, M.S.; Poltronieri, P.; Bahar, M.; Maruccio, G. Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors 2020, 10, 64. https://doi.org/10.3390/bios10060064
Hashemi Tameh M, Primiceri E, Chiriacò MS, Poltronieri P, Bahar M, Maruccio G. Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors. 2020; 10(6):64. https://doi.org/10.3390/bios10060064
Chicago/Turabian StyleHashemi Tameh, Mahdis, Elisabetta Primiceri, Maria Serena Chiriacò, Palmiro Poltronieri, Masoud Bahar, and Giuseppe Maruccio. 2020. "Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato" Biosensors 10, no. 6: 64. https://doi.org/10.3390/bios10060064
APA StyleHashemi Tameh, M., Primiceri, E., Chiriacò, M. S., Poltronieri, P., Bahar, M., & Maruccio, G. (2020). Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors, 10(6), 64. https://doi.org/10.3390/bios10060064







