Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents
Abstract
:1. Introduction
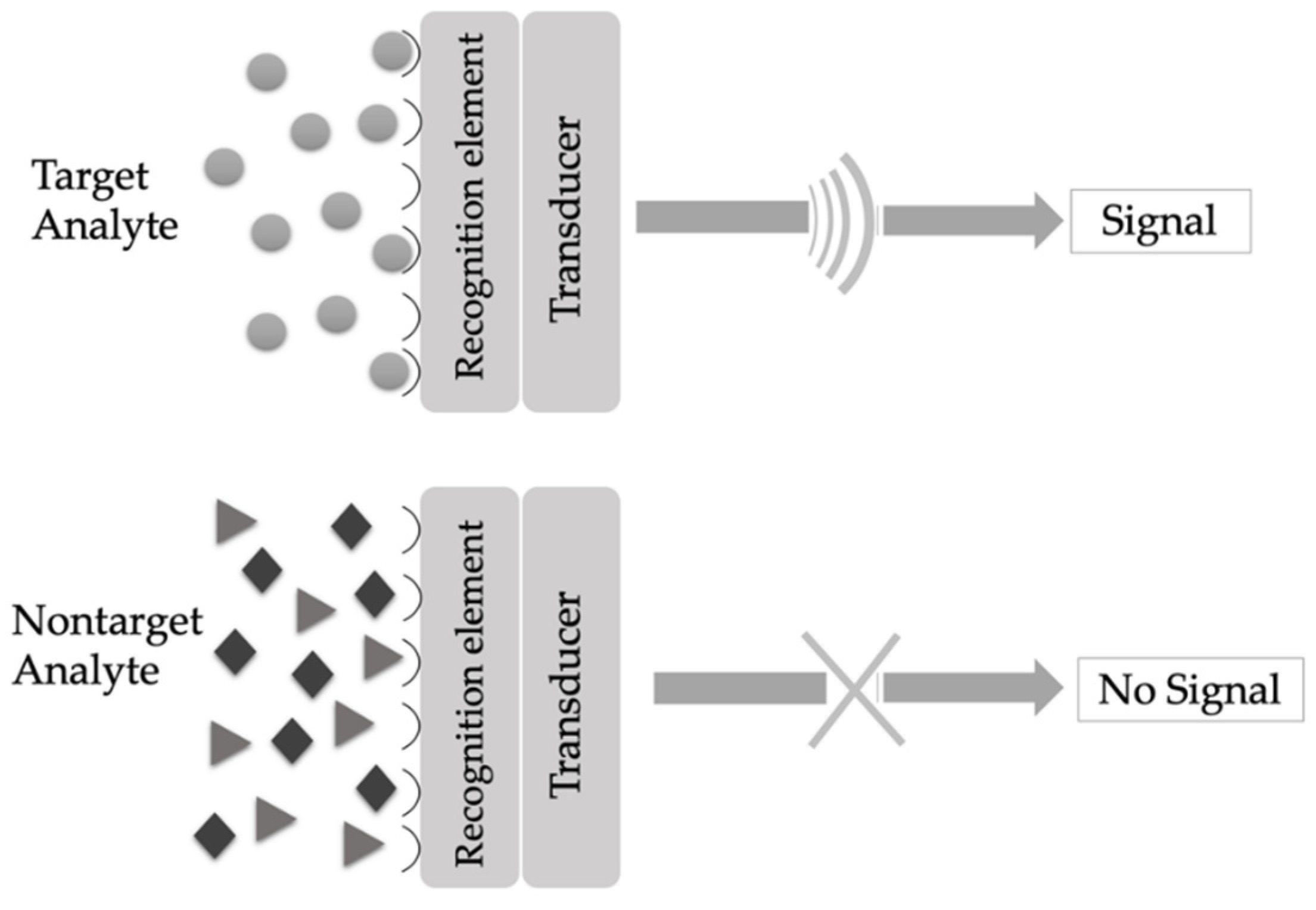
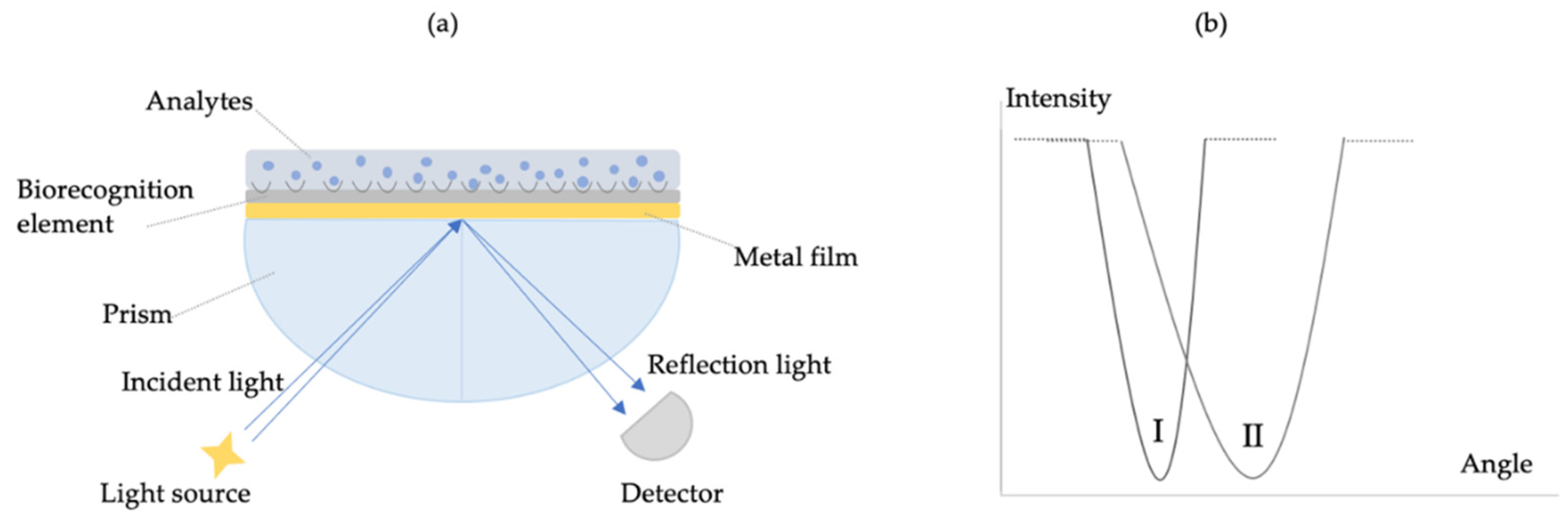
2. Principle of Sensors
3. Monitoring Biological and Chemical Threats Agents
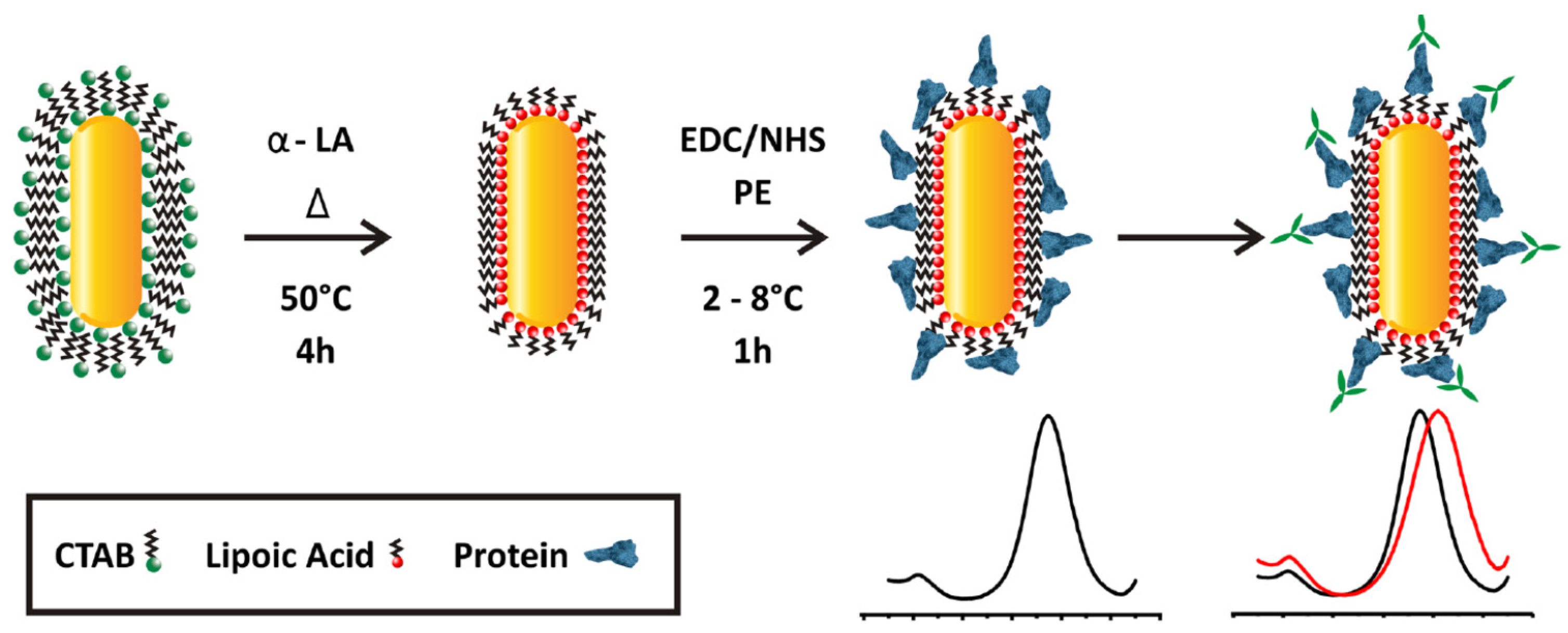
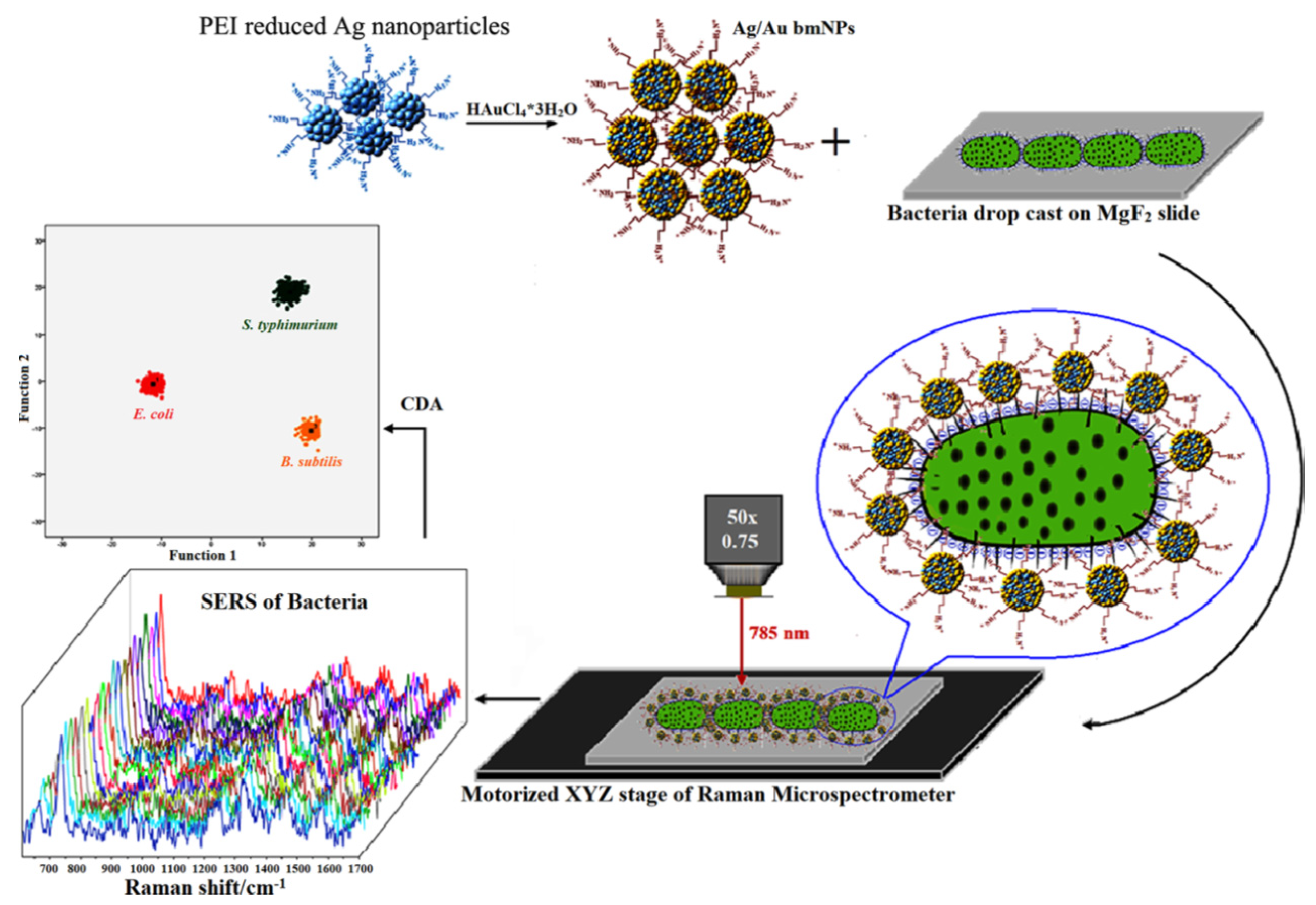
3.1. Biological Threats Agents
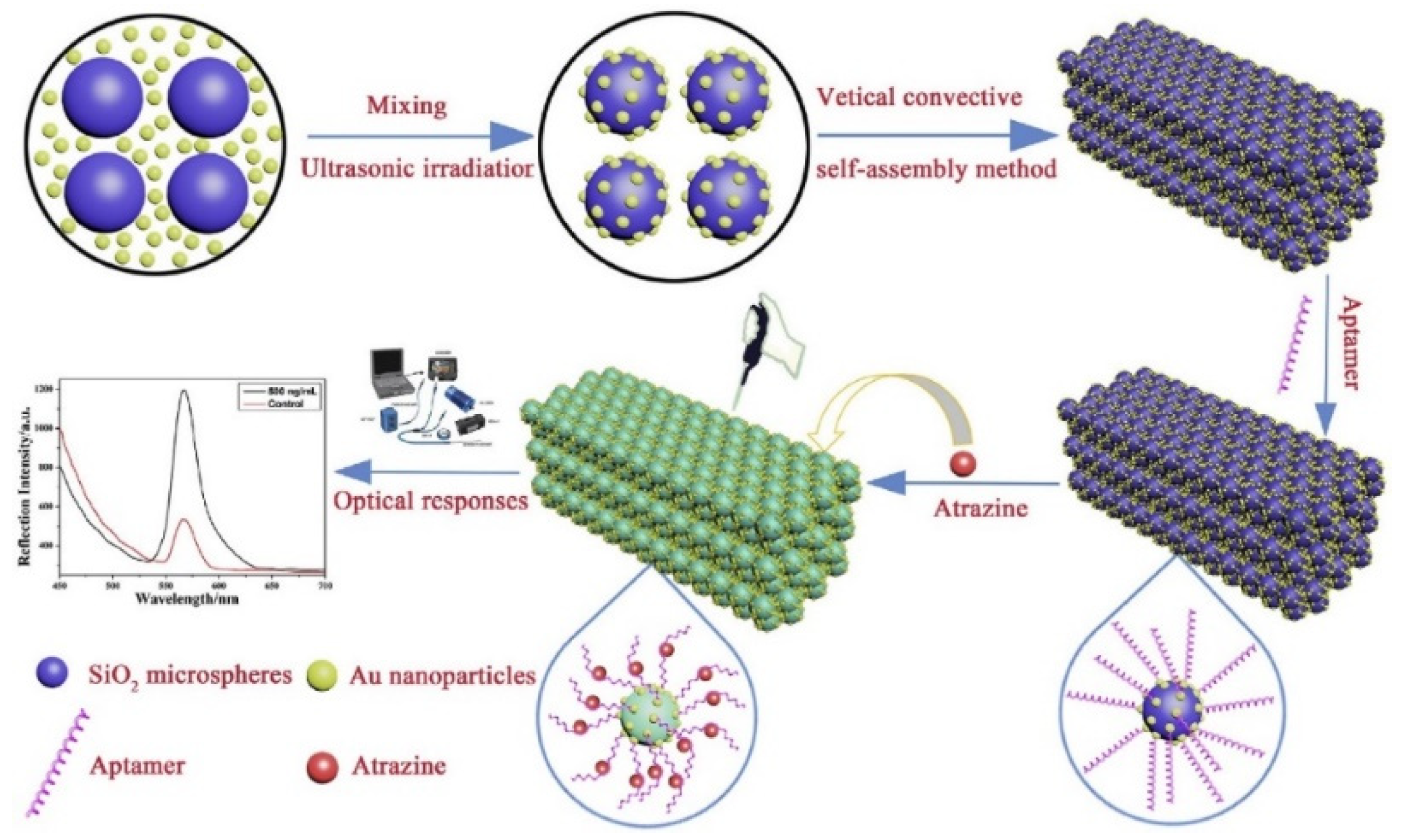
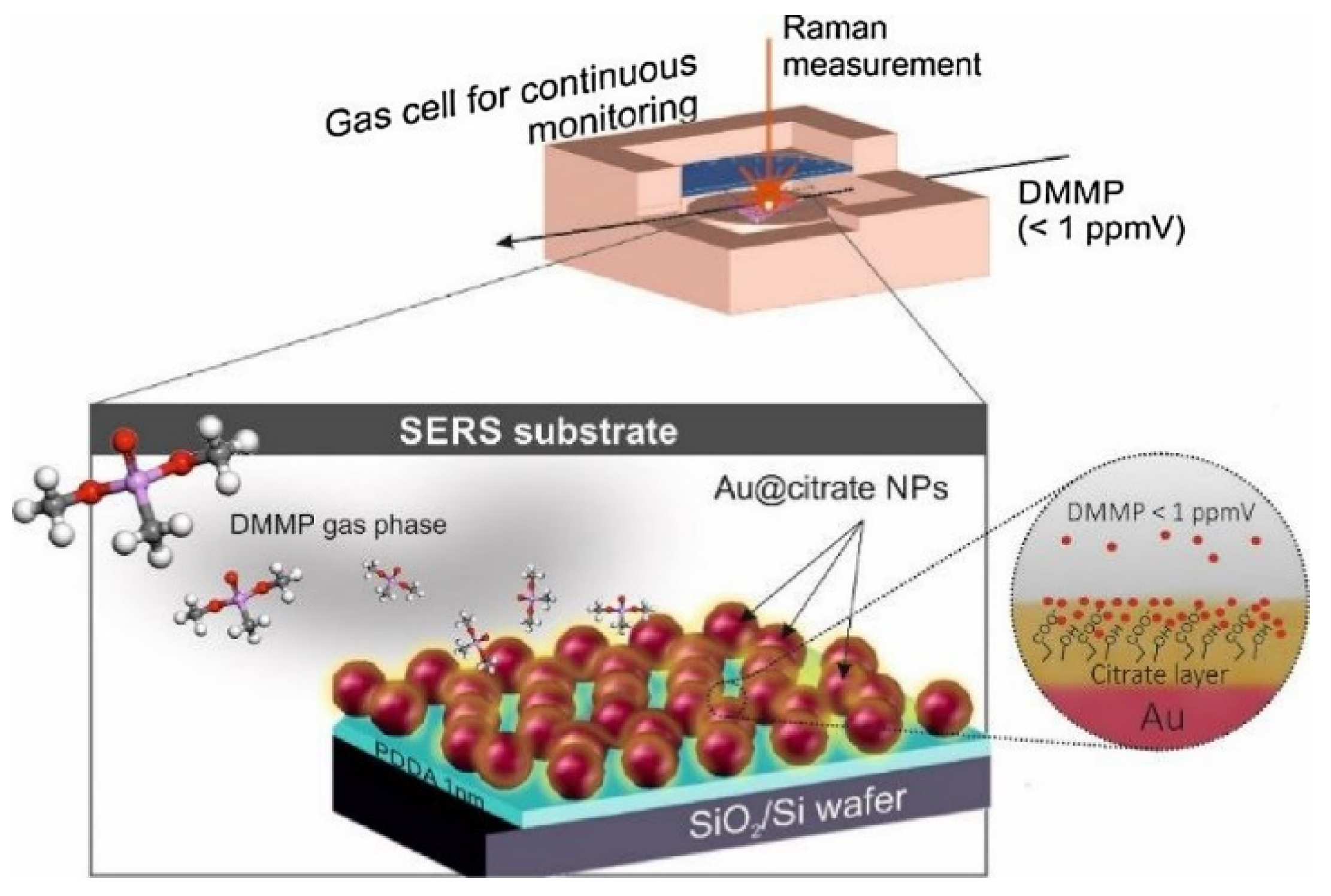
3.2. Chemical Threat Agents
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hakonen, A.; Andersson, P.O.; Schmidt, M.S.; Rindzevicius, T.; Kall, M. Explosive and chemical threat detection by surface-enhanced Raman scattering: A review. Anal. Chim. Acta 2015, 893, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Organisation for the Prohibition of Chemical Weapons. Available online: https://www.nobelprize.org/prizes/peace/2013/opcw/facts/ (accessed on 27 August 2020).
- Green, M.S.; LeDuc, M.; Cohen, D.; Rfranz, D. Confronting the threat of bioterrorism: Realities, challenges, and defensive strategies. Lancet Infect. Dis. 2019, 19, e2–e13. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733–8768. [Google Scholar] [CrossRef] [PubMed]
- Anzai, J.I. Chapter 62: Biosensors for the Detection of OP Nerve Agents. In Handbook of Toxicology of Chemical Warfare Agents, 2nd ed.; Elsevier: London, UK, 2015; pp. 925–934. ISBN 978-0-12-800159-2. [Google Scholar]
- Pohanka, M.; Skládal, M.; Kroèa, M. Biosensors for biological warfare agent detection. Def. Sci. J. 2007, 57, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Walper, S.A.; Aragonés, G.L.; Sapsford, K.E.; Brown, C.W., III; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting biothreat agents: From current diagnostics to developing sensor technologies. ACS Sens. 2018, 3, 1894–2024. [Google Scholar] [CrossRef] [Green Version]
- Sapsford, K.E.; Bradburne, C.; Delehanty, J.B.; Medintz, I.L. Sensors for detecting biological agents. Mater. Today 2008, 11, 38–49. [Google Scholar] [CrossRef]
- Mondal, B.; Bhavanashri, N.; Mounika, S.P.; Tuteja, D.; Tandi, K.; Soniya, H. Chapter 6: Microfluidics application for detection of biological warfare agents. In Handbook on Biological Warfare Preparedness; Academic Press: Cambridge, MA, USA, 2020; pp. 103–131. ISBN 978-0-12-812026-2. [Google Scholar]
- Courtney, B.; Bond, K.C.; Maher, C. Regulatory underpinnings of global health security: FDA’s roles in preventing, detecting, and responding to global health threats. Biosecur. Bioterror. 2014, 12, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B.Ö.; Şehitoğlu, S.K. Pyrene substituted amphiphilic ROMP polymers as nano-sized fluorescence sensors for detection of TNT in water. Polymer 2019, 183, 121868. [Google Scholar] [CrossRef]
- Guo, C.X.; Lei, Y.; Li, C.M. Porphyrin functionalized graphene for sensitive electrochemical detection of ultratrace explosives. Electroanalysis 2011, 23, 885–893. [Google Scholar] [CrossRef]
- Douglas, T.A.; Walsh, M.E.; Weiss, C.A., Jr.; McGrath, C.J.; Trainor, T.P. Desorption and transformation of nitroaromatic (TNT) and nitramine (RDX and HMX) explosive residues on detonated pure mineral phases. Water Air Soil Pollut. 2012, 223, 2189–2200. [Google Scholar] [CrossRef]
- Xin, Y.H.; Wang, Q.; Liu, T.; Wang, L.; Li, J.; Fang, Y. A portable and autonomous multichannel fluorescence detector for on-line and in situ explosive detection in aqueous phase. Lab Chip 2012, 12, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, M.; D’Agostino, G.; Alberti, G.; Biesuz, R.; Merli, D. Voltammetric platform for detection of 2,4,6- trinitrotoluene based on a molecularly imprinted polymer. Anal. Bioanal. Chem. 2013, 405, 3559–3570. [Google Scholar] [CrossRef]
- Leppert, J.; Horner, G.; Rietz, F.; Ringer, J.; Lammers, P.S.; Boeker, P. Near real time detection of hazardous airborne substances. Talanta 2012, 101, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kartha, K.K.; Sandeep, A.; Nair, V.C.; Takeuchi, M.; Ajayaghosh, A. A carbazole-fluorene molecular hybrid for quantitative detection of TNT using a combined fluorescence and quartz crystal microbalance method. Phys. Chem. Chem. Phys. 2014, 16, 18896–18901. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Tsay, O.G.; Murale, D.P.; Jeong, J.A.; Segev, A.; Churchill, D.G. Novel and selective detection of Tabun mimics. Chem. Commun. 2014, 50, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- de Grenu, B.D.; Moreno, D.; Torroba, T.; Berg, A.; Gunnars, J.; Nilsson, T.; Nyman, R.; Persson, M.; Pettersson, J.; Eklind, I.; et al. Fluorescent discrimination between traces of chemical warfare agents and their mimics. J. Am. Chem. Soc. 2014, 136, 4125–4128. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, N.M.; Linnes, J.C.; Fan, A.; Ellenson, C.K.; Pollock, N.R.; Klapperich, C.M. Paper-based RNA extraction, in situ isothermal amplification, and lateral flow detection for low cost, rapid diagnosis of influenza A (H1N1) from clinical specimens. Anal. Chem. 2015, 87, 7872–7879. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.P.; Reddy, P.M. Identification of pathogens by mass spectrometry. Clin. Chem. 2010, 56, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Franciosa, G.; Pourshaban, M.; De Luca, A.; Buccino, A.; Dallapiccola, B.; Aureli, P. Identification of type A, B, E, and F botulinum neurotoxin genes and of botulinum neurotoxigenic clostridia by denaturing high-performance liquid chromatography. Appl. Environ. Microbiol. 2004, 70, 4170–4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, N.O.; Tarasow, T.M.; Tok, J.B. Aptasensors for biosecurity applications. Curr. Opin. Chem. Biol. 2007, 11, 316–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saylan, Y.; Yılmaz, F.; Özgür, E.; Derazshamshir, A.; Bereli, N.; Yavuz, H.; Denizli, A. Chapter 10: Surface Plasmon Resonance Sensors For Medical Diagnosis. In Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 425–458. ISBN 978-3-662-56332-8. [Google Scholar]
- Ferrier, D.C.; Shaver, M.P.; Hands, P.J. Micro-and nanostructure based oligonucleotide sensors. Biosens. Bioelectron. 2015, 68, 798–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkovic, K.; Swallow, A.; Stewart, R.; Gao, Y.; Li, S.; Glenn, F.; Gotama, J.; Dell’Olio, M.; Best, M.; Doward, J.; et al. An integrated portable multiplex microchip device for fingerprinting chemical warfare agents. Micromachines 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.; Yim, C.; Kwon, D.; Lee, J.; Shin, H.H.; Cha, H.J.; Jeon, S. A facile and sensitive detection of pathogenic bacteria using magnetic nanoparticles and optical nanocrystal probes. Analyst 2012, 137, 3609–3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhao, Y.; Zhang, Z.; Zhang, P.; Li, J.; Yang, R.; Yang, C.; Zhou, L. A fiber optic biosensor for specific identification of dead Escherichia coli O157: H7. Sens. Actuators B Chem. 2014, 196, 161–167. [Google Scholar] [CrossRef]
- Mukundan, H.; Kumar, S.; Price, D.N.; Ray, S.M.; Lee, Y.J.; Min, S.; Eum, S.; Kubicek-Sutherland, J.; Resnick, J.M.; Grace, W.K.; et al. Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a waveguide-based optical biosensor. Tuberculosis 2012, 92, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Ohk, S.H.; Bhunia, A.K. Multiplex fiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157: H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol. 2013, 33, 166–171. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef]
- Al-Rekabi, S.H.; Kamil, Y.M.; Bakar, M.H.A.; Fen, Y.W.; Lim, H.N.; Kanagesan, S. Hydrous ferric oxide-magnetite-reduced graphene oxide nanocomposite for optical detection of arsenic using surface plasmon resonance. Opt. Laser Technol. 2019, 111, 417–423. [Google Scholar] [CrossRef]
- Cittadini, M.; Bersani, M.; Perrozzi, F.; Ottaviano, L.; Wlodarski, W.; Martucci, A. Graphene oxide coupled with gold nanoparticles for localized surface plasmon resonance based gas sensor. Carbon N. Y. 2013, 69, 452–459. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet-printed paper-based SERS dipsticks and swabs for trace chemical detection. Analyst 2013, 138, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Rahtuvanoğlu, A.; Akgönüllü, S.; Karacan, S.; Denizli, A. Biomimetic nanoparticles based surface plasmon resonance biosensors for histamine detection in foods. ChemistrySelect 2020, 5, 5683–5692. [Google Scholar] [CrossRef]
- Yılmaz, E.; Özgür, E.; Bereli, N.; Türkmen, D.; Denizli, A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater. Sci. Eng. C 2017, 73, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, C.; Wang, X.; Wang, L.; Chen, A.; Hu, J.; Luo, Z.; Wang, S.; Gambhir, S.S.; Weiss, S. Fabrication of an “ion-imprinting” dual-emission quantum dot nanohybrid for selective fluorescence turn-on and ratiometric detection of cadmium ions. Analyst 2016, 141, 5886–5892. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Ünal, S.; Denizli, A. An alternative medical diagnosis method: Biosensors for virus detection. Biosensors 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Inci, F.; Karaaslan, M.G.; Mataji-Kojouri, A.; Shah, P.A.; Saylan, Y.; Zeng, Y.; Avadhani, A.; Sinclair, R.; Lau, D.T.L.; Demirci, U. Enhancing the nanoplasmonic signal by a nanoparticle sandwiching strategy to detect viruses. Appl. Mater. Today 2020, 20, 100709. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and sensing systems for rapid analysis of phenolic compounds from plants: A comprehensive review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Özgür, E.; Saylan, Y.; Bereli, N.; Türkmen, D.; Denizli, A. Molecularly imprinted polymer integrated plasmonic nanosensor for cocaine detection. J. Biomater. Sci. Polym. Ed. 2020, 31, 1211–1222. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Molecularly imprinted polymers for capturing and sensing proteins: Current progress and future implications. Trends Anal. Chem. 2019, 114, 29–47. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Inci, F.; Denizli, A. Advances in biomimetic systems for molecular recognition and biosensing. Biomimetics 2020, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Denizli, A. Chapter 33: Virus Detection Using Nanosensors. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 501–511. ISBN 978-0-12-819870-4. [Google Scholar]
- Saylan, Y.; Erdem, Ö.; Yılmaz, F.; Cihangir, N.; Denizli, A. Chapter 3: Sensor Application For Environmental Pollutants. In Affinity Sensors; Hacettepe Journal of Biology and Chemistry: Ankara, Turkey, 2018; pp. 53–77. ISBN 978-975-491-466-5. [Google Scholar]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.; Chen, Z.; Xu, D.; Jiang, L.; Chen, X.; Yi, Z.; Wu, P.; Li, G.; Yi, Y. High quality factor, high sensitivity metamaterial graphene—Perfect absorber based on critical coupling theory and ımpedance matching. Nanomaterials 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Masson, J.F. Portable and field-deployed surface plasmon resonance and plasmonic sensor. Analyst 2020, 145, 3776–3800. [Google Scholar] [CrossRef]
- Zhan, C.; Liu, B.W.; Tian, Z.Q.; Ren, B. Determining the interfacial refractive index via ultrasensitive plasmonic sensors. J. Am. Chem. Soc. 2020, 142, 10905–10909. [Google Scholar] [CrossRef]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu+2 in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Demirel, G.; Gieseking, R.L.M.; Ozdemir, R.; Kahmann, S.; Loi, M.A.; Schatz, G.C.; Facchetti, A.; Usta, H. Molecular engineering of organic semiconductors enables noble metal-comparable SERS enhancement and sensitivity. Nat. Commun. 2019, 10, 5502. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Liu, W.; Fu, L.; Wang, Y.; Li, J.; Chen, L. Molecular imprinting based hybrid ratiometric fluorescence sensor for the visual determination of bovine hemoglobin. ACS Sens. 2018, 3, 378–385. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M. Chapter 3: Surface plasmon resonance instruments. In Handbook of Surface Plasmon Resonance; The Royal Society of Chemistry: Croydon, UK, 2017; pp. 60–105. ISBN 978-1-87262-730-2. [Google Scholar]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Couture, M.; Zhao, S.S.; Masson, J.F. Modern surface plasmon resonance for bioanalytics and biophysics. Phys. Chem. Chem. Phys. 2013, 15, 11190–11216. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Inci, F.; Saylan, Y.; Kojouri, A.M.; Ogut, M.G.; Denizli, A.; Demirci, U. A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl. Mater. Today 2020, 18, 100478. [Google Scholar] [CrossRef]
- Liu, L.; Moore, M.D. Survey of analytical techniques for noroviruses. Foods 2020, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Saylan, Y.; Erdem, Ö.; Cihangir, N.; Denizli, A. Detecting fingerprints of waterborne bacteria on a sensor. Chemosensors 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Zhang, F.; Chen, C.; Cai, C. Visual simultaneous detection of hepatitis A and B viruses based on a multifunctional molecularly imprinted fluorescence sensor. Anal. Chem. 2019, 91, 15748–15756. [Google Scholar] [CrossRef] [PubMed]
- Monfared, Y.E. Overview of recent advances in the design of plasmonic fiber-optic biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef]
- Hassanpour, S.; Baradaran, B.; Hejazi, M.; Hasanzadeh, M.; Mokhtarzadeh, A.; de la Guardia, M. Recent trends in rapid detection of influenza infections by bio and nanobiosensor. Trends Anal. Chem. 2018, 98, 201–215. [Google Scholar] [CrossRef]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K. Functionalized gold nanoparticle supported sensory mechanisms applied in detection of chemical and biological threat agents: A review. Anal. Chim. Acta 2012, 715, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.F.; Pan, M.M.; Yu, X.; Xu, L. The recent advances of fluorescent sensors based on molecularly imprinted fluorescent nanoparticles for pharmaceutical analysis. Curr. Med. Sci. 2020, 40, 407–421. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, S.Y.; Min, N.G.; Lee, S.Y.; Kim, S.H. Plasmonic janus microspheres created from pickering emulsion drops. Adv. Mater. 2020, 32, 2001384. [Google Scholar] [CrossRef]
- Redding, B.; Schwab, M.J.; Pan, Y.L. Raman spectroscopy of optically trapped single biological micro-particles. Sensors 2015, 15, 19021–19046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoleli, G.P.; Nikolelis, D.P.; Tzamtzis, N. Portable biosensors for the rapid detection of biochemical weapons of terrorism. In Portable Chemical Sensors Weapons Against Bioterrorism; Springer: Dordrecht, The Netherlands, 2012; pp. 1–14. ISBN 978-94-007-2872-1. [Google Scholar]
- Dembek, Z.P.; Pavlin, J.A.; Siwek, M.; Kortepeter, M.G. Epidemiology of biowarfare and bioterrorism. In Medical Aspects of Biological Warfare; Office of The Surgeon General Borden Institute US Army Medical Department Center and School Health Readiness Center of Excellence Fort Sam Houston: San Antonio, TX, USA, 2018; pp. 37–70. ISBN 978-01-609-4159. [Google Scholar]
- Primmerman, C.A. Detection of biological agents. Linc. Lab. J. 2000, 12, 3–32. [Google Scholar]
- Bogomolova, A. Sensing of biowarfare agents. In Sensors for Chemical and Biological Applications; CRC Press: Boca Raton, FL, USA, 2010; pp. 333–350. ISBN 978-1-4200-0504-2. [Google Scholar]
- Carus, W.S. The history of biological weapons use: What we know and what we don’t. Health Secur. 2015, 13, 219–255. [Google Scholar] [CrossRef]
- Marston, H.D.; Folkers, G.K.; Morens, D.M.; Fauci, A.S. Emerging viral diseases: Confronting threats with new technologies. Sci. Transl. Med. 2014, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Centers for Diseases Control and Prevention (CDC). Bioterrorism Agents/Diseases. Available online: http://www.bt.cdc.gov/agent/agentlist-category.asp (accessed on 31 August 2020).
- Sharma, P.K.; Kumar, J.S.; Singh, V.V.; Biswas, U.; Sarkar, S.S.; Alam, S.I.; Dash, P.K.; Boopathi, M.; Ganesan, K.; Jain, R. Surface plasmon resonance sensing of Ebola virus: A biological threat. Anal. Bioanal. Chem. 2020, 412, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Sikarwar, B.; Singh, V.V.; Sharma, P.K.; Kumar, A.; Thavaselvam, D.; Boopathi, M.; Singh, B.; Jaiswal, Y.K. DNA-probe-target interaction based detection of Brucella melitensis by using surface plasmon resonance. Biosens. Bioelectron. 2017, 87, 964–969. [Google Scholar] [CrossRef]
- Patel, K.; Halevi, S.; Melman, P.; Schwartz, J.; Cai, S.; Singh, B.R. A novel surface plasmon resonance biosensor for the rapid detection of botulinum neurotoxins. Biosensors 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Versiani, A.F.; Martins, E.M.N.; Andrade, L.M.; Cox, L.; Pereira, G.C.; Barbosa-Stancioli, E.F.; Nogueira, M.L.; Ladeira, L.O.; da Fonseca, F.G. Nanosensors based on LSPR are able to serologically differentiate dengue from Zika infections. Sci. Rep. 2020, 10, 11302. [Google Scholar] [CrossRef] [PubMed]
- Dab, C.; Thomas, R.; Ruediger, A. Design of a plasmonic platform to improve the SERS sensitivity for molecular detection. Photonic Sens. 2020, 10, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, O.; Sil, S.; Verma, T.; Umapathy, S. Direct detection of bacteria using positively charged ag/au bimetallic nanoparticles: A label-free surface-enhanced raman scattering study coupled with multivariate analysis. J. Phys. Chem. C 2020, 124, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; HoJeon, J.; Rhie, G.H.; Choo, R. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Yue, G.; Su, S.; Li, N.; Shuai, M.; Lai, X.; Astruc, D.; Zhao, P. Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coord. Chem. Rev. 2016, 311, 75–84. [Google Scholar] [CrossRef]
- Smith, R.G.; D’Souza, N.; Nicklin, S. A review of biosensors and biologically inspired systems for explosives detection. Analyst 2008, 133, 571–584. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Guo, Z.Z.; Chen, Y.; Cao, Y.P. Nanomaterial based biosensors for detection of biomarkers of exposure to op pesticides and nerve agents: A review. Electroanalysis 2017, 29, 1206–1213. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wu, D.; Yoon, J. Recent advances in the development of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bai, J.; Zhang, R.; Wu, E.; Wang, J.; Li, S.; Ning, B.; Wang, M.; Gao, X.; Peng, Y. LSPR-enhanced photonic crystal allows ultrasensitive and label-free detection of hazardous chemicals. Sens. Actuators B Chem. 2020, 310, 127671. [Google Scholar] [CrossRef]
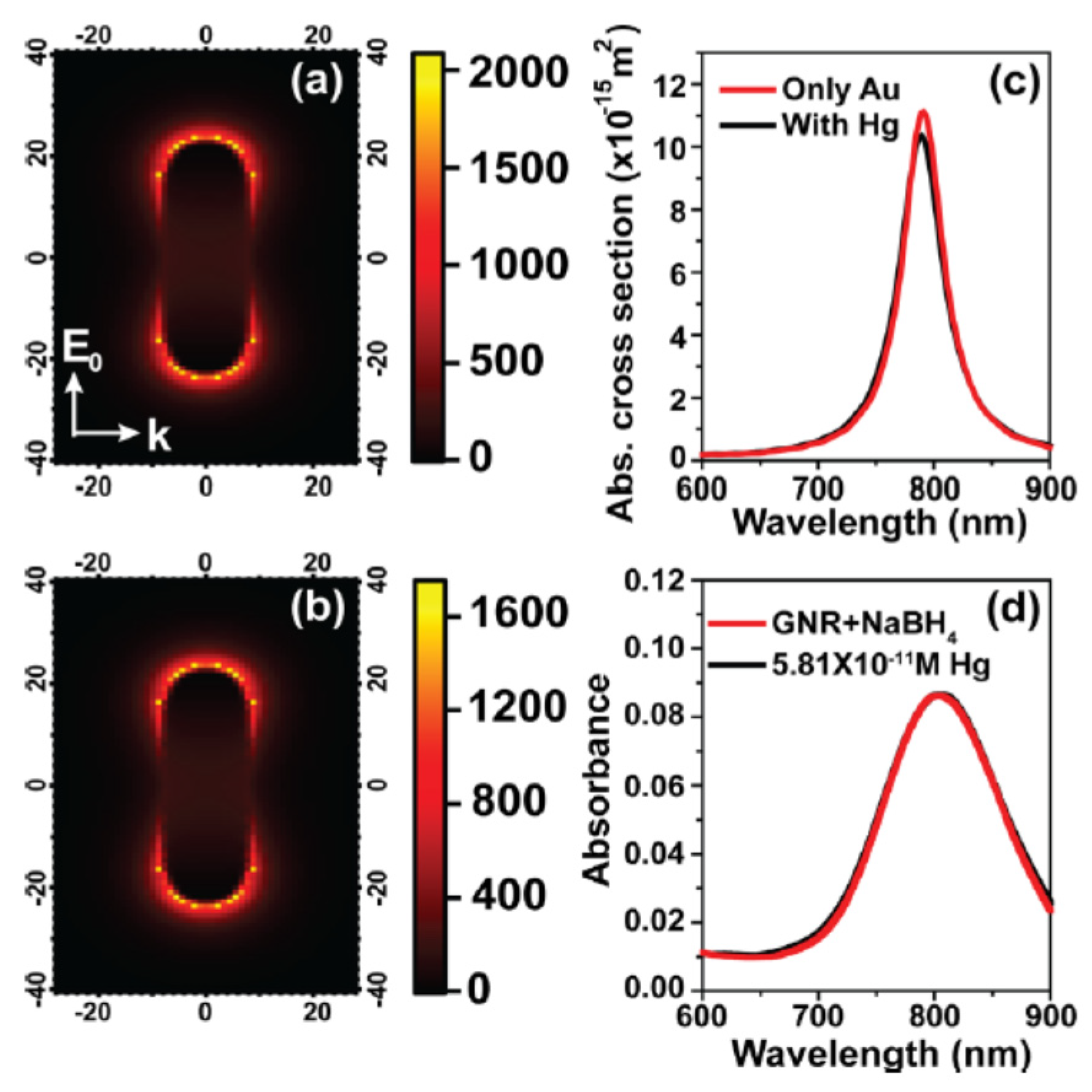
- Verma, M.S.; Chandra, M. Nonlinear plasmonic sensing for label-free and selective detection of mercury at picomolar level. ACS Sens. 2020, 5, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Haghshenas, A.F. Ag nanostructures as the surface plasmon resonance (SPR)˗based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B Chem. 2018, 273, 1768–1779. [Google Scholar] [CrossRef]
- Thenmozhi, H.; Rajana, M.S.M.; Ahmed, K. D-shaped PCF sensor based on SPR for the detection of carcinogenic agents in food and cosmetics. Optik 2019, 180, 264–270. [Google Scholar] [CrossRef]
- Heleg-Shabtai, V.; Sharabi, H.; Zaltsman, A.; Ron, I.; Pevzner, A. Surface-enhanced Raman spectroscopy (SERS) for detection of VX and HD in the gas phase using a hand-held Raman spectrometer. Analyst 2020, 145, 6334–6341. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Pellejero, I.; Sebastián, V.; Urbiztondo, M.A.; Mallada, R.; Pina, M.P.; Santamaría, J. Highly sensitive SERS quantification of organophosphorous chemical warfare agents: A major step towards the real time sensing in the gas phase. Sens. Actuators B Chem. 2018, 267, 457–466. [Google Scholar] [CrossRef] [Green Version]

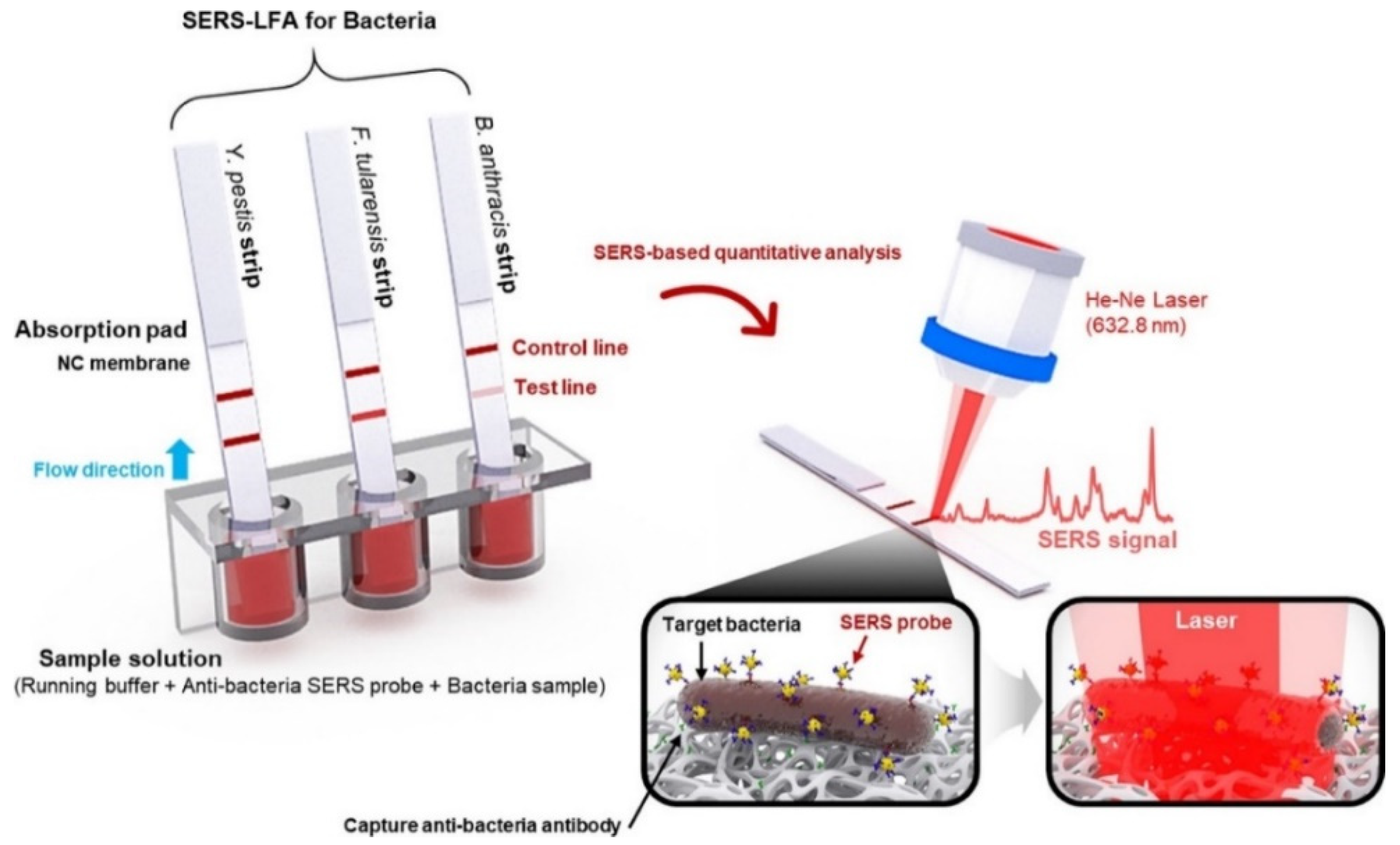

| Group | Diseases | Agents |
|---|---|---|
| A | Anthrax | Bacillus anthracis |
| Botulism | Clostridium botulinum toxin | |
| Plague | Yersinia pestis | |
| Smallpox | Variola major | |
| Tularemia | Francisella tularensis | |
| Viral hemorrhagic fevers | Filoviruses and Arenaviruses | |
| B | Brucellosis | Brucella spp. |
| Epsilon toxin | Clostridium perfringens | |
| Food safety threats | Salmonella spp., E.coli O157:H7, Shigella | |
| Glanders | Burkholderia mallei | |
| Melioidosis | Burkholderia pseudomallei | |
| Psittacosis | Chlamydia psittaci | |
| Q fever | Coxiella burnetii | |
| Ricin toxin | Ricinus communis | |
| Staphylococcal enterotoxin | Staphylococcus spp. | |
| Typhus fever | Rickettsia prowazekii | |
| Viral encephalitis | Alphaviruses | |
| Water safety threats | Vibrio cholera, Cryptosporidium parvum | |
| C | Emerging infectious diseases | Nipahvirus and Hantavirus |
| Group | Agent | Action Mode |
|---|---|---|
| Nerve agents | G-group (Tabun, soman, sarin) V-group (VX, Vx, CVx, VR) A-group (Novichoks) | Inactivates the enzyme acetylcholinesterase, preventing the breakdown of the neurotransmitter acetylcholine in the victim’s synapses and causing both muscarinic and nicotinic effects. |
| Vesicant/Blister | Sulfur mustard Nitrogen mustard | Agents are acid-forming compounds that damage skin and respiratory system, resulting in burns and respiratory problems. |
| Blood/suffocating | Cyanogen chloride Hydrogen cyanide | Cyanide directly prevents cells from using oxygen. The cells then use anaerobic respiration, creating excess lactic acid and metabolic acidosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saylan, Y.; Akgönüllü, S.; Denizli, A. Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents. Biosensors 2020, 10, 142. https://doi.org/10.3390/bios10100142
Saylan Y, Akgönüllü S, Denizli A. Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents. Biosensors. 2020; 10(10):142. https://doi.org/10.3390/bios10100142
Chicago/Turabian StyleSaylan, Yeşeren, Semra Akgönüllü, and Adil Denizli. 2020. "Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents" Biosensors 10, no. 10: 142. https://doi.org/10.3390/bios10100142
APA StyleSaylan, Y., Akgönüllü, S., & Denizli, A. (2020). Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents. Biosensors, 10(10), 142. https://doi.org/10.3390/bios10100142







