One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acryloyl Chloride Modified 8-Armed Polyethylene Glycol (PEGOA)
2.3. Synthesis of 8-Armed Polyethylene Glycol (PEGOMA) Capped with Methacrylate (2-Isocyanoethyl) Ester
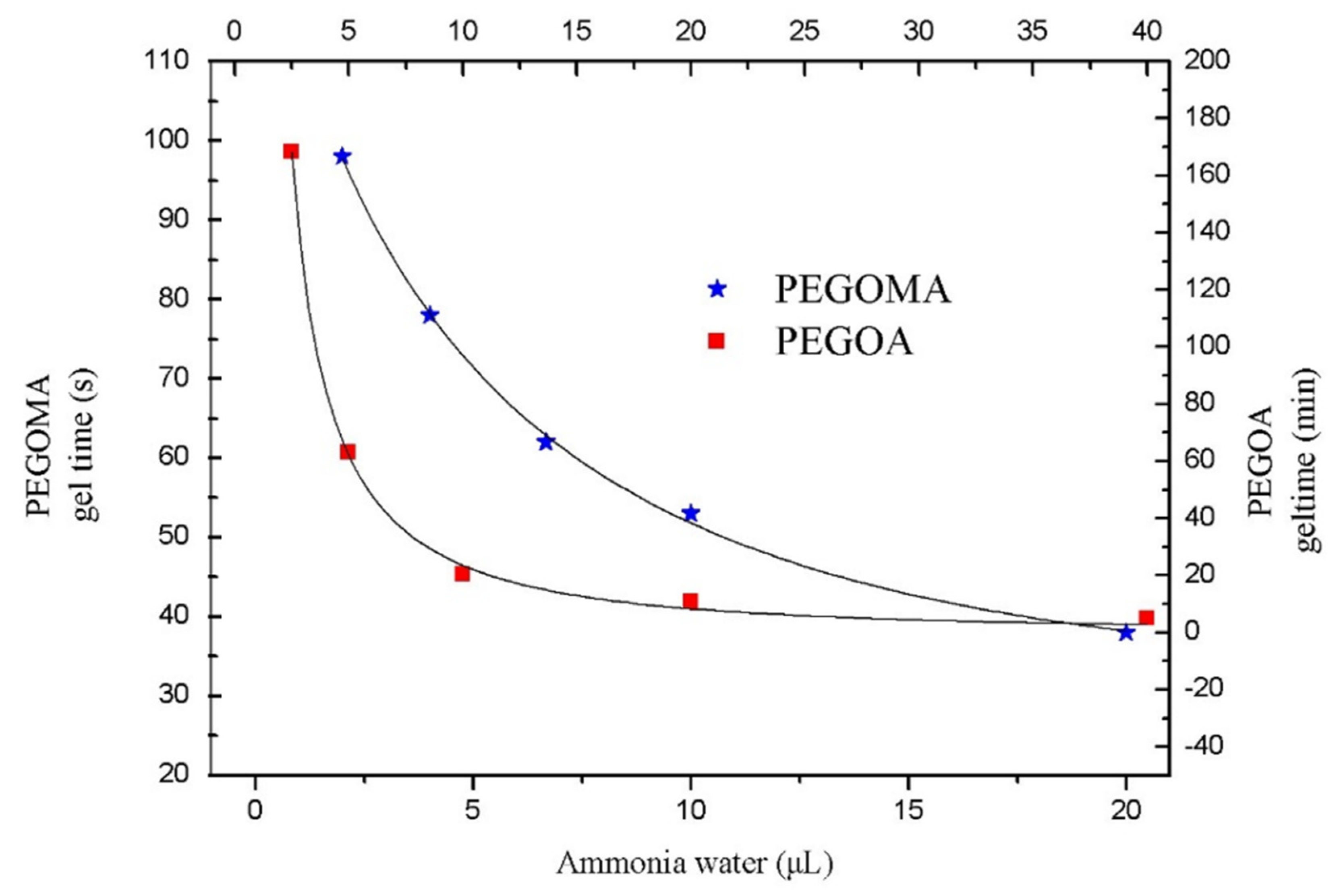
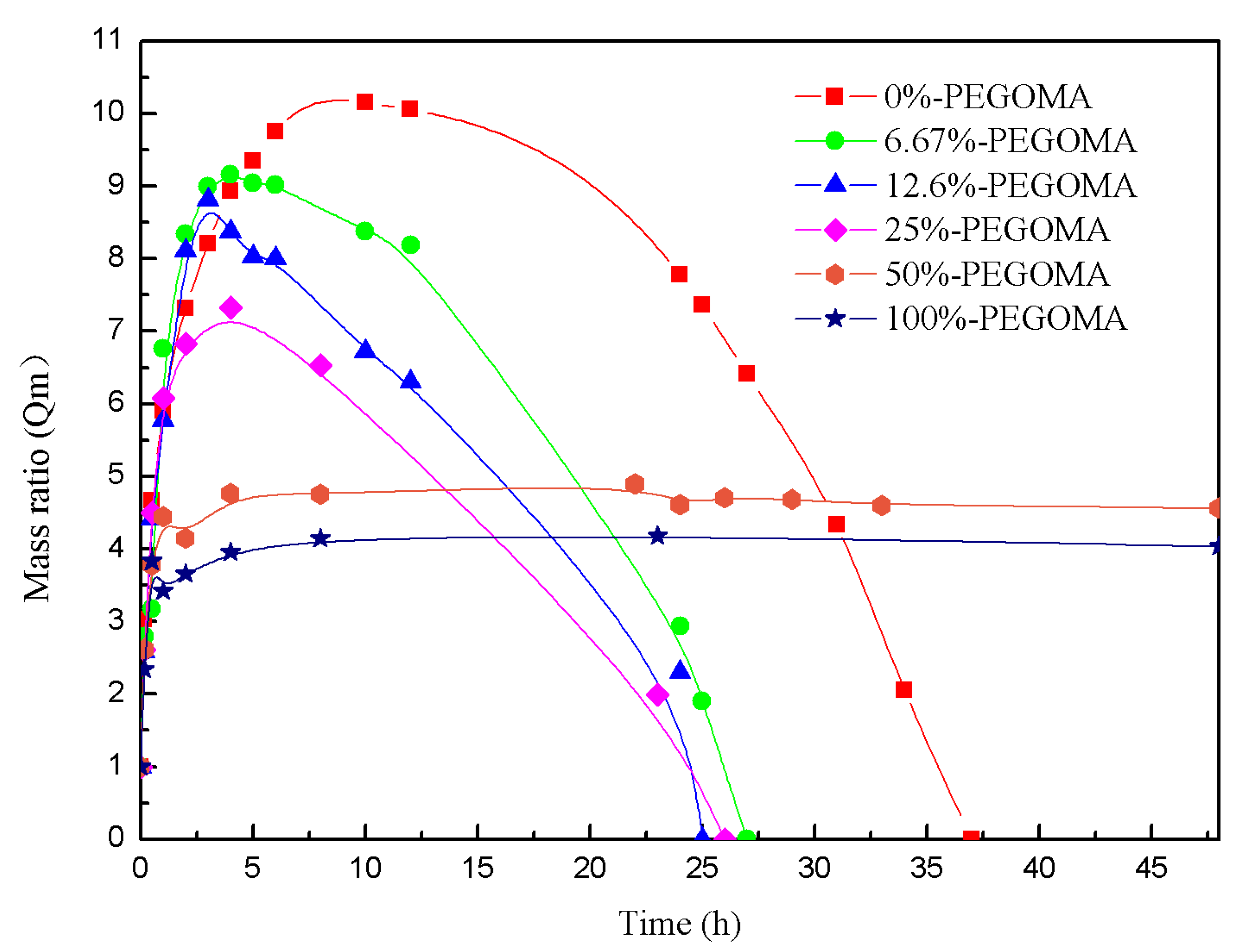
2.4. Determination of the Critical Gelation Concentration of PEGOMA and PEGOA Hydrogels
2.5. Determination of Gelation Time of PEGOMA and PEGOA Hydrogels
2.6. In-Situ Synthesis of AgNCs Using 8PEG-NH3 Hydrogel as Template
2.7. Detection of the Cu2+ Ions Using the 8PEG-AgNCs Nanohybrids
2.8. Instruments and Measurements
3. Results and Discussion
3.1. Analysis of the Gelling Process of PEGOMA and PEGOA Hydrogel Templates
3.2. Fabrication of 8PEG-AgNCs Nanohybrids
3.3. Fluorescent Properties of 8PEG-AgNCs Nanohybrids
3.4. Fluorescent Sensor for Detection of Cu2+ Ions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Basu, K.; Ghosh, S.; Bhattacharyya, K.; Roy, S.; Datta, A.; Banerjee, A. Size specific emission in peptide capped gold quantum clusters with tunable photoswitching behavior. Nanoscale 2017, 9, 4419–4429. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Yu, X.; Liu, W.; Deng, X.; Yan, S.; Su, Z. Gold nanocluster embedded bovine serum albumin nanofibers-graphene hybrid membranes for the efficient detection and separation of mercury ion. Chem. Eng. J. 2018, 335, 176–184. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Su, Z.; Wei, G. Design, fabrication, and biomedical applications of bioinspired peptide-inorganic nanomaterial hybrids. J. Mater. Chem. B 2017, 5, 1130–1142. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, D.; Wang, H.; Li, J.; Nienhaus, G.U.; Su, Z.; Wei, G.; Shang, L. Supramolecular self-assembly bioinspired synthesis of luminescent gold nanocluster-embedded peptide nanofibers for temperature sensing and cellular imaging. Bioconjugate Chem. 2017, 28, 2224–2229. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, C.M.; Johnsen, K.R.; Kiser, J.R.; Antoku, Y.; Dickson, R.M.; Petty, J.T. Ag nanocluster formation using a cytosine oligonucleotide template. J. Phys. Chem. C 2007, 111, 175–181. [Google Scholar] [CrossRef] [Green Version]
- New, S.; Lee, S.; Su, X. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef]
- Sharma, J.; Yeh, H.-C.; Yoo, H.; Werner, J.H.; Martinez, J.S. A complementary palette of fluorescent silver nanoclusters. Chem. Commun. 2010, 46, 3280–3282. [Google Scholar] [CrossRef]
- Mei, L.; Teng, Z.; Zhu, G.; Liu, Y.; Zhang, F.; Zhang, J.; Li, Y.; Guan, Y.; Luo, Y.; Chen, X. Silver nanocluster-embedded zein films as antimicrobial coating materials for food packaging. ACS Appl. Mater. Interfaces 2017, 9, 35297–35304. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.-F.; Liu, F.; Pang, Y.-H. Colorimetric detection toward halide ions by a silver nanocluster hydrogel. Talanta 2020, 211, 120717. [Google Scholar] [PubMed]
- Ershov, B.; Janata, E.; Henglein, A.; Fojtik, A. Silver atoms and clusters in aqueous solution: Absorption spectra and the particle growth in the absence of stabilizing Ag+ ions. J. Phys. Chem. 1993, 97, 4589–4594. [Google Scholar]
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar]
- Udayabhaskararao, T.; Pradeep, T. New protocols for the synthesis of stable Ag and Au nanocluster molecules. J. Phys. Chem. Lett. 2013, 4, 1553–1564. [Google Scholar] [PubMed]
- Kumar, S.; Bolan, M.D.; Bigioni, T.P. Glutathione-stabilized magic-number silver cluster compounds. J. Am. Chem. Soc. 2010, 132, 13141–13143. [Google Scholar]
- Kitazawa, H.; Albrecht, K.; Yamamoto, K. Synthesis of a dendrimer reactor for clusters with a magic number. Chem. Lett. 2012, 41, 828–830. [Google Scholar]
- Xu, H.; Suslick, K.S. Sonochemical synthesis of highly fluorescent Ag nanoclusters. ACS Nano 2010, 4, 3209–3214. [Google Scholar] [PubMed]
- Adhikari, B.; Banerjee, A. Short-peptide-based hydrogel: A template for the in situ synthesis of fluorescent silver nanoclusters by using sunlight. Chem. A Eur. J. 2010, 16, 13698–13705. [Google Scholar]
- Yu, J.; Patel, S.A.; Dickson, R.M. In vitro and intracellular production of peptide-encapsulated fluorescent silver nanoclusters. Angew. Chem. Int. Ed. 2007, 46, 2028–2030. [Google Scholar]
- Copp, S.M.; Bogdanov, P.; Debord, M.; Singh, A.; Gwinn, E. Base motif recognition and design of DNA templates for fluorescent silver clusters by machine learning. Adv. Mater. 2014, 26, 5839–5845. [Google Scholar]
- Tan, R.; He, Y.; Zhu, Y.; Xu, B.; Cao, L. Hydrothermal preparation of mesoporous TiO2 powder from Ti(SO4)2 with poly (ethylene glycol) as template. J. Mater. Sci. 2003, 38, 3973–3978. [Google Scholar] [CrossRef]
- Shen, Z.; Duan, H.; Frey, H. Water-soluble fluorescent Ag nanoclusters obtained from multiarm star poly (acrylic acid) as “molecular hydrogel” templates. Adv. Mater. 2007, 19, 349–352. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, X.; Wang, C.; Chen, D.; Jiang, X.; Zhu, C.; Yu, B.; Qiu, J. Fluorescent Ag nanoclusters in glass induced by an infrared femtosecond laser. Chem. Phys. Lett. 2007, 439, 81–84. [Google Scholar] [CrossRef]
- Huang, Z.; Pu, F.; Lin, Y.; Ren, J.; Qu, X. Modulating DNA-templated silver nanoclusters for fluorescence turn-on detection of thiol compounds. Chem. Commun. 2011, 47, 3487–3489. [Google Scholar]
- Sharma, J.; Rocha, R.C.; Phipps, M.L.; Yeh, H.-C.; Balatsky, K.A.; Vu, D.M.; Shreve, A.P.; Werner, J.H.; Martinez, J.S. A DNA-templated fluorescent silver nanocluster with enhanced stability. Nanoscale 2012, 4, 4107–4110. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S. Facile preparation of water-soluble fluorescent silver nanoclusters using a polyelectrolyte template. Chem. Commun. 2008, 1088–1090. [Google Scholar] [CrossRef]
- Barthel, M.J.; Angeloni, I.; Petrelli, A.; Avellini, T.; Scarpellini, A.; Bertoni, G.; Armirotti, A.; Moreels, I.; Pellegrino, T. Synthesis of highly fluorescent copper clusters using living polymer chains as combined reducing agents and ligands. ACS Nano 2015, 9, 11886–11897. [Google Scholar] [CrossRef]
- Shang, G.; Li, C.; Wen, G.; Zhang, X.; Liang, A.; Jiang, Z. A new silver nanochain SERS analytical platform to detect trace hexametaphosphate with a rhodamine S molecular probe. Luminescence 2016, 31, 640–648. [Google Scholar]
- Schmidt, E.W. Hydrazine and Its Derivatives: Preparation, Properties, Applications, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Choi, S.; Dickson, R.M.; Yu, J. Developing luminescent silver nanodots for biological applications. Chem. Soc. Rev. 2012, 41, 1867–1891. [Google Scholar]
- Wang, S.; Meng, X.; Das, A.; Li, T.; Song, Y.; Cao, T.; Zhu, X.; Zhu, M.; Jin, R. A 200-fold quantum yield boost in the photoluminescence of silver-doped AgxAu25-x nanoclusters: The 13th silver atom matters. Angew. Chem. 2014, 126, 2408–2412. [Google Scholar]
- Zinchenko, A.; Miwa, Y.; Lopatina, L.I.; Sergeyev, V.G.; Murata, S. DNA hydrogel as a template for synthesis of ultrasmall gold nanoparticles for catalytic applications. ACS Appl. Mater. Interfaces 2014, 6, 3226–3232. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wei, G.; Su, Z. Reduced graphene oxide-based double network polymeric hydrogels for pressure and temperature sensing. Sensors 2018, 18, 3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, W.; Yu, X.; Zhang, G.; Su, Z. Synthesis and biomedical applications of fluorescent nanogels. Polym. Chem. 2016, 7, 5749–5762. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Kumacheva, E. Photogeneration of fluorescent silver nanoclusters in polymer microgels. Adv. Mater. 2005, 17, 2336–2340. [Google Scholar] [CrossRef]
- Chakraborty, I.; Udayabhaskararao, T.; Pradeep, T. Luminescent sub-nanometer clusters for metal ion sensing: A new direction in nanosensors. J. Hazard. Mater. 2012, 211, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Dang, W.; Yang, Z.; Chang, J.; Wu, C. MoS2 Nanoclusters-based biomaterials for disease-impaired wound therapy. Appl. Mater. Today 2020, 20, 100735. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, A. Amino acid based smart hydrogel: Formation, characterization and fluorescence properties of silver nanoclusters within the hydrogel matrix. Soft Matter 2011, 7, 5300–5308. [Google Scholar] [CrossRef]
- Guo, S.R.; Gong, J.Y.; Jiang, P.; Wu, M.; Lu, Y.; Yu, S.H. Biocompatible, luminescent silver@ phenol formaldehyde resin core/shell nanospheres: Large-scale synthesis and application for in vivo bioimaging. Adv. Funct. Mater. 2008, 18, 872–879. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Yesildag, C.; Bartsch, C.; Zhang, X.; Liu, W.; Loebus, A.; Su, Z.; Lensen, M.C. Influence of network structure on the crystallization behavior in chemically crosslinked hydrogels. Polymers 2018, 10, 970. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Loebus, A.; de Vicente, G.; Ren, F.; Arafeh, M.; Ouyang, Z.; Lensen, M.C. Synthesis of poly (ethylene glycol)-based hydrogels via amine-michael type addition with tunable stiffness and postgelation chemical functionality. Chem. Mater. 2014, 26, 3624–3630. [Google Scholar] [CrossRef]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Functional PEG-hydrogels convey gold nanoparticles from silicon and aid cell adhesion onto the nanocomposites. Chem. Mater. 2017, 29, 2008–2015. [Google Scholar]
- Liz-Marzán, L.M.; Lado-Touriño, I. Reduction and stabilization of silver nanoparticles in ethanol by nonionic surfactants. Langmuir 1996, 12, 3585–3589. [Google Scholar]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly (ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar]
- Kumar, R.; Chaudhary, P.; Nimesh, S.; Chandra, R. Polyethylene glycol as a non-ionic liquid solvent for Michael addition reaction of amines to conjugated alkenes. Green Chem. 2006, 8, 356–358. [Google Scholar]
- Zheng, J.; Dickson, R.M. Individual water-soluble dendrimer-encapsulated silver nanodot fluorescence. J. Am. Chem. Soc. 2002, 124, 13982–13983. [Google Scholar]
- Shang, L.; Dong, S. Silver nanocluster-based fluorescent sensors for sensitive detection of Cu(II). J. Mater. Chem. 2008, 18, 4636–4640. [Google Scholar]
- Wang, C.; Huang, Y. Facile preparation of fluorescent Ag-clusters-chitosan-hybrid nanocomposites for bio-applications. New J. Chem. 2014, 38, 657–662. [Google Scholar]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar]
- Karaoglu, K.; Yilmaz, F.; Menteşe, E. A new fluorescent “turn-off” coumarin-based chemosensor: Synthesis, structure and Cu-selective Fluorescent sensing in water samples. J. Fluoresc. 2017, 27, 1293–1298. [Google Scholar]
- Dalmieda, J.; Kruse, P. Metal cation detection in drinking water. Sensors 2019, 19, 5134. [Google Scholar]
- Liu, Y.; Zhu, T.; Deng, M.; Tang, X.; Han, S.; Liu, A.; Bai, Y.; Qu, D.; Huang, X.; Qiu, F. Selective and sensitive detection of copper (II) based on fluorescent zinc-doped AgInS2 quantum dots. J. Lumin. 2018, 201, 182–188. [Google Scholar] [CrossRef]
- Kaewnok, N.; Petdum, A.; Sirirak, J.; Charoenpanich, A.; Panchan, W.; Sahasithiwat, S.; Sooksimuang, T.; Wanichacheva, N. Novel Cu2+-specific “Turn-ON” fluorescent probe based on [5] helicene with very large Stokes shift and its potential application in living cells. New J. Chem. 2018, 42, 5540–5547. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Velmathi, S.; Sung, Y.-M.; Wu, S.-P. Highly fluorescent probe for copper (II) ion based on commercially available compounds and live cell imaging. Sens. Actuators B Chem. 2014, 198, 285–293. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wang, Z.; Liu, J.; Lu, Y. Highly sensitive and selective colorimetric sensors for uranyl (UO22+): Development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, T.; Zhang, M.; Shi, G. A DNA–scaffolded silver nanocluster/Cu2+ ensemble as a turn-on fluorescent probe for histidine. Analyst 2014, 139, 3122–3126. [Google Scholar] [CrossRef]
- Su, Y.-T.; Lan, G.-Y.; Chen, W.-Y.; Chang, H.-T. Detection of copper ions through recovery of the fluorescence of DNA-templated copper/silver nanoclusters in the presence of mercaptopropionic acid. Anal. Chem. 2010, 82, 8566–8572. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Fitriani, I.; Wyantuti, S.; Hartati, Y.W.; Khaydarov, R.; McAlister, J.A.; Obata, H.; Gamo, T. Colorimetric detection of mercury (II) ion in aqueous solution using silver nanoparticles. Anal. Sci. 2017, 33, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Liu, H.; Lan, C.; Fu, Q.; Huang, C.; Luo, Z.; Jiang, T.; Tang, Y. Silver nanoparticle enhanced Raman scattering-based lateral flow immunoassays for ultra-sensitive detection of the heavy metal chromium. Nanotechnology 2014, 25, 495501. [Google Scholar] [CrossRef]







| Materials | Cu2+ Selectivity | Detection Limit | Proposed Mechanism | Ref. |
|---|---|---|---|---|
| Helicene dye bearing hydrazine | good | 2.6 ppb | photoinduced electron transfer | [53] |
| 2,3-diaminophenazine, 1,2-diamino-anthraquinone, 2,4-dinitrophenylhydrazine | good | 0.15 × 10−9 M/L | paramagnetic effect | [54] |
| Coumarin-based fluorogenic probe bearing the 2-picolyl unit | good | 0.5 µM | quenching mechanism | [55] |
| Zinc-doped AgInS2 quantum dots | good | 27.3 nM | electron transfer | [52] |
| Poly (methacrylic acid) (PMAA)-templated AgNCs | good | 8 nM | quenching mechanism | [47] |
| DNA–AgNCs | / | 1.4 mM | marvelous fluorescent enhancement in the presence of histidine | [57] |
| DNA-Cu/AgNCs | good | 2.7 nM | static quenching mechanism | [58] |
| 8PEG-AgNCs | good | 50 nM | quenching mechanism | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, G.; Wei, G.; Su, Z. One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+. Biosensors 2020, 10, 131. https://doi.org/10.3390/bios10100131
Zhang X, Zhang G, Wei G, Su Z. One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+. Biosensors. 2020; 10(10):131. https://doi.org/10.3390/bios10100131
Chicago/Turabian StyleZhang, Xiaoyuan, Guanghua Zhang, Gang Wei, and Zhiqiang Su. 2020. "One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+" Biosensors 10, no. 10: 131. https://doi.org/10.3390/bios10100131
APA StyleZhang, X., Zhang, G., Wei, G., & Su, Z. (2020). One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+. Biosensors, 10(10), 131. https://doi.org/10.3390/bios10100131








