Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs
Abstract
:1. Introduction
2. Experimental
2.1. Materials
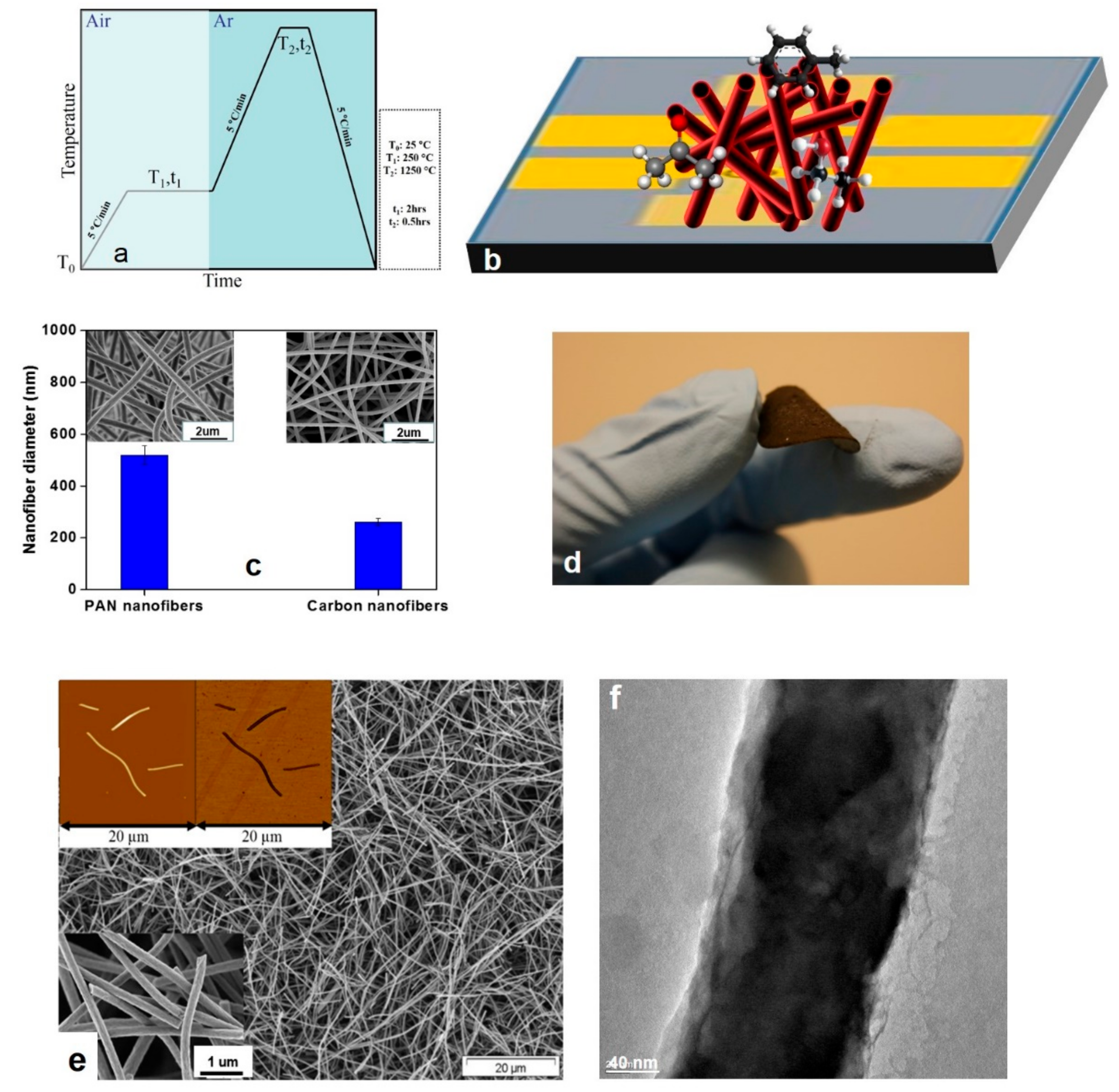
2.2. Sample Preparation
2.3. Structural Characterizations
2.4. Measurement of Gas Responsiveness
3. Results and Discussion
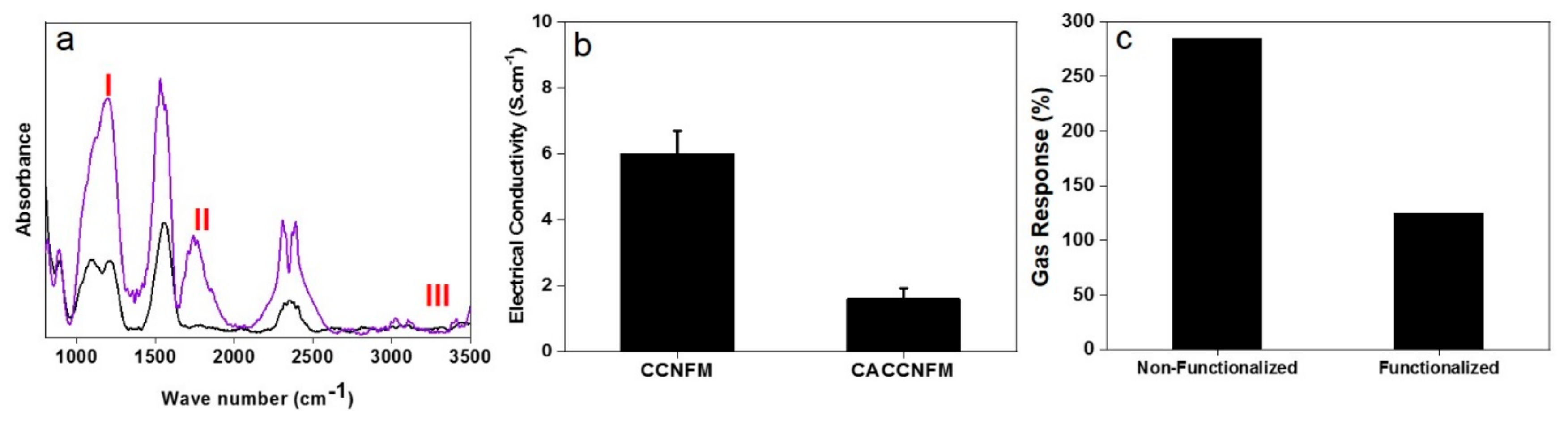
3.1. Morphological Characteristics
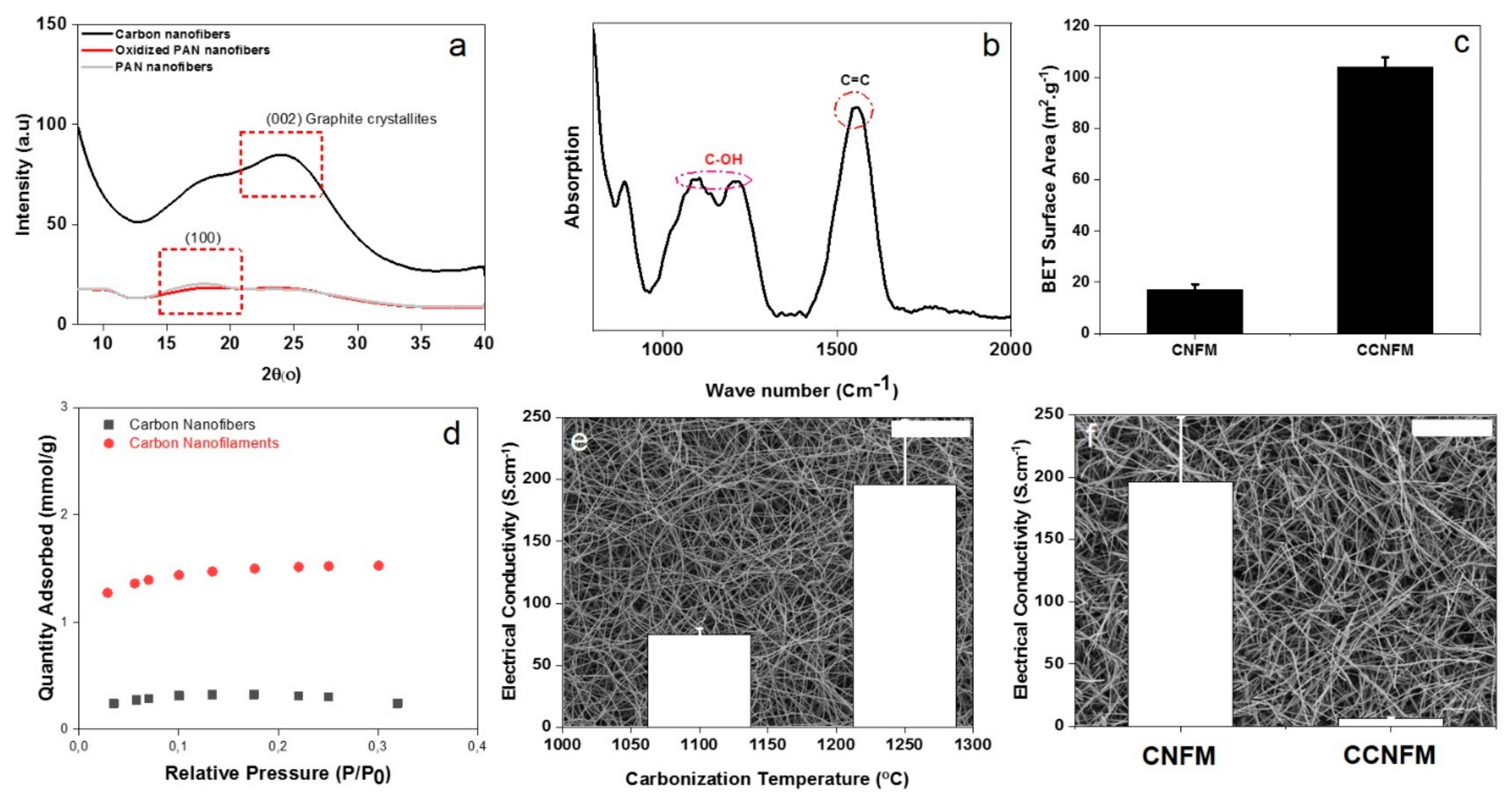
3.2. Structural Characteristics
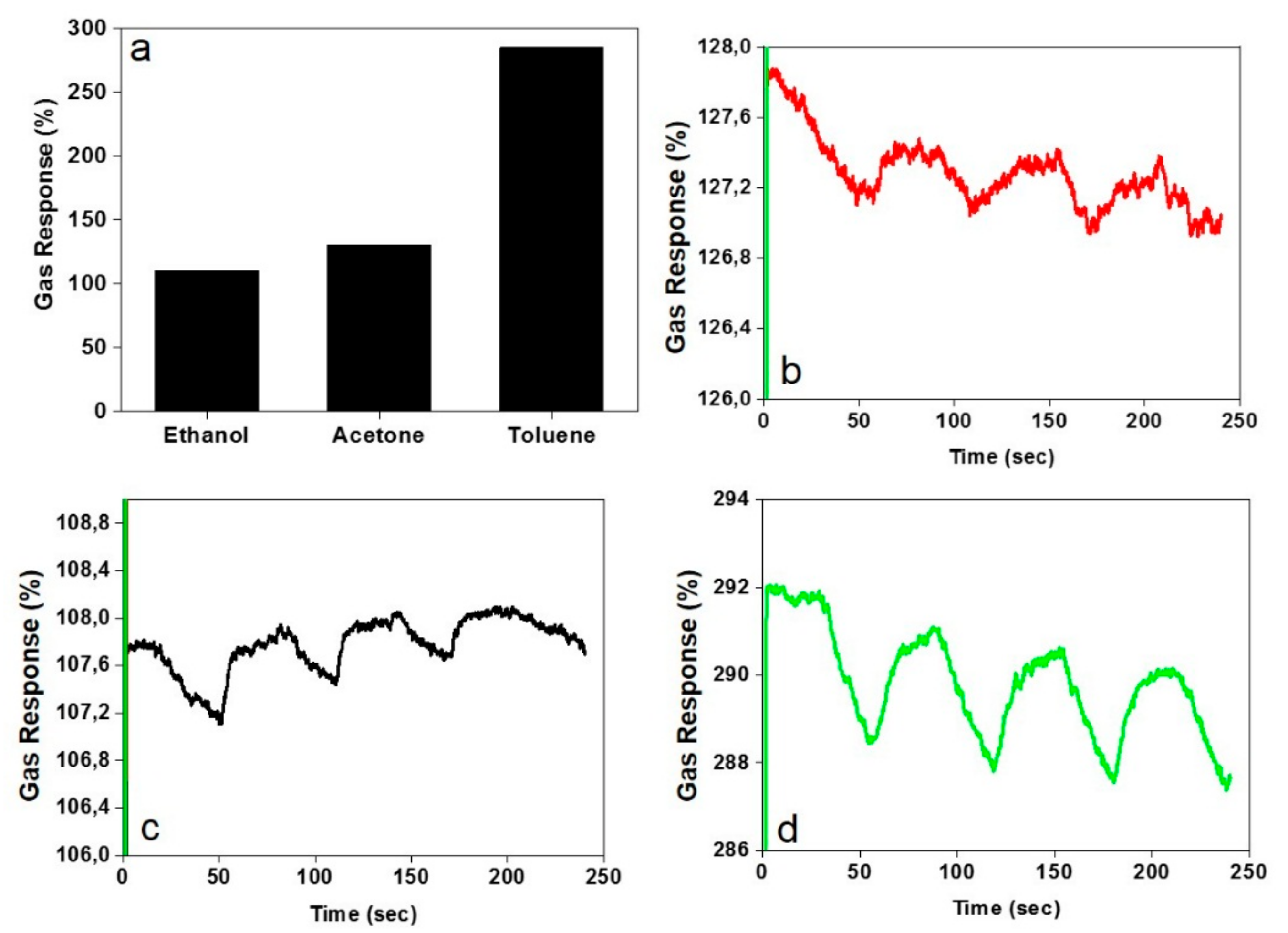
3.3. Gas Sensing Characteristics
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Dai, T.; Elbahri, M. Biofunctionalized nanofibrous membranes as super separators of protein and enzyme from water. J. Colloid Interface Sci. 2013, 406, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- WHO. Indoor Air Quality: Organic Pollutants. Report on a WHO Meeting. Euro Report and Studies; WHO: Geneva, Switzerland, 1989; pp. 1–70. [Google Scholar]
- USEPA. Reduscing Risk: Setting Priorities and Strategies for Environmental Protection; USEPA: Washington, DC, USA, 1990.
- Little, J.C.; Hodgson, A.T.; Gadgil, A.J. Modeling emissions of volatile organic compounds from new carpets. Atmos. Environ. 1994, 28, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Harrad, S.; Harrison, R.M. Concentrations and Sources of VOCs in Urban Domestic and Public Microenvironments. Environ. Sci. Technol. 2001, 35, 997–1004. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef]
- OEHHA. California’s Office of Environmental Health Hazard Assessment. 2007. Available online: http://www.oehha.ca.gov/air.html (accessed on 20 September 2007).
- Díaz, E.; Ordóñez, S.; Vega, A. Adsorption of volatile organic compounds onto carbon nanotubes, carbon nanofibers, and high-surface-area graphites. J. Colloid Interface Sci. 2007, 305, 7–16. [Google Scholar]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas sensors based on electrospun nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. A facile nanocasting synthesis of mesoporous Ag-doped SnO2 nanostructures with enhanced humidity sensing performance. Sens. Actuators B Chem. 2016, 223, 750–760. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Davoudpour, Y.; Habibi, Y.; Elbahri, M. The Electrospun Ceramic Hollow Nanofibers. Nanomaterials 2017, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Koresh, J.E.; Soffer, A. The Carbon Molecular-Sieve Membranes - General-Properties and the Permeability of Ch4/H-2 Mixture. Sep. Sci. Technol. 1987, 22, 973–982. [Google Scholar] [CrossRef]
- Jones, C.W.; Koros, W.J. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon 1994, 32, 1419–1425. [Google Scholar] [CrossRef]
- Yari Sadi, A.; Shokrgozar, M.A.; Homaeigohar, S.S.; Khavandi, A. Biological evaluation of partially stabilized zirconia added HA/HDPE composites with osteoblast and fibroblast cell lines. J. Mater. Sci. Mater. Med. 2008, 19, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Im, J.S.; Lee, S.-H.; Bae, T.-S.; Lee, Y.-S. High-sensitivity gas sensor using electrically conductive and porosity-developed carbon nanofiber. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 297–303. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO 2/SnO 2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Lee, S.H.; Im, J.S.; Kang, S.C.; Bae, T.S.; In, S.J.; Jeong, E.; Lee, Y.S. An increase in gas sensitivity and recovery of an MWCNT-based gas sensor system in response to an electric field. Chem. Phys. Lett. 2010, 497, 191–195. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Joshi, N.; Dankwort, T.; Lin, L.; Kienle, L. Au–TiO2-Loaded Cubic g-C3N4 Nanohybrids for Photocatalytic and Volatile Organic Amine Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 34087–34097. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.K.; Malik, R.; Chaudhary, V.; Mishra, Y.K.; Kienle, L.; Ahuja, R.; Lin, L. Superior visible light photocatalysis and low-operating temperature VOCs sensor using cubic Ag(0)-MoS2 loaded g-CN 3D porous hybrid. Appl. Mater. Today 2019, 16, 193–203. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Mao, X.; Simeon, F.; Rutledge, G.C.; Hatton, T.A. Electrospun Carbon Nanofiber Webs with Controlled Density of States for Sensor Applications. Adv. Mater. 2013, 25, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Strunskus, T.; Strobel, J.; Kienle, L.; Elbahri, M. A Flexible Oxygenated Carbographite Nanofilamentous Buckypaper as an Amphiphilic Membrane. Adv. Mater. Interfaces 2018, 5, 1800001. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. An Amphiphilic, Graphitic Buckypaper Capturing Enzyme Biomolecules from Water. Water 2019, 11, 2. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.-Y.; Young, T.-H.; Yang, H.J.; Ji, Y.-R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym. 2019, 224, 115112. [Google Scholar] [CrossRef] [PubMed]
- Koivusaari, K.J.; Rantala, T.T.; Leppävuori, S. Calculated electronic density of states and structural properties of tetrahedral amorphous carbon. Diam. Relat. Mater. 2000, 9, 736–740. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Z.; Guo, Y.; Hou, H.; Shao, S. Electrospun rhodium nanoparticle-loaded carbon nanofibers for highly selective amperometric sensing of hydrazine. Electrochem. Commun. 2010, 12, 422–426. [Google Scholar] [CrossRef]
- Song, Y.; He, Z.; Xu, F.; Hou, H.; Wang, L. pH-controlled electrocatalysis of amino acid based on electrospun cobalt nanoparticles-loaded carbon nanofibers. Sens. Actuators B Chem. 2012, 166, 357–364. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Xu, L.; Hou, H.; You, T. A novel and simple route to prepare a Pt nanoparticle-loaded carbon nanofiber electrode for hydrogen peroxide sensing. Biosens. Bioelectron. 2011, 26, 4585–4590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiong, W.; Zhang, Y.; Yang, D.; Wang, T.; Che, Y.; Zhao, J. Internanofiber Spacing Adjustment in the Bundled Nanofibers for Sensitive Fluorescence Detection of Volatile Organic Compounds. Anal. Chem. 2017, 89, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Lee, M.-G.; Yun, J.; Lee, J.-S.; Lim, S.H.; Yi, G.-R. Chemically Resistant Perfluoroalkoxy Nanoparticle-Packed Porous Substrates and Their Use in Colorimetric Sensor Arrays. Langmuir 2018, 34, 13014–13024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Suslick, K.S. Colorimetric Sensor Array for Monitoring CO and Ethylene. Anal. Chem. 2019, 91, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Martino, R.; Anikst, V.; Xu, Z.; Mix, S.; Benjamin, R.; Schub, H.; Eiden, M.; Rhodes, P.A.; Banaei, N. Rapid Diagnosis of Tuberculosis from Analysis of Urine Volatile Organic Compounds. ACS Sens. 2016, 1, 852–856. [Google Scholar] [CrossRef] [Green Version]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef]
- Khalil, R.; Homaeigohar, S.; Häußler, D.; Elbahri, M. A shape tailored gold-conductive polymer nanocomposite as a transparent electrode with extraordinary insensitivity to volatile organic compounds (VOCs). Sci. Rep. 2016, 6, 33895. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Elbahri, M. Switchable Plasmonic Nanocomposites. Adv. Opt. Mater. 2019, 7, 1801101. [Google Scholar] [CrossRef]
- Elbahri, M.; Abdelaziz, M.; Homaeigohar, S.; Elsharawy, A.; Keshavarz Hedayati, M.; Röder, C.; El Haj Assad, M.; Abdelaziz, R. Plasmonic Metaparticles on a Blackbody Create Vivid Reflective Colors for Naked-Eye Environmental and Clinical Biodetection. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Serrano, S.; Santiago-Aviles, J.J. Raman characterization of carbon nanofibers prepared using electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Z.; Fong, H. Continuous Nanoscale Carbon Fibers with Superior Mechanical Strength. Small 2009, 5, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Ren, J.; Wu, Q.L. Preparation and structures of electrospun PAN nanofibers as a precursor of carbon nanofibers. Synth. Met. 2005, 155, 157–161. [Google Scholar] [CrossRef]
- Kim, C.; Yang, K. Electrochemical properties of carbon nanofiber web as an electrode for supercapacitor prepared by electrospinning. Appl. Phys. Lett. 2003, 83, 1216–1218. [Google Scholar] [CrossRef]
- Kim, D.K.; Park, S.H.; Kim, B.C.; Chin, B.D.; Jo, S.M.; Kim, D.Y. Electrospun polyacrylonitrile based carbon nanofibers and their hydrogen storages. Macromol. Res. 2005, 13, 521–528. [Google Scholar] [CrossRef]
- Mattia, D.; Rossi, M.P.; Kim, B.M.; Korneva, G.; Bau, H.H.; Gogotsi, Y. Effect of graphitization on the wettability and electrical conductivity of CVD-carbon nanotubes and films. J. Phys. Chem. B 2006, 110, 9850–9855. [Google Scholar] [CrossRef]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P.; Ruoff, R.S. Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Shabafrooz, V.; Mozafari, M.; Vashae, D.; Tayebi, L. Performance enhancement of electrospun carbon fibrous nanostructures. J. Appl. Polym. Sci. 2012, 129, 3077. [Google Scholar] [CrossRef]
- Zhou, Z.; Lai, C.; Zhang, L.; Qian, Y.; Hou, H.; Reneker, D.H.; Fong, H. Development of carbon nanofibers from aligned electrospun polyacrylonitrile nanofiber bundles and characterization of their microstructural, electrical, and mechanical properties. Polymer 2009, 50, 2999–3006. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhang, X. Facile fabrication of freestanding ultrathin reduced graphene oxide membranes for water purification. Adv. Mater. 2015, 27, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Rana, D.; Matsuura, T.; Ramakrishna, S.; Narbaitz, R.M.; Tabe, S. Removal of disinfection byproducts from water by carbonized electrospun nanofibrous membranes. Sep. Purif. Technol. 2010, 74, 202–212. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A. Development and characterization of polyacrylonitrile (PAN)based carbon hollow fiber membrane. Songklanakarin J. Sci. Technol. 2003, 24, 843–854. [Google Scholar]
- Introduction to IR Spectra. Available online: http://www.chem.ucla.edu/~webspectra/irintro.html (accessed on 15 October 2013).
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-Graphene Core-Sheath Microfibers for All-Solid-State, Stretchable Fibriform Supercapacitors and Wearable Electronic Textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable One-Step Wet-Spinning of Graphene Fibers and Yarns from Liquid Crystalline Dispersions of Graphene Oxide: Towards Multifunctional Textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Singhal, R.; Chung, S.-H.; Manthiram, A.; Kalra, V. A free-standing carbon nanofiber interlayer for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.T.; Xu, X.J.; Lu, H.; Zhang, T. Synthesis and gas sensing characteristics of LaxSr1-xFeO3 nanofibers via electrospinning. Solid State Electron. 2013, 79, 87–91. [Google Scholar] [CrossRef]
- Choi, J.; Park, D.W.; Shim, S.E. Electrospun PEDOT:PSS/carbon nanotubes/PVP nanofibers as chemiresistors for aromatic volatile organic compounds. Synth. Met. 2012, 162, 1513–1518. [Google Scholar] [CrossRef]
- Li, W.; Kim, D. Polyaniline/multiwall carbon nanotube nanocomposite for detecting aromatic hydrocarbon vapors. J. Mater. Sci. 2011, 46, 1857–1861. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Liu, L.; Zheng, X. Synthesis and toluene sensing properties of SnO2 nanofibers. Sens. Actuators B Chem. 2009, 137, 471–475. [Google Scholar] [CrossRef]
- Abraham, J.K.; Philip, B.; Witchurch, A.; Varadan, V.K.; Reddy, C.C. A compact wireless gas sensor using a carbon nanotube/PMMA thin film chemiresistor. Smart Mater. Struct. 2004, 13, 1045–1049. [Google Scholar] [CrossRef]
- Lala, N.; Thavasi, V.; Ramakrishna, S. Preparation of Surface Adsorbed and Impregnated Multi-walled Carbon Nanotube/Nylon-6 Nanofiber Composites and Investigation of their Gas Sensing Ability. Sensors 2009, 9, 86–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, P.C.; Mureau, N.; Tang, Z.; Miyajima, Y.; Carey, J.D.; Silva, S.R.P. The importance of oxygen-containing defects on carbon nanotubes for the detection of polar and non-polar vapours through hydrogen bond formation. Nanotechnol. 2007, 18, 175701. [Google Scholar] [CrossRef]
- Plank, N.O.V.; Forrest, G.A.; Cheung, R.; Alexander, A.J. Electronic Properties of n-Type Carbon Nanotubes Prepared by CF4 Plasma Fluorination and Amino Functionalization. J. Phys. Chem. B 2005, 109, 22096–22101. [Google Scholar] [CrossRef]
- Castillejos-Lopéz, E.; Nevskaia, D.M.; Muñoz, V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Specific Interactions between Aromatic Electrons of Organic Compounds and Graphite Surfaces As Detected by Immersion Calorimetry. Langmuir 2004, 20, 1013–1015. [Google Scholar]
- Fu, Q.; Wang, X.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Scalable Fabrication of Electrospun Nanofibrous Membranes Functionalized with Citric Acid for High-Performance Protein Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 11819–11829. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.K.; Lim, S.H.; Pan, H.; Lin, J.; Lee, J.Y. Citric acid functionalized carbon materials for fuel cell applications. J. Power Sources 2008, 176, 70–75. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homaeigohar, S. Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs. Nanomaterials 2019, 9, 1343. https://doi.org/10.3390/nano9091343
Homaeigohar S. Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs. Nanomaterials. 2019; 9(9):1343. https://doi.org/10.3390/nano9091343
Chicago/Turabian StyleHomaeigohar, Shahin. 2019. "Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs" Nanomaterials 9, no. 9: 1343. https://doi.org/10.3390/nano9091343
APA StyleHomaeigohar, S. (2019). Amphiphilic Oxygenated Amorphous Carbon-Graphite Buckypapers with Gas Sensitivity to Polar and Non-Polar VOCs. Nanomaterials, 9(9), 1343. https://doi.org/10.3390/nano9091343




