Synthesis of Highly Photoluminescent All-Inorganic CsPbX3 Nanocrystals via Interfacial Anion Exchange Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Cesium-Oleate Solution
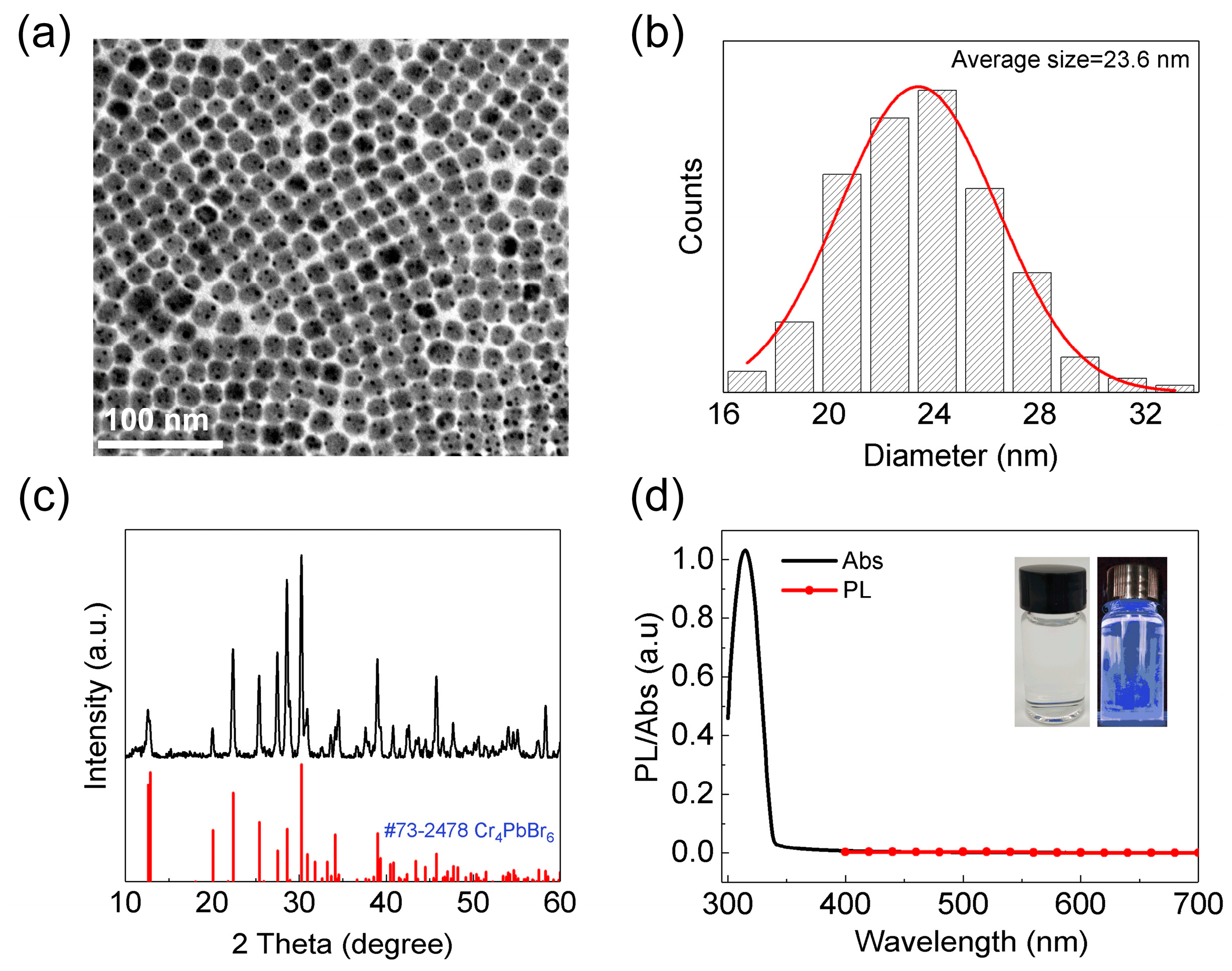
2.3. Preparation of Cs4PbX6 NCs
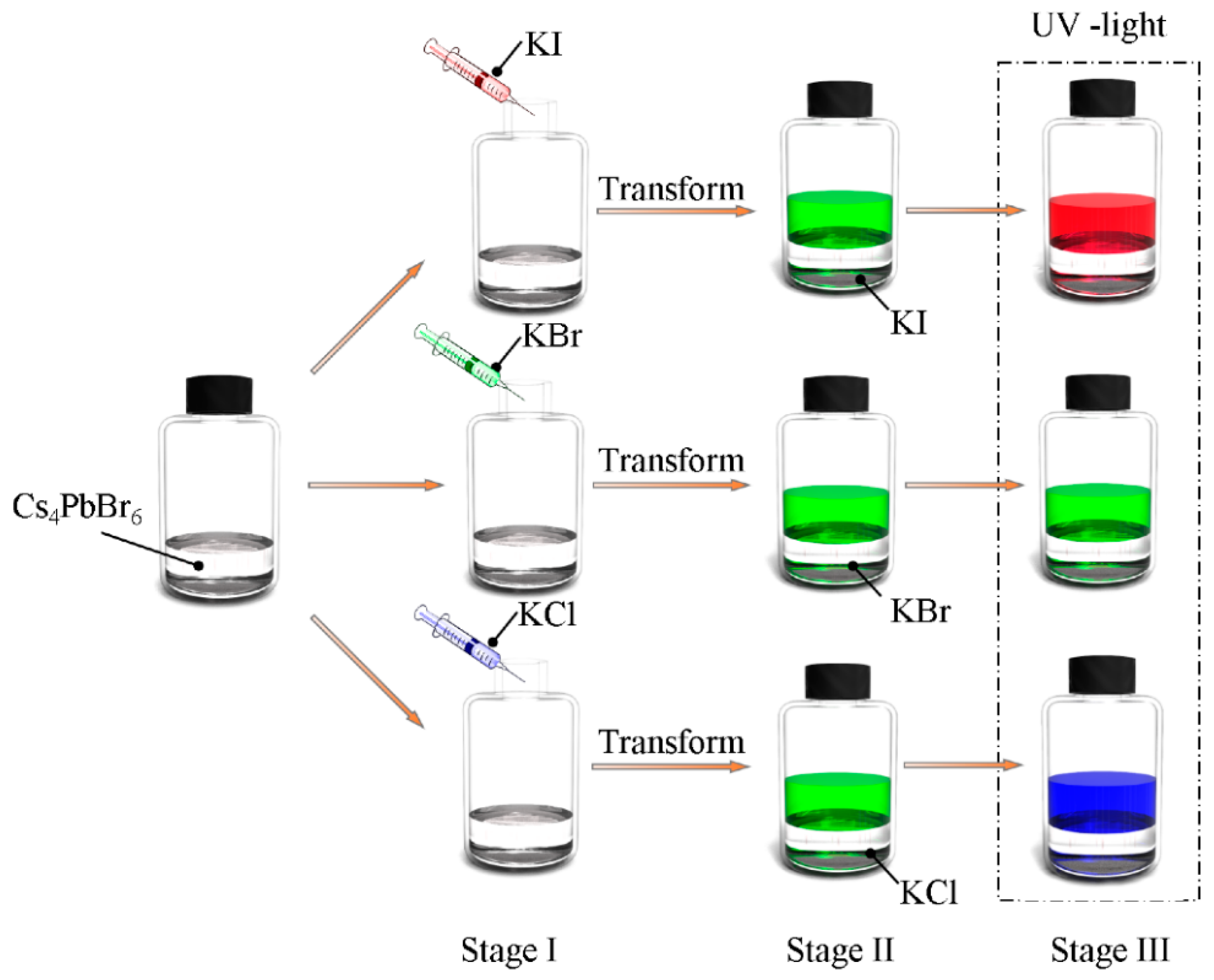
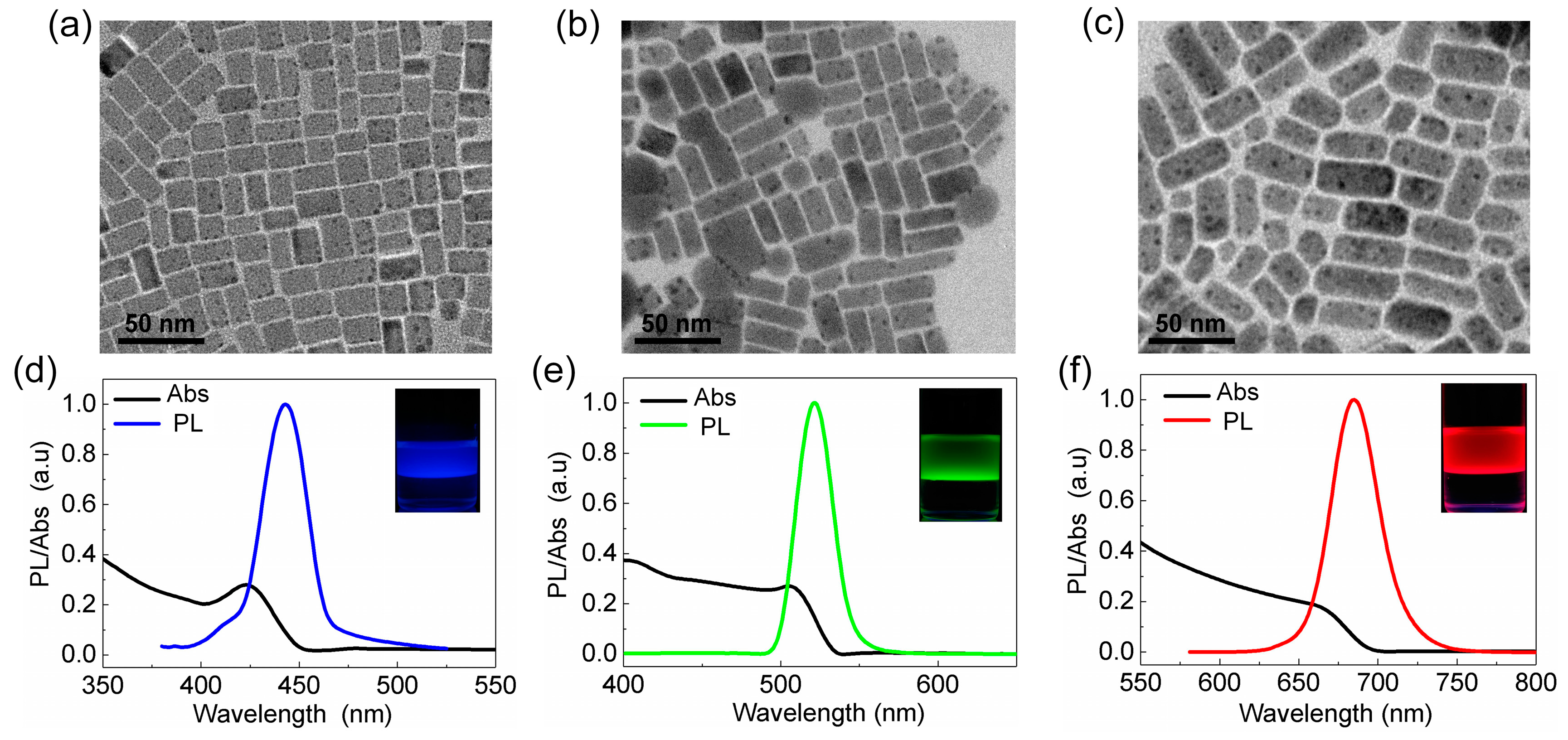
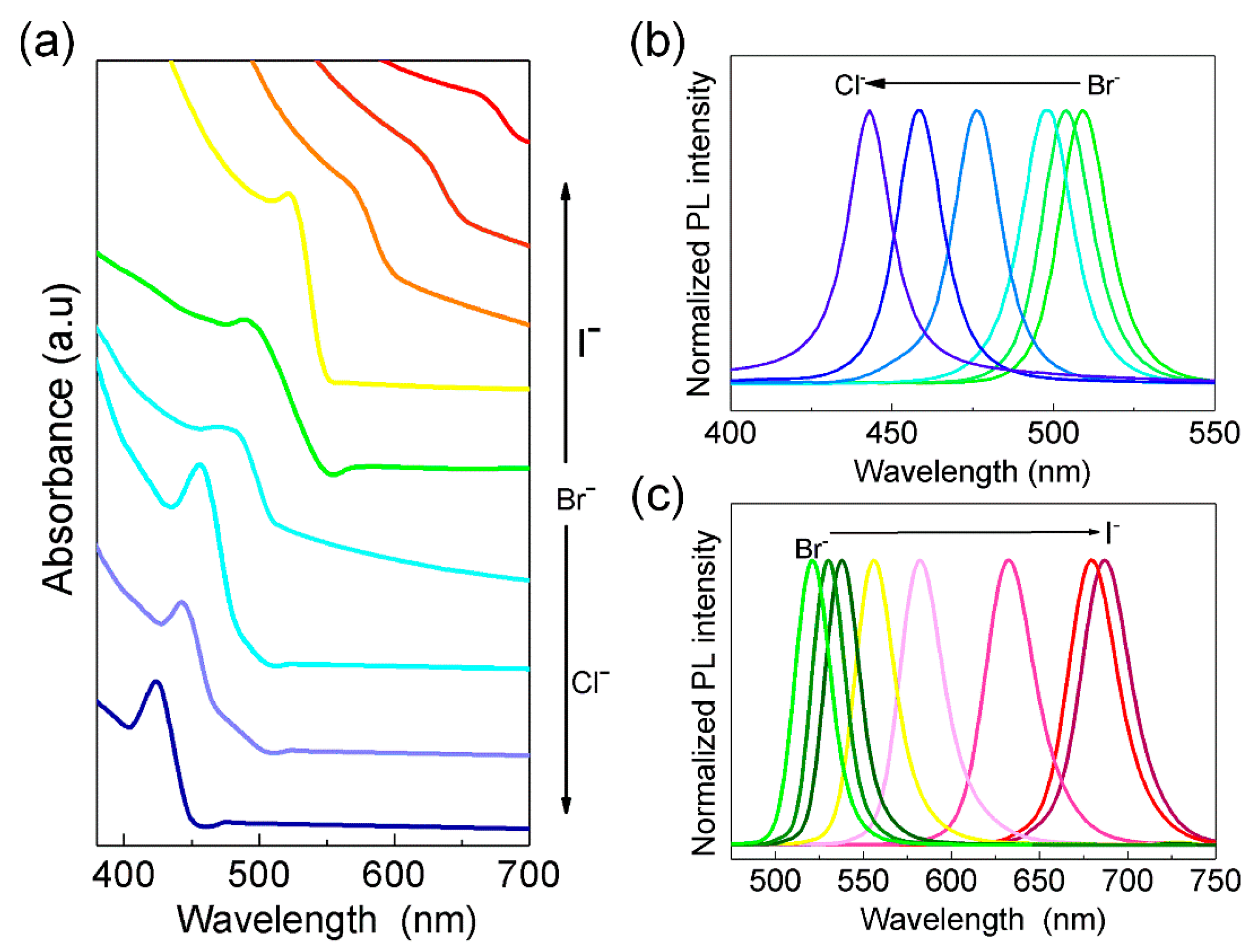
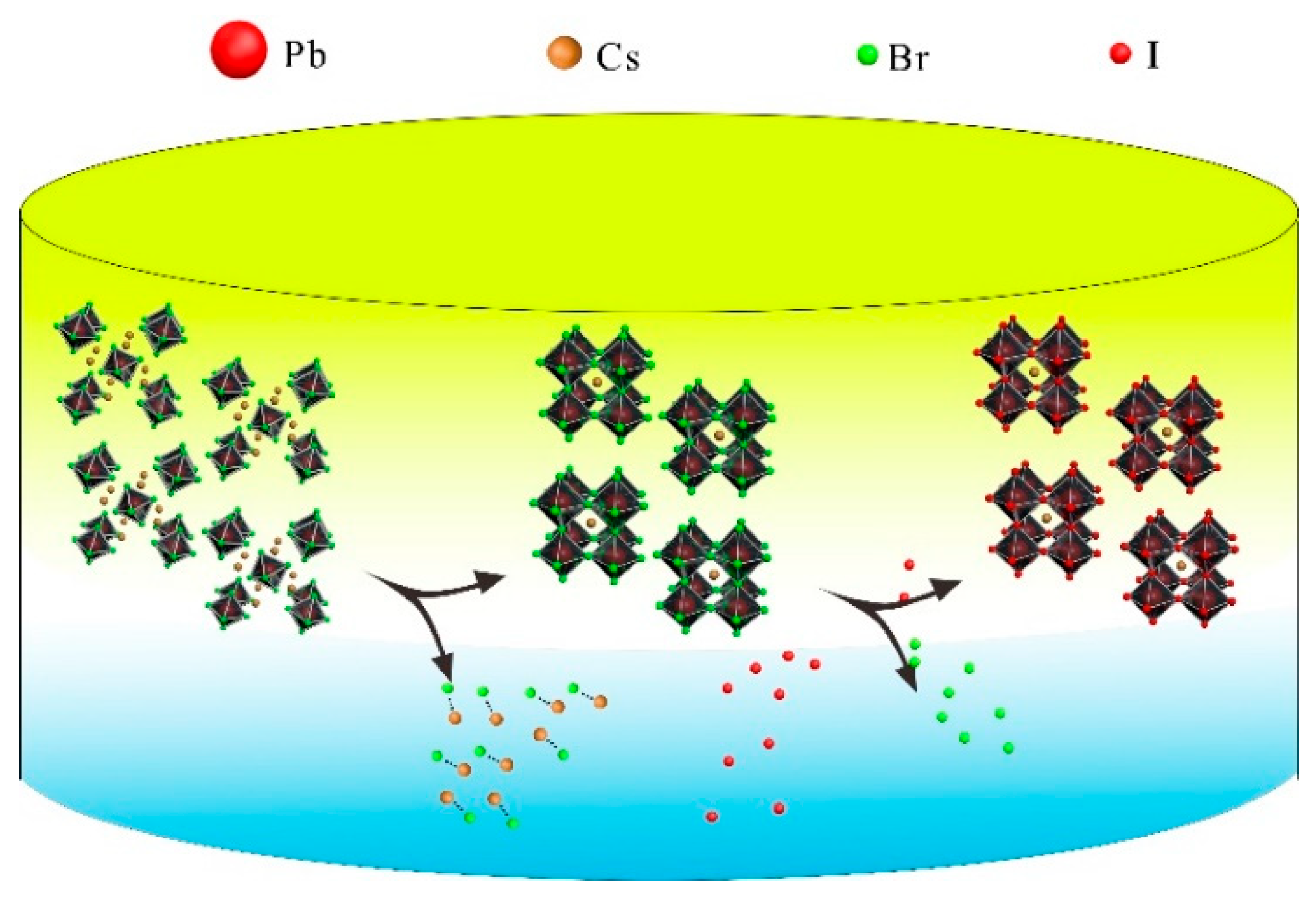
2.4. Interfacial Anion Exchange Reactions
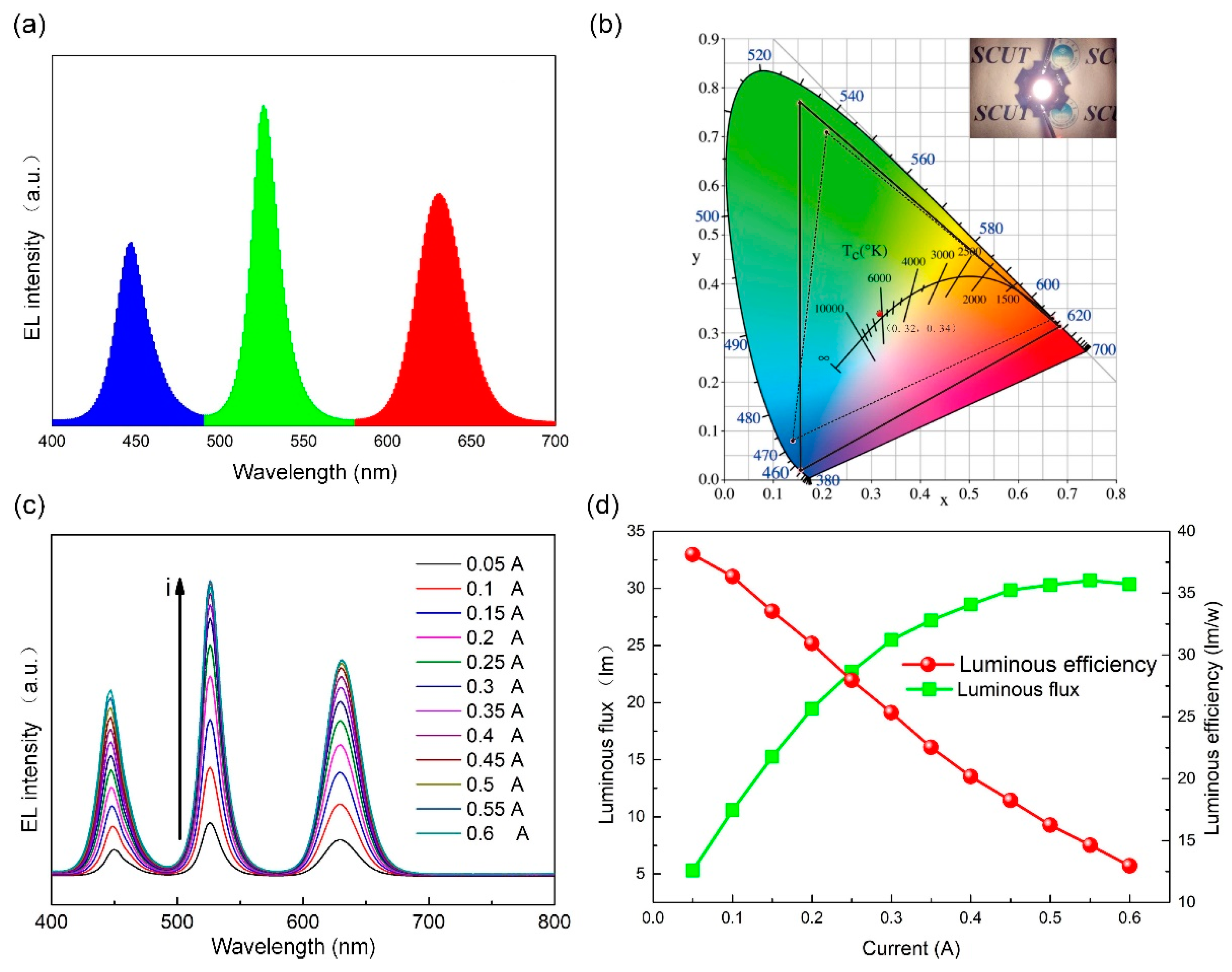
2.5. Fabrication of WLEDs Devices
2.6. Characterizations
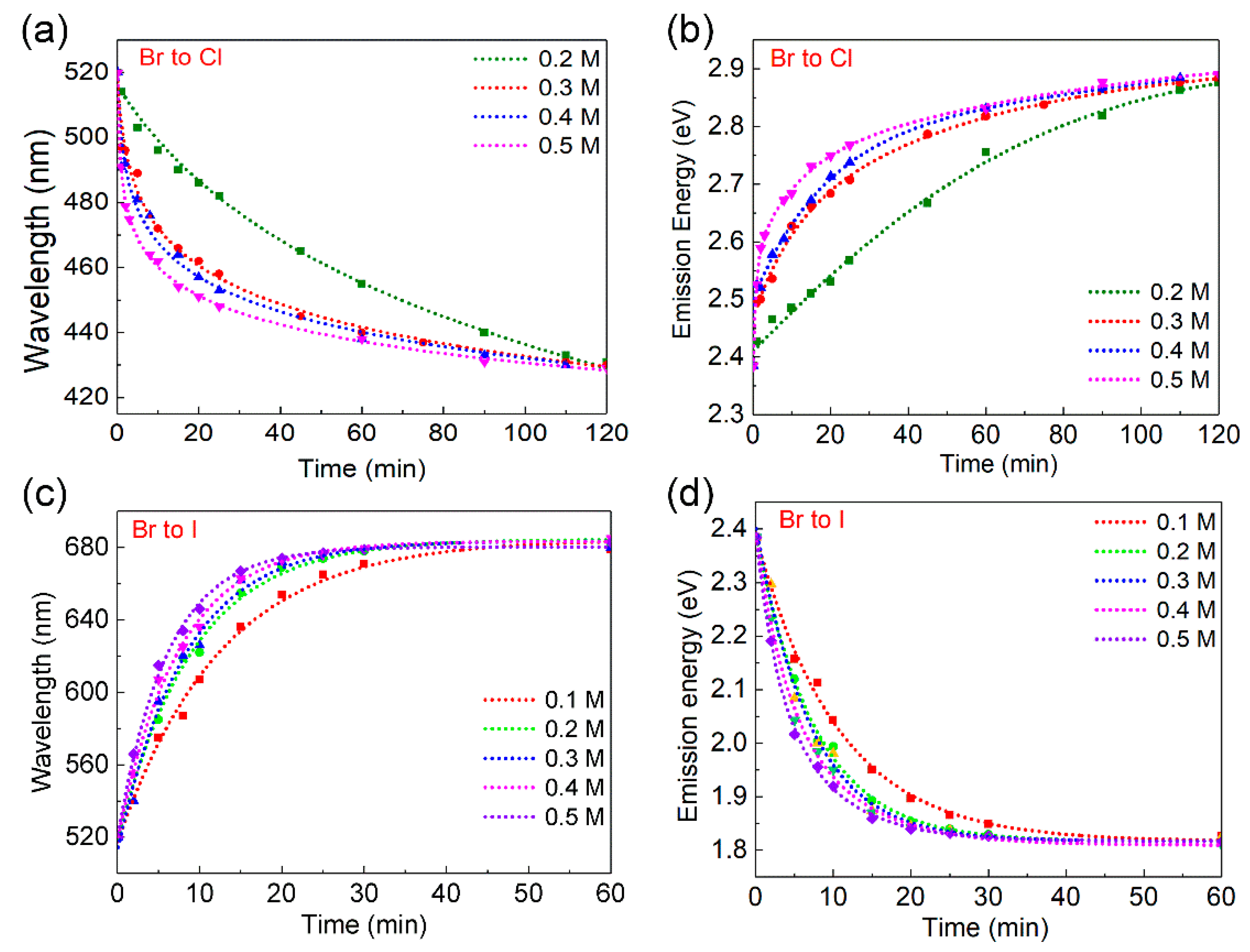
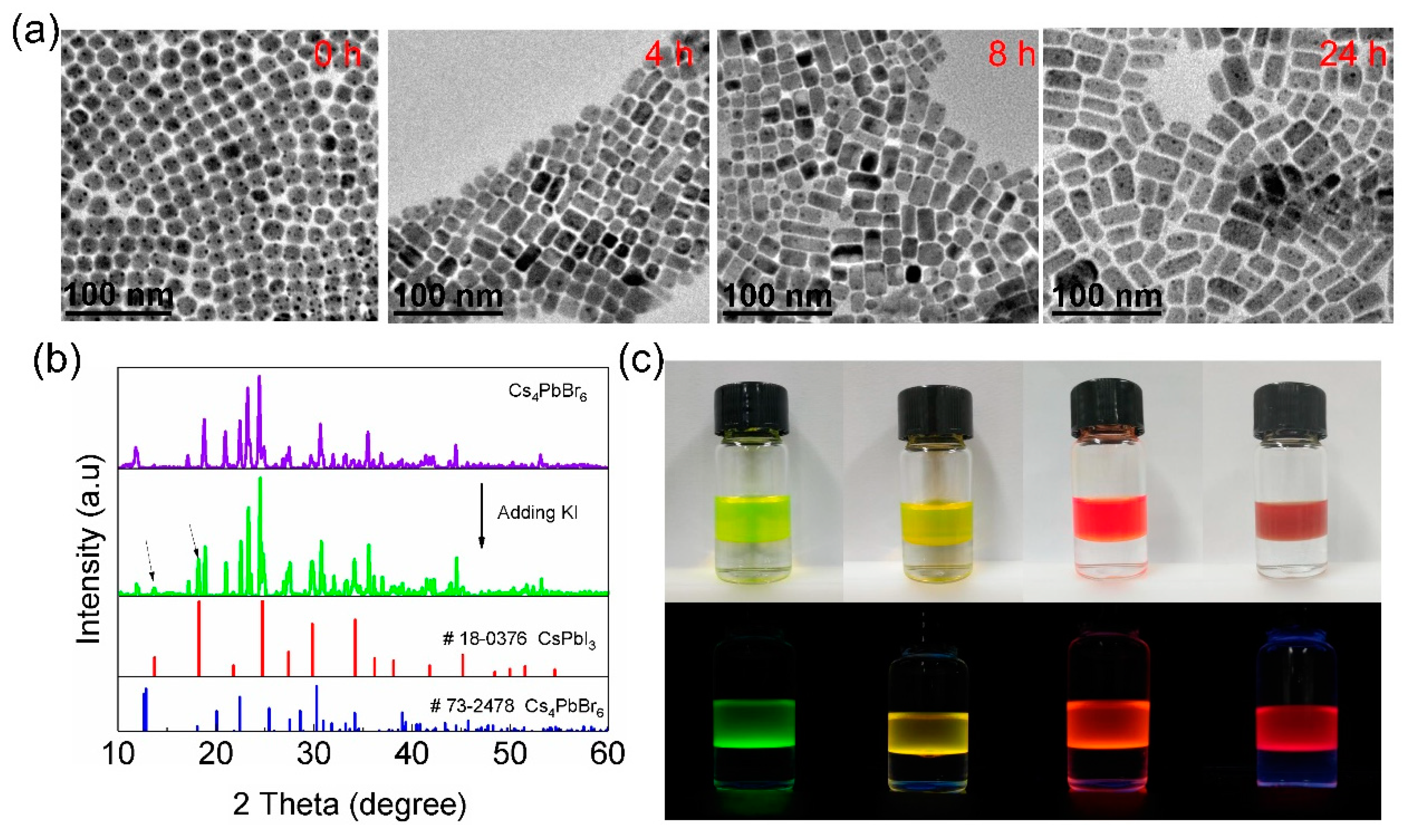
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, X.; Qiu, Y.; Yang, S. Fully-inorganic trihalide perovskite nanocrystals: A new research frontier of optoelectronic materials. Adv. Mater. 2017, 29, 1700775. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Tang, Y.; Song, C.; Xu, K.; Vickers, E.T.; Bonabi Naghadeh, S.; Ding, X.; Li, Z.; Zhang, J.Z. Polar-solvent-free synthesis of highly photoluminescent and stable CsPbBr3 nanocrystals with controlled shape and size by ultrasonication. Chem. Mater. 2018, 31, 365–375. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Li, W.; Zhu, L.; Liu, H.; Geng, H.; Xiang, S.; Liu, J.; Chen, H. Carbon-based CsPbBr3 perovskite solar cells: All-ambient processes and high thermal stability. ACS Appl. Mater. Interfaces 2016, 8, 33649–33655. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.W.; Kim, J.D.; Han, N.S.; Jang, D.M.; Jeong, S.; Park, J.; Song, J.K. Light-matter interactions in cesium lead halide perovskite nanowire lasers. J. Phys. Chem. Lett. 2016, 7, 3703–3710. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, X.; Yu, D.; Huo, C.; Ji, J.; Li, X.; Zhang, S.; Zou, Y.; Zhu, G.; Wang, Y. Boosting two-dimensional MoS2/CsPbBr3 photodetectors via enhanced light absorbance and interfacial carrier separation. ACS Appl. Mater. Interfaces 2018, 10, 2801–2809. [Google Scholar] [CrossRef]
- Rao, L.; Tang, Y.; Yan, C.; Li, J.; Zhong, G.; Tang, K.; Yu, B.; Li, Z.; Zhang, J.Z. Tuning the emission spectrum of highly stable cesium lead halide perovskite nanocrystals through poly (lactic acid)-assisted anion-exchange reactions. J. Mater. Chem. C 2018, 6, 5375–5383. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zhang, T.; Mi, L.; Zhang, Y.; Zhang, Z.; Zhang, W.; Jiang, Y. Solvent-polarity-engineered controllable synthesis of highly fluorescent cesium lead halide perovskite quantum dots and their use in white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 8478–8486. [Google Scholar] [CrossRef]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal halide perovskites for energy applications. Nat. Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Mittal, M.; Singla, A.; Sapra, S. Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. 2017, 53, 3046–3049. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.; Kovalenko, M. Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuan, S.; Wang, X.; Xu, T.; Wang, X.; Li, H.; Zheng, W.; Fan, P.; Li, Y.; Sun, L. Vapor growth and tunable lasing of band gap engineered cesium lead halide perovskite micro/nanorods with triangular cross section. ACS Nano 2016, 11, 1189–1195. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6 (X = Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef]
- Jing, Q.; Su, Y.; Xing, X.; Lu, Z. Highly luminescent CsPbBr3 nanorods synthesized by a ligand-regulated reaction at the water-oil interface. J. Mater. Chem. C 2019, 7, 1854–1858. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Li, Z.; Cao, K.; Li, J.; Tang, Y.; Xu, L.; Ding, X.; Yu, B. Investigation of light-extraction mechanisms of multiscale patterned arrays with rough morphology for gan-based thin-film LEDs. IEEE Access 2019, 7, 73890–73898. [Google Scholar] [CrossRef]
- Rao, L.; Ding, X.; Du, X.; Liang, G.; Tang, Y.; Tang, K.; Zhang, J.Z. Ultrasonication-assisted synthesis of CsPbBr3 and Cs4PbBr6 perovskite nanocrystals and their reversible transformation. Beilstein J. Nanotech. 2019, 10, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.-T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand mediated transformation of cesium lead bromide perovskite nanocrystals to lead depleted Cs4PbBr6 nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, Y.; Bruno, A.; Soci, C.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Intrinsic lead ion emissions in zero-dimensional Cs4PbBr6 Nanocrystals. ACS Energy Lett. 2017, 2, 2805–2811. [Google Scholar] [CrossRef]
- Zhang, Y.; Saidaminov, M.I.; Dursun, I.; Yang, H.; Murali, B.; Alarousu, E.; Yengel, E.; Alshankiti, B.A.; Bakr, O.M.; Mohammed, O.F. Zero-dimensional Cs4PbBr6 perovskite nanocrystals. J. Phys. Chem. Lett. 2017, 8, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Abdelhady, A.L.; Manna, L. Zero-dimensional cesium lead halides: History, properties, and challenges. J. Phys. Chem. Lett. 2018, 9, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.; Wu, H.; Zhang, X.; Sun, C.; Zhang, Y.; Zhang, W.; Zheng, W.; Yu, W.W.; Rogach, A.L. Water-assisted size and shape control of CsPbBr3 perovskite nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Pushkarev, A.P.; Korolev, V.I.; Markina, D.I.; Komissarenko, F.E.; Naujokaitis, A.; Drabavičius, A.; Pakštas, V.; Franckevičius, M.; Khubezhov, S.A.; Sannikov, D.A. A few-minute synthesis of CsPbBr3 nanolasers with a high quality factor by spraying at ambient conditions. ACS Appl. Mater. Interfaces 2018, 11, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Turedi, B.; Lee, K.J.; Dursun, I.; Alamer, B.; Wu, Z.; Alarousu, E.; Mohammed, O.F.; Cho, N.; Bakr, O.M. Water-induced dimensionality reduction in metal-halide perovskites. J. Phys. Chem. C 2018, 122, 14128–14134. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, P.; Gao, D.; Xu, X. Shape-controlled synthesis of all-inorganic CsPbBr3 perovskite nanocrystals with bright blue emission. ACS Appl. Mater. Interfaces 2016, 8, 28824–28830. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Ishida, H.; Bünzli, J.; Beeby, A. Guidelines for measurement of luminescence spectra and quantum yields of inorganic and organometallic compounds in solution and solid state (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 701–711. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B. From nonluminescent Cs4PbX6 (X = Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: Water-triggered transformation through a CsX-stripping mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, C.; Qiu, Z.; Li, J.; Cao, K.; Ding, X.; Tang, Y. Study on the thermal and optical performance of quantum dot white light-emitting diodes using metal-based inverted packaging structure. IEEE Trans. Electron Devices 2019, 66, 3020–3027. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Li, Z.; Ding, X.; Rao, L.; Yu, B. Effect of quantum dot scattering and absorption on the optical performance of white light-emitting diodes. IEEE Trans. Electron Devices 2018, 65, 2877–2884. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H.; Li, J.; Li, Z.; Du, X.; Ding, X.; Yu, B. Improvement of optical and thermal properties for quantum dots WLEDs by controlling layer location. IEEE Access 2019, 7, 77642–77648. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Song, C.; Rao, L.; Lu, H.; Yan, C.; Cao, K.; Ding, X.; Yu, B.; Tang, Y. Synthesis of Highly Photoluminescent All-Inorganic CsPbX3 Nanocrystals via Interfacial Anion Exchange Reactions. Nanomaterials 2019, 9, 1296. https://doi.org/10.3390/nano9091296
Li Z, Song C, Rao L, Lu H, Yan C, Cao K, Ding X, Yu B, Tang Y. Synthesis of Highly Photoluminescent All-Inorganic CsPbX3 Nanocrystals via Interfacial Anion Exchange Reactions. Nanomaterials. 2019; 9(9):1296. https://doi.org/10.3390/nano9091296
Chicago/Turabian StyleLi, Zongtao, Cunjiang Song, Longshi Rao, Hanguang Lu, Caiman Yan, Kai Cao, Xinrui Ding, Binhai Yu, and Yong Tang. 2019. "Synthesis of Highly Photoluminescent All-Inorganic CsPbX3 Nanocrystals via Interfacial Anion Exchange Reactions" Nanomaterials 9, no. 9: 1296. https://doi.org/10.3390/nano9091296
APA StyleLi, Z., Song, C., Rao, L., Lu, H., Yan, C., Cao, K., Ding, X., Yu, B., & Tang, Y. (2019). Synthesis of Highly Photoluminescent All-Inorganic CsPbX3 Nanocrystals via Interfacial Anion Exchange Reactions. Nanomaterials, 9(9), 1296. https://doi.org/10.3390/nano9091296





