Evaporation Rate of Colloidal Droplets of Jet Fuel and Carbon-Based Nanoparticles: Effect of Thermal Conductivity
Abstract
1. Introduction
2. Materials and Methods
3. Experimental Methods
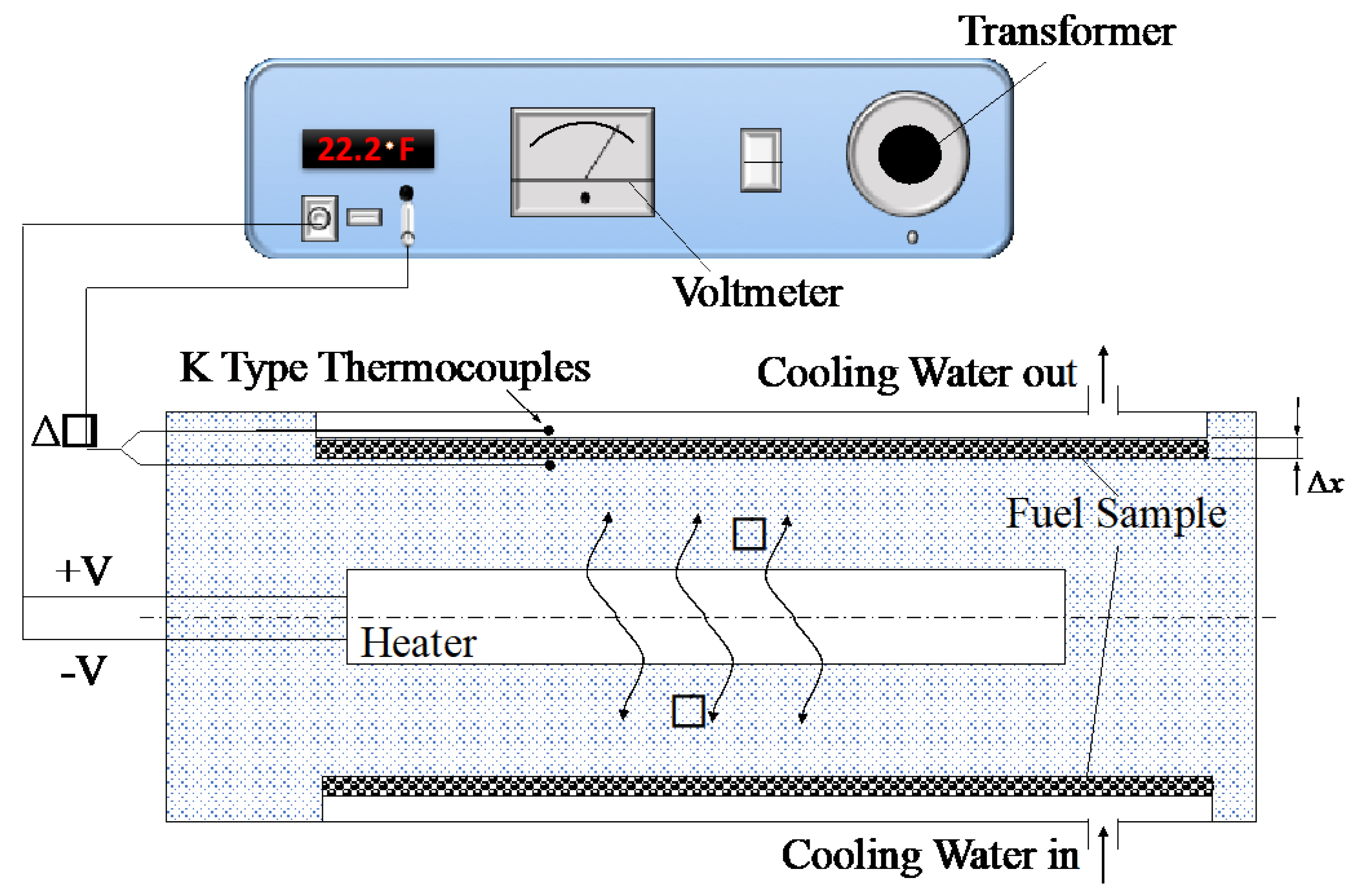
3.1. Thermal Conductivity of Nanofuels
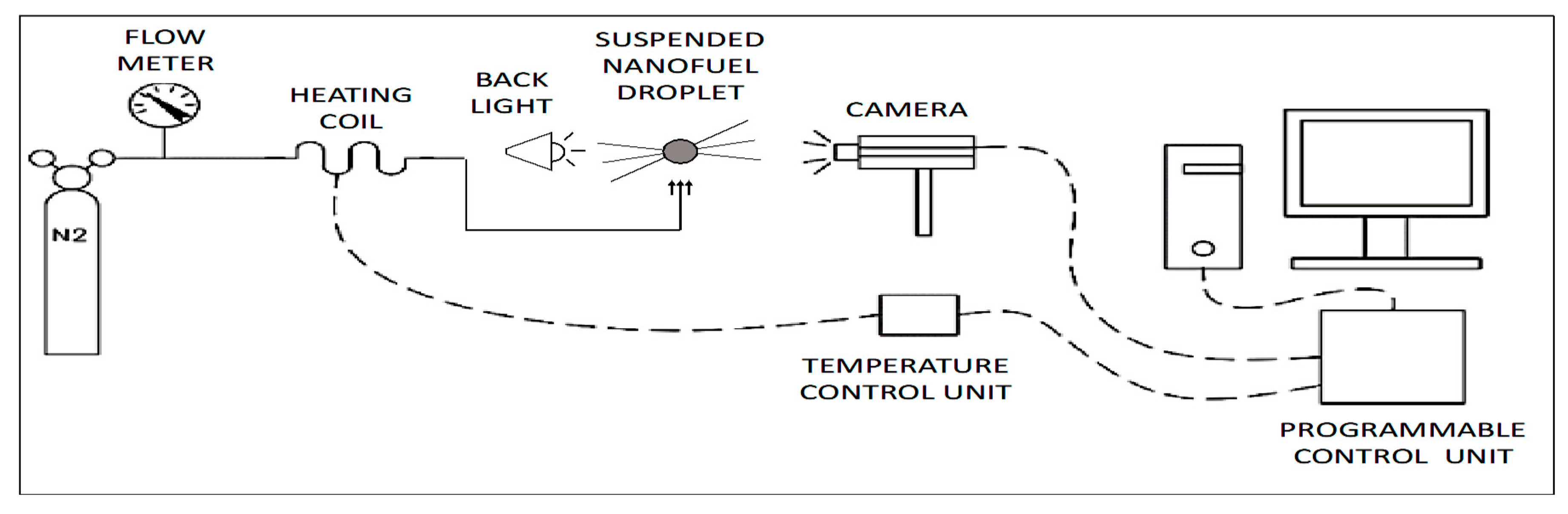
3.2. Evaporation Rate of Nanofuel Droplet
4. Results and Discussion
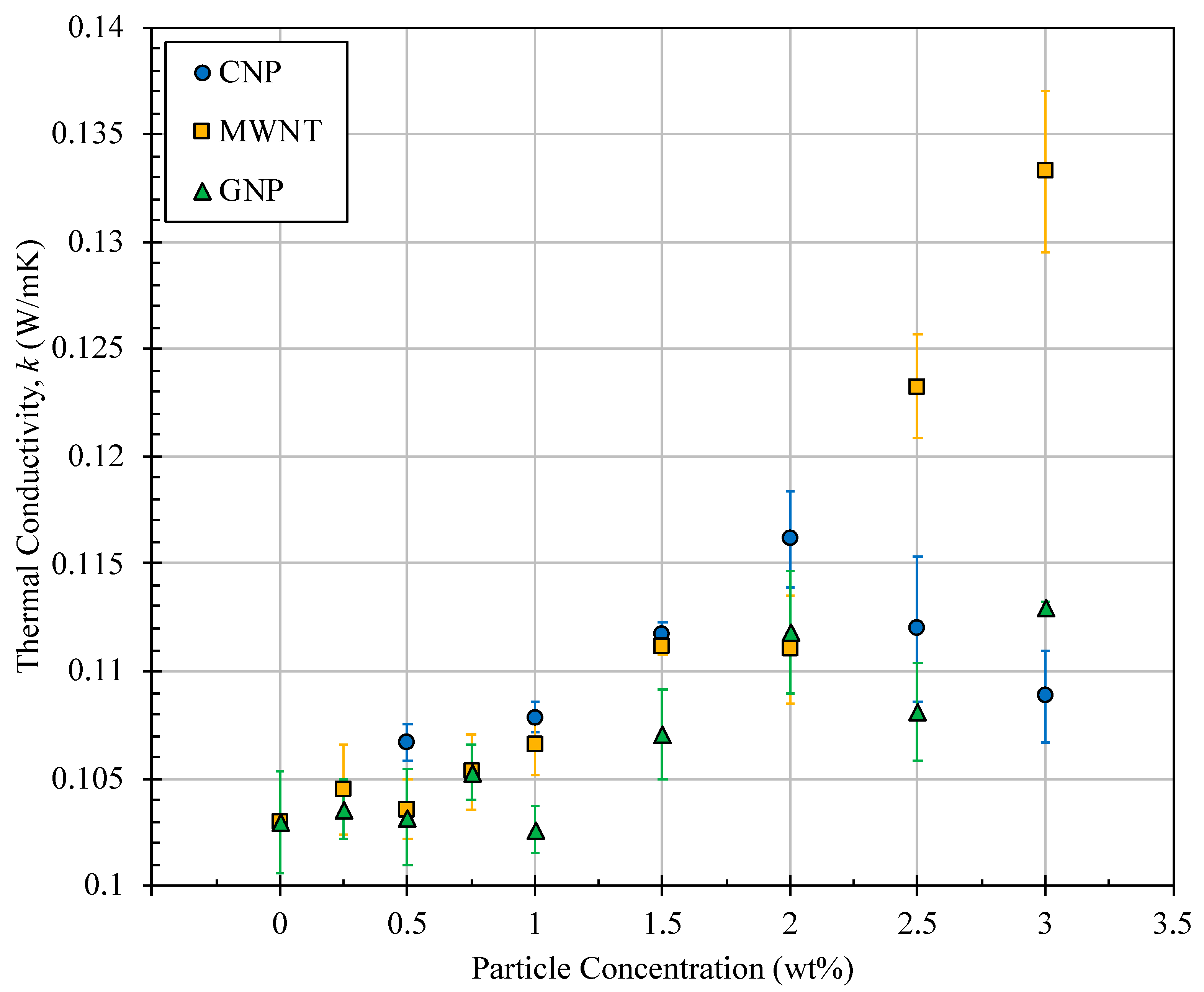
4.1. Thermal Conductivity of Nanofuels
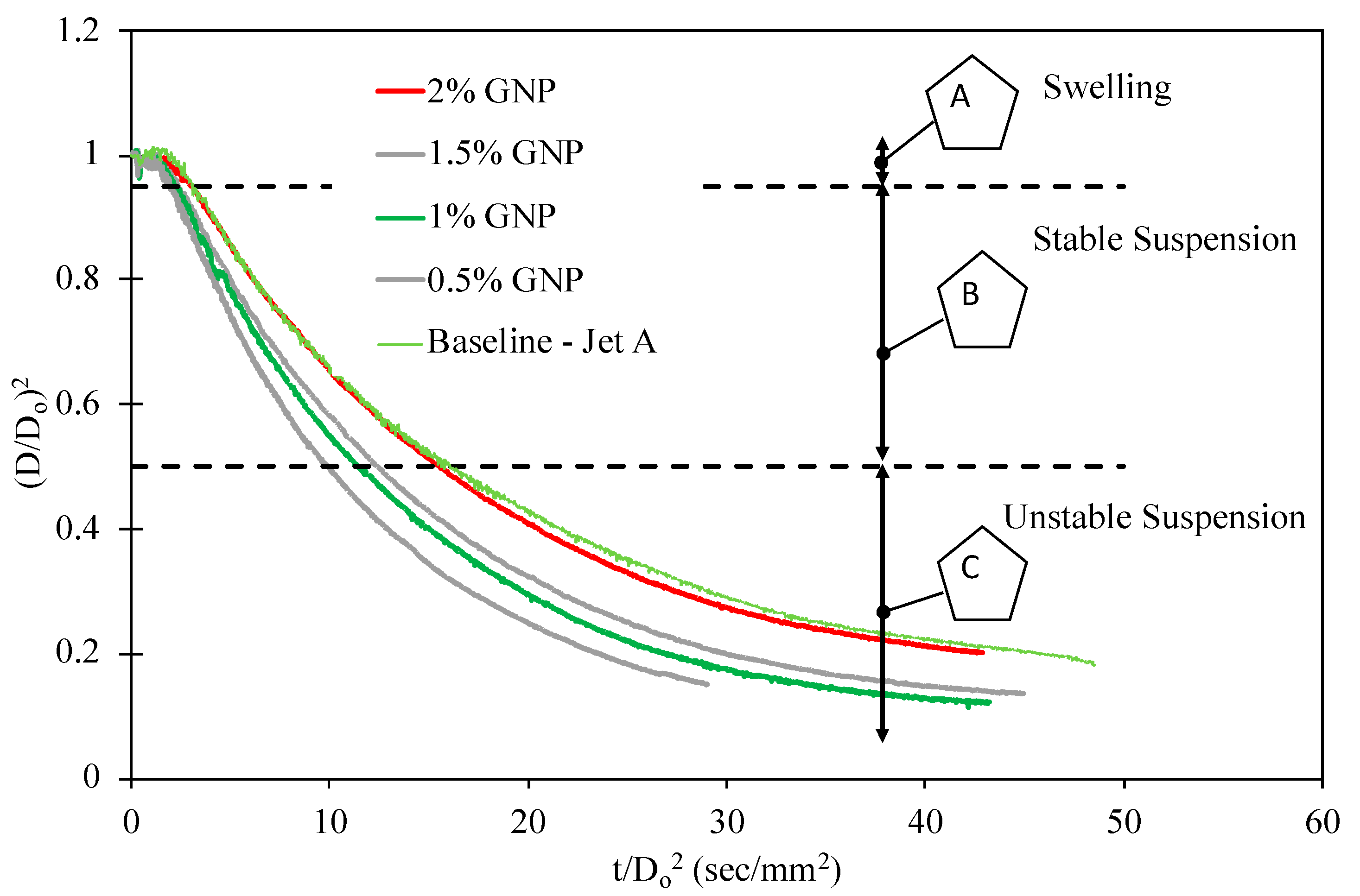
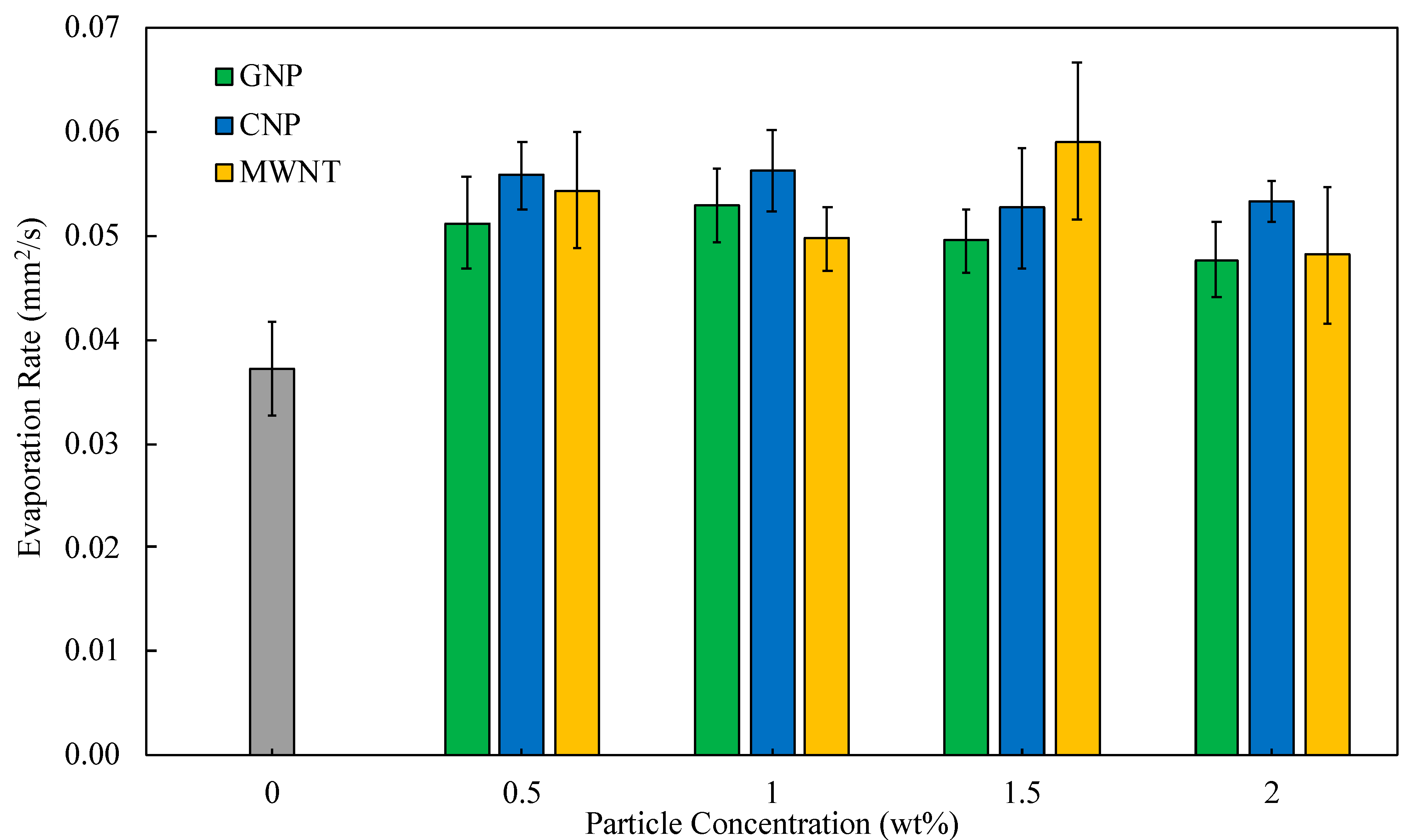
4.2. Evaporation Rate of Nanofuels
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eastman, J.A.; Phillpot, S.; Choi, S.; Keblinski, P. Thermal transport in nanofluids. Annu. Rev. Mater. Res. 2004, 34, 219–246. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Li, S.; Eastman, J. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y.; Ai, F.; Wu, Q. Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys. 2002, 91, 4568–4572. [Google Scholar] [CrossRef]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Albadr, J.; Tayal, S.; Alasadi, M. Heat transfer through heat exchanger using Al2O3 nanofluid at different concentrations. Case Stud. Therm. Eng. 2013, 1, 38–44. [Google Scholar] [CrossRef]
- Seibert, S. Alleviation of Hyperthermia Through Enhanced Heat Transfer via Microchannels: A Simulated Study. Master’s Dissertation, Wilkes University, Wilkes-Barre, PA, USA, 2019. [Google Scholar]
- Kaufui, V.W.; Omar, D.L. Applications of nanofluids: Current and future. Adv. Mech. Eng. 2010. [Google Scholar] [CrossRef]
- Choi, S.U. Nanofluids: From vision to reality through research. J. Heat Transf. 2009, 131, 033106. [Google Scholar] [CrossRef]
- Allen, C.; Mittal, G.; Sung, C.-J.; Toulson, E.; Lee, T. An aerosol rapid compression machine for studying energetic-nanoparticle-enhanced combustion of liquid fuels. Proc. Combust. Inst. 2011, 33, 3367–3374. [Google Scholar] [CrossRef]
- Jones, M.; Li, C.H.; Afjeh, A.; Peterson, G. Experimental study of combustion characteristics of nanoscale metal and metal oxide additives in biofuel (ethanol). Nanoscale Res. Lett. 2011, 6, 246. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Yetter, R.A.; Asay, B.W.; Lloyd, J.M.; Sanders, V.E.; Risha, G.A.; Son, S.F. Effect of Nano-Aluminum and Fumed Silica Particles on Deflagration and Detonation of Nitromethane. Propellants Explos. Pyrotech. 2009, 34, 385–393. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.E.; Prasher, R.; Peck, R.; Lee, T.; Pacheco, J.R.; Arentzen, P. Increased hot-plate ignition probability for nanoparticle-laden diesel fuel. Nano Lett. 2008, 8, 1410–1416. [Google Scholar] [CrossRef]
- Singh, G.; Esmaeilpour, M.; Ratner, A. The effect of acetylene black on droplet combustion and flame regime of petrodiesel and soy biodiesel. Fuel 2019, 246, 108–116. [Google Scholar] [CrossRef]
- Ghamari, M.; Ratner, A. Combustion characteristics of colloidal droplets of jet fuel and carbon based nanoparticles. Fuel 2017, 188, 182–189. [Google Scholar] [CrossRef]
- Tanvir, S.; Qiao, L. Droplet burning rate enhancement of ethanol with the addition of graphite nanoparticles: Influence of radiation absorption. Combust. Flame 2016, 166, 34–44. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Optical properties and radiation-enhanced evaporation of nanofluid fuels containing carbon-based nanostructures. Energy Fuels 2012, 26, 4224–4230. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Evaporation characteristics of fuel droplets with the addition of nanoparticles under natural and forced convections. Int. J. Heat Mass Transf. 2011, 54, 4913–4922. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Yetter, R.A.; Parimi, V.S. Exploring the effects of nanostructured particles on liquid nitromethane combustion. J. Propuls. Power 2010, 26, 1006–1015. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar] [CrossRef]
- Beloni, E.; Hoffmann, V.K.; Dreizin, E.L. Combustion of decane-based slurries with metallic fuel additives. J. Propuls. Power 2008, 24, 1403–1411. [Google Scholar] [CrossRef]
- Antaki, P.; Williams, F. Observations on the combustion of boron slurry droplets in air. Combust. Flame 1987, 67, 1–8. [Google Scholar] [CrossRef]
- Gan, Y.; Lim, Y.S.; Qiao, L. Combustion of nanofluid fuels with the addition of boron and iron particles at dilute and dense concentrations. Combust. Flame 2012, 159, 1732–1740. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Dabbs, D.M.; Yetter, R.A.; Dryer, F.L.; Aksay, I.A. Functionalized graphene sheet colloids for enhanced fuel/propellant combustion. ACS Nano 2009, 3, 3945–3954. [Google Scholar] [CrossRef]
- Finigan, D.J.; Dohm, B.D.; Mockelman, J.A.; Oehlschlaeger, M.A. Deflagration-to-detonation transition via the distributed photo ignition of carbon nanotubes suspended in fuel/oxidizer mixtures. Combust. Flame 2012, 159, 1314–1320. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.-K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef]
- Graphene Nanoplatelets Technical Note [Online]. Available online: https://www.strem.com/catalog/v/06-0210/12/carbon_1034343-98-0 (accessed on 26 July 2019).
- Prasher, R.; Bhattacharya, P.; Phelan, P.E. Thermal conductivity of nanoscale colloidal solutions (nanofluids). Phys. Rev. Lett. 2005, 94, 025901. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.P.; Choi, S.U. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Hamilton-Crosser model. J. Nanoparticle Res. 2004, 6, 355–361. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, L. Radiation-Induced Droplet Breakup of Nano-Dispersed Coal-in-Water Colloidal Fuels. In Proceedings of the AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 14–17 July 2013; pp. 1–14. [Google Scholar]
- Sahoo, B.C.; Das, D.K.; Vajjha, R.S.; Satti, J.R. Measurement of the thermal conductivity of silicon dioxide nanofluid and development of correlations. J. Nanotechnol. Eng. Med. 2012, 3, 041006. [Google Scholar] [CrossRef]
- Ghamari, M.; Aboalhamayie, A. Thermal Conductivity of Colloidal Suspensions of Jet Fuel and Carbon-Based Nanoparticles and its Effect on Evaporation Rate. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018; p. V08AT10A025. [Google Scholar]
- Wang, G.; Xu, A.; He, P.; Guo, Q.; Liu, Z.; Wang, Z.; Li, J.; Hu, X.; Wang, Z.; Chen, D.; et al. Green preparation of lattice phosphorus doped graphene quantum dots with tunable emission wavelength for bio-imaging. Mater. Lett. 2019, 242, 156–159. [Google Scholar] [CrossRef]
- Zora, A.; PTriberis, G.; Simserides, C. Near-field optical properties of quantum dots, applications and perspectives. Recent Pat. Nanotechnol. 2011, 5, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, M.; Ratner, A. Combustion characteristics of diesel and Jet-A droplets blended with polymeric additive. Fuel 2016, 178, 63–70. [Google Scholar] [CrossRef]
- Hicks, M.C.; Nayagam, V.; Williams, F.A. Methanol droplet extinction in carbon-dioxide-enriched environments in microgravity. Combust. Flame 2010, 157, 1439–1445. [Google Scholar] [CrossRef]
- Klimek, R.; Wright, T. Spotlight: Image Analysis and Object Tracking Software [Online]. 2004. Available online: https://ntrs.nasa.gov/search.jsp?R=20060011194 (accessed on 26 July 2019).
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502/1–215502/4. [Google Scholar] [CrossRef] [PubMed]
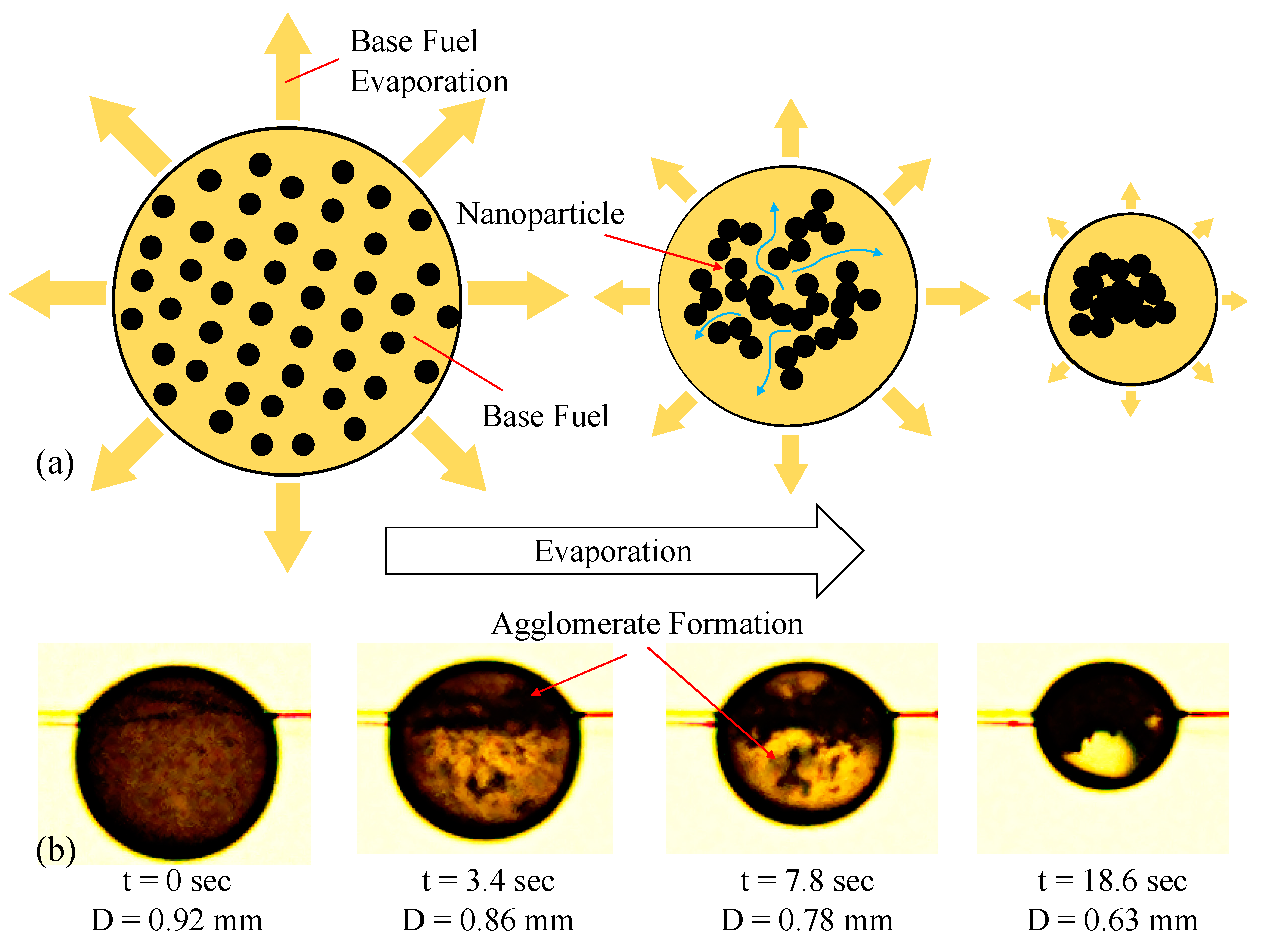
| Particle Type | CNP | MWNT | GNP |
|---|---|---|---|
| Size (nm) | 100 | OD 8–15; ID 3–5; length 3-5 | 6–8 thick; 5000 wide |
| Bulk Density (g/cm3) | 0.37 | 0.36–0.42 | 0.03–0.1 |
| SSA (m2/g) | ~162 | >233 | 120–150 |
| C% | 88.1 | >95 | >99.5 |
| Particle Type | K (mm2/s) | % Increase | Optimal Concentration |
|---|---|---|---|
| Baseline | 0.0372 | - | - |
| MWNT | 0.0591 | 58.8 | 1.5 |
| CNP | 0.0563 | 51.2 | 1.0 |
| GNP | 0.0530 | 42.3 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboalhamayie, A.; Festa, L.; Ghamari, M. Evaporation Rate of Colloidal Droplets of Jet Fuel and Carbon-Based Nanoparticles: Effect of Thermal Conductivity. Nanomaterials 2019, 9, 1297. https://doi.org/10.3390/nano9091297
Aboalhamayie A, Festa L, Ghamari M. Evaporation Rate of Colloidal Droplets of Jet Fuel and Carbon-Based Nanoparticles: Effect of Thermal Conductivity. Nanomaterials. 2019; 9(9):1297. https://doi.org/10.3390/nano9091297
Chicago/Turabian StyleAboalhamayie, Ahmed, Luigi Festa, and Mohsen Ghamari. 2019. "Evaporation Rate of Colloidal Droplets of Jet Fuel and Carbon-Based Nanoparticles: Effect of Thermal Conductivity" Nanomaterials 9, no. 9: 1297. https://doi.org/10.3390/nano9091297
APA StyleAboalhamayie, A., Festa, L., & Ghamari, M. (2019). Evaporation Rate of Colloidal Droplets of Jet Fuel and Carbon-Based Nanoparticles: Effect of Thermal Conductivity. Nanomaterials, 9(9), 1297. https://doi.org/10.3390/nano9091297






