Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
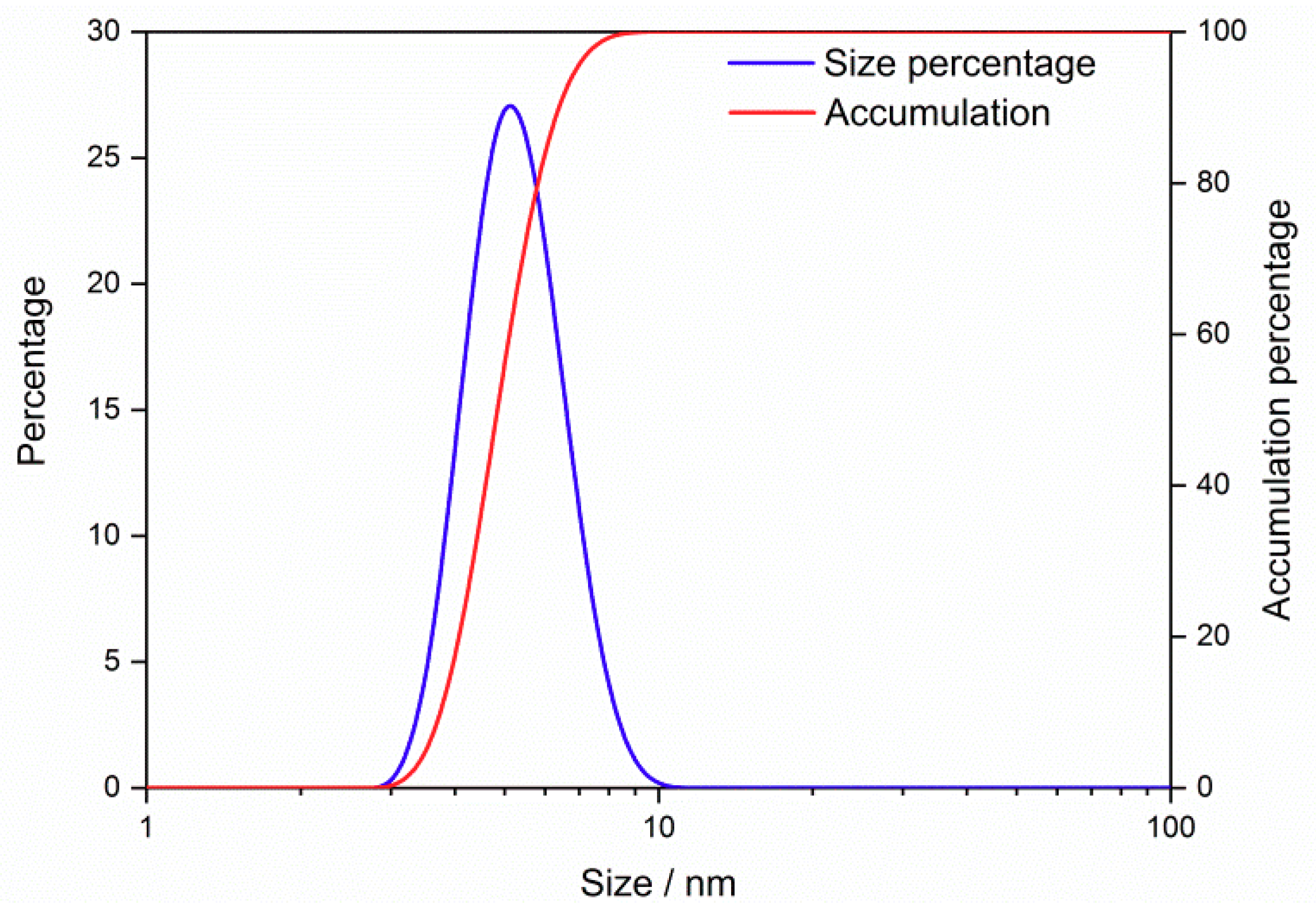
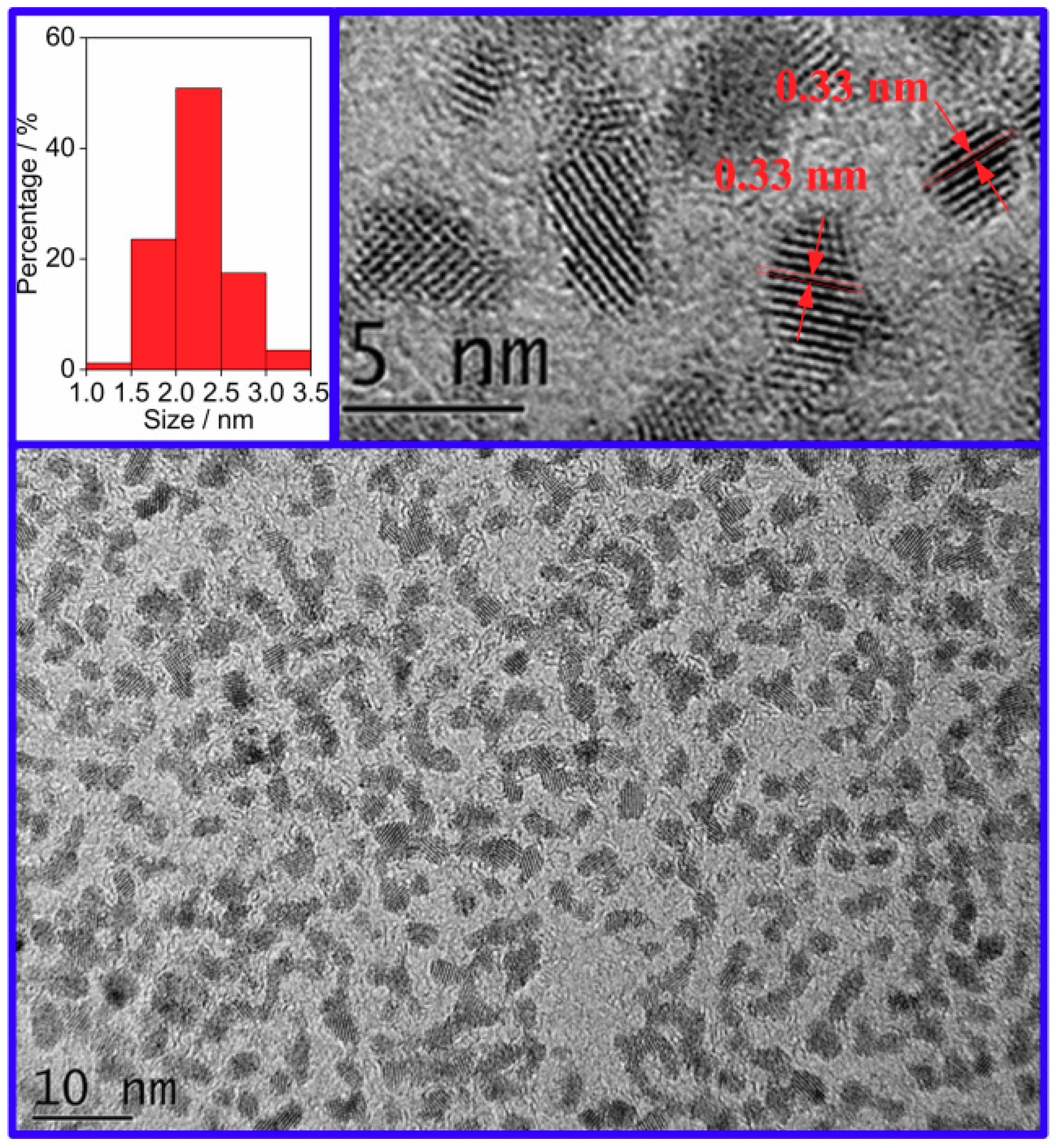
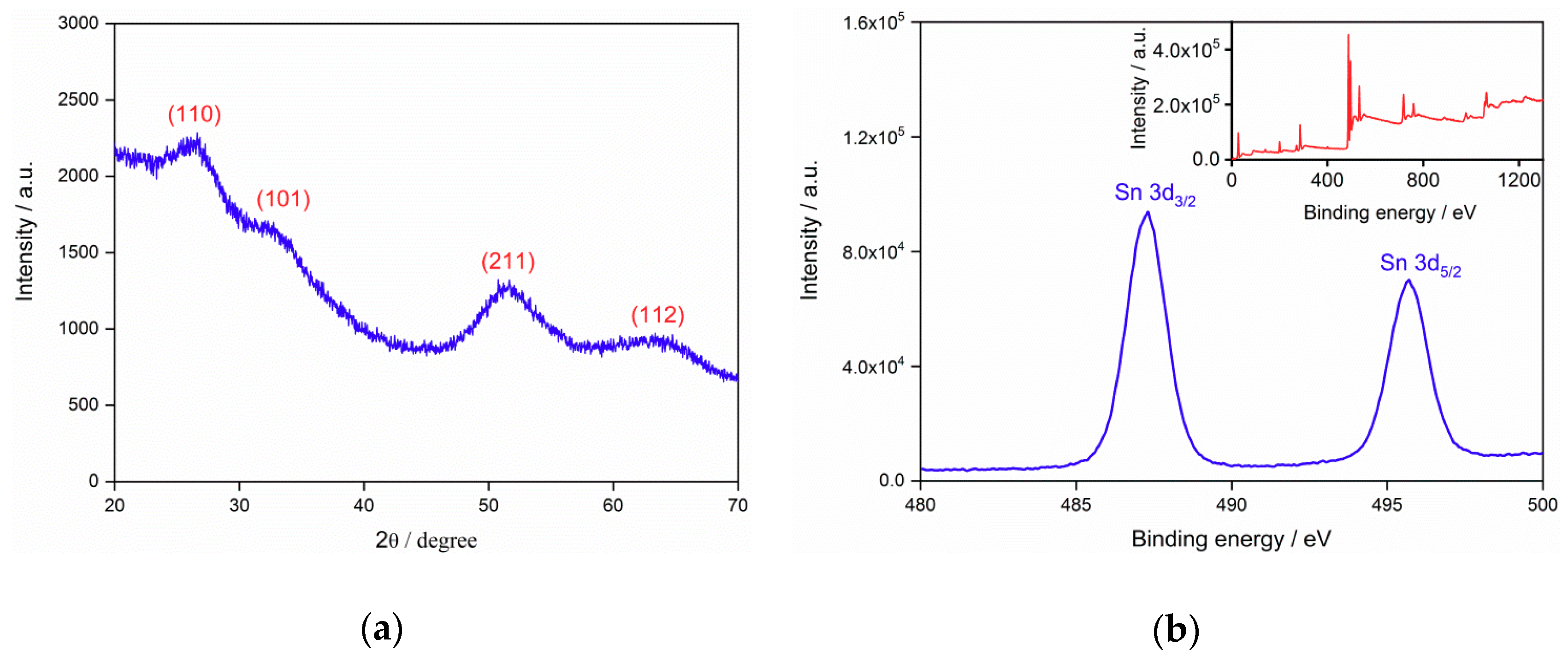
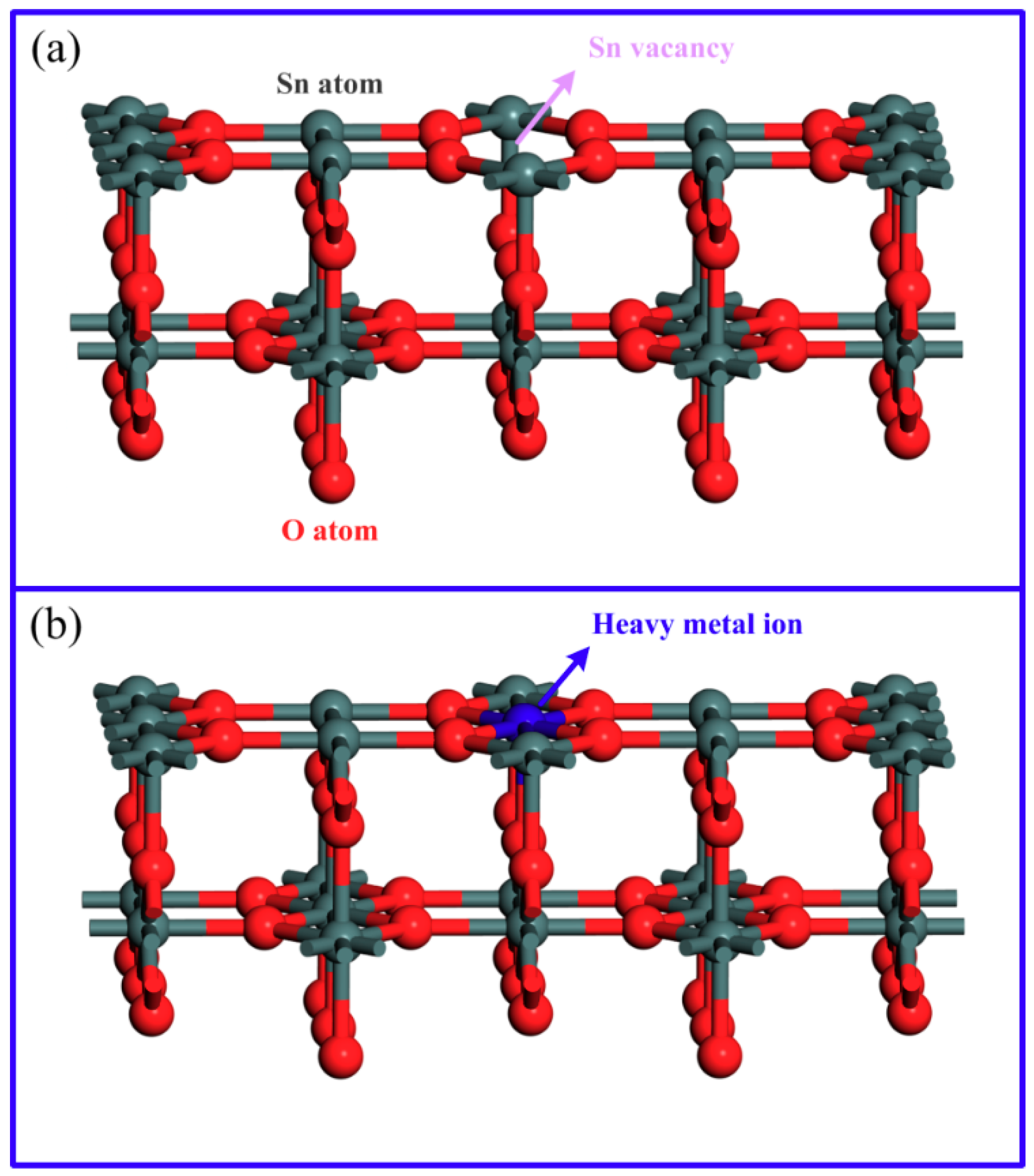
3.1. Structure and Morphology
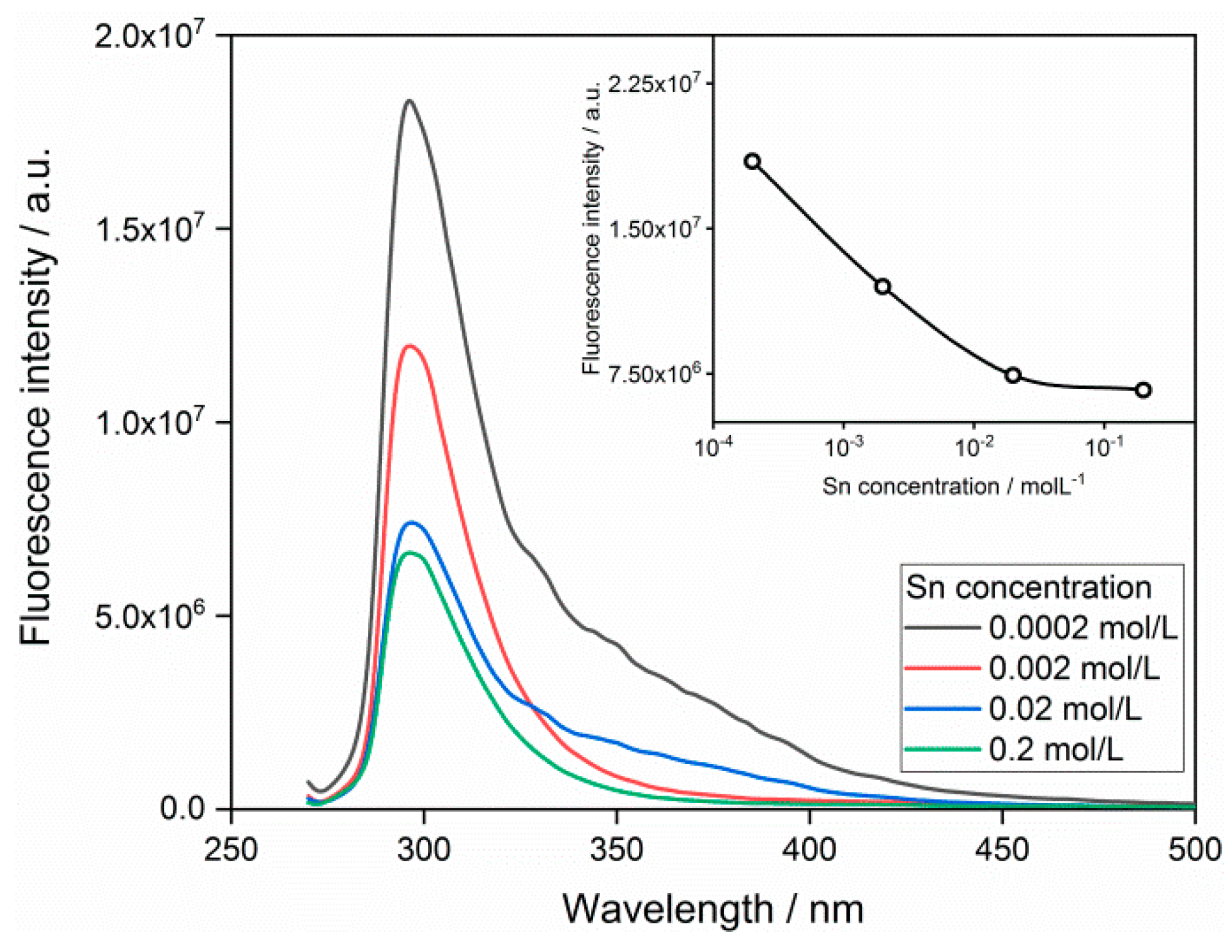
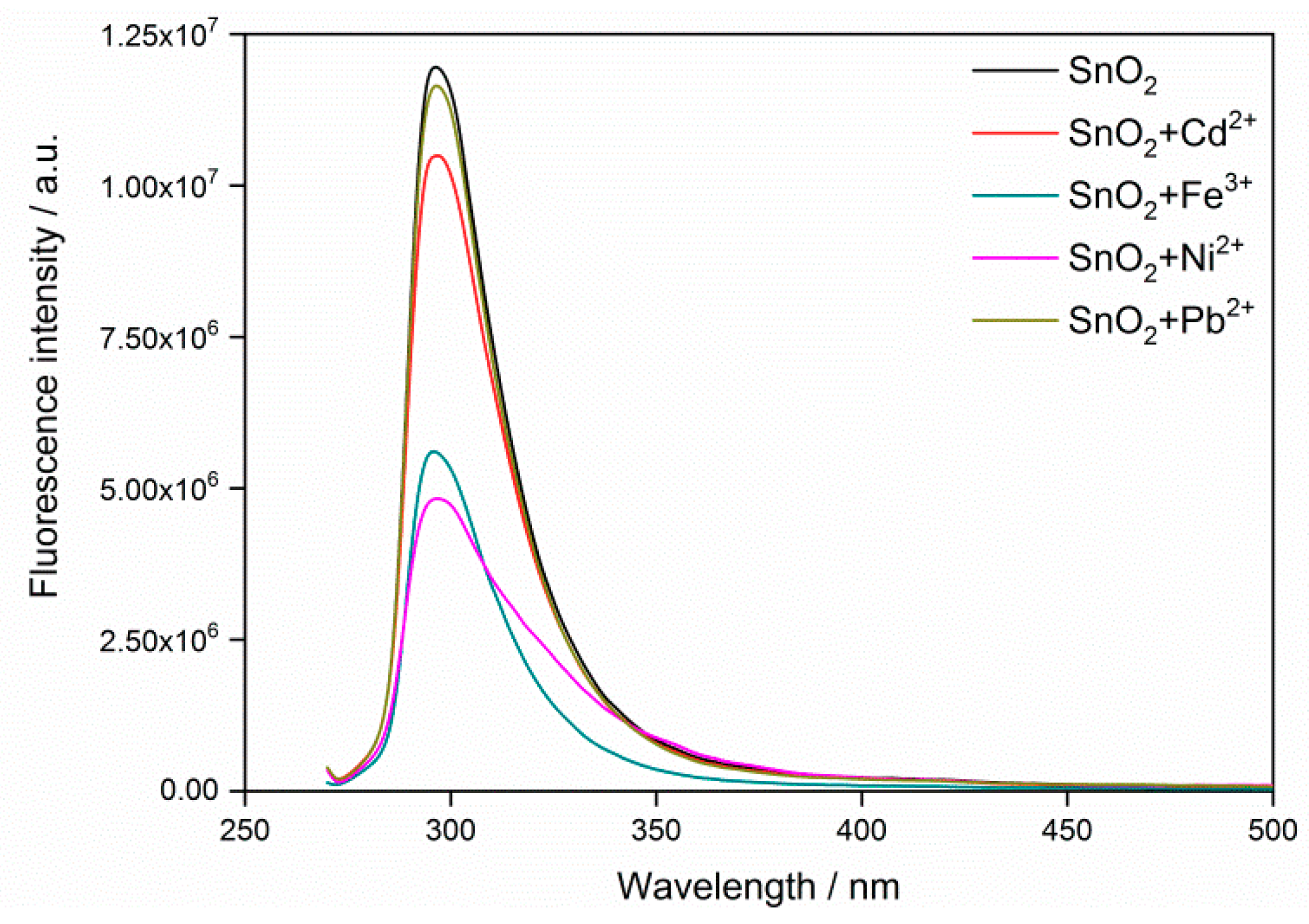
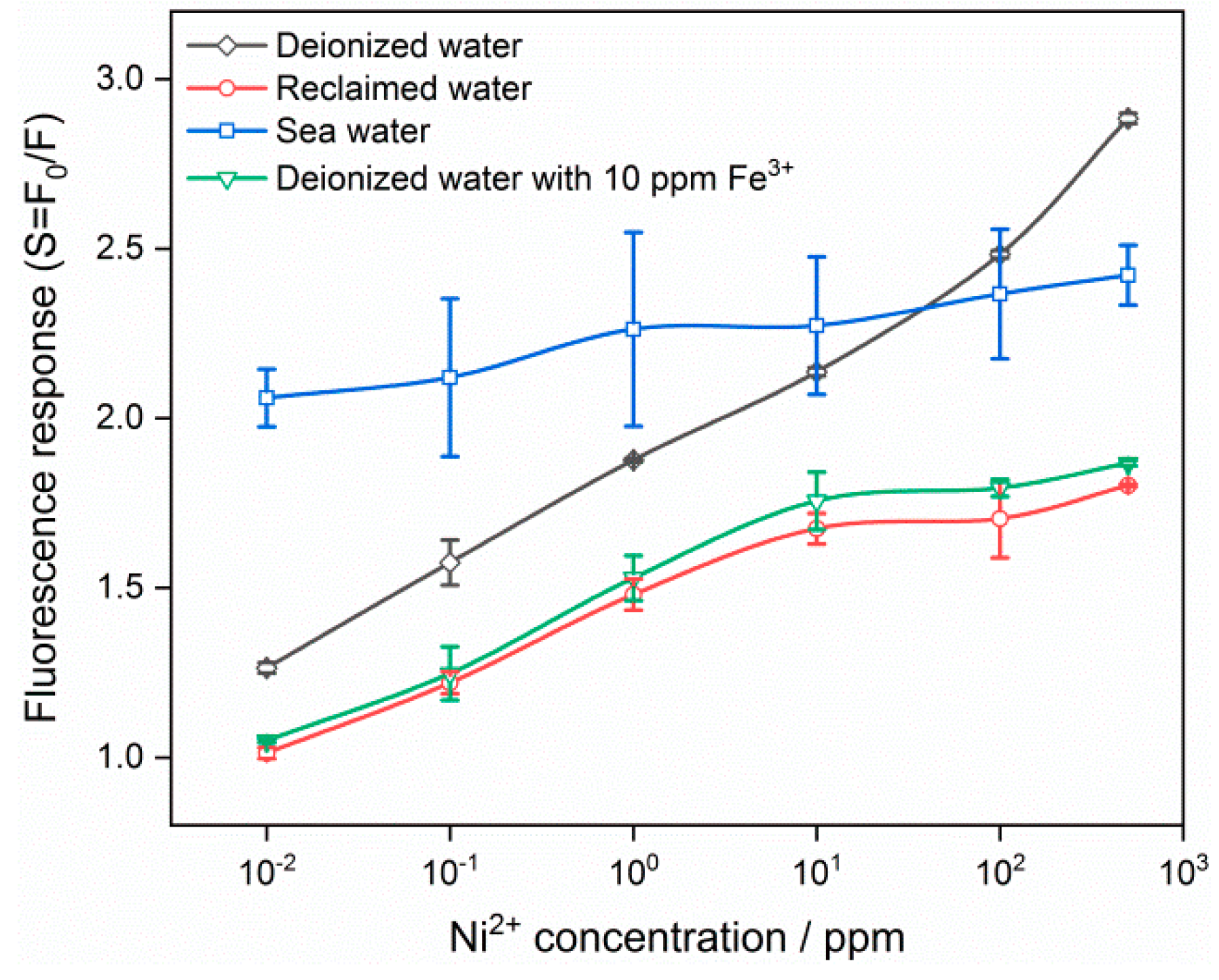
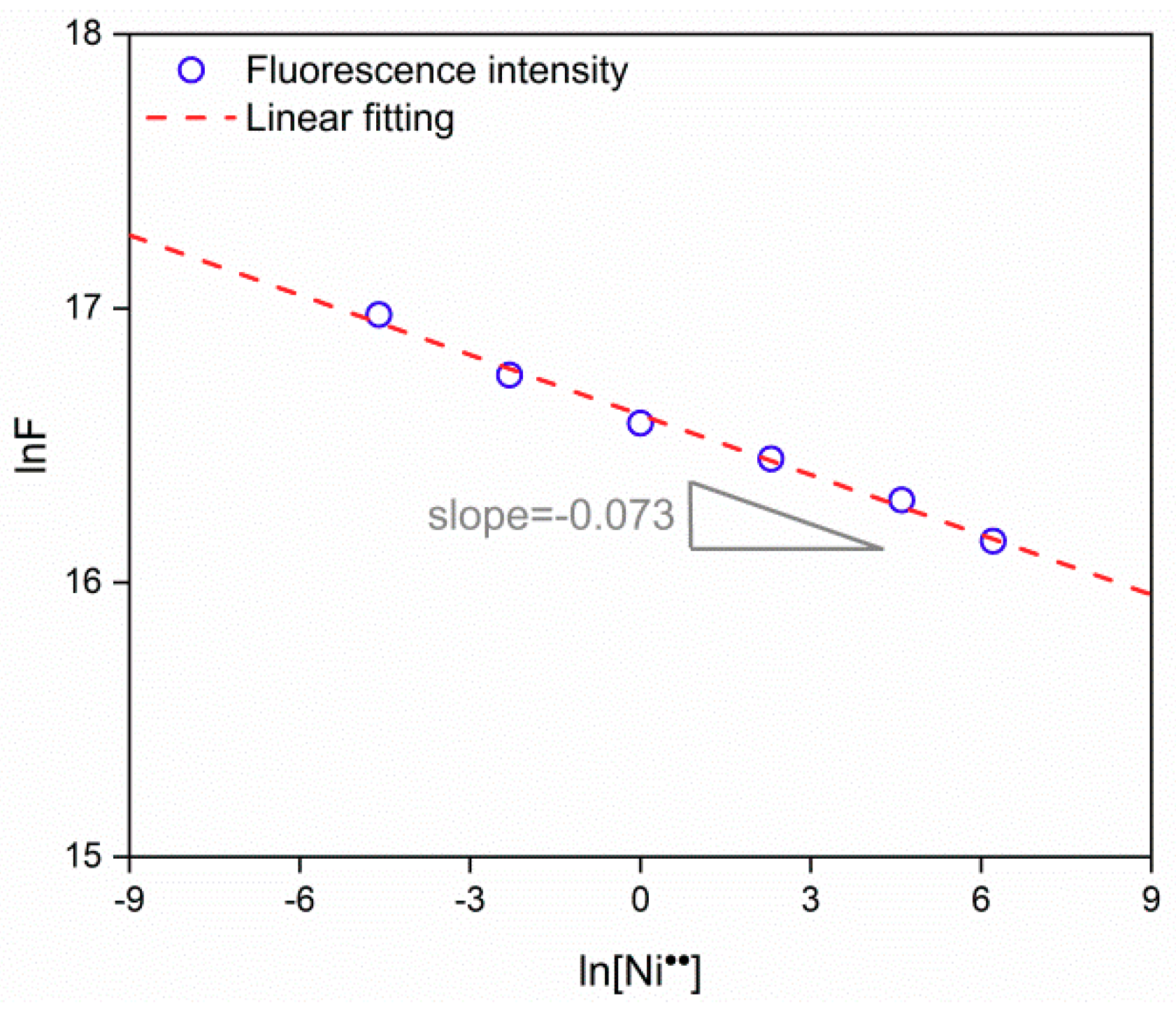
3.2. Fluorescence Response to Heavy Metal Ions
3.3. First Principle Calculation
3.4. Mechanism of Fluorescence Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Agouborde, L.; Navia, R. Heavy metals retention capacity of a non-conventional sorbent developed from a mixture of industrial and agricultural wastes. J. Hazard. Mater. 2009, 167, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Takafuji, M.; Ihara, H. Peptide-based surface modified silica particles: Adsorption materials for dye-loaded wastewater treatment. RSC Adv. 2013, 3, 23664–23672. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Improved removal of lead(II) from water using a polymer-based graphene oxide nanocomposite. J. Mater. Chem. A 2013, 1, 3789–3796. [Google Scholar] [CrossRef]
- Dutta, D.; Thiyagarajan, S.; Bahadur, D. SnO2 quantum dots decorated reduced graphene oxide nanocomposites for efficient water remediation. Chem. Eng. J. 2016, 297, 55–65. [Google Scholar] [CrossRef]
- Suk Hyun, J.; Byung Gil, M.; Young Gyu, J.; Won Seok, L.; Cheol, L.S. Removal of lead ions in aqueous solution by hydroxyapatite/polyurethane composite foams. J. Hazard. Mater. 2008, 152, 1285–1292. [Google Scholar]
- Zepeda, A.M.; Gonzalez, D.; Heredia, L.G.; Marquez, K.; Perez, C.; Pena, E.; Flores, K.; Valdes, C.; Eubanks, T.M.; Parsons, J.G. Removal of Cu2+ and Ni2+ from aqueous solution using SnO2 nanomaterial effect of: pH, time, temperature, interfering cations. Microchem. J. 2018, 141, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, V.; Subray, S.H.; Ramamurthy, P. A green synthesis of highly luminescent carbon dots from itaconic acid and their application as an efficient sensor for Fe3+ ions in aqueous medium. New J. Chem. 2018, 42, 8933–8942. [Google Scholar] [CrossRef]
- Hussein, H.; Farag, S.; Kandil, K.; Moawad, H. Tolerance and uptake of heavy metals by Pseudomonads. Process Biochem. 2005, 40, 955–961. [Google Scholar] [CrossRef]
- Claudio, E.S.; Godwin, H.A.; Magyar, J.S. Fundamental coordination chemistry, environmental chemistry, and biochemistry of lead(II). Cheminform 2003, 34, 1–144. [Google Scholar] [CrossRef]
- Magyar, J.S.; Tsu-Chien, W.; Stern, C.M.; Dye, D.F.; Rous, B.W.; Payne, J.C.; Bridgewater, B.M.; Ana, M.; Gerard, P.; Zaleski, J.M. Reexamination of lead(II) coordination preferences in sulfur-rich sites: Implications for a critical mechanism of lead poisoning. J. Am. Chem. Soc. 2005, 127, 9495–9505. [Google Scholar] [CrossRef] [PubMed]
- Bouton, C.M.; Frelin, L.P.; Forde, C.E.; Arnold, G.H.; Pevsner, J. Synaptotagmin I is a molecular target for lead. J. Neurochem. 2010, 76, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Claudia, C.; Enrico, M.; Catherine, K. Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol. 2004, 134, 716–725. [Google Scholar]
- Ping, L.; Feng, X.; Qiu, G. Methylmercury exposure and health effects from rice and fish consumption: A review. Int. J. Environ. Res. Public Health 2010, 7, 2666–2691. [Google Scholar]
- Tokalioglu, S.; Kartal, S.; Elci, L. Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure. Anal. Chim. Acta 2000, 413, 33–40. [Google Scholar] [CrossRef]
- Rao, G.P.C.; Kalluru, S.; Yerra Koteswara, R.; Wang, M.C. Solid phase extraction of Cd, Cu, and Ni from leafy vegetables and plant leaves using amberlite XAD-2 functionalized with 2-hydroxy-acetophenone-thiosemicarbazone (HAPTSC) and determination by inductively coupled plasma atomic emission spectroscopy. J. Agric. Food Chem. 2006, 54, 2868–2872. [Google Scholar] [CrossRef]
- Zhuo, W.; Zhangrun, X.; Jianhua, W. Flow injection on-line solid phase extraction for ultra-trace lead screening with hydride generation atomic fluorescence spectrometry. Analyst 2005, 131, 141–147. [Google Scholar]
- Prete, A. Study of fluorescence quenching of mercaptosuccinic acid-capped CdS quantum dots in the presence of some heavy metal ions and its application to Hg(II) ion determination. Luminescence 2015, 29, 798–804. [Google Scholar]
- Wang, Y.Q.; Chao, Y.; Zhu, Z.H.; Hu, Y.Z. Cadmium telluride quantum dots as pH-sensitive probes for tiopronin determination. Anal. Chim. Acta 2008, 610, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Rama, M.J.; Hall, E.A. A quantum dot-lucigenin probe for Cl-. Analyst 2008, 133, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, C. Sonochemical synthesis of cyclodextrin-coated quantum dots for optical detection of pollutant phenols in water. Chem. Mater. 2008, 20, 6053–6059. [Google Scholar] [CrossRef]
- Rogach, A.L. Fluorescence energy transfer in hybrid structures of semiconductor nanocrystals. Nano Today 2011, 6, 355–365. [Google Scholar] [CrossRef]
- Luby, B.M.; Charron, D.M.; MacLaughlin, C.M.; Zheng, G. Activatable fluorescence: From small molecule to nanoparticle. Adv. Drug. Deliv. Rev. 2017, 113, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Emril Mohamed, A.; Yuangang, Z.; Hsiao-Hua, Y.; Ying, J.Y. Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal. Chem. 2007, 79, 9452–9458. [Google Scholar] [CrossRef] [PubMed]
- Koneswaran, M.; Narayanaswamy, R. l-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Maria Jose, R.R.; Hall, E.A.H. Azamacrocycle activated quantum dot for zinc ion detection. Anal. Chem. 2008, 80, 8260–8268. [Google Scholar]
- Maria Jose, R.R.; Hall, E.A.H. Multiplexed energy transfer mechanisms in a dual-function quantum dot for zinc and manganese. Analyst 2008, 134, 159–169. [Google Scholar]
- Nagarajan, N.; Paramaguru, G.; Vanitha, G.; Renganathan, R. Photosensitization of Colloidal SnO2 Semiconductor Nanoparticles with Xanthene Dyes. J. Chem. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Comprehensive Screen of Metal Oxide Nanoparticles for DNA Adsorption, Fluorescence Quenching, and Anion Discrimination. ACS Appl. Mat. Interfaces 2015, 7, 24833–24838. [Google Scholar] [CrossRef] [PubMed]
- Suvetha Rani, J.; Ramakrishnan, V. Interaction of Schiff base ligand with tin dioxide nanoparticles: Optical studies. Spectrochim. Acta Part A 2013, 114, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, S.; Liu, J.; Hu, Z.; Gao, N.; Zhang, J.; Yi, F.; Zhang, G.; Jiang, S.; Liu, H. Enhanced catalytic activity of SnO2 quantum dot films employing atomic ligand-exchange strategy for fast response H2S gas sensors. Sens. Actuators B 2018, 271, 147–156. [Google Scholar] [CrossRef]
- Liu, H.; Xu, S.; Li, M.; Shao, G.; Song, H.; Zhang, W.; Wei, W.; He, M.; Gao, L.; Song, H. Chemiresistive gas sensors employing solution-processed metal oxide quantum dot films. Appl. Phys. Lett. 2014, 105, 766. [Google Scholar] [CrossRef]
- Babu, B.; Neelakanta Reddy, I.; Yoo, K.; Kim, D.; Shim, J. Bandgap tuning and XPS study of SnO2 quantum dots. Mater. Lett. 2018, 221, 211–215. [Google Scholar] [CrossRef]
- Liu, J.; Xue, W.; Jin, G.; Zhai, Z.; Lv, J.; Hong, W.; Chen, Y. Preparation of tin oxide quantum dots in aqueous solution and applications in semiconductor gas sensors. Nanomaterials 2019, 9, 240. [Google Scholar] [CrossRef]
- Stranick, M.A.; Moskwa, A. SnO2 by XPS. Surf. Sci. Spectra 1993, 2, 50–54. [Google Scholar] [CrossRef]
- Barreca, D.; Garon, S.; Tondello, E.; Zanella, P. SnO2 nanocrystalline thin films by XPS. Surf. Sci. Spectra 2000, 7, 81–85. [Google Scholar] [CrossRef]
- Moore, D.E.; Patel, K. Q-CdS Photoluminescence Activation on Zn2+ and Cd2+ Salt Introduction. Langmuir 2001, 17, 2541–2544. [Google Scholar] [CrossRef]
- Xie, H.Y.; Liang, J.G.; Zhang, Z.L.; Liu, Y.; He, Z.K.; Pang, D.W. Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2527–2530. [Google Scholar] [CrossRef]
- Henry, J.; Mohanraj, K.; Sivakumar, G.; Umamaheswari, S. Electrochemical and fluorescence properties of SnO2 thin films and its antibacterial activity. Spectrochim. Acta Part A 2015, 143, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Baranov, A.V.; Orlova, A.O.; Maslov, V.G.; Toropova, Y.A.; Berwick, K. Dissociative CdSe/ZnS quantum dot-molecule complex for luminescent sensing of metal ions in aqueous solutions. J. Appl. Phys. 2010, 108, 37. [Google Scholar] [CrossRef]
- Mahapatra, N.; Panja, S.; Mandal, A.; Halder, M. A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co2+ and Ni2+ ions. J. Mater. Chem. C 2014, 2, 7373–7384. [Google Scholar] [CrossRef]
- Sui, C.X.; Liu, Y.F.; Li, P.A.; Zhang, D.; Xia, F. Determination of IO4− and Ni2+ ions using L-cysteine-CdTe/ZnS quantum dots as pH-dependent fluorescent probes. Anal. Methods 2013, 5, 1695–1701. [Google Scholar] [CrossRef]
- Zare, H.; Ghalkhani, M.; Akhavan, O.; Taghavinia, N.; Marandi, M. Highly sensitive selective sensing of nickel ions using repeatable fluorescence quenching-emerging of the CdTe quantum dots. Mater. Res. Bull. 2017, 95, 532–538. [Google Scholar] [CrossRef]
- Gong, Y.; Liang, H. Nickel ion detection by imidazole modified carbon dots. Spectrochim. Acta Part A 2019, 211, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.L.; Roldan, P.d.S.; Giné, M.F. Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. J. Hazard. Mater. 2009, 171, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Khadro, B.; Sikora, A.; Loir, A.S.; Errachid, A.; Garrelie, F.; Donnet, C.; Jaffrezic-Renault, N. Electrochemical performances of B doped and undoped diamond-like carbon (DLC) films deposited by femtosecond pulsed laser ablation for heavy metal detection using square wave anodic stripping voltammetric (SWASV) technique. Sens. Actuators B 2011, 155, 120–125. [Google Scholar] [CrossRef]
- Bansod, B.S.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Milman, V.; Winkler, B.; White, J.A.; Pickard, C.J.; Payne, M.C.; Akhmatskaya, E.V.; Nobes, R.H. Electronic structure, properties, and phase stability of inorganic crystals: A pseudopotential plane-wave study. Int. J. Quantum Chem. 2015, 77, 895–910. [Google Scholar] [CrossRef]
- Wang, D.; Jin, J.; Xia, D.; Ye, Q.; Long, J. The effect of oxygen vacancies concentration to the gas-sensing properties of tin dioxide-doped Sm. Sens. Actuators B 2000, 66, 260–262. [Google Scholar] [CrossRef]
- Mulheran, P.A.; Harding, J.H. The stability of SnO2 surfaces. Modell. Simul. Mater. Sci. Eng. 1992, 1, 39–43. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and structure of stoichiometric SnO2 surfaces studied by first-principles calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Hu, J. CO sensing properties and mechanism of Pd doped SnO2 thick-films. Appl. Surf. Sci. 2018, 428, 207–217. [Google Scholar] [CrossRef]
- Kavarnos, G.J. Fundamental concepts of photoinduced electron transfer. Photoinduced Electron. Transf. I 1990, 21–58. [Google Scholar] [CrossRef]
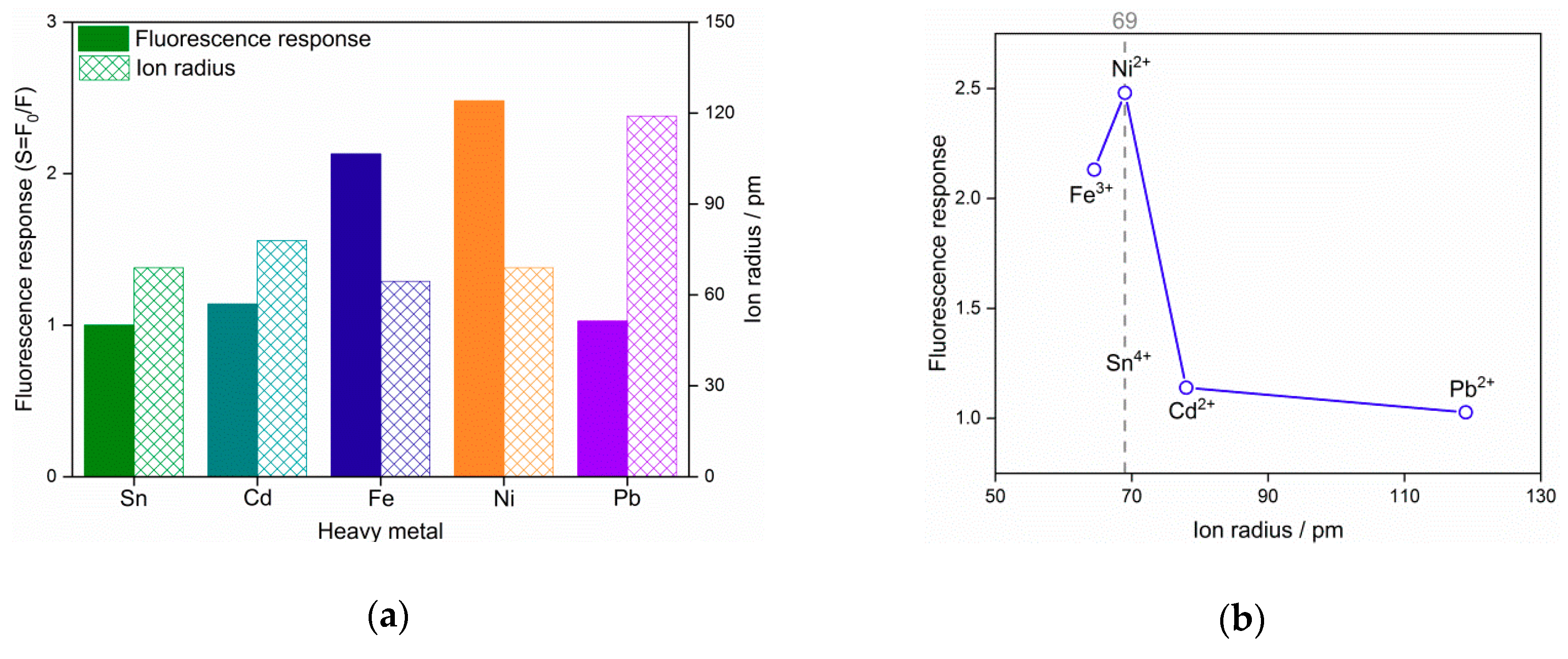
| Heavy Metal Ion | Adsorption Energy (eV) | Ion Radius (Å) | Fluorescence Response |
|---|---|---|---|
| Cd2+ | 4.21 | 78 | 1.14 |
| Fe3+ | 13.46 | 64.5 | 2.13 |
| Ni2+ | 7.65 | 69 | 2.48 |
| Pb2+ | 5.39 | 119 | 1.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Q.; Xue, W.; Zhang, H.; Bai, Y.; Wu, L.; Zhai, Z.; Jin, G. Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water. Nanomaterials 2019, 9, 1294. https://doi.org/10.3390/nano9091294
Liu J, Zhang Q, Xue W, Zhang H, Bai Y, Wu L, Zhai Z, Jin G. Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water. Nanomaterials. 2019; 9(9):1294. https://doi.org/10.3390/nano9091294
Chicago/Turabian StyleLiu, Jianqiao, Qianru Zhang, Weiting Xue, Haipeng Zhang, Yu Bai, Liting Wu, Zhaoxia Zhai, and Guohua Jin. 2019. "Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water" Nanomaterials 9, no. 9: 1294. https://doi.org/10.3390/nano9091294
APA StyleLiu, J., Zhang, Q., Xue, W., Zhang, H., Bai, Y., Wu, L., Zhai, Z., & Jin, G. (2019). Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water. Nanomaterials, 9(9), 1294. https://doi.org/10.3390/nano9091294






