Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review
Abstract
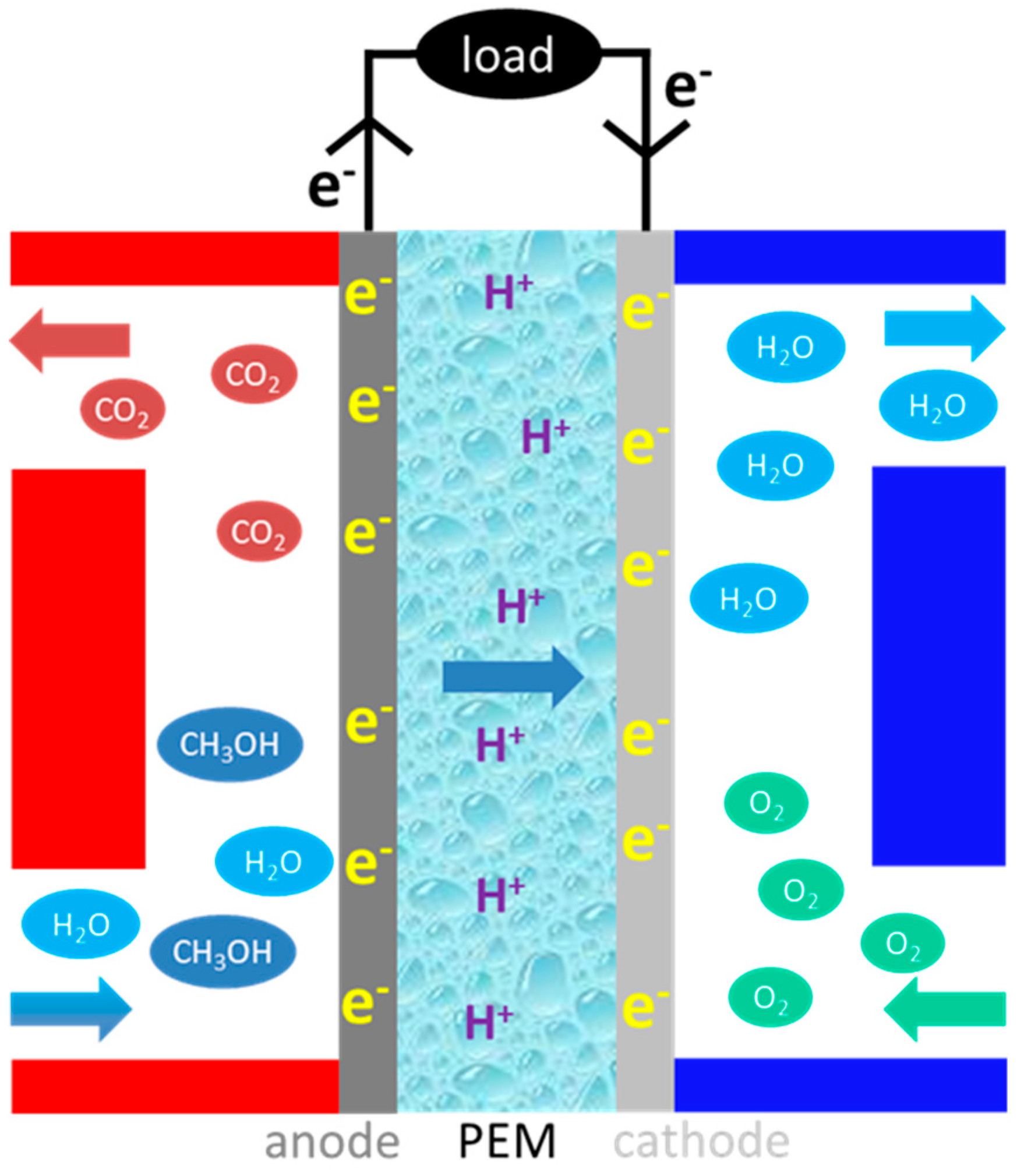
:1. Introduction
- Efficient anode catalyst for complete electro-oxidation of methanol.
- Solid polymer electrolyte with high proton conductivity and low methanol permeability.
- Methanol-tolerant cathode catalyst with high oxygen reduction activity.
2. Carbon Nanomaterials as Additives in PEMs
2.1. Carbon Nanotubes as Additives for PEMs in DMFC
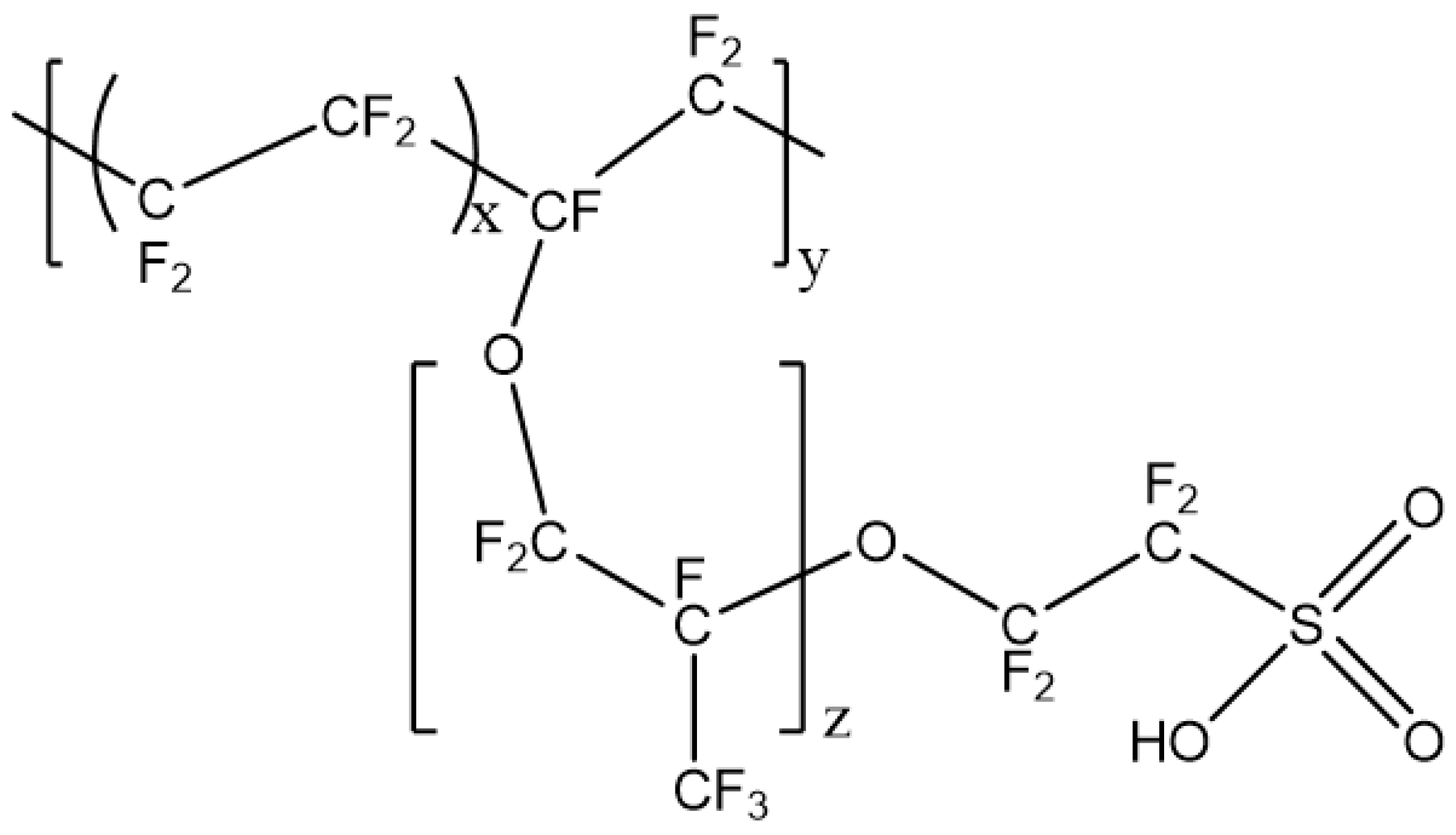
2.1.1. PFSA-CNT Composite PEMs
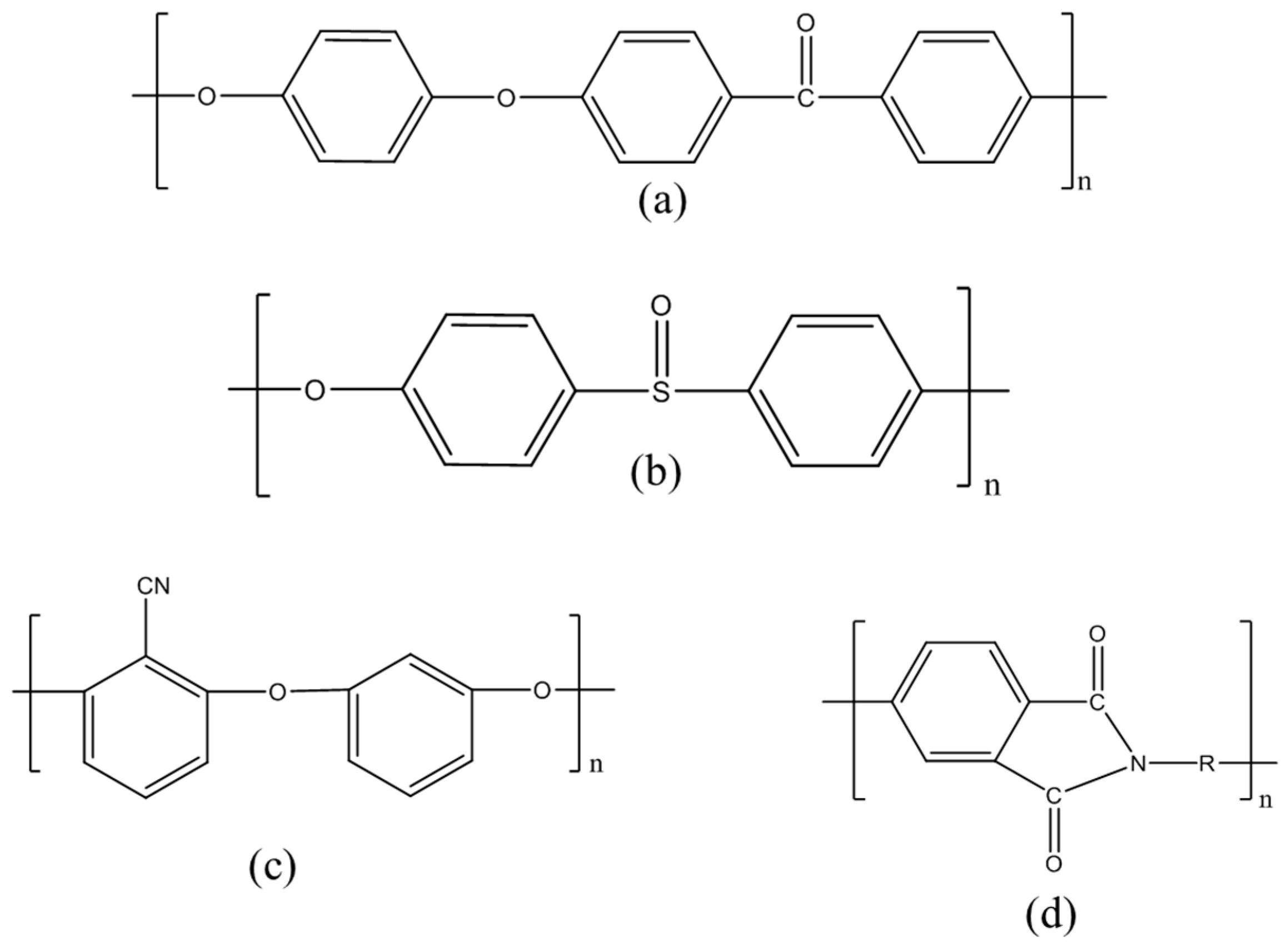
2.1.2. Non-Fluorinated Polymer-CNTs Composites
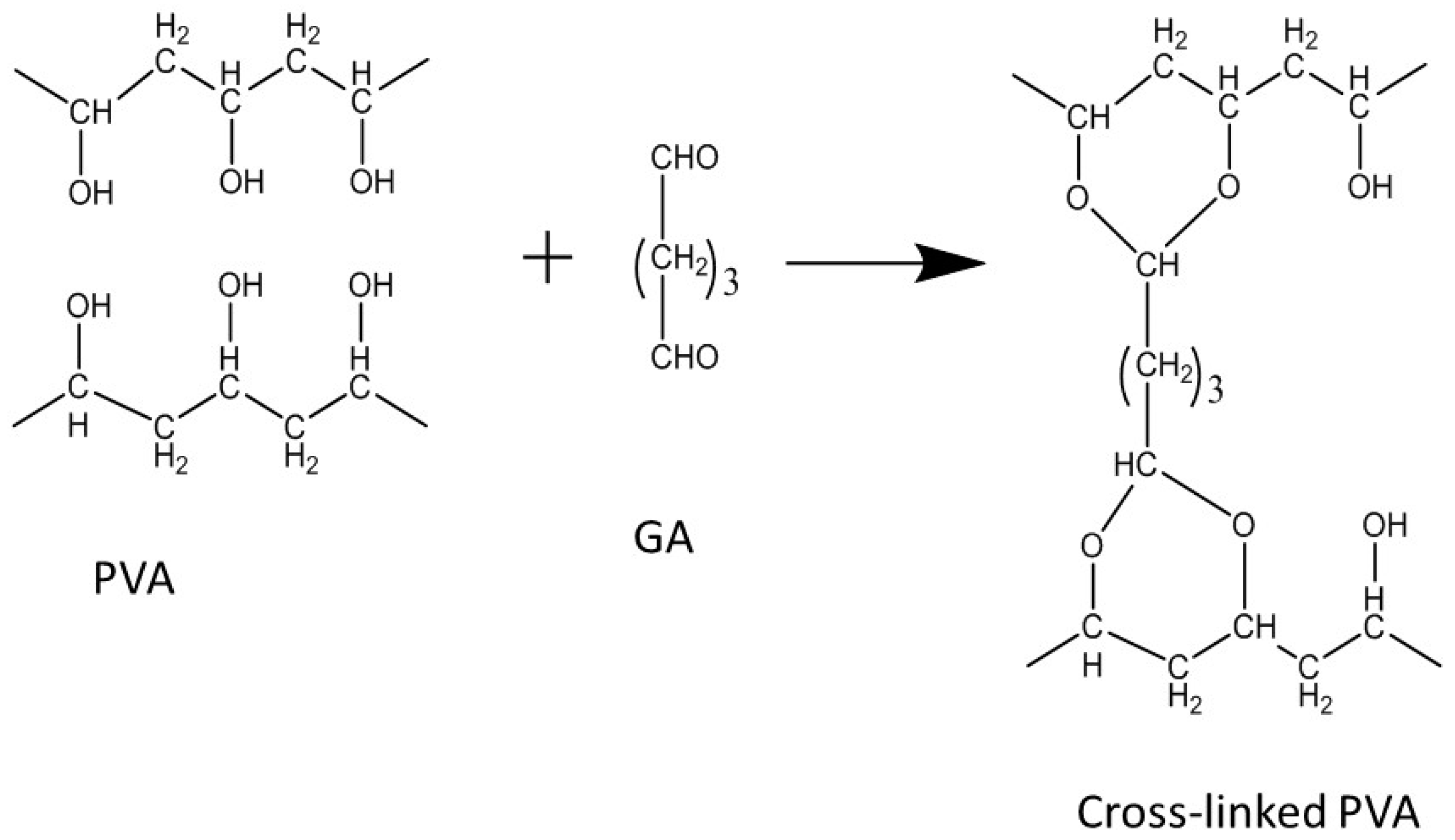
2.1.3. PVA-CNT Composite PEMs
2.2. Graphene Oxide as an Additive for PEMs in DMFCs
2.2.1. PFSA-GO Composites
2.2.2. SPEEK-GO Composite PEMs
2.2.3. Sulfonated Poly Imide-GO Composites
2.2.4. Sulfonated Poly Sulfones-GO Composites
2.2.5. PVA-GO Composite PEMs
2.2.6. GO Free-Standing Membranes
2.3. PEMs with Other Carbon Nanomaterials
3. Conclusions
4. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| PEM | Polymer electrolyte membrane |
| MEA | Membrane electrode assembly |
| DMFC | Direct methanol fuel cells |
| SWCNT | single-wall carbon nanotubes |
| MWCNT | Multi-wall Carbon nanotube |
| GO | Graphene oxide |
| PFSA | Perfluorosulfonic acid |
| SPEEK | Sulfonated poly(ether ether ketone) |
| SFMC | Sulfonated fluorinated multi-block copolymer |
| SDBC | Sulfonated diblock copolymer |
| PES | Poly(ether sulfone) |
| PEI | poly(ether imide) |
| PVA | Polyvinyl alcohol |
| PEN | poly ether nitrile |
| DS | Degree of sulfonation |
| GA | Glutaraldehyde |
| PWA | Phosphotungstic acid |
| PDDA | poly(diallyldimethylammonium chloride) |
| PSSA | poly(styrene sulfonic acid) |
| SDBS | Sulfonated dodecyl benzene sulfonate |
| IL | Ionic liquid |
| CNF | Carbon nanofibre |
| MC | Mesoporous carbon |
| OCV | Open circuit voltage |
| NCD | Nafion modified carbon dots |
References
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- McNicol, B.D.; Rand, D.A.J.; Williams, K.R. Direct methanol-air fuel cells for road transportation. J. Power Sources 1999, 83, 15–31. [Google Scholar] [CrossRef]
- Antonucci, V.; Shukla, A.K.; Arico, A.S. An appraisal of electric automobile power sources. Renew. Sustain. Energy Rev. 2001, 5, 137–155. [Google Scholar]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of Nafion® in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1498–1504. [Google Scholar] [CrossRef]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Piela, P.; Eickes, C.; Brosha, E.; Garzon, F.; Zelenay, P. Ruthenium crossover in direct methanol fuel cell with Pt-Ru black anode. J. Electrochem. Soc. 2004, 151, 2053–2059. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Composite silica/Nafion® membranes prepared by tetraethylorthosilicate sol-gel reaction and solution casting for direct methanol fuel cells. J. Memb. Sci. 2006, 272, 116–124. [Google Scholar] [CrossRef]
- Dimitrova, P.; Friedrich, K.A.; Stimming, U.; Vogt, B. Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ion. 2002, 150, 115–122. [Google Scholar] [CrossRef]
- Tricoli, V.; Nannetti, F. Zeolite-Nafion composites as ion conducting membrane materials. Electrochim. Acta 2003, 48, 2625–2633. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.J.; Hong, W.H.; Lee, H.K. Physical and electrochemical properties of Nafion/polypyrrole composite membrane for DMFC. J. Memb. Sci. 2006, 272, 28–36. [Google Scholar] [CrossRef]
- Tsai, J.C.; Cheng, H.P.; Kuo, J.F.; Huang, Y.H.; Chen, C.Y. Blended Nafion®/SPEEK direct methanol fuel cell membranes for reduced methanol permeability. J. Power Sources 2009, 189, 958–965. [Google Scholar] [CrossRef]
- Cho, K.Y.; Jung, H.Y.; Shin, S.S.; Choi, N.S.; Sung, S.J.; Park, J.K.; Choi, J.H.; Park, K.W.; Sung, Y.E. Proton conducting semi-IPN based on Nafion and crosslinked poly(AMPS) for direct methanol fuel cell. Electrochim. Acta 2004, 50, 588–593. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhao, C.; Fu, T.; Shi, Y.; Na, H. Composite membranes based on highly sulfonated PEEK and PBI: Morphology characteristics and performance. J. Memb. Sci. 2008, 308, 66–74. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Ma, W.; Zhao, C.; Zhang, Y.; Han, M.; Zhu, J.; Liu, Z.; Wu, J.; Na, H. Composite membranes based on a novel benzimidazole grafted PEEK and SPEEK for fuel cells. Int. J. Hydrog. Energy 2010, 35, 11172–11179. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hickner, M.A.; Dong, L.; Pivovar, B.S.; McGrath, J.E. Sulfonated poly(arylene ether sulfone) copolymer proton exchange membranes: Composition and morphology effects on the methanol permeability. J. Memb. Sci. 2004, 243, 317–326. [Google Scholar] [CrossRef]
- Kim, Y.S.; Dong, L.; Hickner, M.A.; Pivovar, B.S.; McGrath, J.E. Processing induced morphological development in hydrated sulfonated poly(arylene ether sulfone) copolymer membranes. Polymer (Guildf) 2003, 44, 5729–5736. [Google Scholar] [CrossRef]
- Every, H.A.; Hickner, M.A.; McGrath, J.E.; Zawodzinski, T.A. An NMR study of methanol diffusion in polymer electrolyte fuel cell membranes. J. Memb. Sci. 2005, 250, 183–188. [Google Scholar] [CrossRef]
- Xu, W.; Liu, C.; Xue, X.; Su, Y.; Lv, Y.; Xing, W.; Lu, T. New proton exchange membranes based on poly (vinyl alcohol) for DMFCs. Solid State Ion. 2004, 171, 121–127. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Bhat, S.D.; Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. A new mixed-matrix membrane for DMFCs. Energy Environ. Sci. 2009, 2, 1210–1216. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Memb. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Zhou, W.; Xiao, J.; Chen, Y.; Zeng, R.; Xiao, S.; Nie, H.; Li, F.; Song, C. Sulfonated carbon nanotubes/sulfonated poly(ether sulfone ether ketone ketone) composites for polymer electrolyte membranes. Polym. Adv. Technol. 2011, 22, 1747–1752. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R.S. Carbon based conductive polymer composites. J. Mater. Sci. 2007, 42, 3408–3418. [Google Scholar] [CrossRef]
- You, P.Y.; Kamarudin, S.K. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shukla, G.; Manohar, M.; Shahi, V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: An overview. Adv. Colloid Interface Sci. 2017, 240, 15–30. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Asgari, M.S.; Nikazar, M.; Molla-Abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/histidine functionalized carbon nanotube: High-performance fuel cell membranes. Int. J. Hydrog. Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Kollar, J.; Caldarella, G.; Germain, A.; Jérôme, R.; Detrembleur, C. Beneficial effect of carbon nanotubes on the performances of Nafion membranes in fuel cell applications. J. Memb. Sci. 2007, 303, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Wu, S.; Bertsch, A.; Moaddel, H.; Renaud, P. Nafion/chitosan-wrapped CNT nanocomposite membrane for high-performance direct methanol fuel cells. RSC Adv. 2013, 3, 7337–7346. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Janghorban, K.; Asgari, M.S. A novel heteropolyacid-doped carbon nanotubes/Nafion nanocomposite membrane for high performance proton-exchange methanol fuel cell applications. Iran. Polym. J. (Engl. Ed.) 2018, 27, 77–86. [Google Scholar] [CrossRef]
- Chang, C.M.; Li, H.Y.; Lai, J.Y.; Liu, Y.L. Nanocomposite membranes of Nafion and Fe3O4-anchored and Nafion-functionalized multiwalled carbon nanotubes exhibiting high proton conductivity and low methanol permeability for direct methanol fuel cells. RSC Adv. 2013, 3, 12895–12904. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Hariprasad, R.; Ramakrishnan, S.; Kim, A.R.; Yoo, D.J. Potential Bifunctional Filler (CeO 2 –ACNTs) for Nafion Matrix toward Extended Electrochemical Power Density and Durability in Proton-Exchange Membrane Fuel Cells Operating at Reduced Relative Humidity. ACS Sustain. Chem. Eng. 2019, 7, 12847–12857. [Google Scholar] [CrossRef]
- Heo, Y.; Yun, S.; Im, H.; Kim, J. Low methanol permeable sulfonated poly(ether imide)/sulfonated multiwalled carbon nanotube membrane for direct methanol fuel cell. J. Appl. Polym. Sci. 2012, 126, E467–E477. [Google Scholar] [CrossRef]
- Joo, S.H.; Pak, C.; Kim, E.A.; Lee, Y.H.; Chang, H.; Seung, D.; Choi, Y.S.; Park, J.B.; Kim, T.K. Functionalized carbon nanotube-poly(arylene sulfone) composite membranes for direct methanol fuel cells with enhanced performance. J. Power Sources 2008, 180, 63–70. [Google Scholar] [CrossRef]
- Yun, S.; Im, H.; Heo, Y.; Kim, J. Crosslinked sulfonated poly(vinyl alcohol)/sulfonated multi-walled carbon nanotubes nanocomposite membranes for direct methanol fuel cells. J. Memb. Sci. 2011, 380, 208–215. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Yoon, Y.S. PVA nano composite membrane for DMFC application. Solid State Ion. 2011, 380, 208–215. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wu, Q.; Xu, X.; Lu, S.; Xiang, Y. A poly(vinyl alcohol)-based composite membrane with immobilized phosphotungstic acid molecules for direct methanol fuel cells. Electrochim. Acta 2017, 224, 369–377. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D. Simultaneous tuning of methanol crossover and ionic conductivity of sPEEK membrane electrolyte by incorporation of PSSA functionalized MWCNTs: A comparative study in DMFCs. Chem. Eng. J. 2014, 243, 517–525. [Google Scholar] [CrossRef]
- Gahlot, S.; Kulshrestha, V. Dramatic improvement in water retention and proton conductivity in electrically aligned functionalized CNT/SPEEK nanohybrid PEM. ACS Appl. Mater. Interfaces 2015, 7, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, H.; Zhang, C.; Li, B.; Fang, F.; Wang, Y. Strengthen the performance of sulfonated poly(ether ether ketone) as proton exchange membranes with phosphonic acid functionalized carbon nanotubes. Ionics (Kiel) 2017, 23, 2103–2112. [Google Scholar] [CrossRef]
- Cui, L.; Geng, Q.; Gong, C.; Liu, H.; Zheng, G.; Wang, G.; Liu, Q.; Wen, S. Novel sulfonated poly (ether ether ketone)/silica coated carbon nanotubes high-performance composite membranes for direct methanol fuel cell. Polym. Adv. Technol. 2015, 26, 457–464. [Google Scholar] [CrossRef]
- Kim, A.R.; Gabunada, J.C.; Yoo, D.J. Amelioration in physicochemical properties and single cell performance of sulfonated poly(ether ether ketone) block copolymer composite membrane using sulfonated carbon nanotubes for intermediate humidity fuel cells. Int. J. Energy Res. 2019, 43, 2974–2989. [Google Scholar] [CrossRef]
- Scipioni, R.; Gazzoli, D.; Teocoli, F.; Palumbo, O.; Paolone, A.; Ibris, N.; Brutti, S.; Navarra, M.A. Preparation and characterization of nanocomposite polymer membranes containing functionalized SnO2 additives. Membranes (Basel) 2014, 4, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Hong, J.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Hammond, P.T.; Park, H. Innovative polymer nanocomposite electrolytes: Nanoscale manipulation of ion channels by functionalized graphenes. ACS Nano 2011, 5, 5167–5174. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Sulfonated graphene oxide-silica for highly selective Nafion-based proton exchange membranes. J. Mater. Chem. A 2014, 2, 16083–16092. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Yan, X.H.; Wu, R.; Xu, J.B.; Luo, Z.; Zhao, T.S. A monolayer graphene-Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Sha Wang, L.; Nan Lai, A.; Xiao Lin, C.; Gen Zhang, Q.; Mei Zhu, A.; Lin Liu, Q. Orderly sandwich-shaped graphene oxide/Nafion composite membranes for direct methanol fuel cells. J. Memb. Sci. 2015, 492, 58–66. [Google Scholar] [CrossRef]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Manthiram, A. Sulfonated poly(ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 5875–5884. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Fu, Y.; Manthiram, A. Composite membranes based on sulfonated poly(ether ether ketone) and SDBS-adsorbed graphene oxide for direct methanol fuel cells. J. Mater. Chem. 2012, 22, 24862–24869. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on Sulfonated Poly (Ether Ether Ketone) membrane for direct methanol fuel cells. J. Memb. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, H.; Cao, L.; Li, Z.; Li, Z.; Gang, M.; Wang, C.; Wu, H.; Jiang, Z.; Zhang, P. Sulfonated poly(ether ether ketone)-based hybrid membranes containing graphene oxide with acid-base pairs for direct methanol fuel cells. Electrochim. Acta 2016, 203, 178–188. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Jiang, Z.; Tian, X.; Luo, L.; Liu, M. Sulfonated Holey Graphene Oxide (SHGO) Filled Sulfonated Poly(ether ether ketone) Membrane: The Role of Holes in the SHGO in Improving Its Performance as Proton Exchange Membrane for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 20046–20056. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Sulfonated fluorinated multi-block copolymer hybrid containing sulfonated(poly ether ether ketone) and graphene oxide: A ternary hybrid membrane architecture for electrolyte applications in proton exchange membrane fuel cells. J. Energy Chem. 2018, 27, 1247–1260. [Google Scholar] [CrossRef]
- Pandey, R.P.; Thakur, A.K.; Shahi, V.K. Sulfonated polyimide/acid-functionalized graphene oxide composite polymer electrolyte membranes with improved proton conductivity and water-retention properties. ACS Appl. Mater. Interfaces 2014, 6, 16993–17002. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Ye, Y.S.; Cheng, M.Y.; Kao, K.Y.; Shen, W.C.; Rick, J.; Chen, J.C.; Hwang, B.J. Sulfonated polyimide proton exchange membranes with graphene oxide show improved proton conductivity, methanol crossover impedance, and mechanical properties. Adv. Energy Mater. 2011, 1, 1220–1224. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Sulphonated imidized graphene oxide (SIGO) based polymer electrolyte membrane for improved water retention, stability and proton conductivity. J. Power Sources 2015, 299, 104–113. [Google Scholar] [CrossRef]
- Gahlot, S.; Sharma, P.P.; Kulshrestha, V.; Jha, P.K. SGO/SPES-based highly conducting polymer electrolyte membranes for fuel cell application. ACS Appl. Mater. Interfaces 2014, 6, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
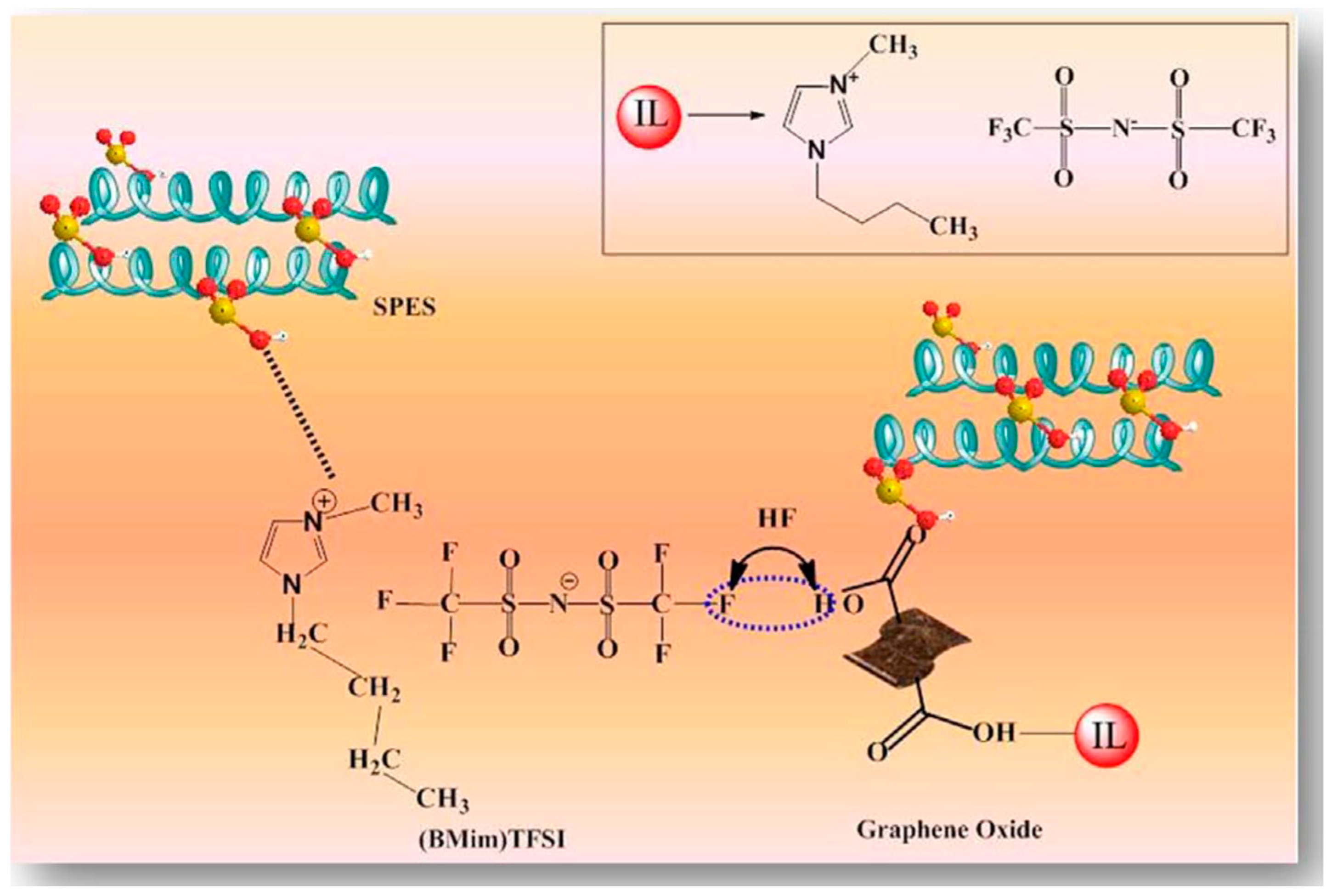
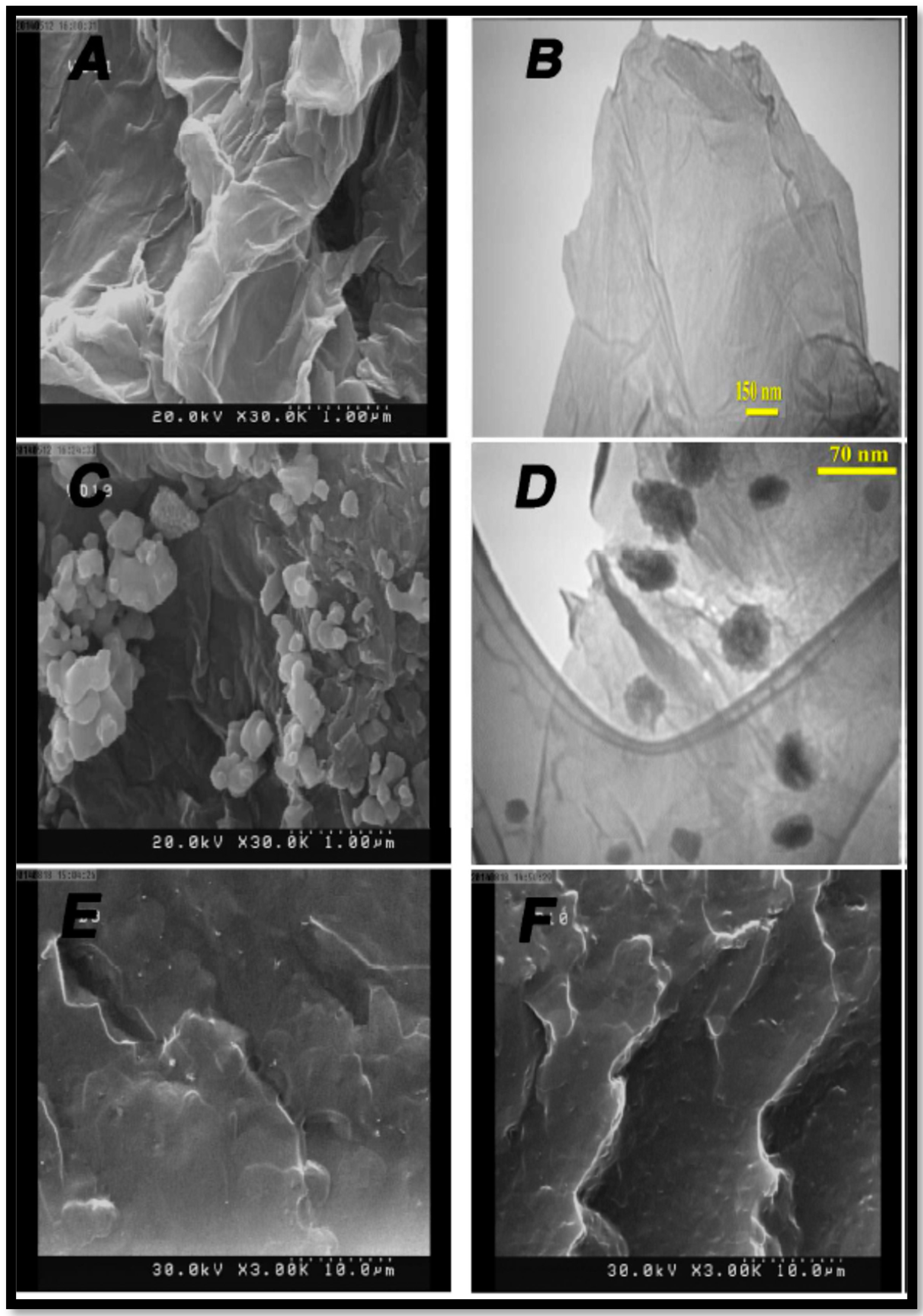
- Gahlot, S.; Sharma, P.P.; Kulshrestha, V. Ionic liquid grafted graphene oxide composite to create proton transfer pathways in polymer electrolyte membrane. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 622–627. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, H.; Li, X.; Wu, Y. A morphology and property study of composite membranes based on sulfonated polyarylene ether sulfone and adequately sulfonated graphene oxide. Int. J. Hydrog. Energy 2016, 41, 331–341. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Saraswathi, M.S.A.; Rana, D.; Nagendran, A. Fabrication and electrochemical properties of highly selective SPES/GO composite membranes for direct methanol fuel cells. J. Environ. Chem. Eng. 2017, 5, 3828–3833. [Google Scholar] [CrossRef]
- Liu, D.; Peng, J.; Li, Z.; Liu, B.; Wang, L. Improvement in the mechanical properties, proton conductivity, and methanol resistance of highly branched sulfonated poly(arylene ether)/graphene oxide grafted with flexible alkylsulfonated side chains nanocomposite membranes. J. Power Sources 2018, 378, 451–459. [Google Scholar] [CrossRef]
- Bunlengsuwan, P.; Paradee, N.; Sirivat, A. Influence of Sulfonated Graphene Oxide on Sulfonated Polysulfone Membrane for Direct Methanol Fuel Cell. Polym.-Plast. Technol. Eng. 2017, 56, 1695–1703. [Google Scholar] [CrossRef]
- Feng, M.; Huang, Y.; Wei, M.; Liu, X. Sulfonated poly(arylene ether nitrile)-based hybrid membranes containing amine-functionalized GO for constructing long-range ionic nanochannels. Int. J. Hydrog. Energy 2018, 43, 11214–11222. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, T.; Zhang, X.; Zhang, W.; Liu, X. Novel composite proton exchange membrane with long-range proton transfer channels constructed by synergistic effect between acid and base functionalized graphene oxide. Polymer (Guildf) 2018, 149, 305–315. [Google Scholar] [CrossRef]
- Feng, M.; Huang, Y.; Cheng, T.; Liu, X. Synergistic effect of graphene oxide and carbon nanotubes on sulfonated poly(arylene ether nitrile)-based proton conducting membranes. Int. J. Hydrog. Energy 2017, 42, 8224–8232. [Google Scholar] [CrossRef]
- Devi, A.U.; Divya, K.; Kaleekkal, N.J.; Rana, D.; Nagendran, A. Tailored SPVdF-co-HFP/SGO nanocomposite proton exchange membranes for direct methanol fuel cells. Polymer (Guildf) 2018, 140, 22–32. [Google Scholar] [CrossRef]
- Lin, C.W.; Lu, Y.S. Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sources 2013, 237, 187–194. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M. Aligned Nanocomposite Membranes Containing Sulfonated Graphene Oxide with Superior Ionic Conductivity for Direct Methanol Fuel Cell Application. Ind. Eng. Chem. Res. 2015, 54, 7028–7037. [Google Scholar]
- Jiang, Z.; Shi, Y.; Jiang, Z.J.; Tian, X.; Luo, L.; Chen, W. High performance of a free-standing sulfonic acid functionalized holey graphene oxide paper as a proton conducting polymer electrolyte for air-breathing direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6494–6503. [Google Scholar] [CrossRef]
- Paneri, A.; Heo, Y.; Ehlert, G.; Cottrill, A.; Sodano, H.; Pintauro, P.; Moghaddam, S. Proton selective ionic graphene-based membrane for high concentration direct methanol fuel cells. J. Memb. Sci. 2014, 467, 217–225. [Google Scholar] [CrossRef]
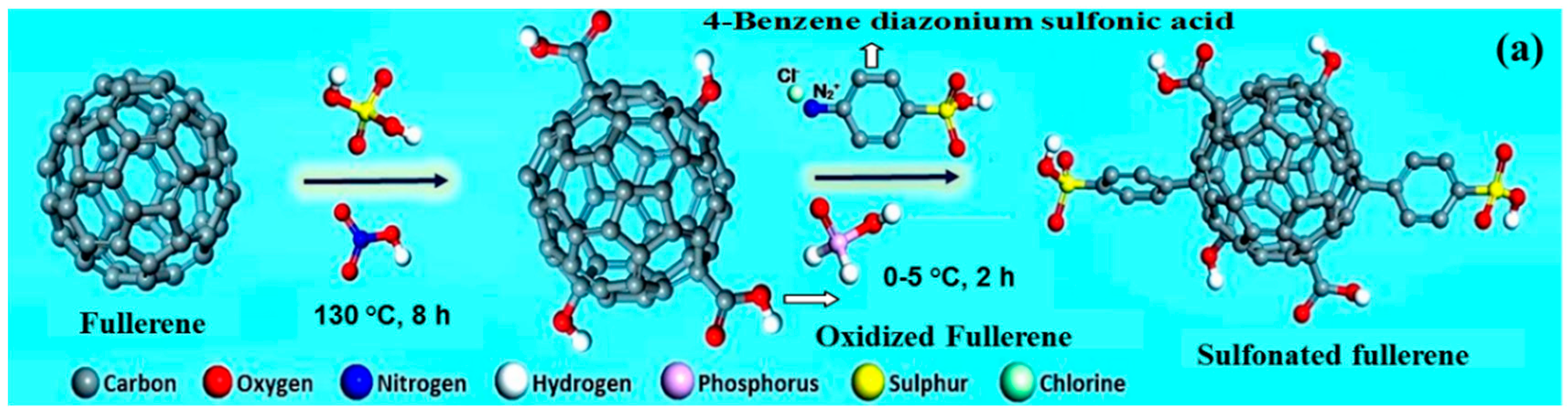
- Rambabu, G.; Bhat, S.D. Sulfonated fullerene in SPEEK matrix and its impact on the membrane electrolyte properties in direct methanol fuel cells. Electrochim. Acta 2015, 299, 104–113. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Zhang, Y.; Li, C.; Dong, J.; Liu, Y.; Cheng, H. Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application. Int. J. Hydrog. Energy 2017, 42, 10275–10284. [Google Scholar] [CrossRef]
- Rambabu, G.; Sasikala, S.; Bhat, S.D. Nanocomposite membranes of sulfonated poly(phthalalizinone ether ketone)–sulfonated graphite nanofibers as electrolytes for direct methanol fuel cells. RSC Adv. 2016, 6, 107507–107518. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Sahu, A.K. Surfactant templated nanoporous carbon-Nafion hybrid membranes for direct methanol fuel cells with reduced methanol crossover. J. Memb. Sci. 2017, 541, 127–136. [Google Scholar] [CrossRef]
- Jia, W.; Tang, B.; Wu, P. Carbon dots with multi-functional groups and the application in proton exchange membranes. Electrochim. Acta 2018, 260, 92–100. [Google Scholar] [CrossRef]
- Sarangdevot, K.; Sonigara, B.S. The wondrous world of carbon nanotubes: Structure, synthesis, properties and applications. J. Chem. Pharm. Res. 2015, 7, 916–933. [Google Scholar]
- Mukherjee, S.; Bates, A.; Lee, S.C.; Lee, D.H.; Park, S. A review of the application of CNTs in PEM fuel cells. Int. J. Green Energy 2015, 12, 787–809. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tang, Q.Q.; Yang, D.; Hua, W.M.; Yue, Y.H.; Wang, B.D.; Zhang, X.H.; Hu, J.H. Preparation of poly(p-styrenesulfonic acid) grafted multi-walled carbon nanotubes and their application as a solid-acid catalyst. Mater. Chem. Phys. 2011, 126, 310–313. [Google Scholar] [CrossRef]
- Cele, N.P.; Sinha Ray, S.; Pillai, S.K.; Ndwandwe, M.; Nonjola, S.; Sikhwivhilu, L.; Mathe, M.K. Carbon nanotubes based nafion compositemembranes for fuel cell applications. Fuel Cells 2010, 10, 64–71. [Google Scholar]
- Miyake, N.; Wainright, J.S.; Savinell, R.F. Evaluation of a Sol-Gel Derived Nafion/Silica Hybrid Membrane for Proton Electrolyte Membrane Fuel Cell Applications: I. Proton Conductivity and Water Content. J. Electrochem. Soc. 2001, 148, A898–A904. [Google Scholar] [CrossRef]
- Miyake, N.; Wainright, J.S.; Savinell, R.F. Evaluation of a Sol-Gel Derived Nafion/Silica Hybrid Membrane for Polymer Electrolyte Membrane Fuel Cell Applications: II. Methanol Uptake and Methanol Permeability. J. Electrochem. Soc. 2001, 148, A905–A909. [Google Scholar] [CrossRef]
- Ruffmann, B.; Silva, H.; Schulte, B.; Nunes, S.P. Organic/inorganic composite membranes for application in DMFC. Solid State Ion. 2003, 163, 269–275. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, W.C.; Woo, S.I.; Hong, W.H. Evaluation of a palladinized NafionTM for direct methanol fuel cell application. Electrochim. Acta 2004, 49, 3227–3234. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Chen, X.; Xin, J.H. Decoration of carbon nanotubes with chitosan. Carbon 2005, 43, 3178–3180. [Google Scholar] [CrossRef]
- Cavalcanti, W.L.; Marschall, R.; Tölle, P.; Köhler, C.; Work, M.; Frauenheim, T. Insight into proton conduction of immobilised imidazole systems via simulations and impedance spectroscopy. Fuel Cells 2008, 8, 244–253. [Google Scholar]
- Sharma, A.; Vijay, Y.K. Effect of electric field variation in alignment of SWNT/PC nanocomposites. Int. J. Hydrog. Energy 2012, 37, 3945–3948. [Google Scholar] [CrossRef]
- Martin, C.A.; Sandler, J.K.W.; Windle, A.H.; Schwarz, M.K.; Bauhofer, W.; Schulte, K.; Shaffer, M.S.P. Electric field-induced aligned multi-wall carbon nanotube networks in epoxy composites. Polymer (Guildf) 2005, 46, 877–886. [Google Scholar] [CrossRef]
- Kim, S.; Fornasiero, F.; Park, H.G.; In, J.B.; Meshot, E.; Giraldo, G.; Stadermann, M.; Fireman, M.; Shan, J.; Grigoropoulos, C.P.; et al. Fabrication of flexible, aligned carbon nanotube/polymer composite membranes by in-situ polymerization. J. Memb. Sci. 2014, 460, 91–98. [Google Scholar] [CrossRef]
- Copyright, C.; Corporation, F.; Only, F.E.; Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Edited by Foxit Reader Graphene-based polymer nanocomposites Edited by Foxit Reader Copyright (C) by Foxit Corporation, 2005-2010. Polymer (Guildf) 2011, 52, 5–25. [Google Scholar]
- Ye, Y.S.; Tseng, C.Y.; Shen, W.C.; Wang, J.S.; Chen, K.J.; Cheng, M.Y.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. A new graphene-modified protic ionic liquid-based composite membrane for solid polymer electrolytes. J. Mater. Chem. 2011, 21, 10448–10453. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. “Evaporating” graphene oxide sheets (GOSs) for rolled up GOSs and its applications in proton exchange membrane fuel cell. ACS Appl. Mater. Interfaces 2013, 5, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Graphene-mediated organic-inorganic composites with improved hydroxide conductivity and outstanding alkaline stability for anion exchange membranes. Compos. Part B Eng. 2019, 164, 324–332. [Google Scholar] [CrossRef]
- Cele, N.; Ray, S.S. Recent progress on nafion-based nanocomposite membranes for fuel cell applications. Macromol. Mater. Eng. 2009, 294, 719–738. [Google Scholar] [CrossRef]
- Burgaz, E.; Lian, H.; Alonso, R.H.; Estevez, L.; Kelarakis, A.; Giannelis, E.P. Nafion-clay hybrids with a network structure. Polymer (Guildf) 2009, 50, 2384–2392. [Google Scholar] [CrossRef]
- Herrera Alonso, R.; Estevez, L.; Lian, H.; Kelarakis, A.; Giannelis, E.P. Nafion-clay nanocomposite membranes: Morphology and properties. Polymer (Guildf) 2009, 50, 2402–2410. [Google Scholar] [CrossRef]
- Chien, H.C.; Tsai, L.D.; Huang, C.P.; Kang, C.Y.; Lin, J.N.; Chang, F.C. Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Affoune, A.M.; Yamada, A.; Umeda, M. Conductivity and surface morphology of Nafion membrane in water and alcohol environments. J. Power Sources 2005, 148, 9–17. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Selective growth of mos2 for proton exchange membranes with extremely high selectivity. ACS Appl. Mater. Interfaces 2013, 5, 13042–13049. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Dong, L.; Hickner, M.A.; Glass, T.E.; Webb, V.; McGrath, J.E. State of water in disulfonated poly(arylene ether sulfone) copolymers and a perfluorosulfonic acid copolymer (nafion) and its effect on physical and electrochemical properties. Macromolecules 2003, 36, 6281–6285. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.S.; Sebastian, D.; Baglio, V. Sulfonated graphene oxide platelets in nafion nanocomposite membrane: Advantages for application in direct methanol fuel cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Peera, S.G.; Meenakshi, S.; Gopi, K.H.; Bhat, S.D.; Sridhar, P.; Pitchumani, S. Impact on the ionic channels of sulfonated poly(ether ether ketone) due to the incorporation of polyphosphazene: A case study in direct methanol fuel cells. RSC Adv. 2013, 3, 14048–14056. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrog. Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D. Amino acid functionalized graphene oxide based nanocomposite membrane electrolytes for direct methanol fuel cells. J. Memb. Sci. 2018, 551, 1–11. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Rambabu, G.; Suganthi, S.; Bhat, S.D.; Vasanthkumar, V.; Anbarasu, V.; Raj, V. Bio-functionalized hybrid nanocomposite membranes for direct methanol fuel cells. RSC Adv. 2016, 6, 57709–57721. [Google Scholar] [CrossRef]
- Galatanu, A.N.; Rollet, A.L.; Porion, P.; Diat, O.; Gebel, G. Study of the casting of sulfonated polyimide ionomer membranes: Structural evolution and influence on transport properties. J. Phys. Chem. B 2005, 109, 11332–11339. [Google Scholar] [CrossRef]
- Asano, N.; Miyatake, K.; Watanabe, M. Hydrolytically stable polyimide ionomer for fuel cell applications. Chem. Mater. 2004, 16, 2841–2843. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Aliphatic-aromatic sulphonated polyimide and acid functionalized polysilsesquioxane composite membranes for fuel cell applications. J. Mater. Chem. A 2013, 1, 14375–14383. [Google Scholar] [CrossRef]
- Hwang, B.J.; Joseph, J.; Zeng, Y.Z.; Lin, C.W.; Cheng, M.Y. Analysis of states of water in poly (vinyl alcohol) based DMFC membranes using FTIR and DSC. J. Memb. Sci. 2011, 369, 88–95. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Na, H. Novel cross-linked sulfonated poly (arylene ether ketone) membranes for direct methanol fuel cell. Int. J. Hydrog. Energy 2010, 35, 2176–2182. [Google Scholar] [CrossRef]
- Wang, E.D.; Zhao, T.S.; Yang, W.W. Poly (vinyl alcohol)/3-(trimethylammonium) propyl-functionalized silica hybrid membranes for alkaline direct ethanol fuel cells. Int. J. Hydrog. Energy 2010, 35, 2183–2189. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Q.; Lv, Z.; Dong, H.; Yu, J.; Dong, L. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrog. Energy 2013, 38, 1413–1418. [Google Scholar] [CrossRef]
- Giovambattista, N.; Rossky, P.J.; Debenedetti, P.G. Phase transitions induced by nanoconfinement in liquid water. Phys. Rev. Lett. 2009, 102, 050603. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science (80-. ). 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Haubold, H.G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 104, 4535–4586. [Google Scholar] [CrossRef]
- Ren, X.; Springer, T.E.; Gottesfeld, S. Water and methanol uptakes in Nafion membranes and membrane effects on direct methanol cell performance. J. Electrochem. Soc. 2000, 147, 92–98. [Google Scholar] [CrossRef]
- Tasaki, K.; Gasa, J.; Wang, H.; DeSousa, R. Fabrication and characterization of fullerene-Nafion composite membranes. Polymer (Guildf) 2007, 48, 4438–4448. [Google Scholar] [CrossRef]
- Saga, S.; Matsumoto, H.; Saito, K.; Minagawa, M.; Tanioka, A. Polyelectrolyte membranes based on hydrocarbon polymer containing fullerene. J. Power Sources 2008, 176, 16–22. [Google Scholar] [CrossRef]
- Ye, Y.S.; Wang, H.; Bi, S.G.; Xue, Y.; Xue, Z.G.; Liao, Y.G.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. Enhanced ion transport in polymer-ionic liquid electrolytes containing ionic liquid-functionalized nanostructured carbon materials. Carbon 2015, 86, 86–97. [Google Scholar] [CrossRef]
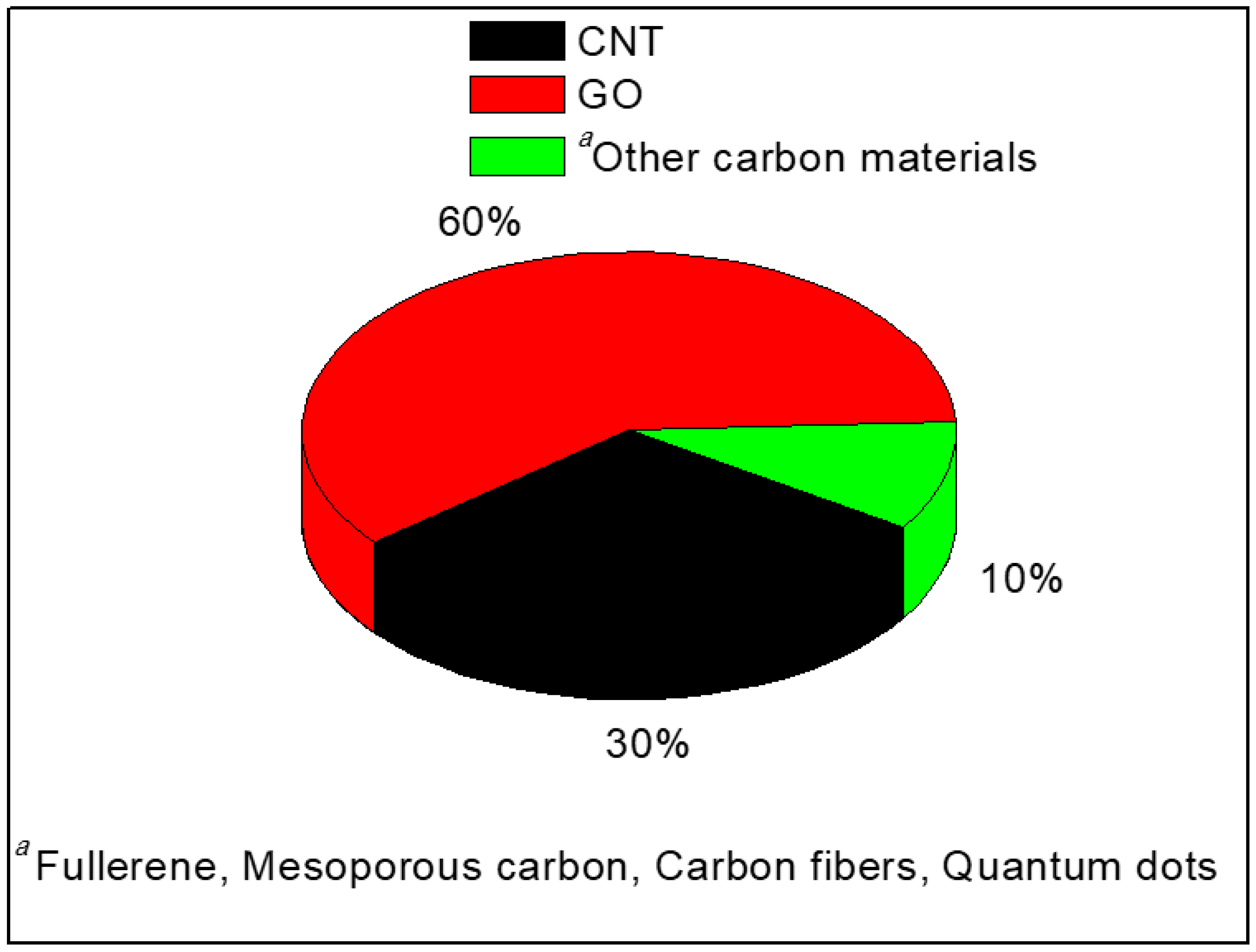
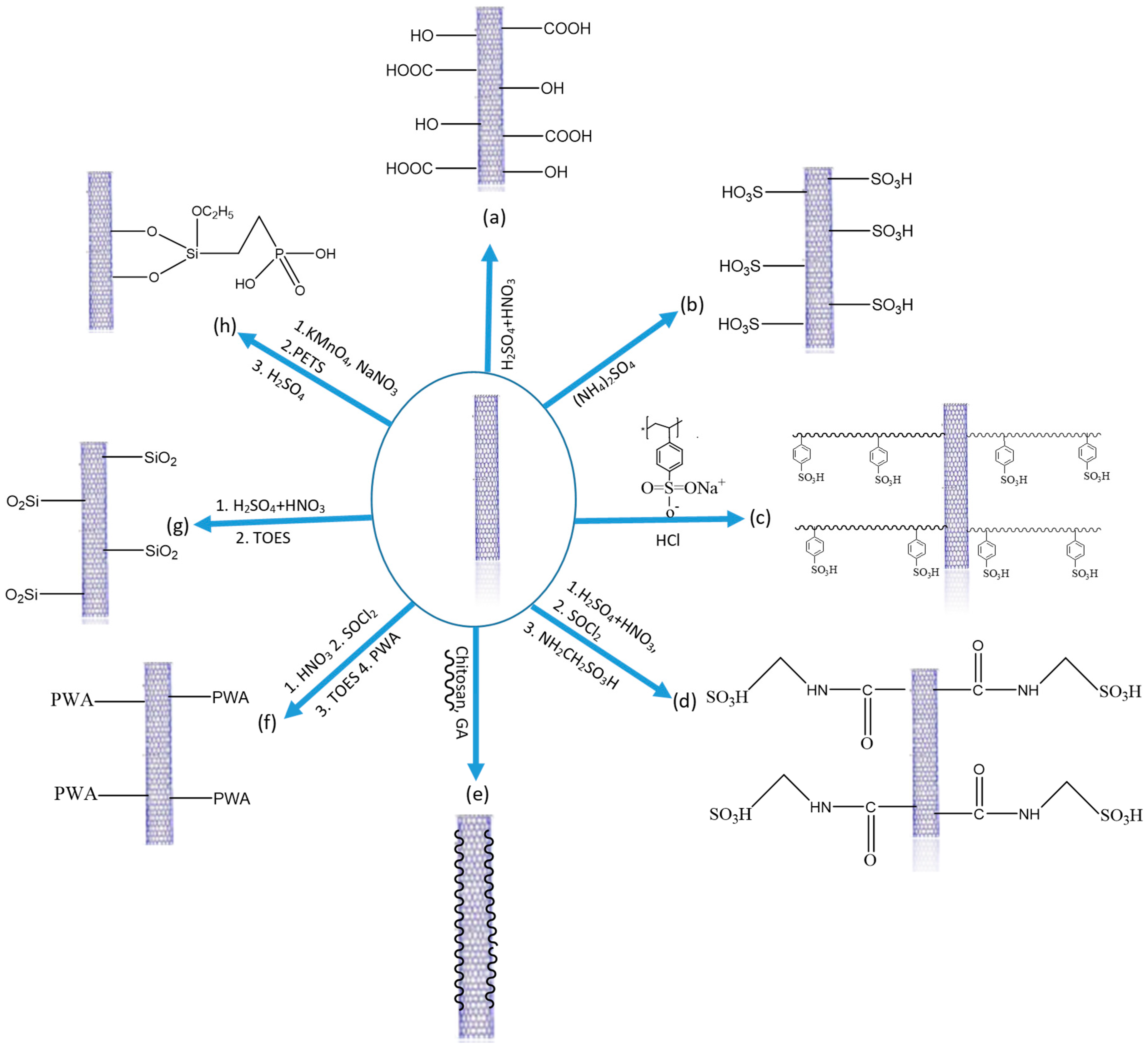
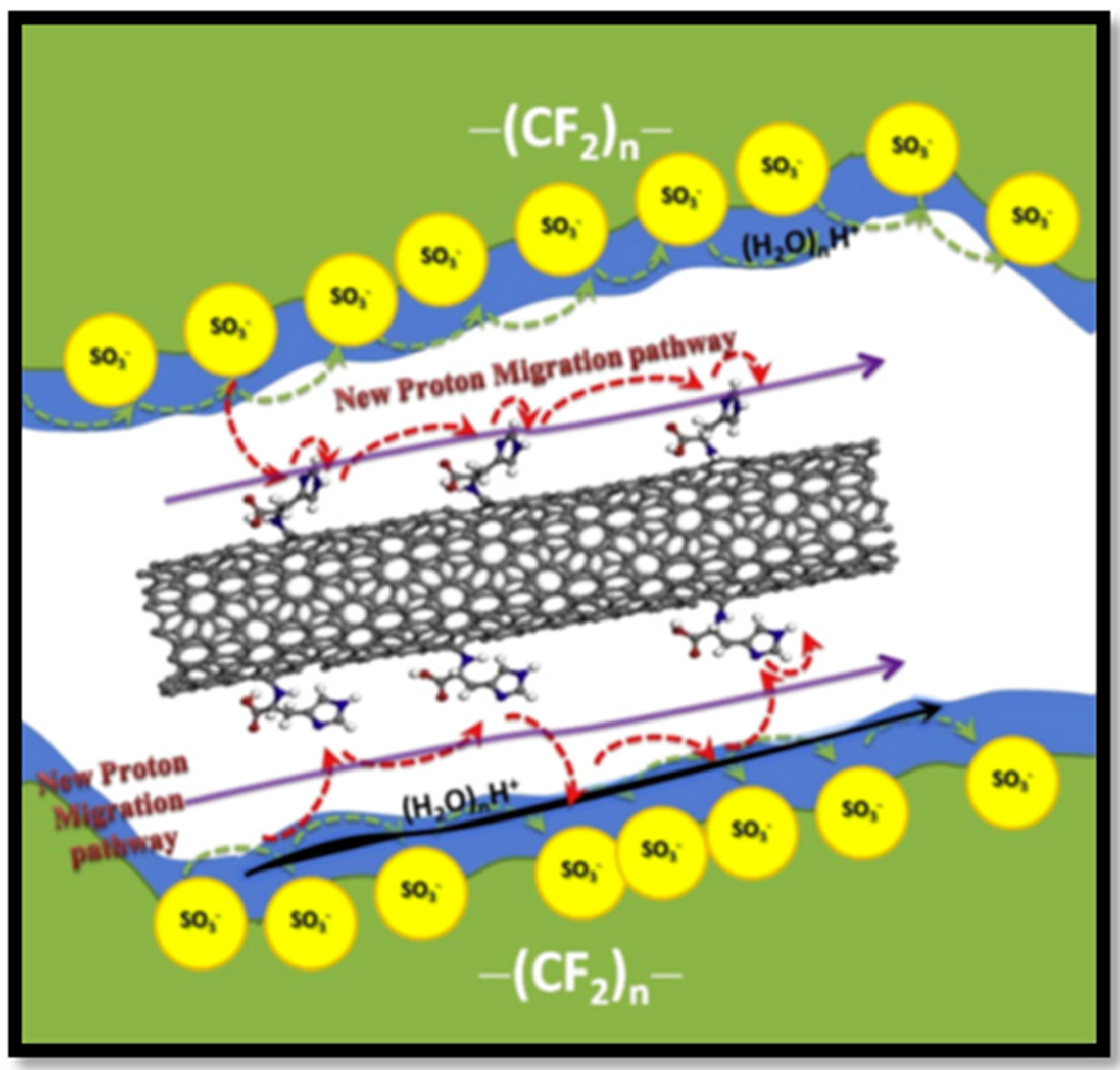
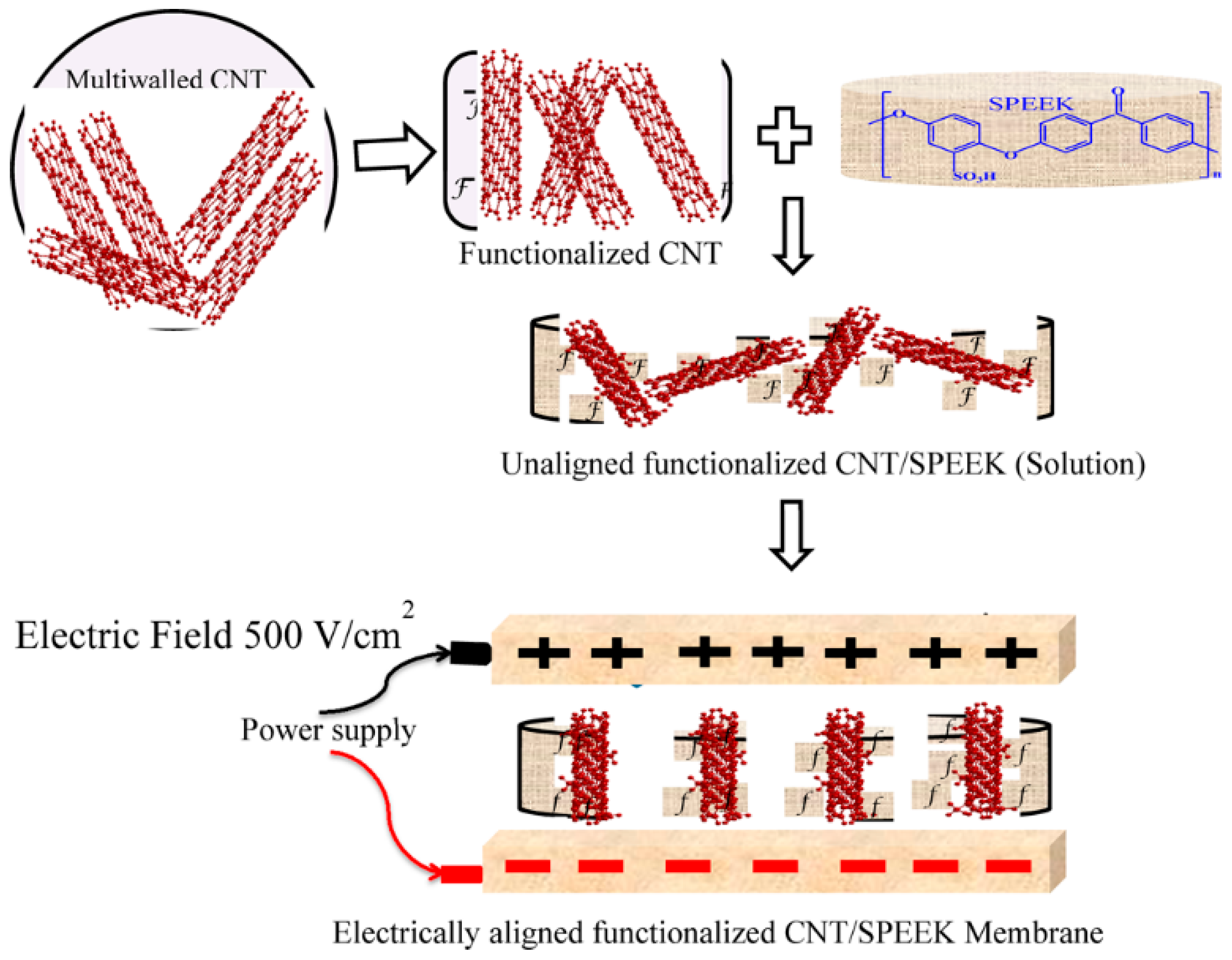
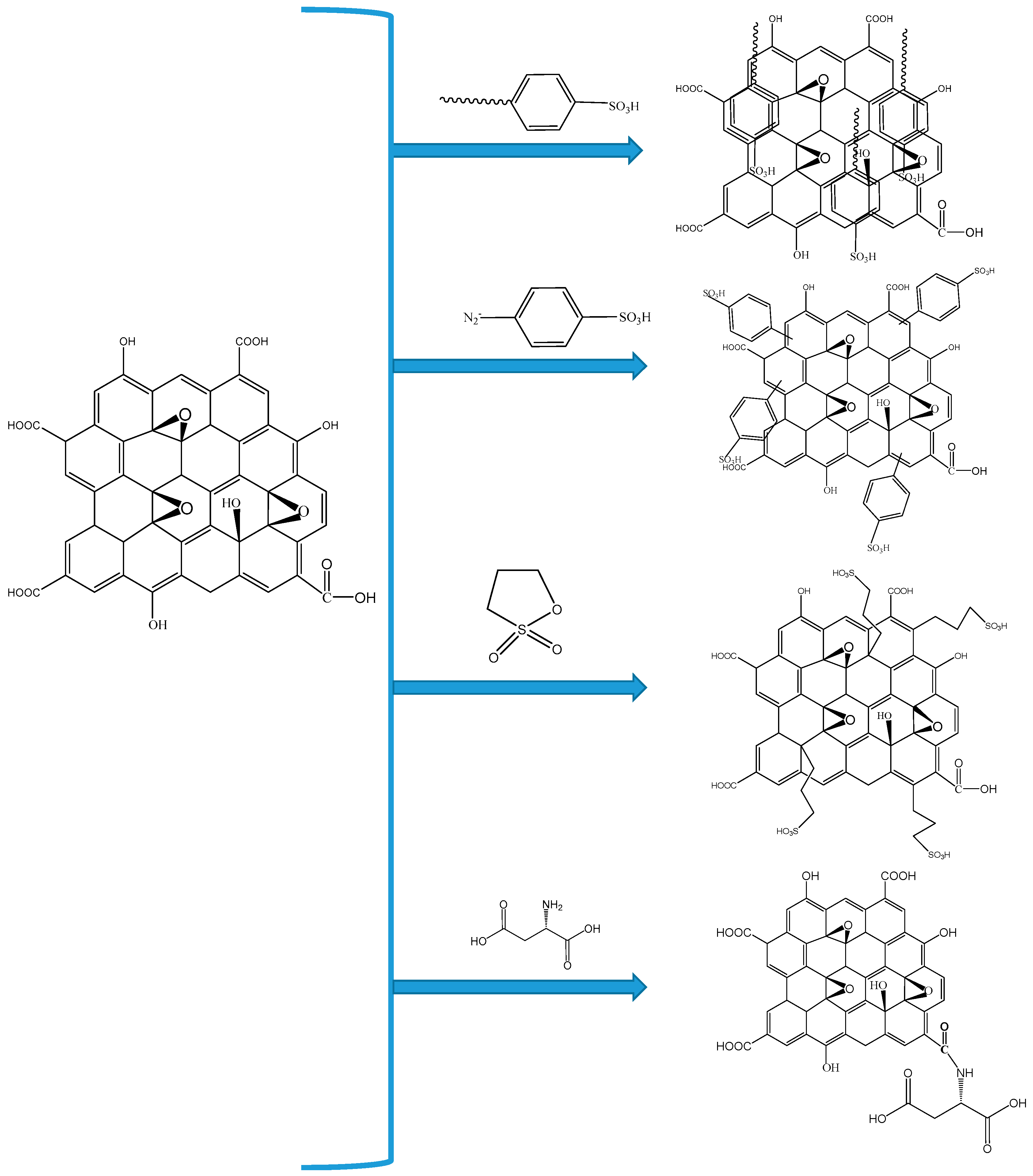
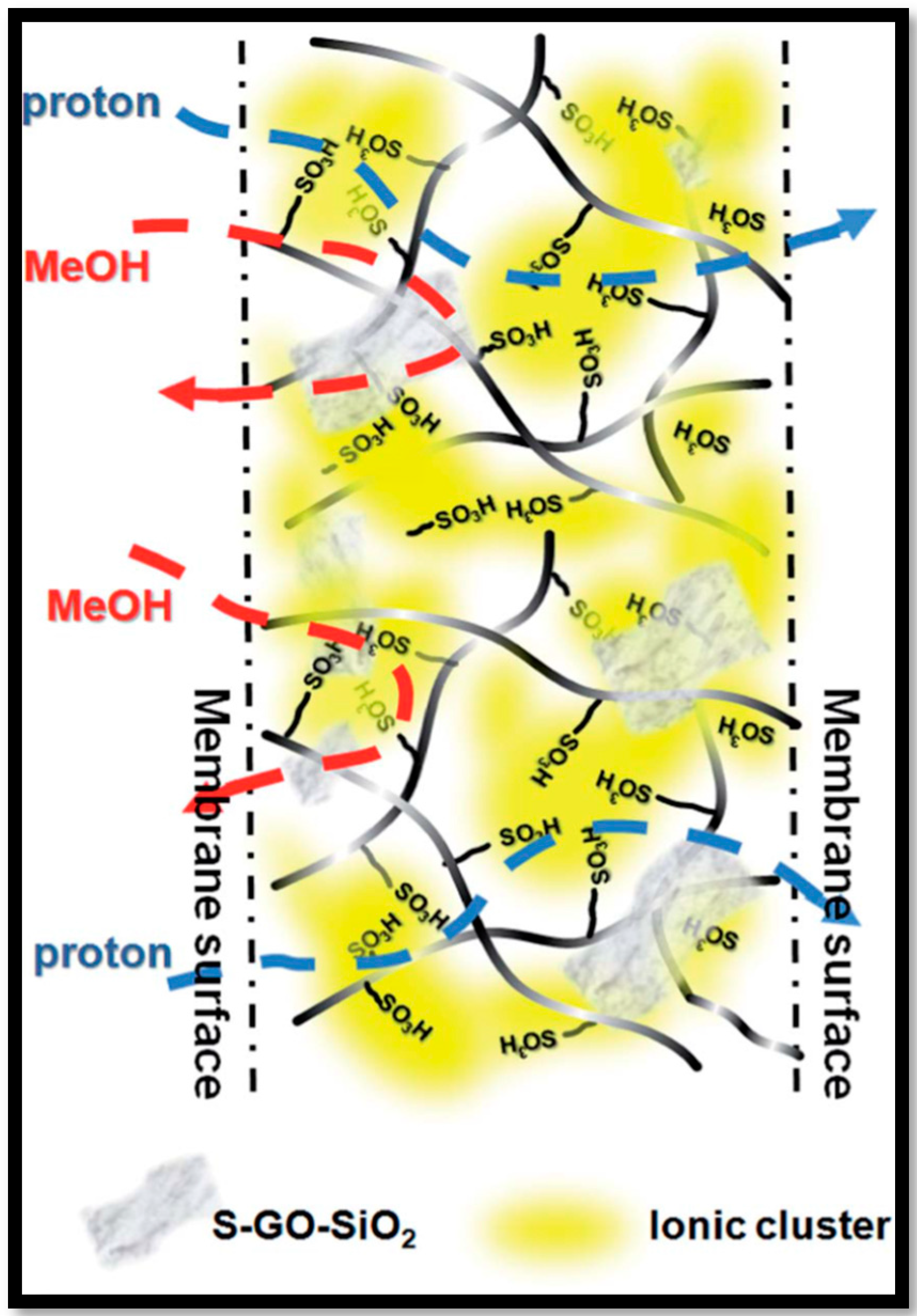
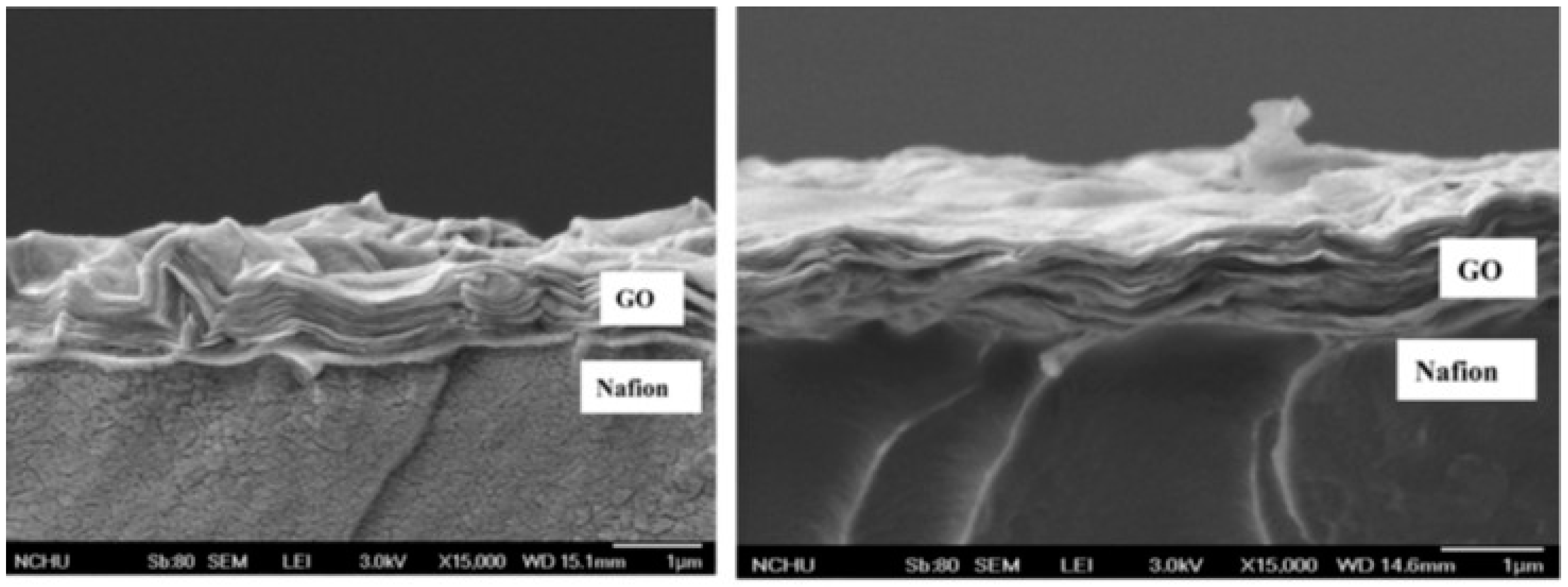
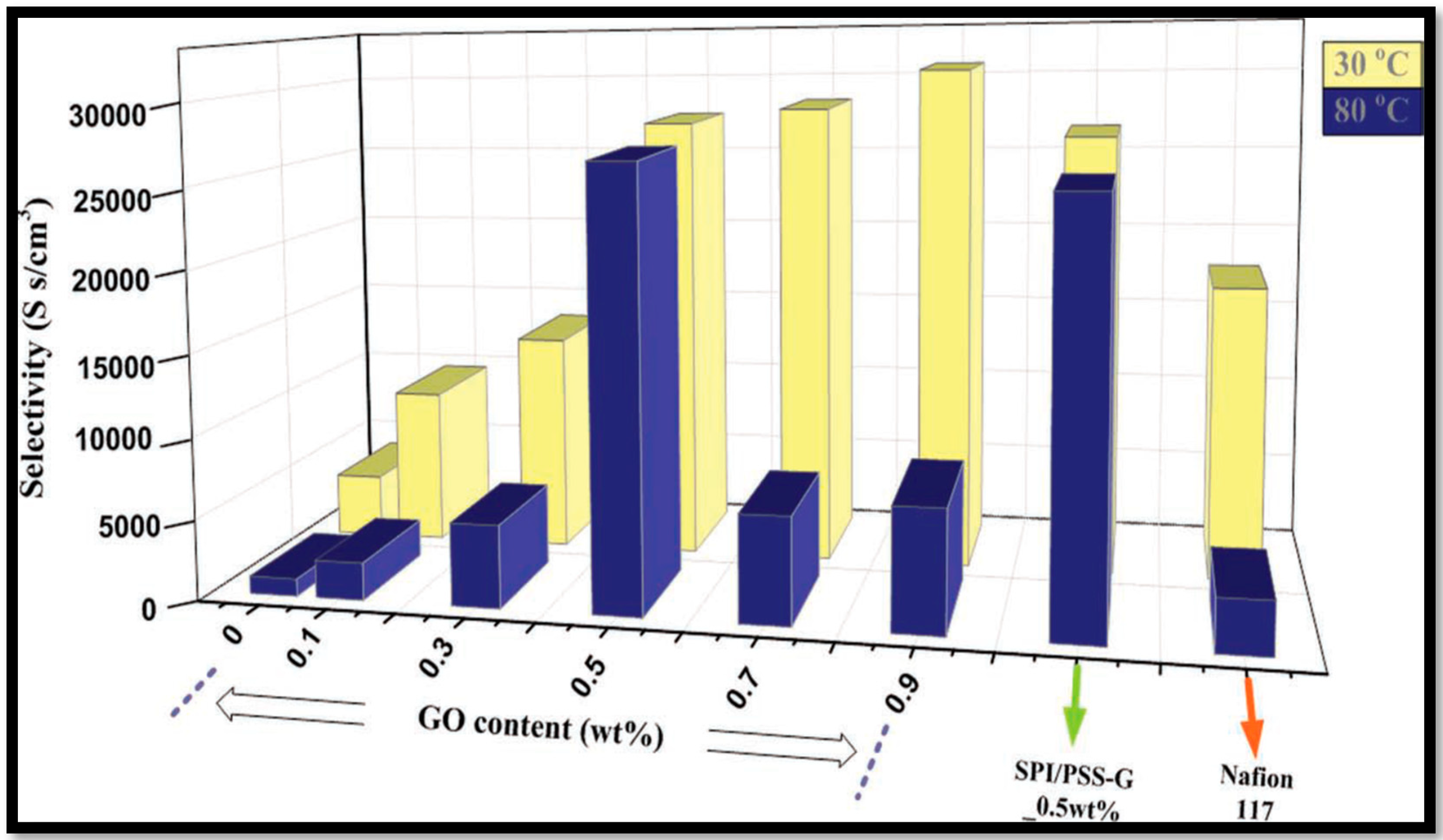

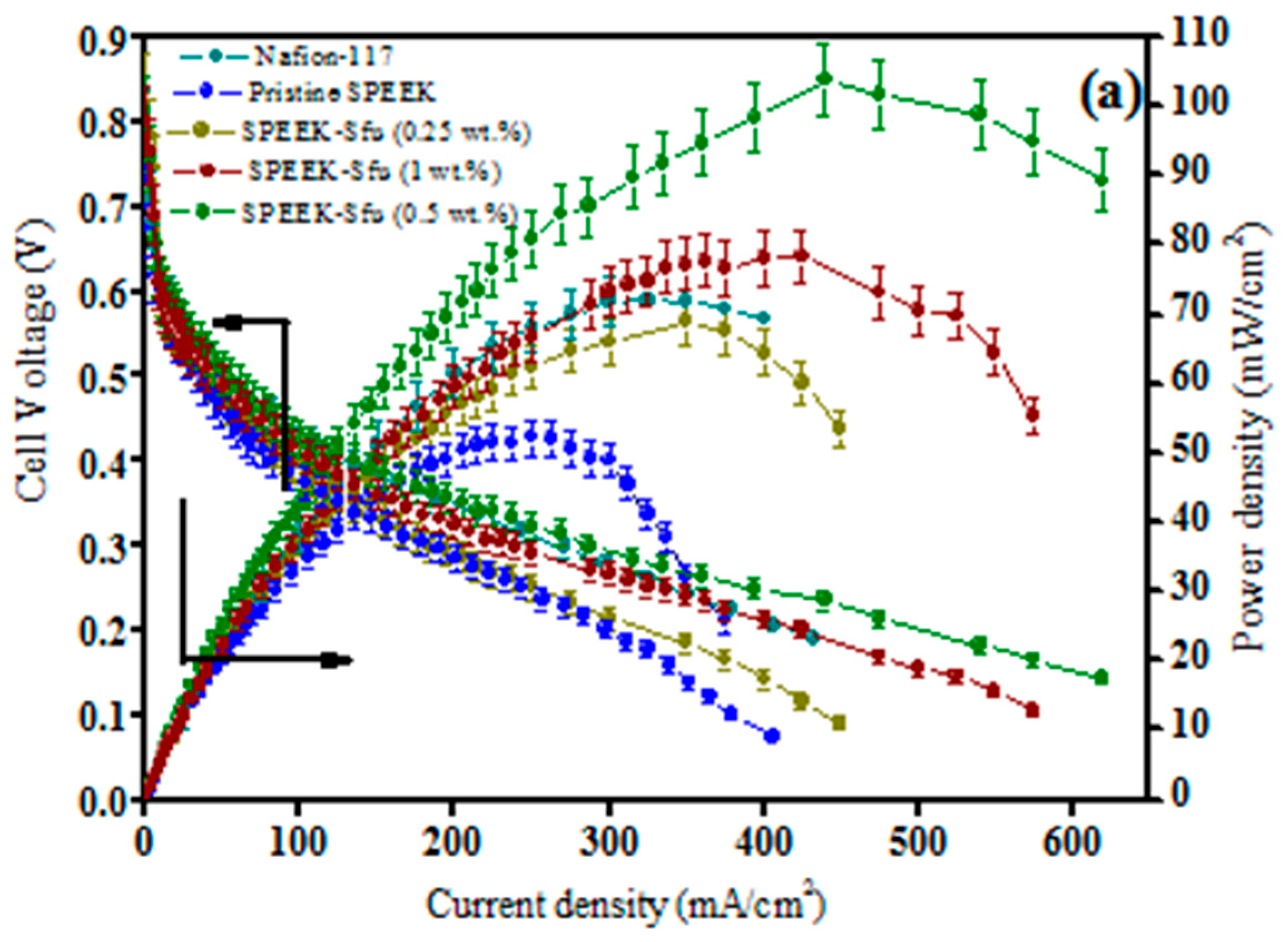
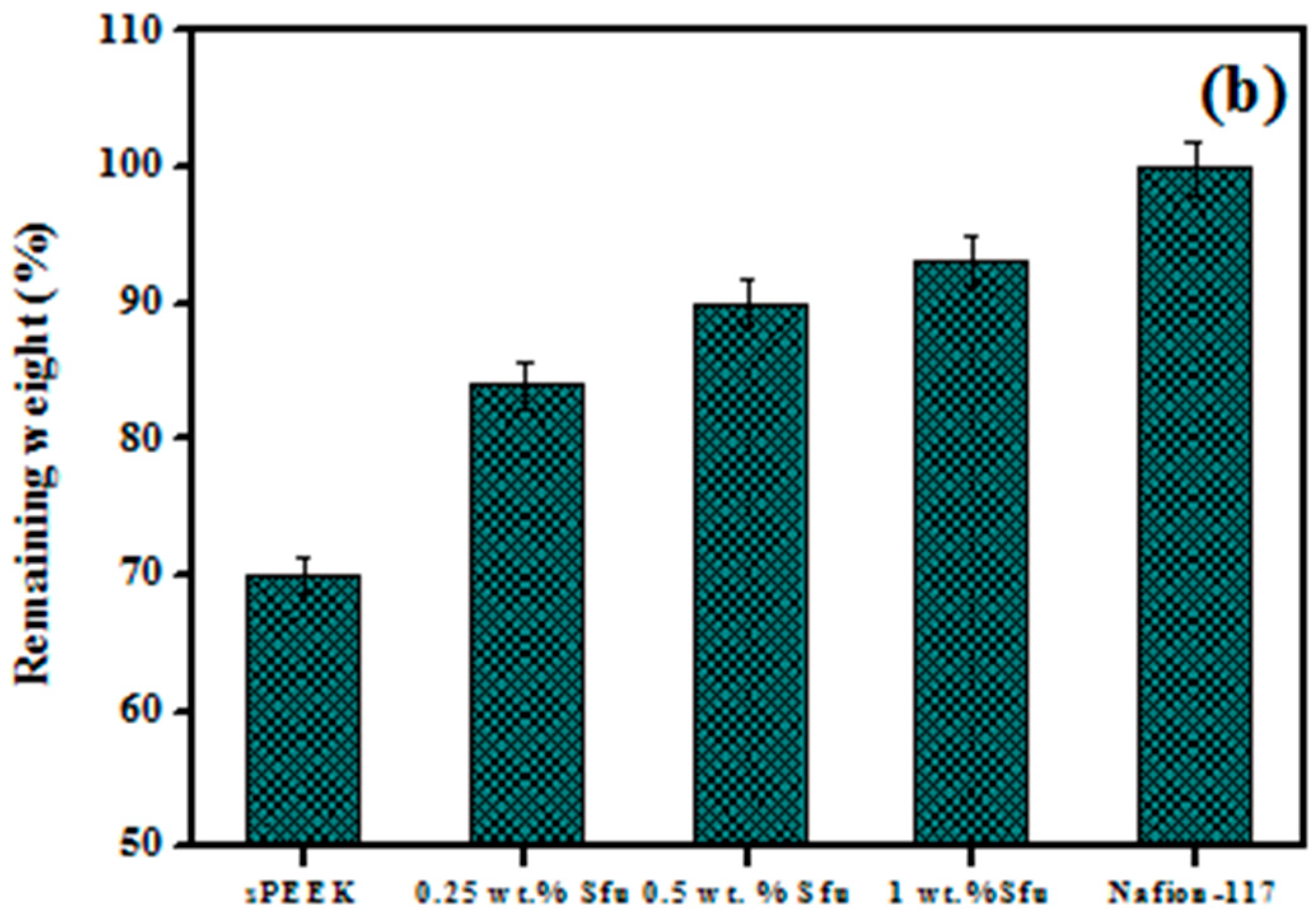

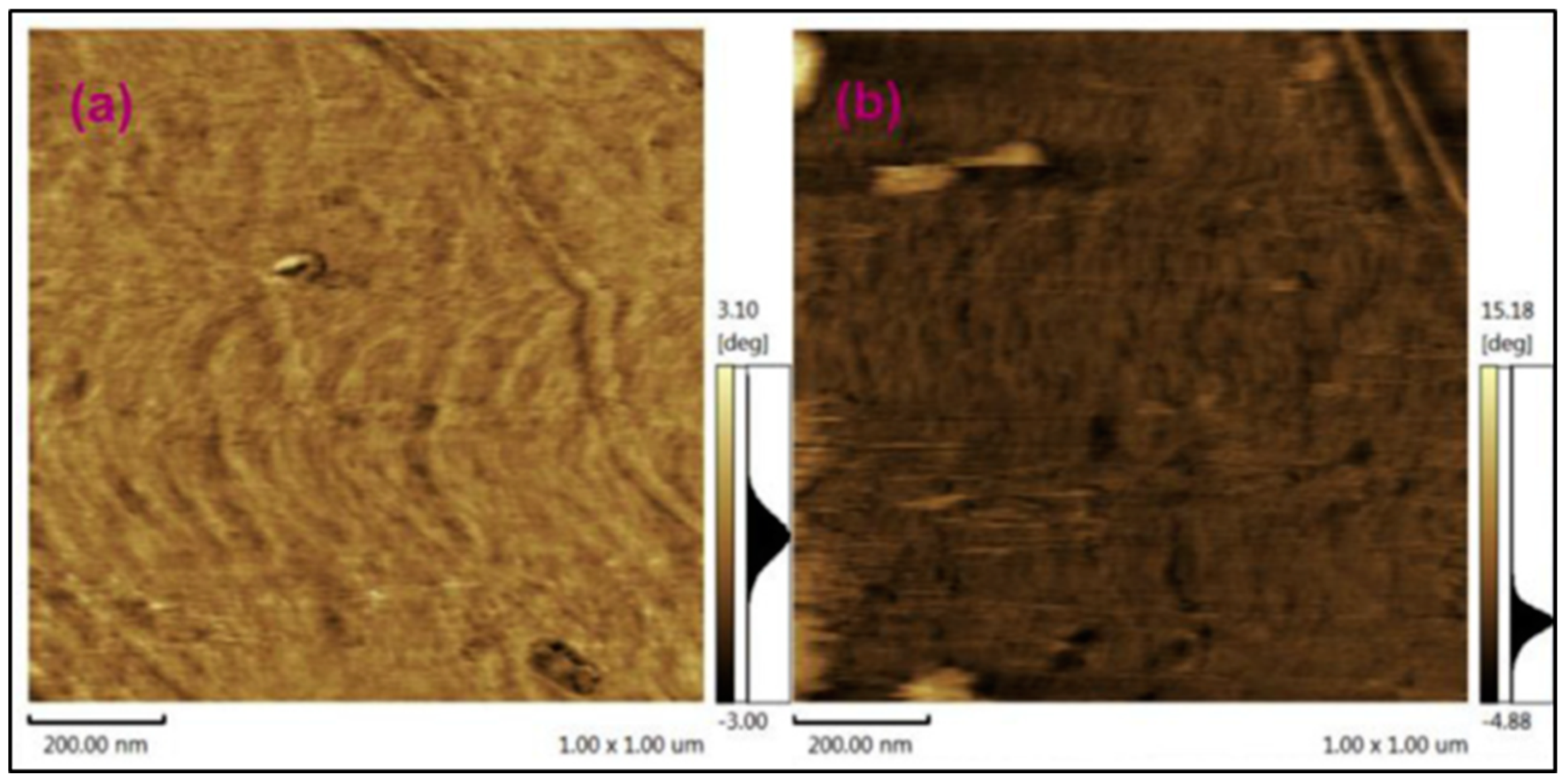
| Polymer Matrix | Carbon Material | Additive Loading wt.% | Proton Conductivity (mS cm−1) | Methanol Permeability (×10−7 cm2 s−1) | Temp. (°C) | Ref. |
|---|---|---|---|---|---|---|
| Nafion® | Im-CNT | 0.5 | 150 | 10.0 | 80 | [29] |
| Nafion® | COOH-MWCNT | 2 | 100 | 11.3 | [30] | |
| Nafion® | Chitosan-CNTs | 0.5 | 104 | 2.03 | 25 | [31] |
| Nafion® | PWA-SiO2-CNT | 1 | 87 | 2.63 | 25 | [32] |
| Nafion® | Fe3O4-CNTs | 0.1 | 85 | 5.4 | 60 | [33] |
| Nafion® | CeO2−ACNTs | 2 | 96 | NR | 60 | [34] |
| SPEI | S-MWCNT | 5 | 3.98 | 11.7 | 80 | [35] |
| SPAS | SO3CNT/PtRu/CNT | 1 | 106 | 23.6 | [36] | |
| PVA | s-MWNTs | 20 | 75 | 0.03 | 60 | [37] |
| PVA | S-MWCNT/F-MMT | 1 | 6 | 20 | 30 | [38] |
| PVA | S-MWCNT | 1 | 4 | 41 | 30 | [38] |
| PVA | CNT-PDDA-HPW | 2 | 9.4 | 4.02 | 30 | [39] |
| SPESEKK | sCNTs | 1.5 | 4.3 | 0.96 | 30 | [24] |
| SPEEK | PSSA-CNTs | 0.5 | 101 | 2.17 | 60 | [40] |
| SPEEK | fCNTs | 0.5 | 43.1 | 1.68 | 30 | [41] |
| SPEEK | POH-CNTs | 2 | 160 | 3.7 | 60 | [42] |
| SPEEK | SiO2-CNTs | 1.5 | 77.8 | 0.72 | RT | [43] |
| SDBC | S-CNTs | 1.5 | 141.7 | NR | 90 | [44] |
| Nafion® | GO | 1.5 | 23.5 | 9.1 | 35 | [45] |
| Nafion® | S-GO | 0.5 | 100 | 19.9 | 60 | [46] |
| Nafion® | GO | 0.5 | 40 | 7.92 | 30 | [47] |
| Nafion® | GO-silica | 0.8 | 48.1 | 0.16 | 50 | [48] |
| Nafion® | S-GO | NR | 89.6 | 8.4 | 30 | [49] |
| Nafion® | Graphene | NR | 40 | 4.4 | 25 | [50] |
| Nafion® | GO | NR | 15 | 0.67 | 30 | [51] |
| Nafion® | PDDA-GO | NR | 25 | 13 | 25 | [52] |
| SPEEK | S-GO | 8 | 162.6 | 13.6 | 65 | [53] |
| SPEEK | SDBS-GO | 8 | 162.6 | 9.5 | 65 | [54] |
| SPEEK | S-GO | 5 | 8.41 | 2.6 | 80 | [55] |
| SPEEK | Histidine-GO | 4 | 69.4 | 1.35 | 25 | [56] |
| SPEEK | SH-GO | 5 | 90.5 | 0.3 | 25 | [57] |
| SF-SPEEK | GO | 5 | 111.90 | NR | 90 | [58] |
| SPI | SPS-GO | 8 | 96.2 | 2.0 | 30 | [59] |
| SPI | PSS-GO | 0.5 | 86 | 4.31 | 60 | [60] |
| SPI | SI-GO | 10 | 113.8 | 10.52 | 30 | [61] |
| SPES | S-GO | 5 | 58 | 1.5 | 30 | [62] |
| SPES | IL-GO | 5 | 72.7 | 0.53 | RT | [63] |
| SPES | S-GO | 15 | 78.2 | 3.82 | 25 | [64] |
| SPES | GO | 1 | 4.3 | 0.49 | RT | [65] |
| SPE | S-GO | 0.75 | 390 | 4.89 | 80 | [66] |
| SPS | S-GO | 3 | 4.27 | 3.48 | RT | [67] |
| SPEN | N-GO | 1 | 104 | 1.74 | 20 | [68] |
| SPEN | S-N-GO | NR | 64 | 1.43 | 20 | [69] |
| SPAEN | CNT-GO | NR | 119.7 | 2.0 | 20 | [70] |
| SPVdF-co-HFP | S-GO | 0.7 | 5.5 | 1.8 | 30 | [71] |
| PVA | GO | 1.5 | 13.5 | 2.0 | 35 | [72] |
| PVA | Fe3O4/S-GO | 5 | 64 | 0.45 | 30 | [73] |
| GO paper | 54.2 | 2.4 | 65 | [74] | ||
| Holey GO paper | 68.4 | 145.5 | 65 | [74] | ||
| SDBS-GO paper | 68.5 | 4.4 | 65 | [74] | ||
| Holey SDBS Holey GO paper | 91.8 | 35.4 | 65 | [74] | ||
| GO paper | 4.9 | 0.16 | 30 | [75] | ||
| SPEEK | S-fullerene | 0.5 | 96 | 2.4 | 60 | [76] |
| Nafion® | S-fullerene | 1 | 97 | 8.5 | 60 | [77] |
| SPEEK | SCNF | 1 | 128 | 5.02 | 60 | [78] |
| SPPEK | SGNF | 0.5 | 85 | 4.5 | 60 | [79] |
| Nafion® | MC | 1 | 75 | 9.8 | 30 | [80] |
| Nafion® | NCD | 0.5 | 21 | 0.12 | 40 | [81] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rambabu, G.; D. Bhat, S.; Figueiredo, F.M.L. Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review. Nanomaterials 2019, 9, 1292. https://doi.org/10.3390/nano9091292
Rambabu G, D. Bhat S, Figueiredo FML. Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review. Nanomaterials. 2019; 9(9):1292. https://doi.org/10.3390/nano9091292
Chicago/Turabian StyleRambabu, Gutru, Santoshkumar D. Bhat, and Filipe M. L. Figueiredo. 2019. "Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review" Nanomaterials 9, no. 9: 1292. https://doi.org/10.3390/nano9091292
APA StyleRambabu, G., D. Bhat, S., & Figueiredo, F. M. L. (2019). Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review. Nanomaterials, 9(9), 1292. https://doi.org/10.3390/nano9091292






