Colorimetric Determination of the Activity of Starch-Debranching Enzyme via Modified Tollens’ Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Debranching of Waxy Maize Starch
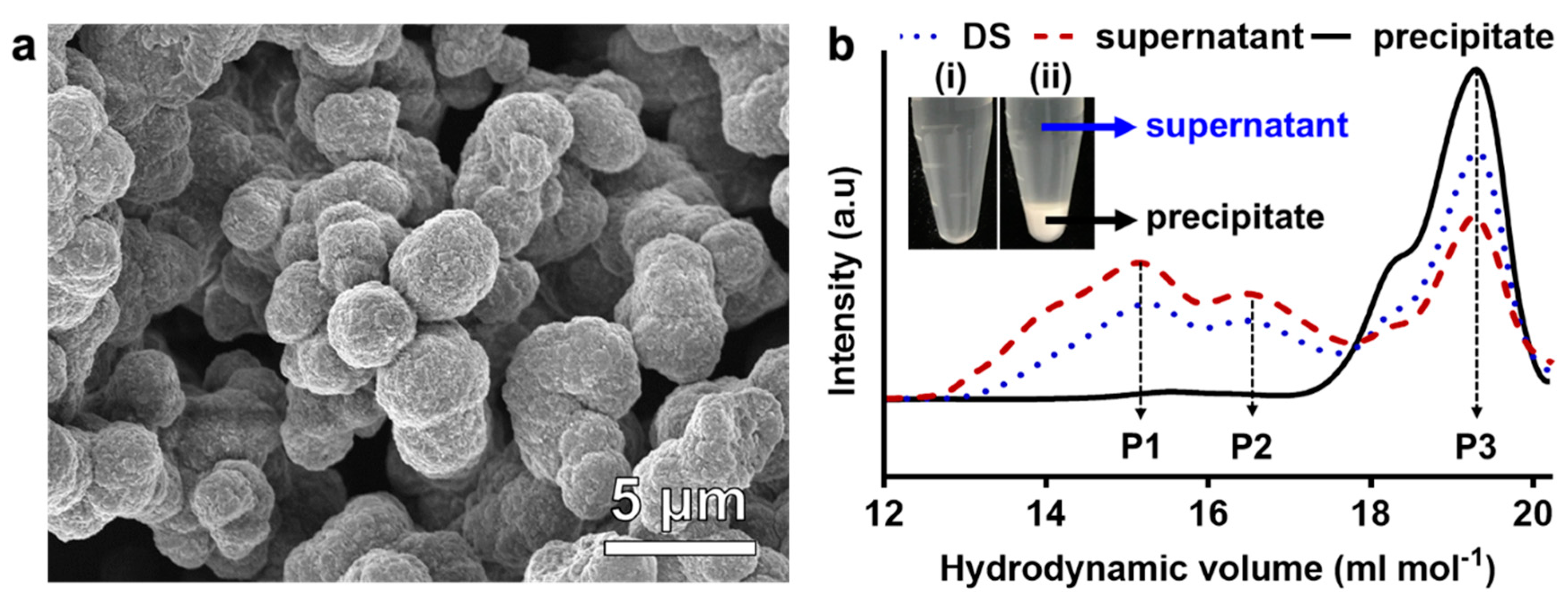
2.3. Separation of SCG From the Debranching Reaction
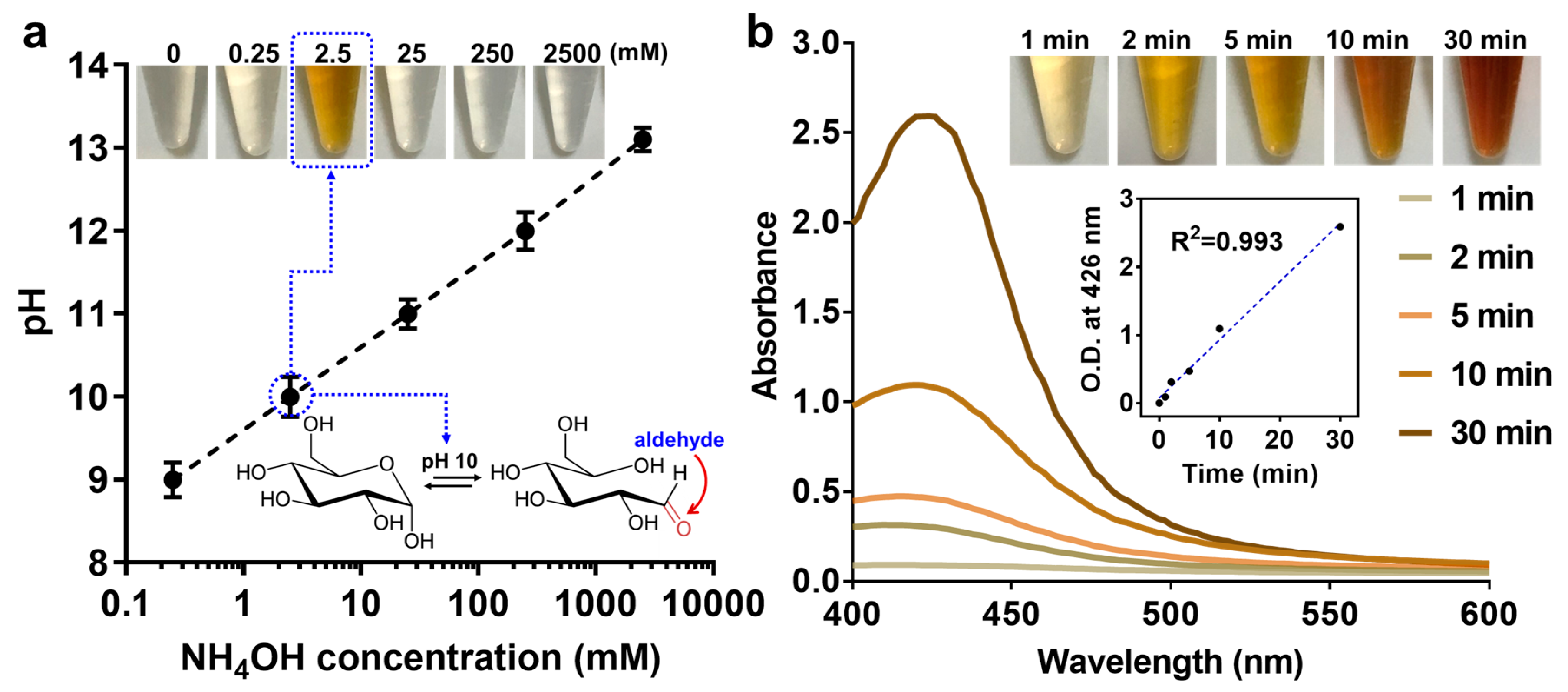
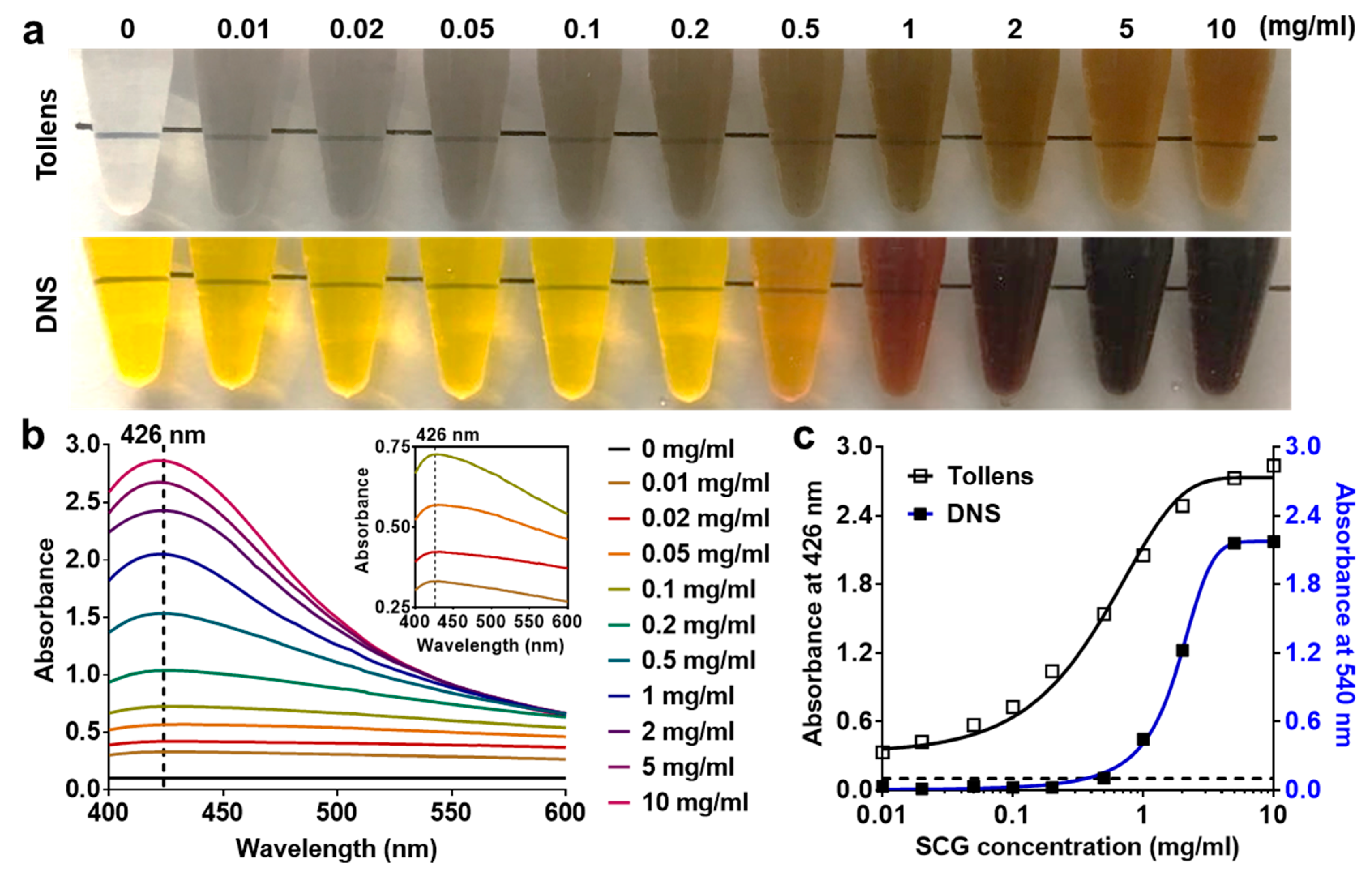
2.4. Measurement of Debranching Enzyme Activity
2.5. Characterization of AgNPs Synthesized During Modified Tollens’ Reaction
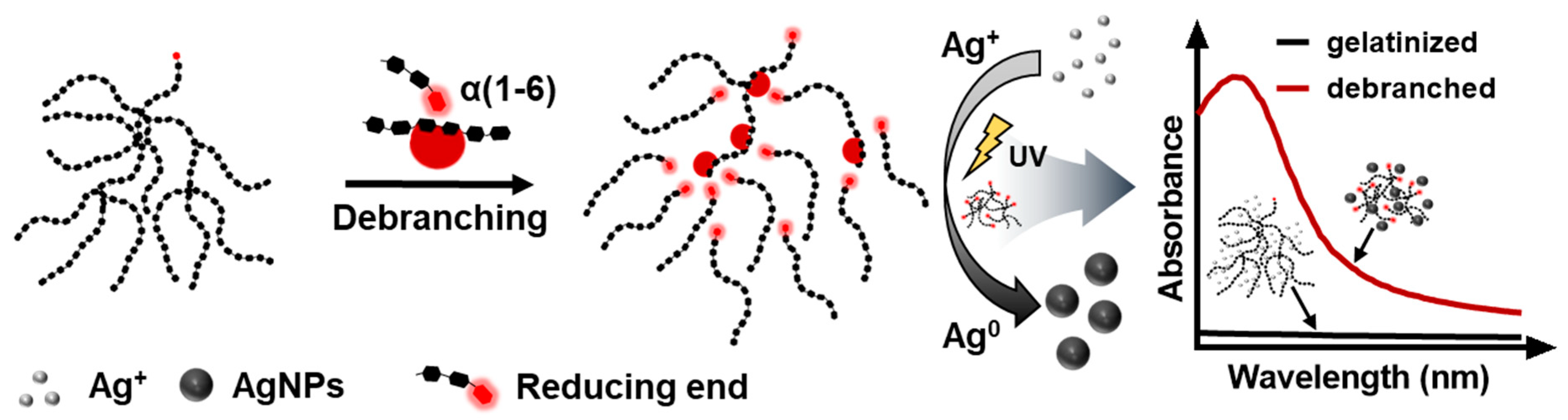
3. Results and Discussion
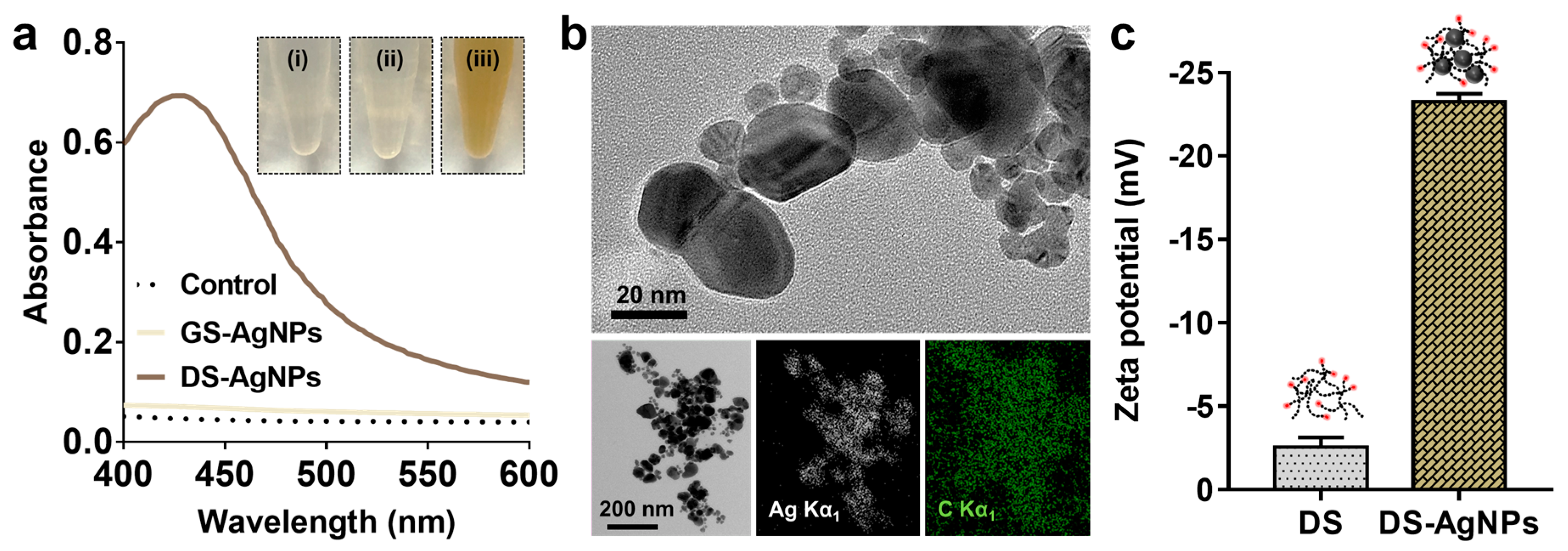
3.1. Synthesis of AgNPs Using Debranched Starch via Modified Tollens’ Reaction
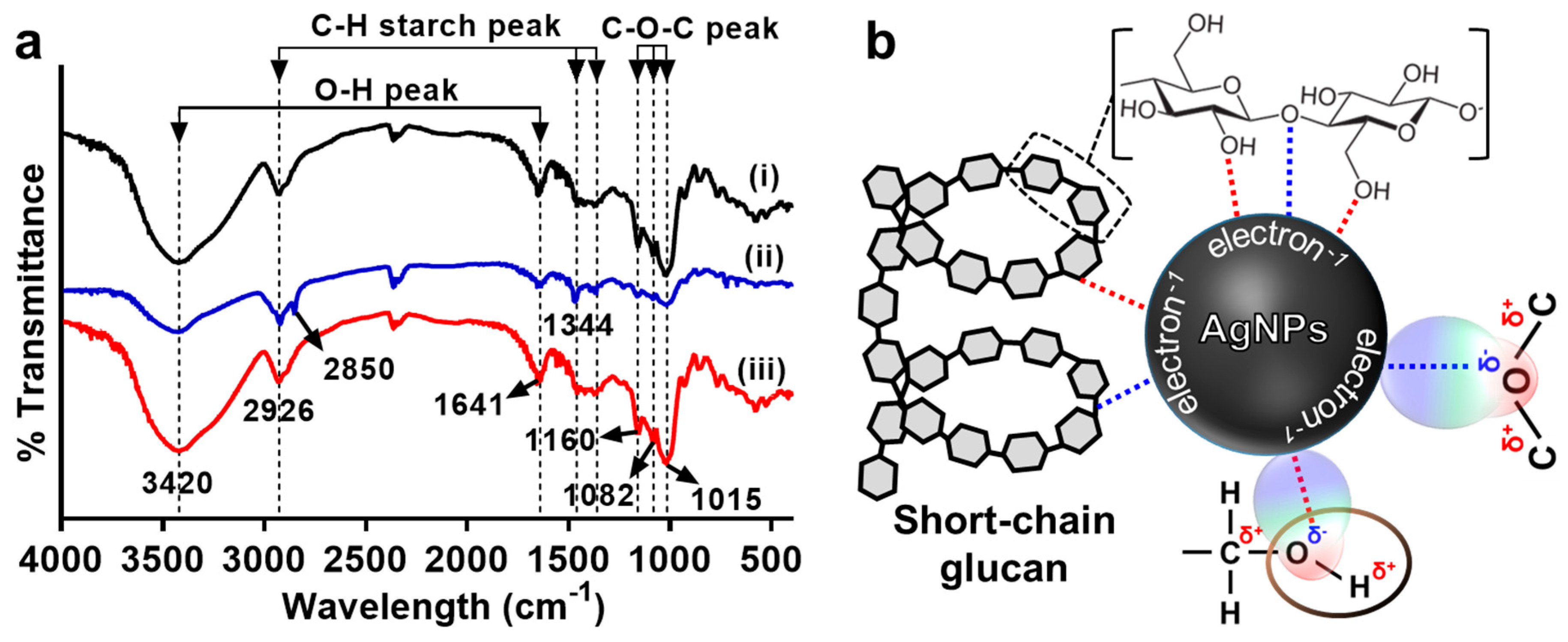
3.2. Characterization of AgNPs Synthesized by Modified Tollens’ Reaction
3.3. Quantification of Debranched SCGs through Modified Tollens’ Reaction
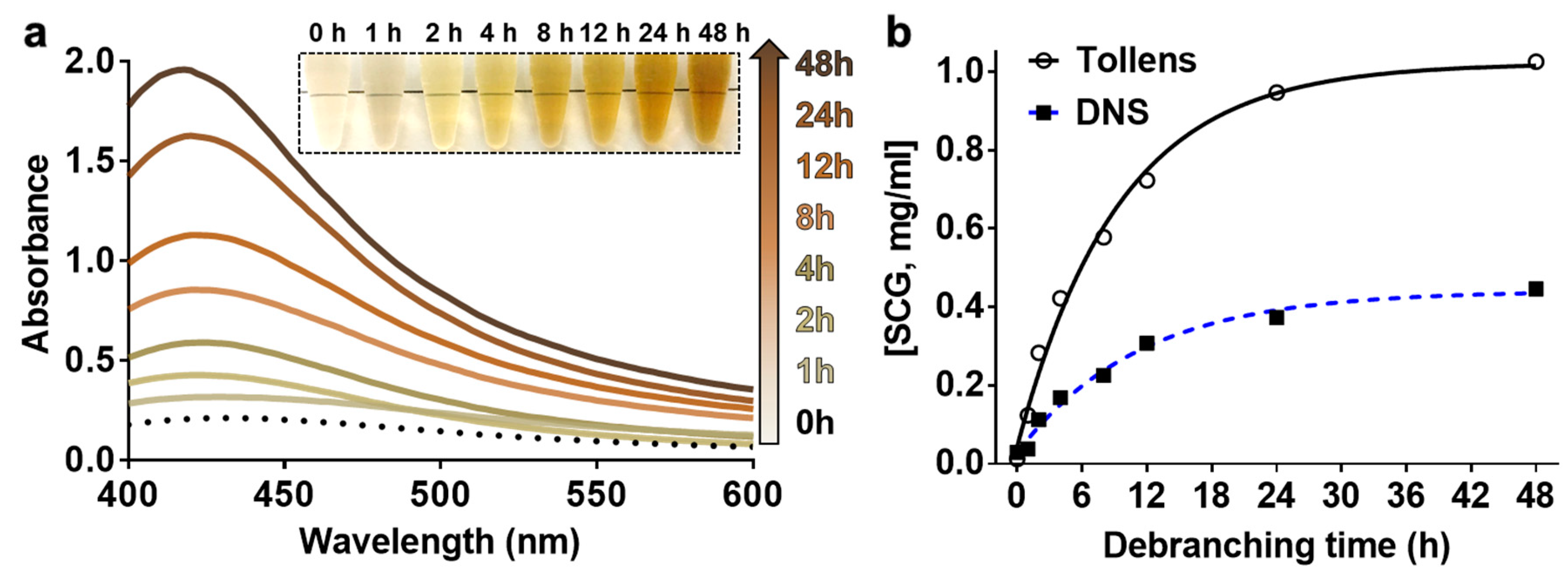
3.4. Investigation of the Debranching Reaction in a Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beatty, M.K.; Rahman, A.; Cao, H.; Woodman, W.; Lee, M.; Myers, A.M.; James, M.G. Purification and Molecular Genetic Characterization of ZPU1, a pullulanase-type starch-debranching enzyme from maize. Plant Physiol. 1999, 119, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Furuta, S.; Suda, I. Sweet potato β-amylase immobilized on chitosan beads and its application in the semi-continuous production of maltose. Carbohydr. Polym. 2001, 44, 189–195. [Google Scholar] [CrossRef]
- Letona, C.A.M.; Luo, K.; Jeong, K.-B.; Adra, H.J.; Park, C.-S.; Kim, Y.-R. Effect of lecithin on the spontaneous crystallization of enzymatically synthesized short-chain amylose molecules into spherical microparticles. Polymers 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Park, K.-H.; Lee, D.-H.; Hong, C.-E.; Song, Y.-W.; Yoo, S.-H.; Kim, Y.-R. Self-assembly kinetics of debranched short-chain glucans from waxy maize starch to form spherical microparticles and its applications. Colloids Surf. B Biointerfaces 2019, 176, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.-C.; Seo, D.-H.; Jung, J.-H.; Park, C.-S.; Kim, Y.-R. Enzymatic synthesis of amylose nanocomposite microbeads using amylosucrase from Deinococcus geothermalis. RSC Adv. 2014, 4, 26421–26424. [Google Scholar] [CrossRef]
- Luo, K.; Jeong, K.-B.; Park, C.-S.; Kim, Y.-R. Biosynthesis of superparamagnetic polymer microbeads via simple precipitation of enzymatically synthesized short-chain amylose. Carbohydr. Polym. 2018, 181, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Jeong, K.-B.; You, S.-M.; Lee, D.-H.; Jung, J.-Y.; Kim, Y.-R. Surface-engineered starch magnetic microparticles for highly effective separation of a broad range of bacteria. ACS Sustain. Chem. Eng. 2018, 6, 13524–13531. [Google Scholar] [CrossRef]
- Luo, K.; Jeong, K.-B.; You, S.-M.; Lee, D.-H.; Kim, Y.-R. Molecular rearrangement of glucans from natural starch to form size-controlled functional magnetic polymer beads. J. Agric. Food Chem. 2018, 66, 6806–6813. [Google Scholar] [CrossRef]
- Lim, M.-C.; Park, K.-H.; Choi, J.-H.; Lee, D.-H.; Letona, C.A.M.; Baik, M.-Y.; Park, C.-S.; Kim, Y.-R. Effect of short-chain fatty acids on the formation of amylose microparticles by amylosucrase. Carbohydr. Polym. 2016, 151, 606–613. [Google Scholar] [CrossRef]
- Letona, C.A.M.; Park, C.S.; Kim, Y.R. Amylosucrase-mediated β-carotene encapsulation in amylose microparticles. Biotechnol. Prog. 2017, 33, 1640–1646. [Google Scholar] [CrossRef]
- Zareian, S.; Khajeh, K.; Ranjbar, B.; Dabirmanesh, B.; Ghollasi, M.; Mollania, N. Purification and characterization of a novel amylopullulanase that converts pullulan to glucose, maltose, and maltotriose and starch to glucose and maltose. Enzym. Microb. Technol. 2010, 46, 57–63. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int. J. Anal. Chem. 2011, 2011, 4. [Google Scholar] [CrossRef]
- Breuil, C.; Saddler, J.N. Comparison of the 3,5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity. Enzym. Microb. Technol. 1985, 7, 327–332. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, S.; Luo, J.; Wang, X.; Sun, R. Green synthesis of silver nanoparticles in xylan solution via Tollens reaction and their detection for Hg2+. Nanoscale 2015, 7, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, D.; Yu, L.; Chang, M.; Ci, L. One-step, room temperature, colorimetric melamine sensing using an in-situ formation of silver nanoparticles through modified Tollens process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-B.; Fan, S.-G.; Zhao, C.-Y.; Wang, Q.-R.; Zhou, T.-Y.; Chen, X.; Yan, Z.-F.; Li, Y.-P.; Xing, W.; Wang, X.-D. A colorimetric agarose gel for formaldehyde measurement based on nanotechnology involving Tollens reaction. Chem. Commun. 2014, 50, 8121–8123. [Google Scholar] [CrossRef]
- Chaiendoo, K.; Sooksin, S.; Kulchat, S.; Promarak, V.; Tuntulani, T.; Ngeontae, W. A new formaldehyde sensor from silver nanoclusters modified Tollens’ reagent. Food Chem. 2018, 255, 41–48. [Google Scholar] [CrossRef]
- Luo, K.; Lee, D.-H.; Adra, H.J.; Kim, Y.-R. Synthesis of monodisperse starch microparticles through molecular rearrangement of short-chain glucans from natural waxy maize starch. Carbohydr. Polym. 2019, 218, 261–268. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Naoi, K.; Ohko, Y.; Tatsuma, T. TiO2 films loaded with silver nanoparticles: Control of multicolor photochromic behavior. J. Am. Chem. Soc. 2004, 126, 3664–3668. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Jung, S.; Park, K.-H.; Kim, Y.-R. Microbial biosynthesis of silver nanoparticles in different culture media. J. Agric. Food Chem. 2018, 66, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Murria, P.; Jiang, Y.; Xiao, W.; Kenttämaa, H.I.; Abu-Omar, M.M.; Mosier, N.S. Maleic acid and aluminum chloride catalyzed conversion of glucose to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media. Green Chem. 2016, 18, 5219–5229. [Google Scholar] [CrossRef]
- Benet, W.E.; Lewis, G.S.; Yang, L.Z.; Hughes, D.E.P. The mechanism of the reaction of the Tollens reagent. J. Chem. Res. 2011, 35, 675–677. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Kim, N.-g.; You, S.-M.; Kim, Y.-R. Colorimetric Determination of the Activity of Starch-Debranching Enzyme via Modified Tollens’ Reaction. Nanomaterials 2019, 9, 1291. https://doi.org/10.3390/nano9091291
Luo K, Kim N-g, You S-M, Kim Y-R. Colorimetric Determination of the Activity of Starch-Debranching Enzyme via Modified Tollens’ Reaction. Nanomaterials. 2019; 9(9):1291. https://doi.org/10.3390/nano9091291
Chicago/Turabian StyleLuo, Ke, Nack-geun Kim, Sang-Mook You, and Young-Rok Kim. 2019. "Colorimetric Determination of the Activity of Starch-Debranching Enzyme via Modified Tollens’ Reaction" Nanomaterials 9, no. 9: 1291. https://doi.org/10.3390/nano9091291
APA StyleLuo, K., Kim, N.-g., You, S.-M., & Kim, Y.-R. (2019). Colorimetric Determination of the Activity of Starch-Debranching Enzyme via Modified Tollens’ Reaction. Nanomaterials, 9(9), 1291. https://doi.org/10.3390/nano9091291




