Morphological Transformation of Silver Nanoparticles from Commercial Products: Modeling from Product Incorporation, Weathering through Use Scenarios, and Leaching into Wastewater
Abstract
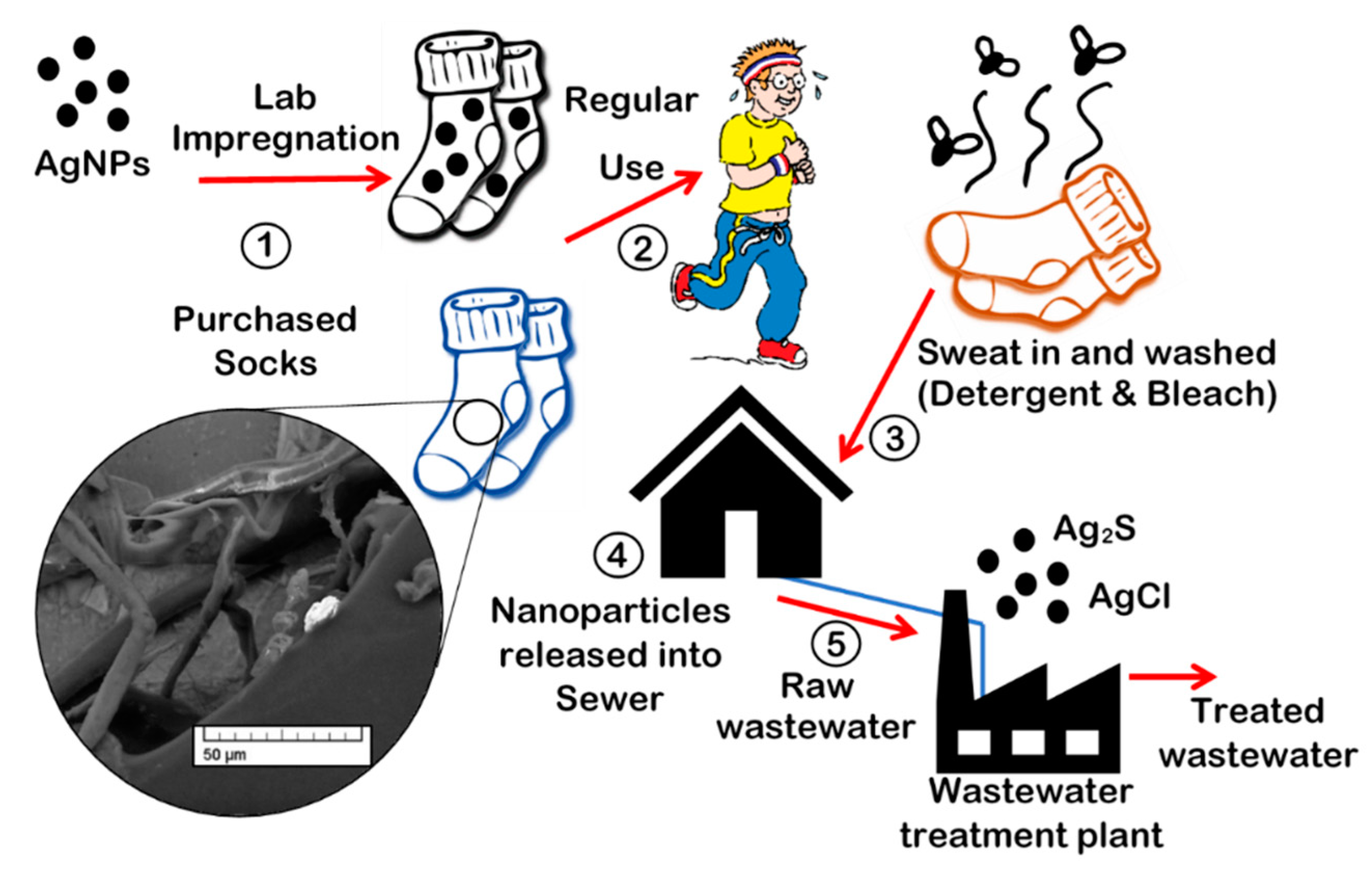
:1. Introduction
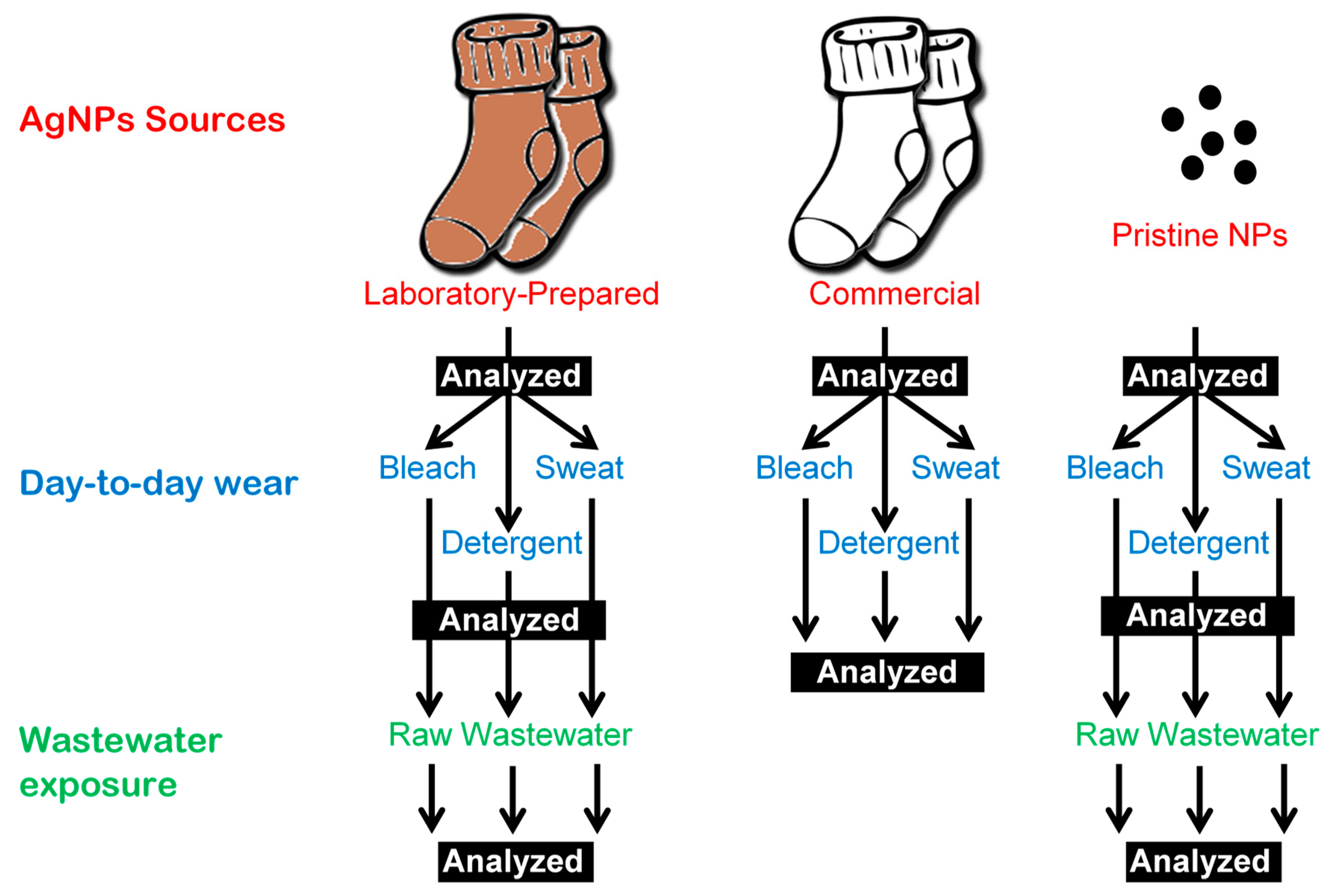
2. Materials and Methods
2.1. Preparing Socks for In-Situ Impregnation (Kier boiling)
2.2. In-Situ Impregnation of Ag Nanoparticles into Socks (Laboratory-Prepared Socks)
2.3. Day-to-day Wear with Sweat, Bleach, and Detergent
2.4. Wastewater Exposure
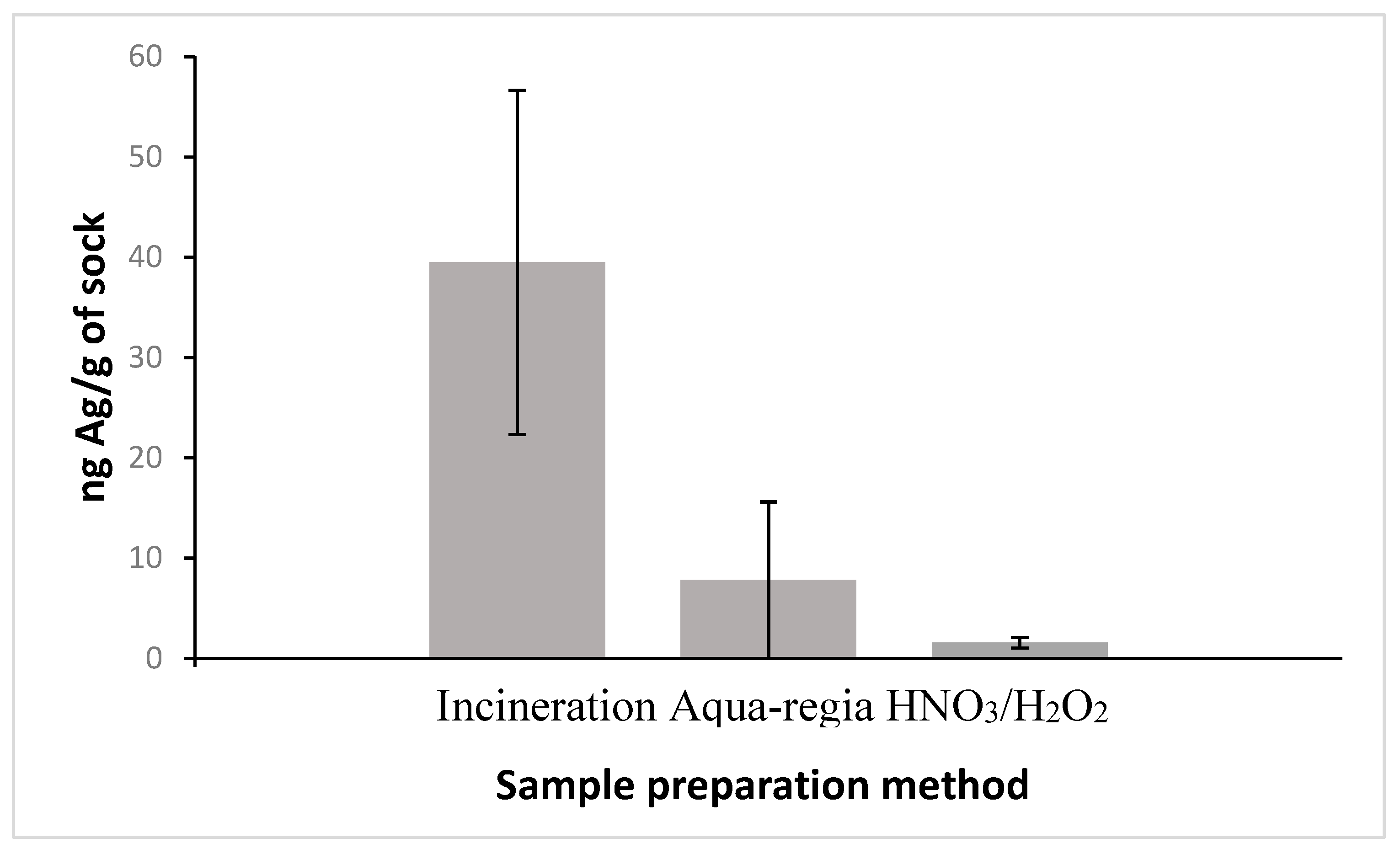
2.5. Sample Preparation for ICP-MS Analysis
2.5.1. Incineration
2.5.2. Aqua-Regia
2.5.3. HNO3/H2O2
2.6. Analysis
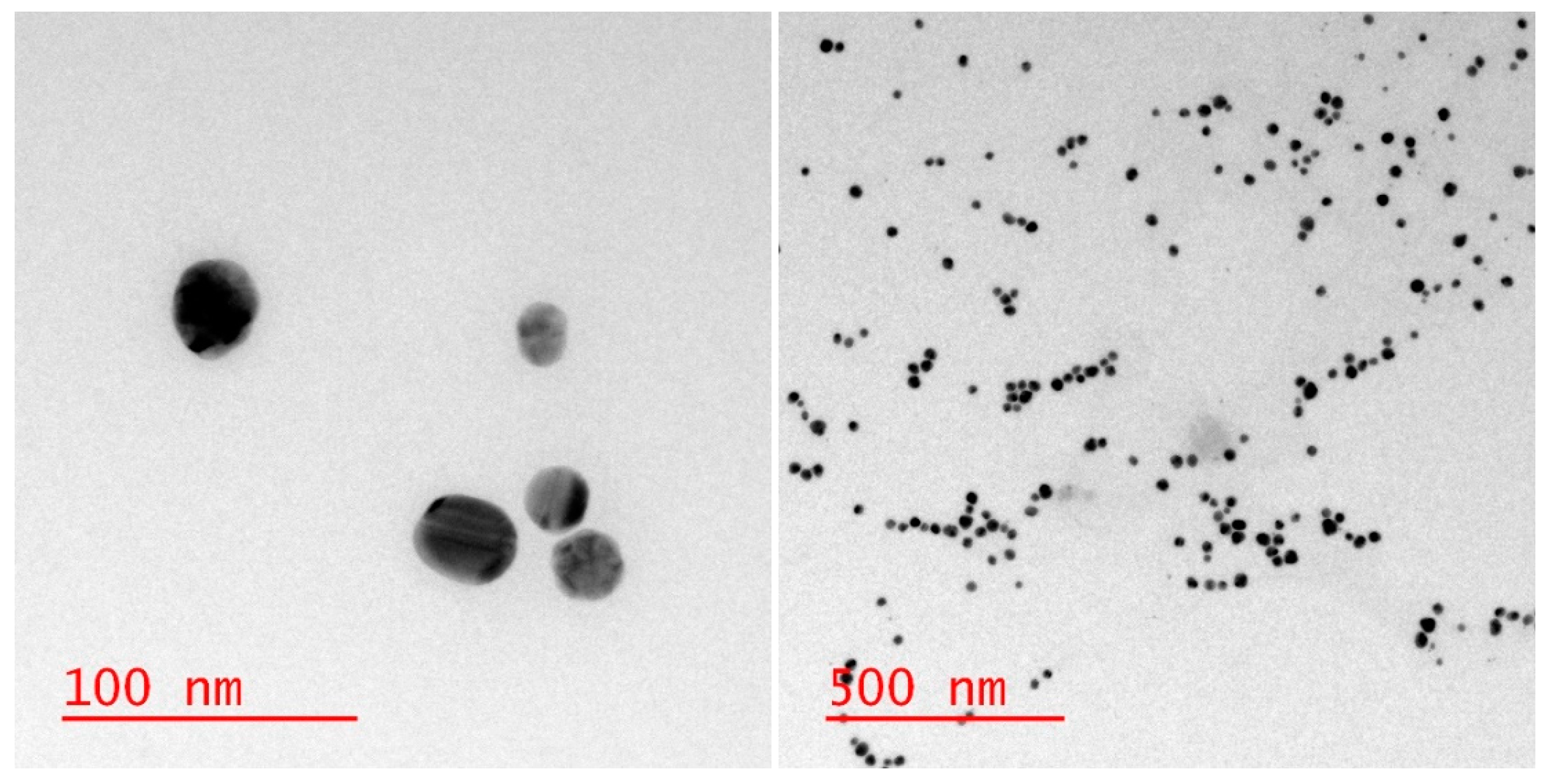
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Lin, P.-C. Noble metal nanoparticles: Synthesis, and biomedical implementations. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 177–233. [Google Scholar]
- Cassano, D.; David, J.; Luin, S.; Voliani, V. Passion fruit-like nano-architectures: A general synthesis route. Sci. Rep. 2017, 7, 43795. [Google Scholar] [CrossRef]
- The Project on Emerging Nanotechnologies. Available online: http://www.nanotechproject.org/cpi/about/analysis/ (accessed on 1 April 2015).
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Walser, T.; Demou, E.; Lang, D.J.; Hellweg, S. Prospective environmental life cycle assessment of nanosilver T-shirts. Environ. Sci. Technol. 2011, 45, 4570–4578. [Google Scholar] [CrossRef]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella, M.F.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J.; et al. Low Concentrations of Silver Nanoparticles in Biosolids Cause Adverse Ecosystem Responses under Realistic Field Scenario. PLoS ONE 2013, 8, e57189. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Miseljic, M.; Olsen, S.I. Life-cycle assessment of engineered nanomaterials: A literature review of assessment status. J. Nanoparticle Res. 2014, 16, 2427. [Google Scholar] [CrossRef]
- Diez-Ortiz, M.; Lahive, E.; George, S.; Ter Schure, A.; Van Gestel, C.A.M.; Jurkschat, K.; Svendsen, C.; Spurgeon, D.J. Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils. Environ. Pollut. 2015, 203, 191–198. [Google Scholar] [CrossRef]
- Fernández, M.D.; Alonso-Blázquez, M.N.; García-Gómez, C.; Babin, M. Evaluation of zinc oxide nanoparticle toxicity in sludge products applied to agricultural soil using multispecies soil systems. Sci. Total Environ. 2014, 497–498, 688–696. [Google Scholar] [CrossRef]
- Fabrega, J.; Fawcett, S.R.; Renshaw, J.C.; Lead, J.R. Silver nanoparticle impact on bacterial growth: Effect of pH, concentration, and organic matter. Environ. Sci. Technol. 2009, 43, 7285–7290. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Wei, A.; Sepúlveda, M.S. Protein Corona Analysis of Silver Nanoparticles Exposed to Fish Plasma. Environ. Sci. Technol. Lett. 2017, 4, 174–179. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S.; Mishra, S.R. Synthesis and Characterization of Silver Sulfide Nanoparticles Containing Sol-Gel Derived HPC-Silica Film for Ion-Selective Electrode Application. J. Sol-Gel Sci. Technol. 2002, 23, 151–164. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, S.-J.; Wu, X.; Sauve, J.; Lee, I.; Nam, J.; Kim, J.; Dordick, J.S. Selective Killing of Pathogenic Bacteria by Antimicrobial Silver Nanoparticle—Cell Wall Binding Domain Conjugates. ACS Appl. Mater. Interfaces 2018, 10, 13317–13324. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Laban, G.; Nies, L.F.; Turco, R.F.; Bickham, J.W.; Sepúlveda, M.S. The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos. Ecotoxicology 2010, 19, 185–195. [Google Scholar] [CrossRef]
- Dhas, S.P.; Shiny, P.J.; Khan, S.; Mukherjee, A.; Chandrasekaran, N. Toxic behavior of silver and zinc oxide nanoparticles on environmental microorganisms. J. Basic Microbiol. 2013, 916–927. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Asiri, A.M.; Abdelwahed, N.A.M.; Al-Otaibi, M.M. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Servin, A.D.; White, J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. NanoImpact 2016, 1, 9–12. [Google Scholar] [CrossRef]
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Rimmele, E.; Wichser, A.; Erni, R.; Height, M.; Nowack, B. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 2014, 8, 7208–7219. [Google Scholar] [CrossRef]
- Garg, S.; Rong, H.; Miller, C.J.; Waite, T.D. Oxidative Dissolution of Silver Nanoparticles by Chlorine: Implications to Silver Nanoparticle Fate and Toxicity. Environ. Sci. Technol. 2016, 50, 3890–3896. [Google Scholar] [CrossRef]
- Meier, C.; Voegelin, A.; Pradas Del Real, A.; Sarret, G.; Mueller, C.R.; Kaegi, R. Transformation of Silver Nanoparticles in Sewage Sludge during Incineration. Environ. Sci. Technol. 2016, 50, 3503–3510. [Google Scholar] [CrossRef]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Modeling nanosilver transformations in freshwater sediments. Environ. Sci. Technol. 2013, 47, 12920–12928. [Google Scholar] [CrossRef]
- Chambers, B.A.; Afrooz, A.R.M.N.; Bae, S.; Aich, N.; Katz, L.; Saleh, N.B.; Kirisits, M.J. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ. Sci. Technol. 2014, 48, 761–769. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical transformations of nanosilver in biological environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar] [CrossRef]
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Lombi, E.; Dasilva, Y.A.R.; Nowack, B. Unraveling the Complexity in the Aging of Nanoenhanced Textiles: A Comprehensive Sequential Study on the Effects of Sunlight and Washing on Silver Nanoparticles. Environ. Sci. Technol. 2016, 50, 5790–5799. [Google Scholar] [CrossRef]
- Hedberg, J.; Skoglund, S.; Karlsson, M.E.; Wold, S.; Odnevall Wallinder, I.; Hedberg, Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Environ. Sci. Technol. 2014, 48, 7314–7322. [Google Scholar] [CrossRef]
- Kulthong, K.; Srisung, S.; Boonpavanitchakul, K.; Kangwansupamonkon, W.; Maniratanachote, R. Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fibre Toxicol. 2010, 7, 8. [Google Scholar] [CrossRef]
- Lorenz, C.; Windler, L.; von Goetz, N.; Lehmann, R.P.; Schuppler, M.; Hungerbühler, K.; Heuberger, M.; Nowack, B. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere 2012, 89, 817–824. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Y. Effects of silver nanoparticles on wastewater biofilms. Water Res. 2011, 45, 6039–6050. [Google Scholar] [CrossRef]
- Hou, L.; Li, K.; Ding, Y.; Li, Y.; Chen, J.; Wu, X.; Li, X. Removal of silver nanoparticles in simulated wastewater treatment processes and its impact on COD and NH 4 reduction. Chemosphere 2012, 87, 248–252. [Google Scholar] [CrossRef]
- García, A.; Delgado, L.; Torà, J.A.; Casals, E.; González, E.; Puntes, V.; Font, X.; Carrera, J.; Sánchez, A. Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. J. Hazard. Mater. 2012, 199–200, 64–72. [Google Scholar] [CrossRef]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wick, P. Human health risk of ingested nanoparticles that are added as multifunctional agents to paints: An in vitro study. PLoS ONE 2013, 8, e83215. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef]
- Martin, L.; Kelso, G. Use of Biosolids in Agriculture. In Primefacts; NSW DPI: Sydney, Australia, 2009; Volume 859. [Google Scholar]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Kim, B.; Park, C.S.; Murayama, M.; Hochella, M.F. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ. Sci. Technol. 2010, 44, 7509–7514. [Google Scholar] [CrossRef]
- Karande, V.S.; Bharimalla, A.K.; Vigneshwaran, N.; Kadam, P.G.; Mhaske, S.T. Cotton linter nano-fibers as the potential reinforcing agent for guar gum. Iran. Polym. J. 2014, 23, 869–879. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.; Varadarajan, P.; Nachane, R.; Balasubramanya, R. Functional Finishing of Cotton Fabrics Using Silver Nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 1893–1897. [Google Scholar] [CrossRef]
- Mwilu, S.K.; Siska, E.; Baig, R.B.N.; Varma, R.S.; Heithmar, E.; Rogers, K.R. Separation and measurement of silver nanoparticles and silver ions using magnetic particles. Sci. Total Environ. 2014, 472, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Nanotex, L.L.C. Nanoparticle-Based Permanent Treatments for Textiles. U.S. Patent 6607994B2, 18 August 2003. [Google Scholar]
- Geganken, A.; Nitzan, Y.; Perelshtein, I.; Perkas, N.; Applerot, G. Sonochemical Coating of Textiles with Metal Oxide Nanoparticles for Antimicrobial Fabrics. EP Patent 2294260A1, 16 March 2011. [Google Scholar]
- Eastern Research Group. State of the Science Literature Review: Nano Titanium Dioxide Environmental Matters; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Kapo, K.E.; Paschka, M.; Vamshi, R.; Sebasky, M.; McDonough, K. Estimation of U.S. sewer residence time distributions for national-scale risk assessment of down-the-drain chemicals. Sci. Total Environ. 2017, 603–604, 445–452. [Google Scholar] [CrossRef]
- Fox, B.S.; Beyer, M.K.; Bondybey, V.E. Coordination chemistry of silver cations. J. Am. Chem. Soc. 2002, 124, 13613–13623. [Google Scholar] [CrossRef]
- Hu, P.; Hou, D.; Huang, Y.; Hu, X.; Chen, C. Biomaterial-assisted synthesis of AgCl@Ag concave cubes with efficient visible-light-driven photocatalytic activity. CrystEngComm 2013, 16, 649–653. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron Spectros. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. The behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Impellitteri, C.A.; Tolaymat, T.M.; Scheckel, K.G. The Speciation of Silver Nanoparticles in Antimicrobial Fabric Before and After Exposure to a Hypochlorite/Detergent Solution. J. Environ. Qual. 2009, 38, 1528–1530. [Google Scholar] [CrossRef]
- Hemraj-Benny, T.; Banerjee, S.; Sambasivan, S.; Balasubramanian, M.; Fischer, D.A.; Eres, G.; Puretzky, A.A.; Geohegan, D.B.; Lowndes, D.H.; Han, W.; et al. Near-Edge X-ray Absorption Fine Structure Spectroscopy as a Tool for Investigating Nanomaterials. Small 2006, 2, 26–35. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Huang, Y.; Keller, A.A. Incidence and persistence of silver nanoparticles throughout the wastewater treatment process. Water Res. 2019, 156, 188–198. [Google Scholar] [CrossRef]
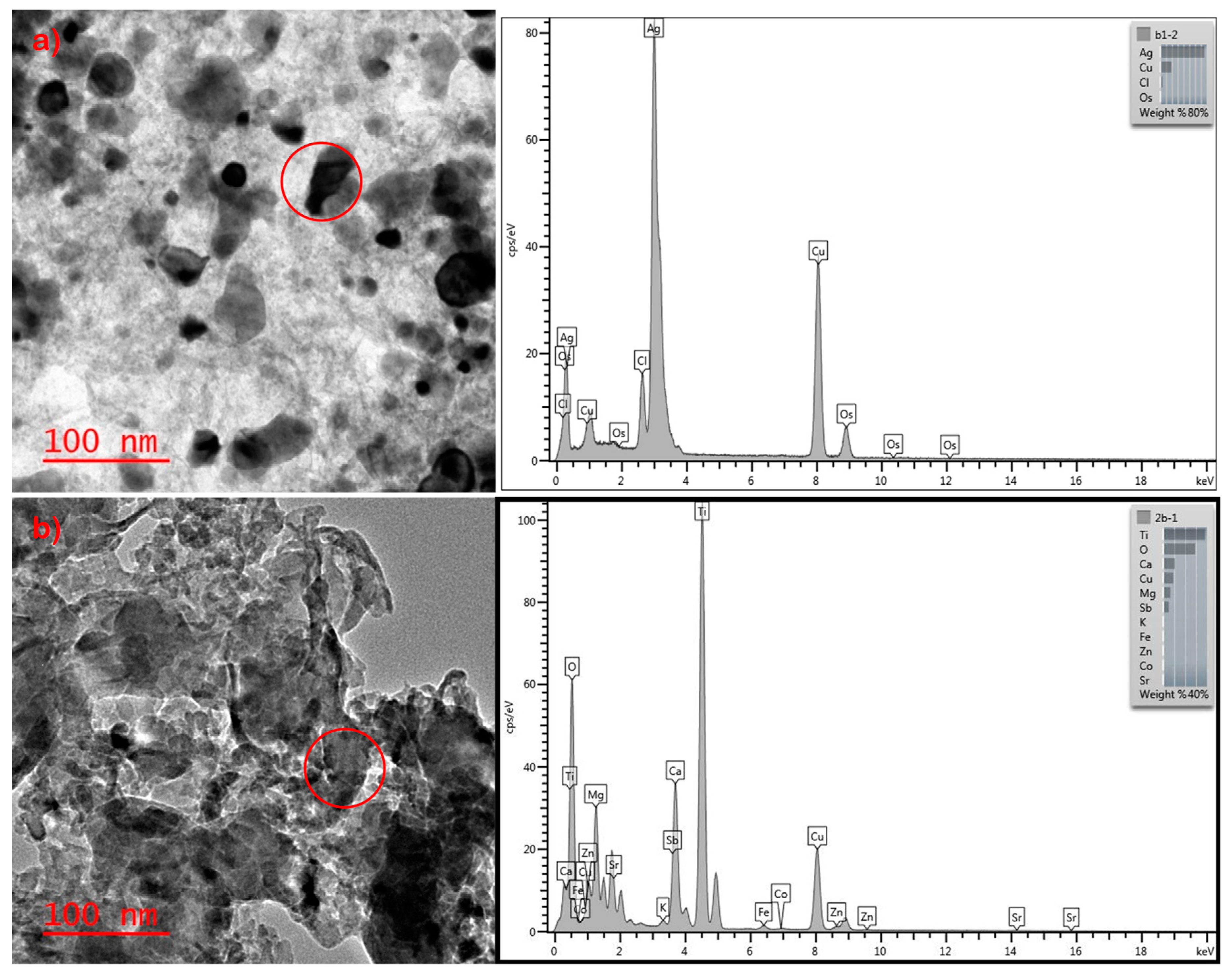

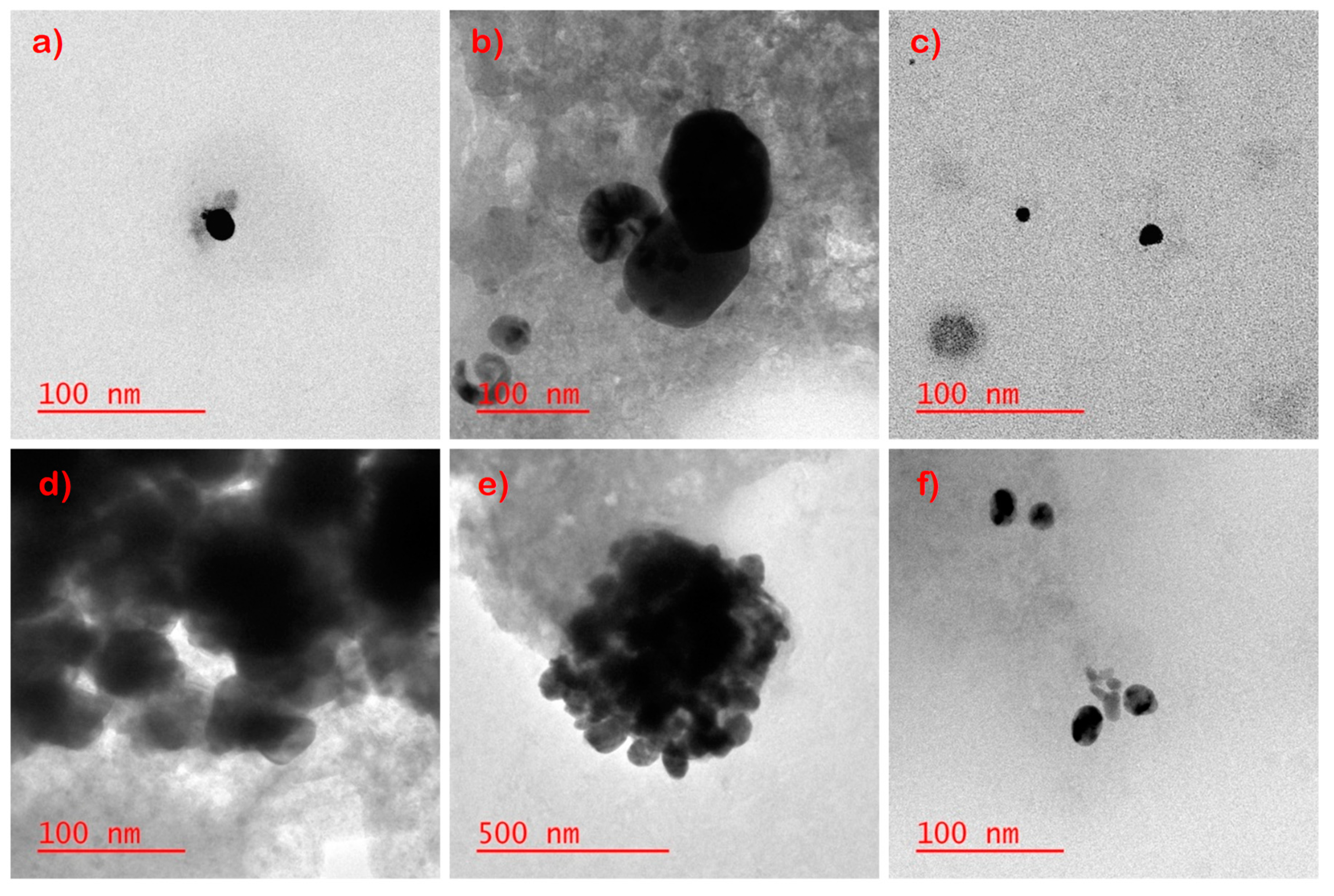
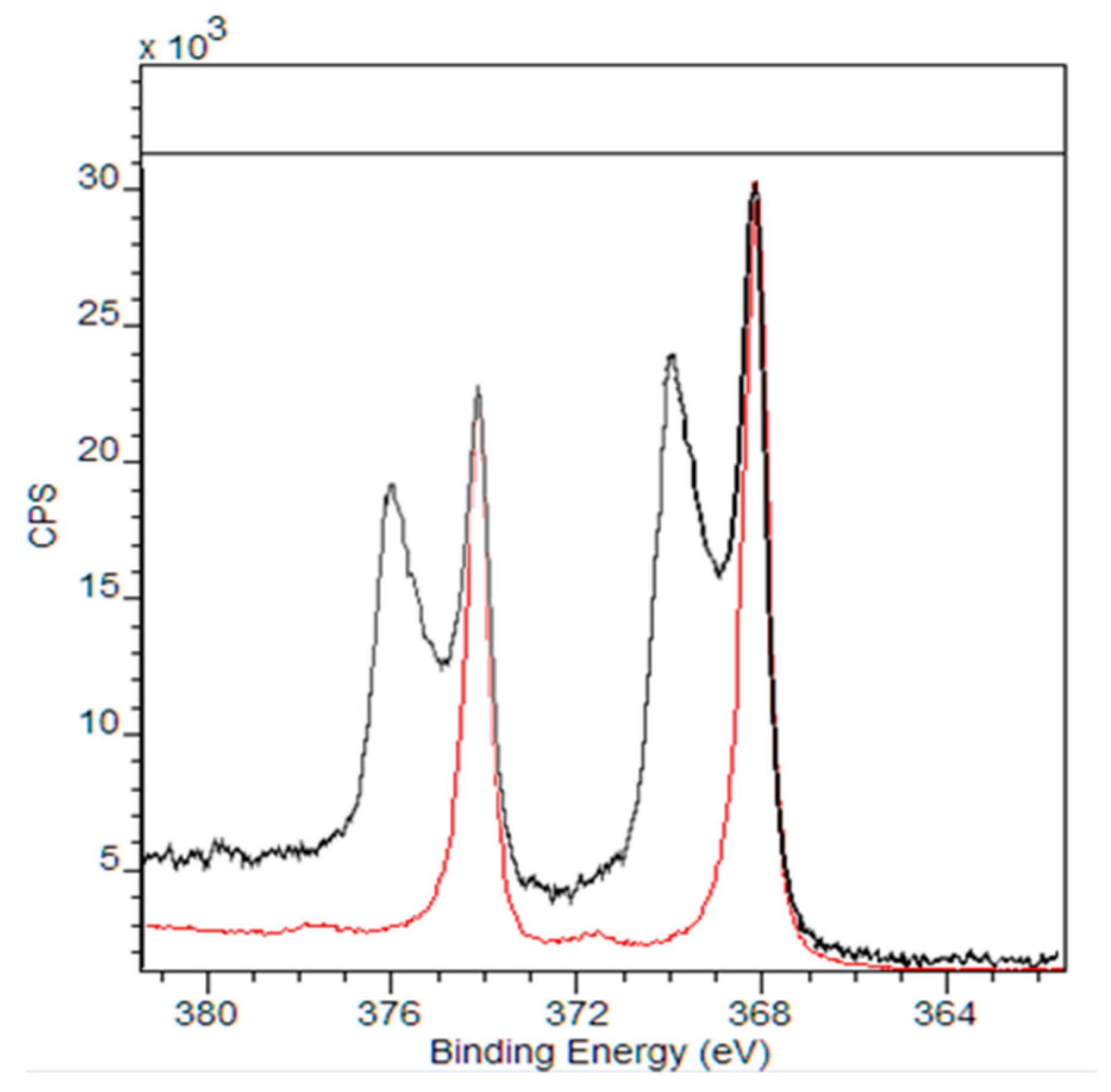
| Sample | mg/g of Sock |
|---|---|
| Commercial socks | 0.0063 ± 0.0040 |
| Laboratory-prepared socks | 2.84 ± 0.47 |
| Ag Concentration | |||
|---|---|---|---|
| Day-To-Day Use | Sweat | Bleach | Detergent |
| Commercial socks (ng/L) (± 5 ng/L) | 202 | 5 | 158 |
| Laboratory-prepared socks (mg/L) (± 10 mg/L) | 441 | 564 | 620 |
| Samples | Conditions | Changes | ||
|---|---|---|---|---|
| Agglomeration [23] | Aggregation [23] | Elemental Association | ||
| Laboratory-prepared Socks | Sweat | No | No | Cl, S |
| Bleach | Yes | Yes | Cl | |
| Detergent | No | No | Cl, S | |
| Sweat + Wastewater | Yes | Yes | Cl, S | |
| Bleach + Wastewater | Yes | Yes | Cl, S | |
| Detergent + Wastewater | Yes | Yes | Cl, S | |
| Pristine Nanoparticles | Sweat | No | Yes | Cl |
| Bleach | Yes | Yes | Cl | |
| Detergent | No | Yes | Cl, S | |
| Sweat + Wastewater | Yes | Yes | Cl, S | |
| Bleach + Wastewater | Yes | Yes | Cl, S | |
| Detergent + Wastewater | Yes | Yes | Cl, S | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, S.; Princz, J.; Ormeci, B.; DeRosa, M.C. Morphological Transformation of Silver Nanoparticles from Commercial Products: Modeling from Product Incorporation, Weathering through Use Scenarios, and Leaching into Wastewater. Nanomaterials 2019, 9, 1258. https://doi.org/10.3390/nano9091258
Mohan S, Princz J, Ormeci B, DeRosa MC. Morphological Transformation of Silver Nanoparticles from Commercial Products: Modeling from Product Incorporation, Weathering through Use Scenarios, and Leaching into Wastewater. Nanomaterials. 2019; 9(9):1258. https://doi.org/10.3390/nano9091258
Chicago/Turabian StyleMohan, Selvan, Juliska Princz, Banu Ormeci, and Maria C. DeRosa. 2019. "Morphological Transformation of Silver Nanoparticles from Commercial Products: Modeling from Product Incorporation, Weathering through Use Scenarios, and Leaching into Wastewater" Nanomaterials 9, no. 9: 1258. https://doi.org/10.3390/nano9091258






