Preparation of CuCrO2 Hollow Nanotubes from an Electrospun Al2O3 Template
Abstract
:1. Introduction
2. Materials and Methods
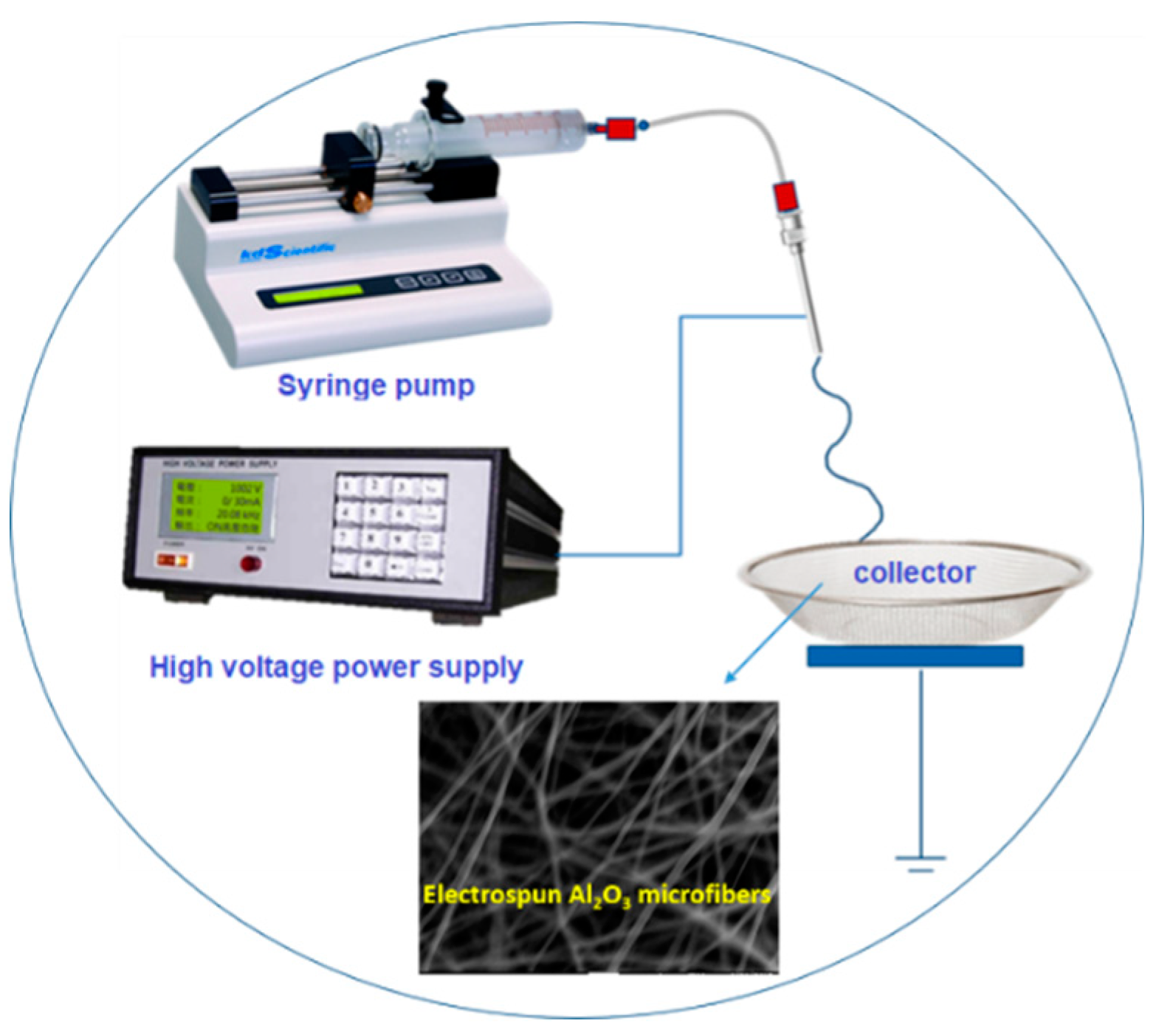
2.1. Preparation of CuCrO2 Hollow Nanotube
2.2. Characterization
3. Results
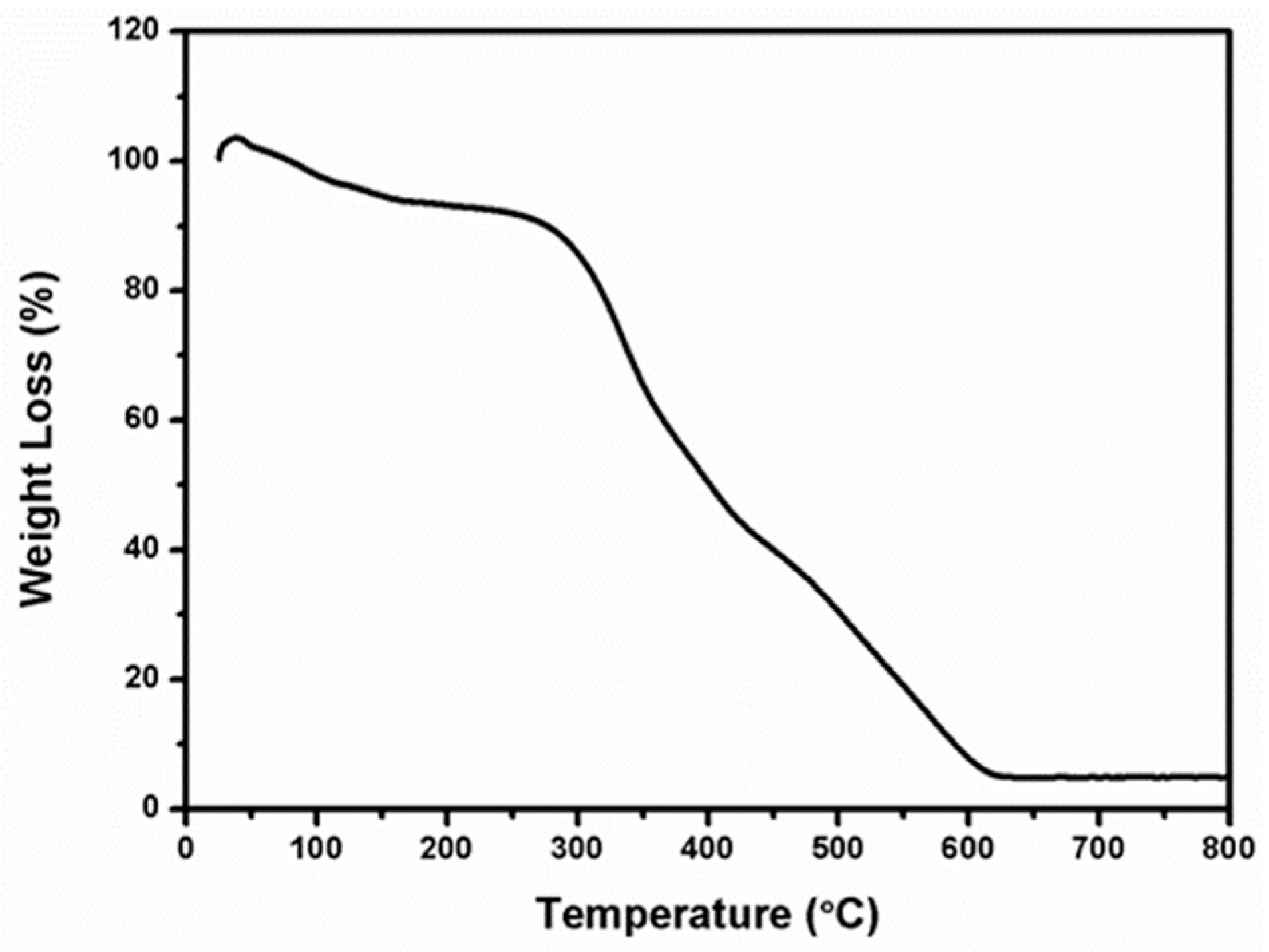
3.1. TGA Analysis
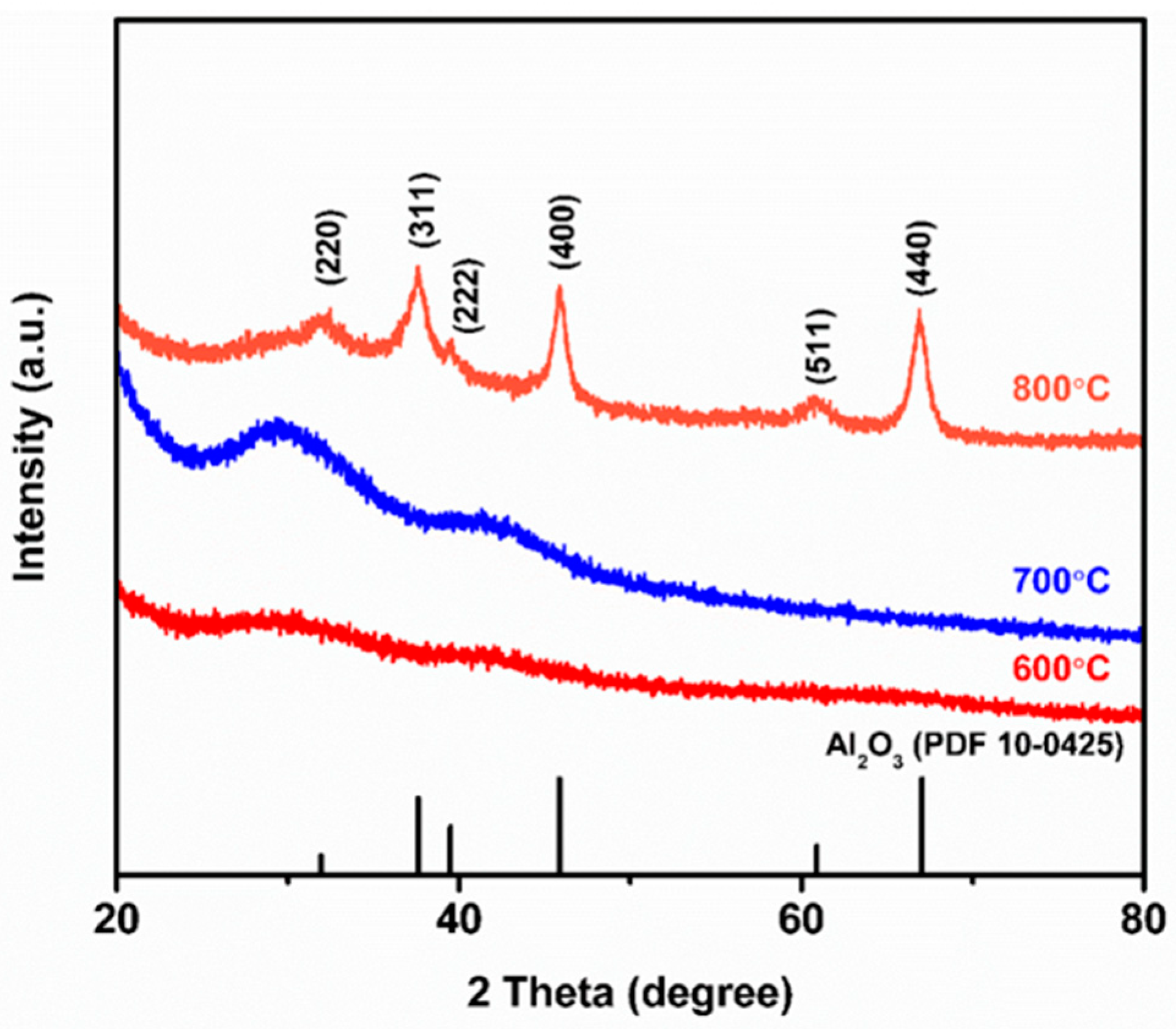
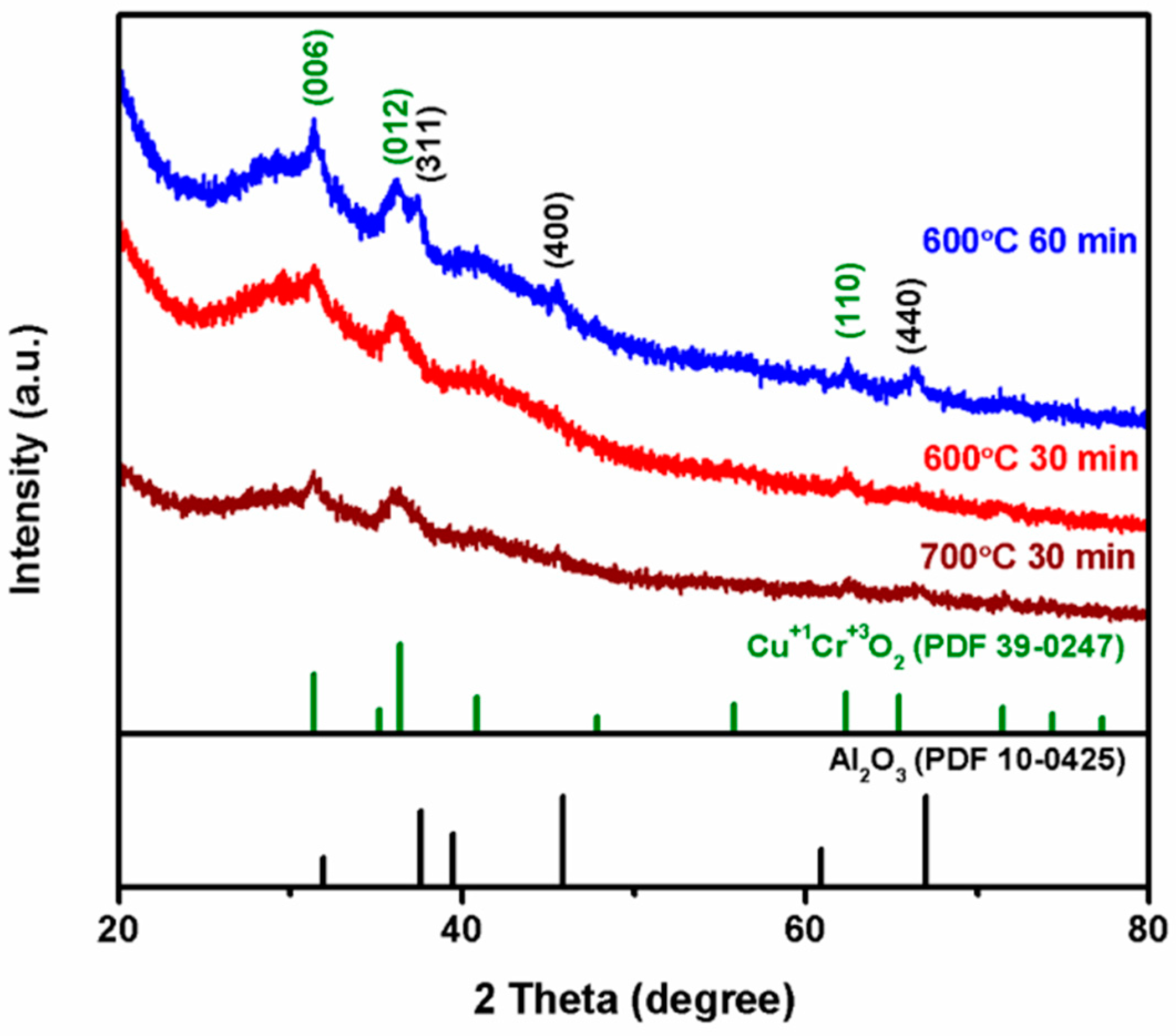
3.2. X-ray Diffraction Investigation
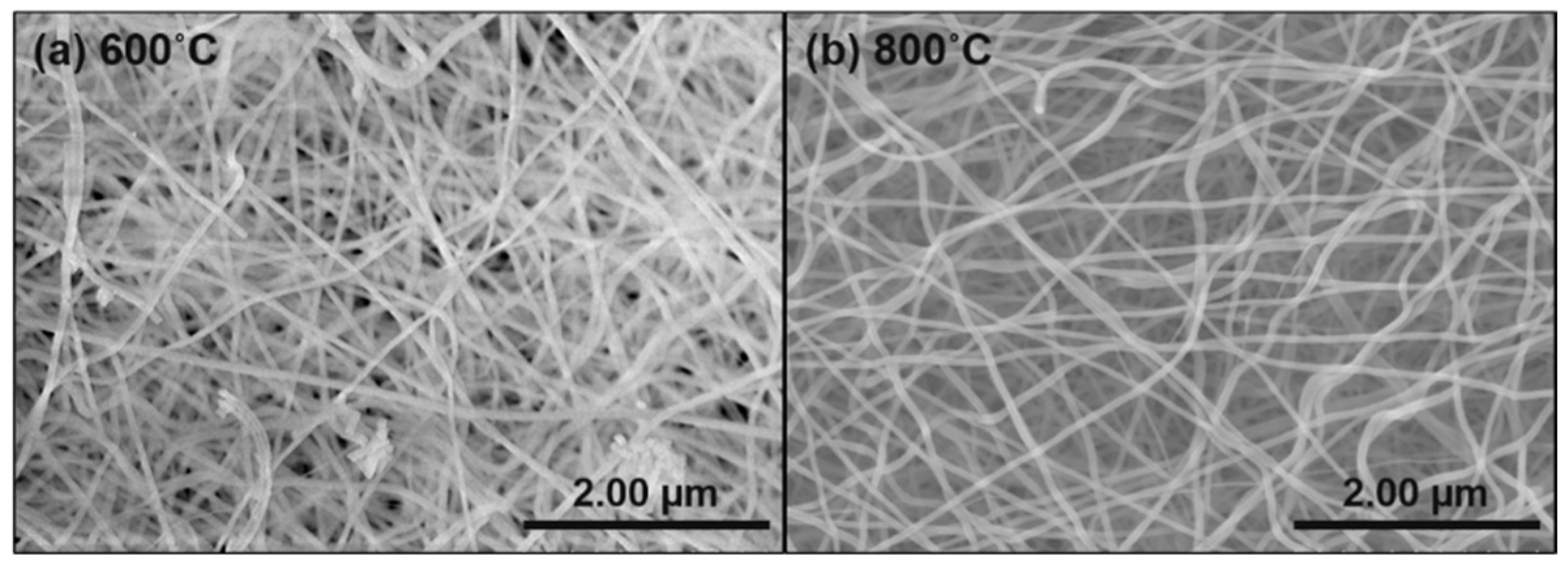
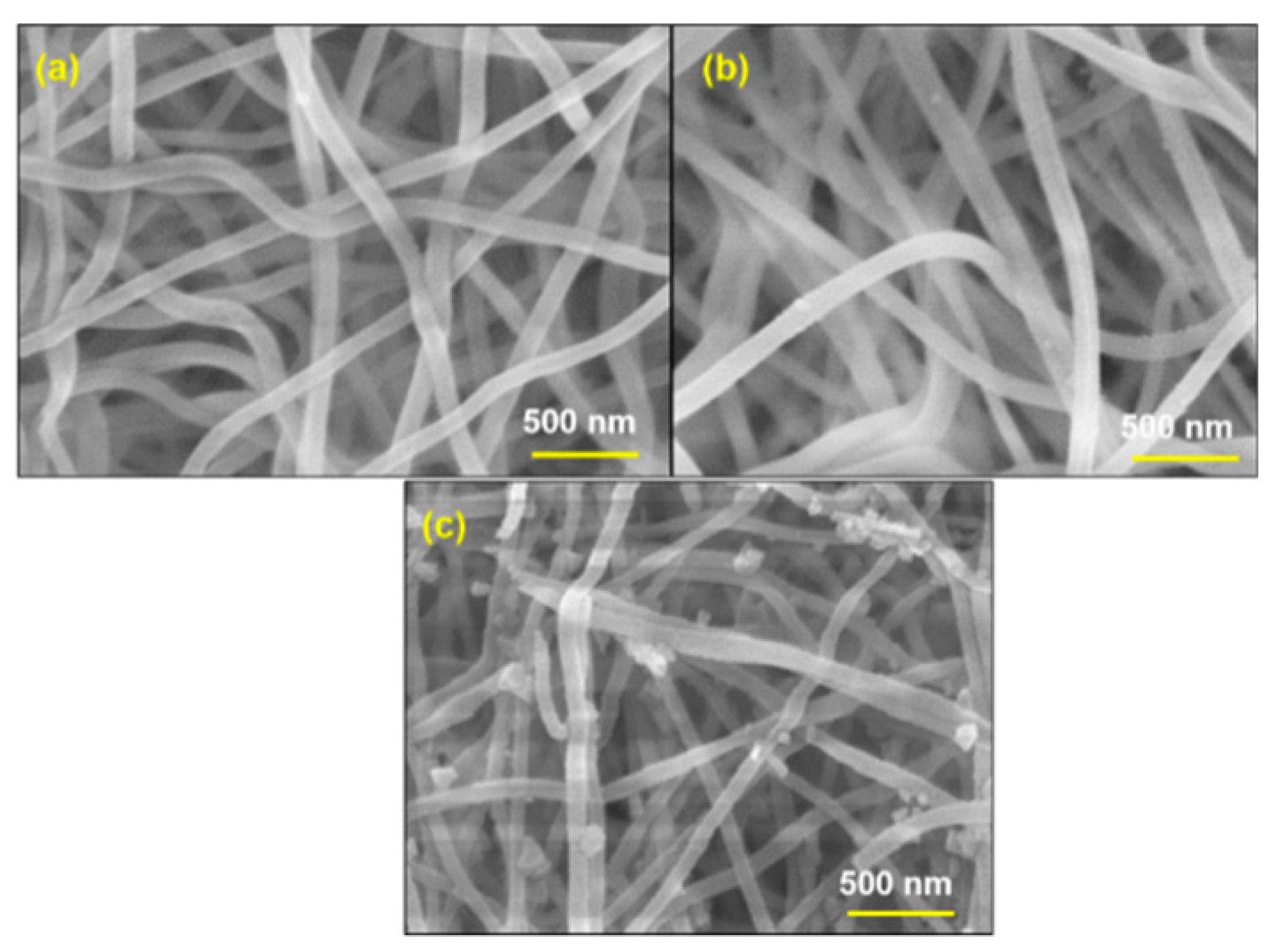
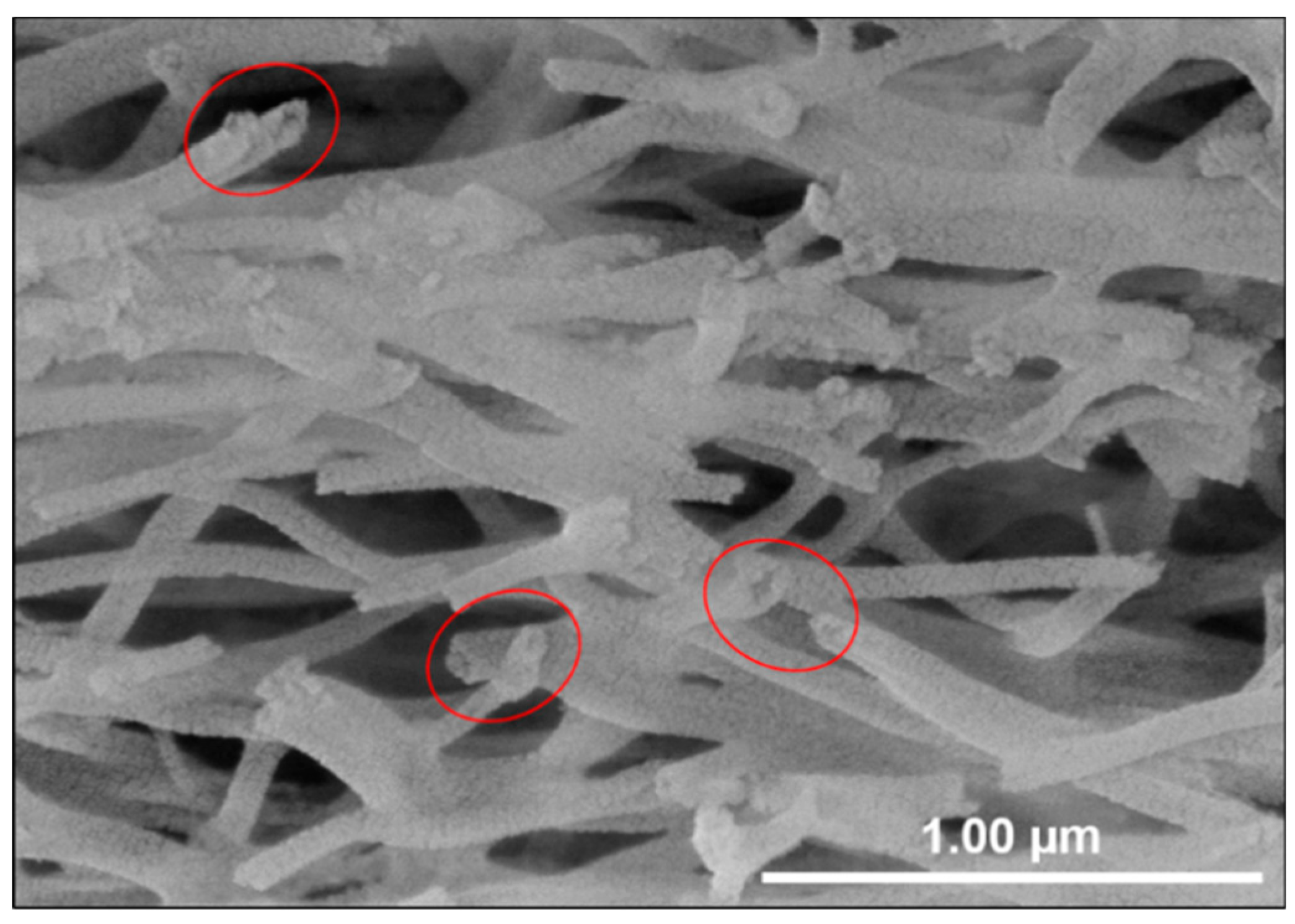
3.3. SEM Analysis
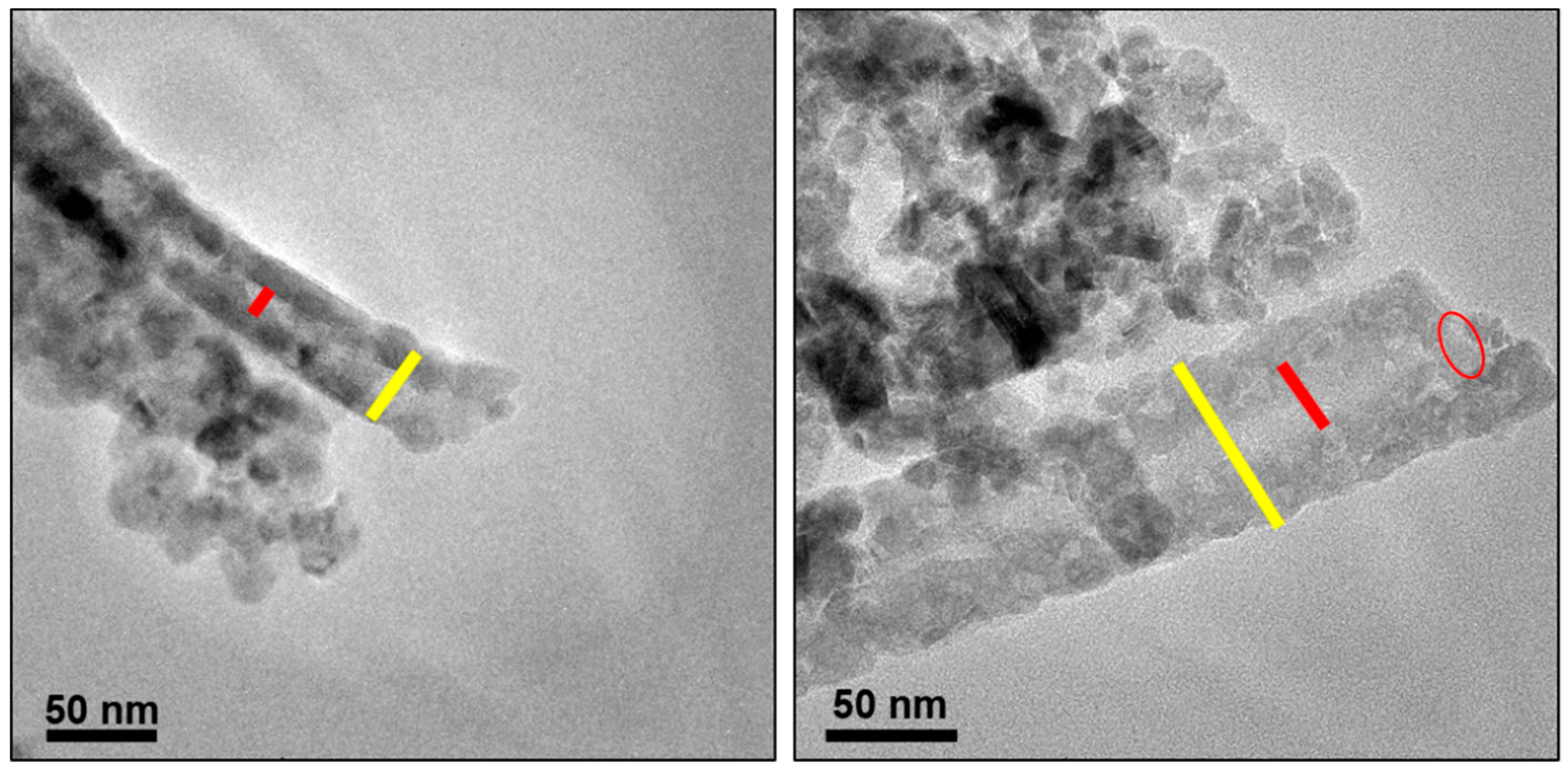
3.4. TEM Analysis
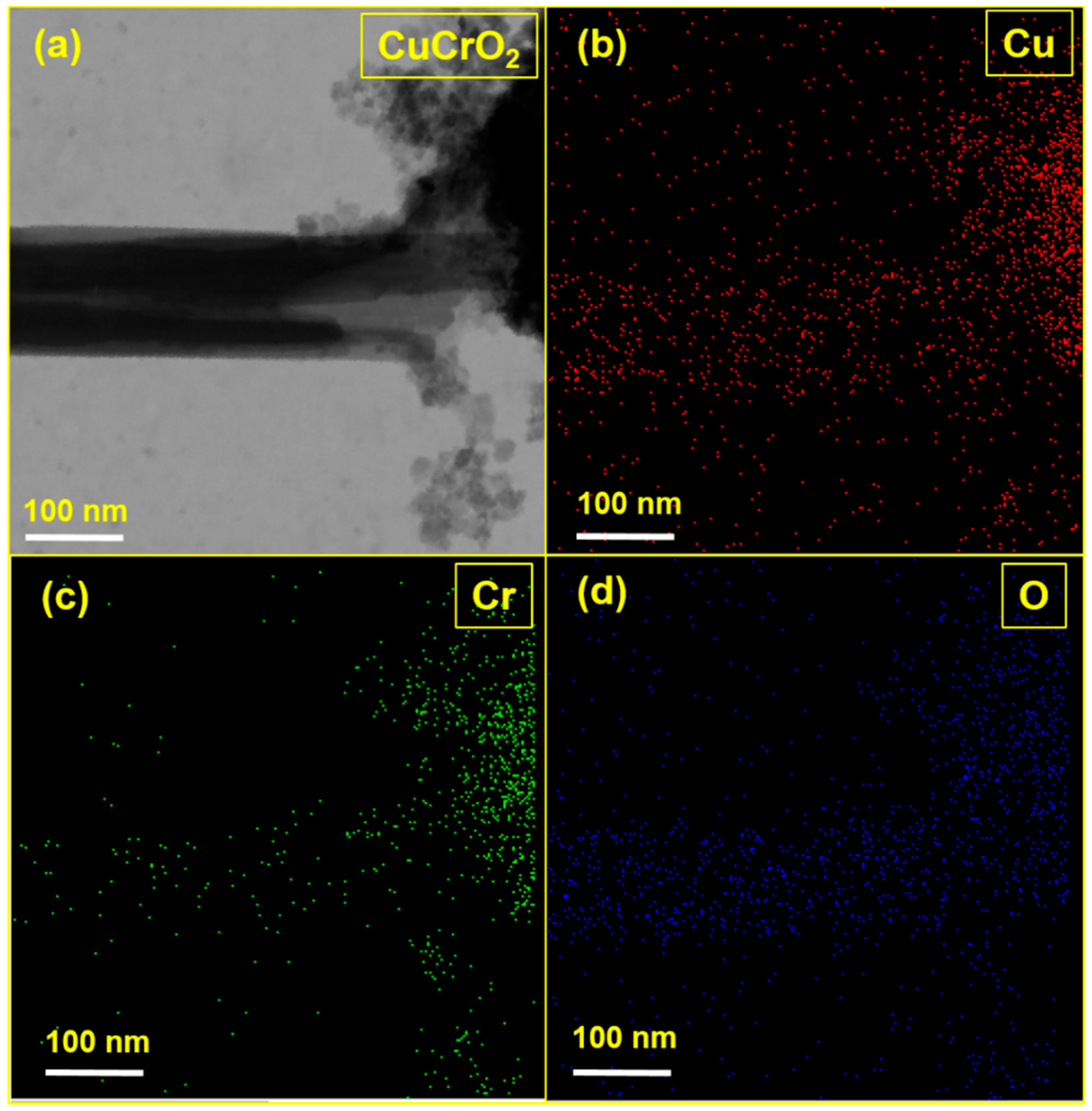
3.5. STEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, J.Y.; Zhong, L.; Wang, C.M.; Sullivan, J.P.; Xu, W.; Zhang, L.Q.; Mao, S.X.; Hudak, N.S.; Liu, X.H.; Subramanian, A.; et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 2010, 330, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Lin, G.; Zhang, Y.; Gunawan, P.; Xu, R. A new approach to synthesize uniform metal oxide hollow nanospheres via controlled precipitation. Nanotechnology 2007, 18, 355602–355608. [Google Scholar] [CrossRef]
- Zhan, G.; Zeng, H.C. General strategy for preparation of carbon-nanotube-supported nanocatalysts with hollow cavities and mesoporous shells. Chem. Mater. 2015, 27, 726–734. [Google Scholar] [CrossRef]
- Choi, S.W.; Park, J.Y.; Kim, S.S. Growth behavior and sensing properties of nanograins in CuO nanofibers. Chem. Eng. J. 2011, 172, 550–556. [Google Scholar]
- Chiu, T.W.; Tu, C.H.; Chen, Y.T. Fabrication of electrospun CuCr2O4 fibers. Ceram. Int. 2015, 41, S399–S406. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Acik, M.; Baristiran, C.; Sonmez, G. Highly surfaced polypyrrole nano-networks and nano-fibers. J. Mater. Sci. 2006, 41, 4678–4683. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; Ning, L.; He, H.; Jia, J.; Zhang, L.; Li, X. Synthesis and optical properties of Mg-doped ZnO nanofibers prepared by electrospinning. J. Alloys Compd. 2010, 507, 97–100. [Google Scholar] [CrossRef]
- Wang, J.; Daunis, T.B.; Cheng, L.; Zhang, B.; Kim, J.; Hsu, J.W.P. Combustion synthesis of p-type transparent conducting CuCrO2+x and Cu: CrOx thin films at 180 °C. ACS Appl. Mater. Interfaces 2018, 10, 3732–3738. [Google Scholar] [CrossRef]
- Shohl, W.A.D.; Daunis, T.B.; Wang, X.; Wang, J.; Zhang, B.; Barrera, D.; Yan, Y.; Hsu, J.W.P.; Mitzi, D.B. Room-temperature fabrication of a delafossite CuCrO2 hole transport layer for perovskite solar cells. J. Mater. Chem. A 2018, 6, 469–477. [Google Scholar] [CrossRef]
- Chiu, T.W.; Yang, Y.C.; Yeh, A.C.; Wang, Y.P.; Feng, Y.W. Antibacterial property of CuCrO2 thin films prepared by RF magnetron sputtering deposition. Vacuum 2013, 87, 174–177. [Google Scholar] [CrossRef]
- Tong, B.; Deng, Z.; Xu, B.; Meng, G.; Shao, J.; Liu, H.; Dai, T.; Shan, X.; Dong, W.; Wang, S.; et al. Oxygen vacancy defects boosted high performance p-Type delafossite CuCrO2 gas sensors. ACS Appl. Mater. Interfaces 2018, 10, 34727–34734. [Google Scholar] [CrossRef] [PubMed]
- Sathiskumar, P.S.; Thomas, C.R.; Madras, G. Solution combustion synthesis of nanosized copper chromite and its use as a burn rate modifier in solid propellants. Ind. Eng. Chem. Res. 2012, 51, 10108–10116. [Google Scholar] [CrossRef]
- Chiu, T.W.; Hong, R.T.; Yu, B.S.; Huang, Y.H.; Kameoka, S.; Tsai, A.P. Improving steam-reforming performance by nanopowdering CuCrO2. Int. J. Hydrogen Energy 2014, 39, 14222–14226. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Sakthinathan, S.; Chiu, T.W.; Chuang, C.H.; Fu, Y.; Yu, B.S. Production of hydrogen from steam reforming of methanol carried out by self-combusted CuCr1-xFexO2 (x = 0–1) nanopowders catalyst. Int. J. Hydrogen Energy 2019, 44, 2848–2856. [Google Scholar] [CrossRef]
- Amrute, A.P.; Larrazabal, G.O.; Mondelli, C.; Ramirez, J.P. CuCrO2 delafossite: A stable copper catalyst for chlorine production. Angew. Chem. Int. Ed. 2013, 52, 9772–9775. [Google Scholar] [CrossRef]
- Fang, M.; He, H.; Lu, B.; Zhang, W.; Zhao, B.; Ye, Z.; Huang, J. Optical properties of p-type CuAlO2 thin film grown by rf magnetron sputtering. Appl. Surf. Sci. 2011, 257, 8330–8333. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J. Transparent conductive CuFeO2 thin films prepared by sol gel processing. Appl. Surf. Sci. 2012, 258, 4844–4847. [Google Scholar] [CrossRef]
- Ueda, K.; Hase, T.; Yanagi, H.; Kawazoe, H.; Hosono, H.; Ohta, H.; Orita, M.; Hirano, M. Epitaxial growth of transparent p-type conducting CuGaO2 thin films on sapphire (001) substrates by pulsed laser deposition. J. Appl. Phys. 2001, 89, 1790–1793. [Google Scholar] [CrossRef]
- Lee, J.; Heo, Y.; Lee, J.; Kim, J. Growth of CuInO2 thin fi lm using highly dense Cu2O-In2O3 composite targets. Thin Solid Films 2009, 518, 1234–1237. [Google Scholar] [CrossRef]
- Liu, F.; Makino, T.; Hiraga, H.; Fukumura, T.; Kong, Y.; Kawasaki, M. Ultrafast dynamics of excitons in delafossite CuScO2 thin films. Appl. Phys. Lett. 2010, 96, 211904. [Google Scholar] [CrossRef]
- Lim, W.T.; Sadik, P.W.; Norton, D.P.; Pearton, S.J.; Ren, F. Dry etching of CuCrO2 thin films. Appl. Surf. Sci. 2008, 254, 2359–2363. [Google Scholar] [CrossRef]
- Chiu, T.W.; Shih, J.H.; Chang, C.H. Preparation and properties of CuCr1-xFexO2 thin films prepared by chemical solution deposition with two-step annealing. Thin Solid Films 2016, 618, 151–158. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Wang, T.; Shi, W. Structural, optical and electrical properties of Mg-doped CuCrO2 thin films by sol gel processing. J. Alloys Compd. 2011, 509, 5897–5902. [Google Scholar] [CrossRef]
- Nagarajan, R.; Draeseke, A.D.; Sleight, A.W.; Tate, J. p-type conductivity in CuCr1-xMgxO2 films and powders. J. Appl. Phys. 2001, 89, 8022–8025. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Saadi, S.; Bouguelia, A.; Trari, M. Photocatalytic hydrogen evolution over CuCrO2. Sol. Energy 2006, 80, 272–280. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-Xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Rushi, A.D.; Datta, K.P.; Ghosh, P.S.; Mulchandani, A.; Shirsat, M.D. Selective discrimination among benzene, toluene, and xylene: Probing metalloporphyrin-functionalized single-walled carbon nanotube-based field effect transistors. J. Phys. Chem. C 2014, 118, 24034–24041. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Shi, H.; Wu, Q.; Bu, X.; Yang, Y.; Zhang, J.; Huang, Y. MOF-derived CeO2/Au@SiO2 hollow nanotubes and their catalytic activity toward 4-nitrophenol reduction. New J. Chem. 2019, 43, 4581–4589. [Google Scholar] [CrossRef]
- Cho, N.G.; Woo, H.S.; Lee, J.H.; Kim, I.D. Thin-walled NiO tubes functionalized with catalytic Pt for highly selective C2H5OH sensors using electrospun fibers as a sacrificial template. Chem. Commun. 2011, 47, 11300–11302. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, T.; Yuan, F.; Zhu, Y. Preparation of hollow CuO@SiO2 spheres and its catalytic performances for the NO+CO and CO oxidation. Sci. Rep. 2015, 5, 9153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Williams, P.T. Carbon nanotubes and hydrogen production from the pyrolysis catalysis or catalytic-steam reforming of waste tyres. J. Anal. Appl. Pyrolysis 2016, 122, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Shang, Z.; Cui, K.; Zhang, H.; Qiao, Z.; Zhu, C.; Zhao, N.; Xu, J. Coaxial electrospinning core-shell fibers for self-healing scratch on coatings. Chin. Chem. Lett. 2019, 30, 157–159. [Google Scholar] [CrossRef]
- Chiu, T.W.; Yu, B.S.; Wang, Y.R.; Chen, K.T.; Lin, Y.T. Synthesis of nanosized CuCrO2 porous powders via a self-combustion glycine nitrate process. J. Alloys Compd. 2011, 509, 2933–2935. [Google Scholar] [CrossRef]
- Cetin, C.; Akyildiz, H. Production and characterization of CuCrO2 nanofibers. Mater. Chem. Phys. 2016, 70, 138–144. [Google Scholar] [CrossRef]
- Tan, Y.; Jia, Z.; Sun, J.; Wang, Y.; Cui, Z.; Guo, X. Controllable synthesis of hollow copper oxide encapsulated into N-doped carbon nanosheets as high-stability anodes for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 24139–24144. [Google Scholar] [CrossRef]
- Su, S.Y.; Wang, S.S.; Sakthinathan, S.; Chiu, T.W.; Park, J.H. Preparation of CuAl2O4 submicron tubes from electrospun Al2O3 fibers. Ceram. Int. 2019, 45, 1439–1442. [Google Scholar] [CrossRef]
- Chiu, T.W.; Chen, Y.T. Preparation of CuCrO2 nanowires by electrospinning. Ceram. Int. 2015, 41, S407–S413. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Synthesis of ultra-fine α-Al2O3 fibers via electrospinning method. Ceram. Int. 2011, 37, 2329–2333. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, S.J.; Kwak, D.H.; Jung, H.J.; Kim, T.Y.; Park, K.H.; Lee, J.W. Characterization and application of electrospun alumina nanofibers. Nanoscale Res. Lett. 2014, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Cheng, B.; Li, Q.; Zhuang, X.; Ren, Y. A new method for preparing alumina nanofibers by electrospinning technology. Text. Res. J. 2011, 81, 148–155. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.C.; Liu, C.; Dong, J.W.; Chow, P. Annealing of Al2O3 thin films prepared by atomic layer deposition. J. Phys. D Appl. Phys. 2007, 40, 3707–3713. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-J.; Fan, Y.-J.; Wang, S.-S.; Sakthinathan, S.; Chiu, T.-W.; Li, S.-S.; Park, J.-H. Preparation of CuCrO2 Hollow Nanotubes from an Electrospun Al2O3 Template. Nanomaterials 2019, 9, 1252. https://doi.org/10.3390/nano9091252
Wu H-J, Fan Y-J, Wang S-S, Sakthinathan S, Chiu T-W, Li S-S, Park J-H. Preparation of CuCrO2 Hollow Nanotubes from an Electrospun Al2O3 Template. Nanomaterials. 2019; 9(9):1252. https://doi.org/10.3390/nano9091252
Chicago/Turabian StyleWu, Hsin-Jung, Yu-Jui Fan, Sheng-Siang Wang, Subramanian Sakthinathan, Te-Wei Chiu, Shao-Sian Li, and Joon-Hyeong Park. 2019. "Preparation of CuCrO2 Hollow Nanotubes from an Electrospun Al2O3 Template" Nanomaterials 9, no. 9: 1252. https://doi.org/10.3390/nano9091252





